Abstract
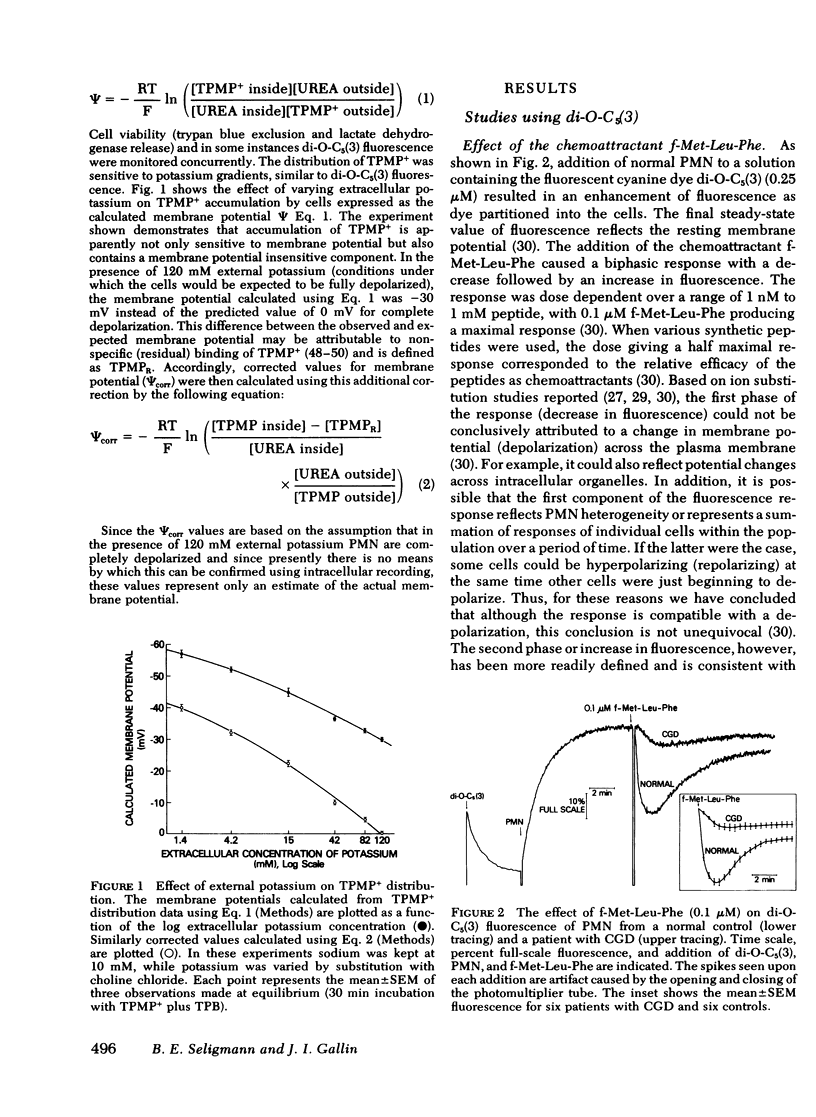
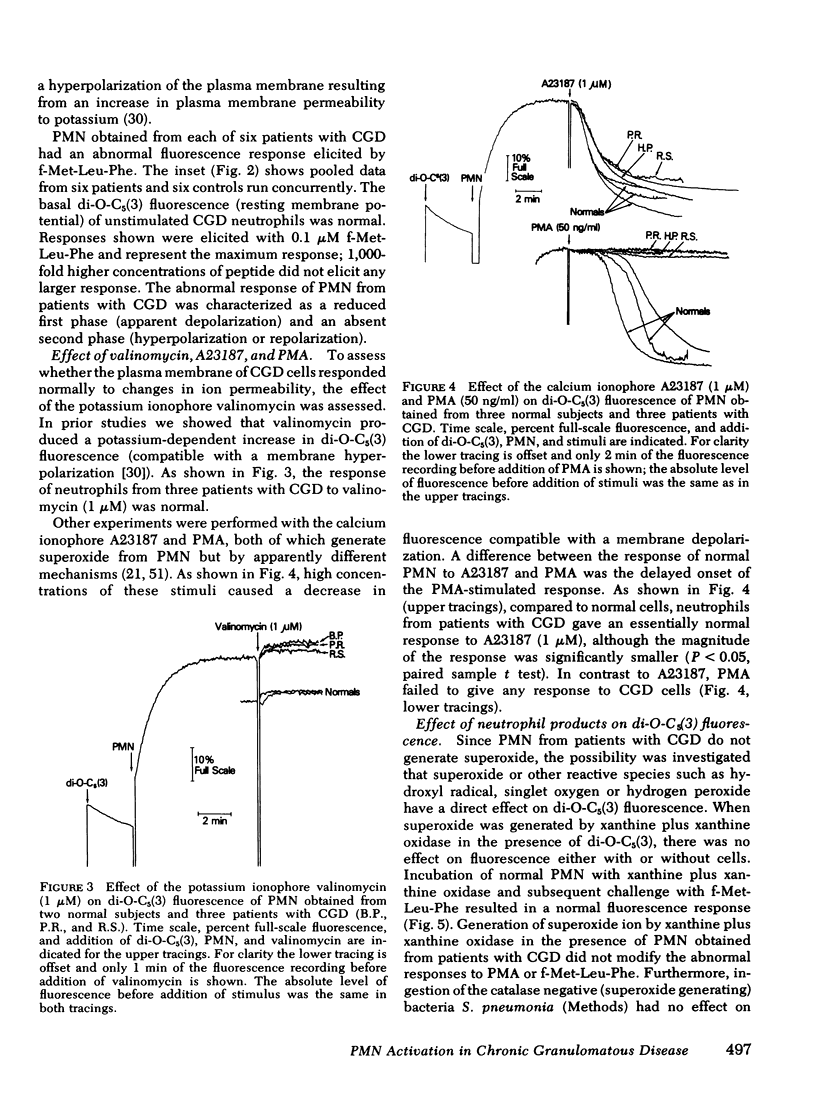
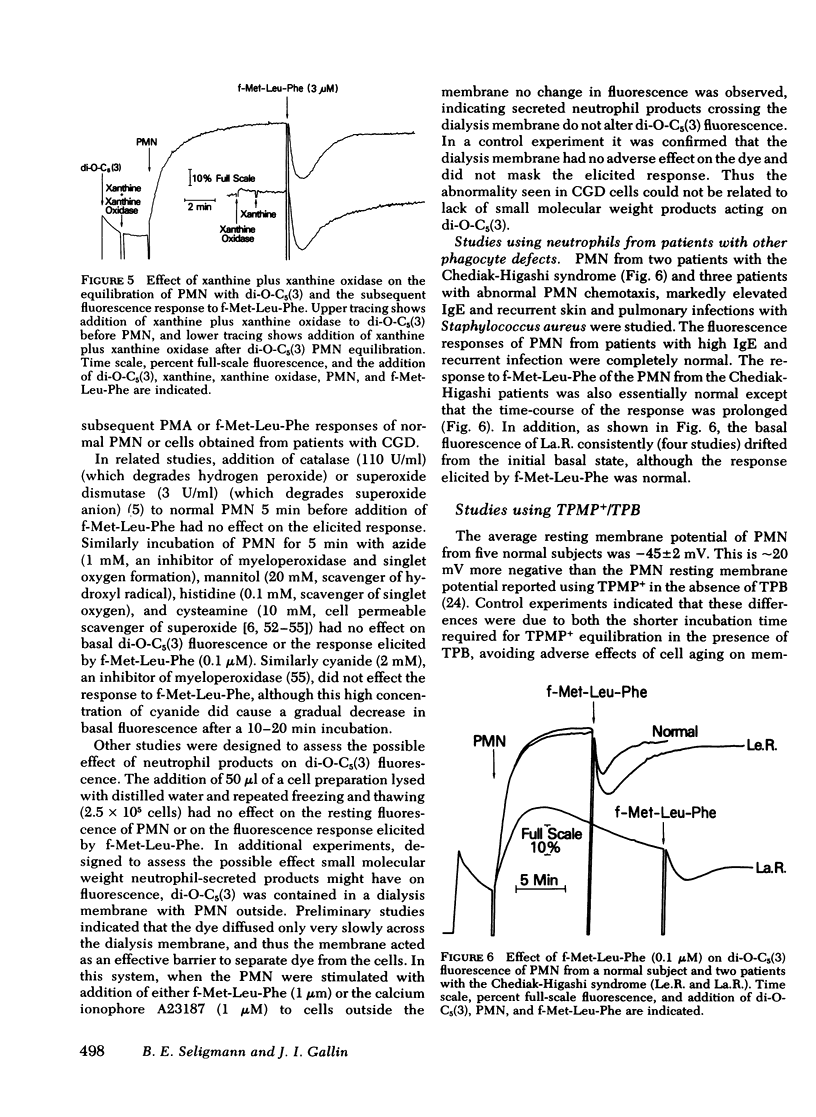
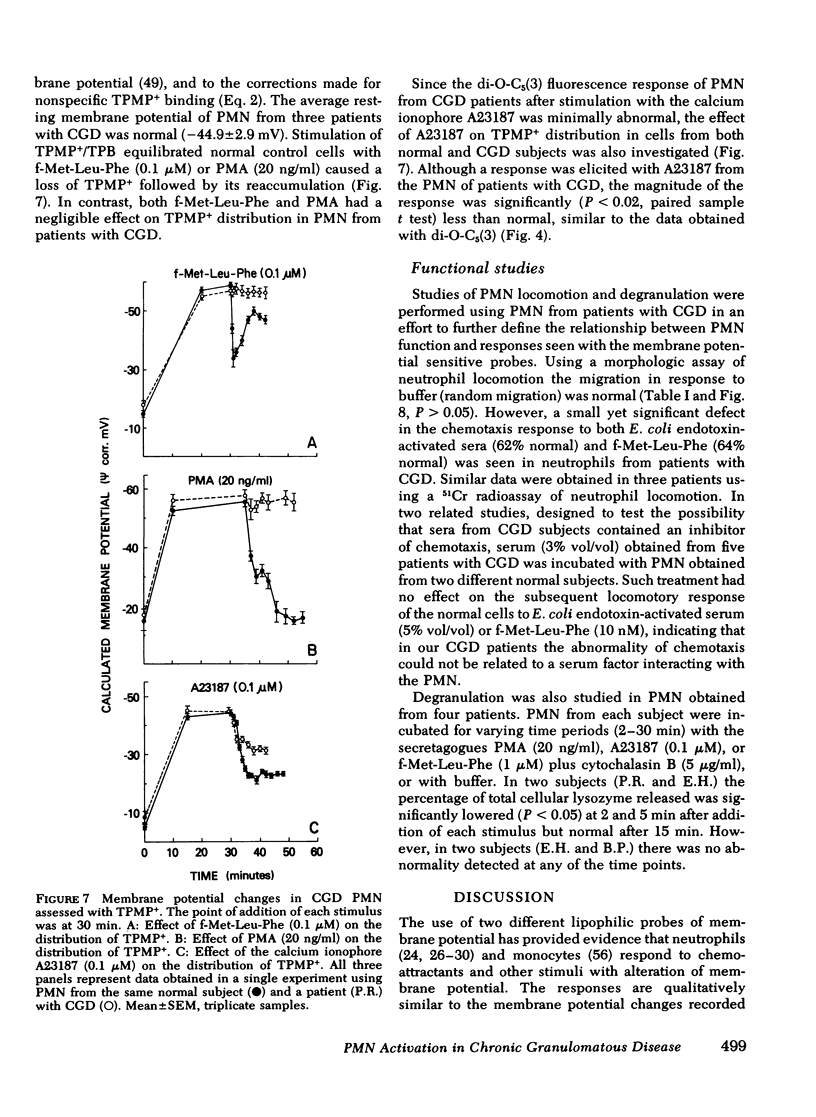
Previous studies using membrane potential sensitive probes have provided evidence that chemotactic factors elicit membrane potential changes in normal human neutrophils (PMN). In addition to stimulation of PMN motility, chemotactic factors also stimulate degranulation and superoxide ion (O-2) generation and it has been suggested that alteration of membrane potential activates these events (Korchak, H. M., and G. Weissmann. 1978. Proc, Natl, Acad, Sci. U. S. A. 75: 3818--3822). To further define the inter-relationship of these functions, studies were done with two indirect probes of membrane potential, 3-3'-dipentyloxacarbocyanine and triphenylmethylphosphonium ion (TPMP+) using PMN from normal subjects, from patients with abnormal O-2 production (chronic granulomatous disease [CGD]), and from patients with defective degranulation and/or chemotaxis (Cheddiak-Higashi syndrome and patients with elevated immunoglobulin (Ig)E and recurrent staphylococcal infections). The stimuli used were the chemoattractant N-formyl-methionyl-leucyl-phenylalanine (f-Met-Leu-Phe) and the secretagogues ionophore A23187 and phorbol myristate acetate (PMA). The results obtained with 3-3'-dipentyloxacarbocyanine and TPMP+ were comparable. The apparent membrane potential changes elicited by f-Met-Leu-Phe and PMA in normal PMN were reduced or entirely absent in PMN obtained from patients with CGD but normal in PMN from other patients. PMN from patients with CGD had normal calculated resting membrane potentials and normal responses elicited by the potassium ionophore valinomycin. The responses to calcium ionophore A23187 were only slightly impaired. The abnormality of the elicited response of CGD cells of f-Met-Leu-Phe and PMA could not be attributed to the absence of O-2, hydroxyl radical, singlet oxygen, or hydrogen peroxide acting on the probes. Instead this abnormality appears to be associated with a dysfunction in the normal molecular mechanism(s) stimulated upon neutrophil activation. The data suggest chemoattractant alteration of membrane potential in normal PMN is related to activation of oxidative metabolism but the relationship to chemotaxis and degranulation remains to be established.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERENDES H., BRIDGES R. A., GOOD R. A. A fatal granulomatosus of childhood: the clinical study of a new syndrome. Minn Med. 1957 May;40(5):309–312. [PubMed] [Google Scholar]
- Babior B. M., Curnutte J. T., McMurrich B. J. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J Clin Invest. 1976 Oct;58(4):989–996. doi: 10.1172/JCI108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Karnovsky M. J., Karnovsky M. L. Degranulation of leukocytes in chronic granulomatous disease. J Clin Invest. 1969 Jan;48(1):187–192. doi: 10.1172/JCI105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N Engl J Med. 1968 May 2;278(18):971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- Becker E. L., Sigman M., Oliver J. M. Superoxide production induced in rabbit polymorphonuclear leukocytes by synthetic chemotactic peptides and A23187. Am J Pathol. 1979 Apr;95(1):81–97. [PMC free article] [PubMed] [Google Scholar]
- Becker E. L., Talley V., Showell H. J., Naccache P. H., Sha'afi R. I. Activation of the rabbit polymorphonuclear leukocyte membrane "Na+, K+"-ATPase by chemotactic factor. J Cell Biol. 1978 May;77(2):329–333. doi: 10.1083/jcb.77.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume R. S., Wolff S. M. The Chediak-Higashi syndrome: studies in four patients and a review of the literature. Medicine (Baltimore) 1972 Jul;51(4):247–280. [PubMed] [Google Scholar]
- Boxer L. A., Yoder M., Bonsib S., Schmidt M., Ho P., Jersild R., Baehner R. L. Effects of a chemotactic factor, N-formylmethionyl peptide, on adherence, superoxide anion generation, phagocytosis, and microtubule assembly of human polymorphonuclear leukocytes. J Lab Clin Med. 1979 Mar;93(3):506–514. [PubMed] [Google Scholar]
- Bujak J. S., Ottesen E. A., Dinarello C. A., Brenner V. J. Nocardiosis in a child with chronic granulomatous disease. J Pediatr. 1973 Jul;83(1):98–100. doi: 10.1016/s0022-3476(73)80325-7. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cheson B. D., Curnette J. T., Babior B. M. The oxidative killing mechanisms of the neutrophil. Prog Clin Immunol. 1977;3:1–65. [PubMed] [Google Scholar]
- Chusid M. J., Gelfand J. A., Nutter C., Fauci A. S. Letter: Pulmonary aspergillosis, inhalation of contaminated marijuana smoke, chronic granulomatous disease. Ann Intern Med. 1975 May;82(5):682–683. doi: 10.7326/0003-4819-82-5-682. [DOI] [PubMed] [Google Scholar]
- Clark F. A., Klebanoff S. J. Chronic granulomatous disease: studies of a family with impaired neutrophil chemotactic, metabolic and bactericidal function. Am J Med. 1978 Dec;65(6):941–948. doi: 10.1016/0002-9343(78)90745-3. [DOI] [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide production by digitonin-stimulated guinea pig granulocytes. The effects of N-ethyl maleimide, divalent cations; and glycolytic and mitochondrial inhibitors on the activation of the superoxide generating system. J Clin Invest. 1978 Apr;61(4):1088–1096. doi: 10.1172/JCI109008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch C. J., Holian A., Holian S. K., Daniele R. P., Wilson D. F. Transmembrane electrical and pH gradients across human erythrocytes and human peripheral lymphocytes. J Cell Physiol. 1979 Apr;99(1):79–93. doi: 10.1002/jcp.1040990110. [DOI] [PubMed] [Google Scholar]
- Edelson P. J., Stites D. P., Gold S., Fudenberg H. H. Disorders of neutrophil function. Defects in the early stages of the phagocytic process. Clin Exp Immunol. 1973 Jan;13(1):21–28. [PMC free article] [PubMed] [Google Scholar]
- Gallin E. K., Gallin J. I. Interaction of chemotactic factors with human macrophages. Induction of transmembrane potential changes. J Cell Biol. 1977 Oct;75(1):277–289. doi: 10.1083/jcb.75.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin E. K., Wiederhold M. L., Lipsky P. E., Rosenthal A. S. Spontaneous and induced membrane hyperpolarizations in macrophages. J Cell Physiol. 1975 Dec;86 (Suppl 2)(3 Pt 2):653–661. doi: 10.1002/jcp.1040860510. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Bujak J. S., Patten E., Wolff S. M. Granulocyte function in the Chediak-Higashi syndrome of mice. Blood. 1974 Feb;43(2):201–206. [PubMed] [Google Scholar]
- Gallin J. I., Clark R. A., Kimball H. R. Granulocyte chemotaxis: an improved in vitro assay employing 51 Cr-labeled granulocytes. J Immunol. 1973 Jan;110(1):233–240. [PubMed] [Google Scholar]
- Gallin J. I., Rosenthal A. S. The regulatory role of divalent cations in human granulocyte chemotaxis. Evidence for an association between calcium exchanges and microtubule assembly. J Cell Biol. 1974 Sep;62(3):594–609. doi: 10.1083/jcb.62.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold S. B., Hanes D. M., Stites D. P., Fudenberg H. H. Abnormal kinetics of degranulation in chronic granulomatous disease. N Engl J Med. 1974 Aug 15;291(7):332–337. doi: 10.1056/NEJM197408152910704. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Hoffstein S. T., Weissmann G. Mechanisms of lysosomal enzyme release from human polymorphonuclear leukocytes. Effects of phorbol myristate acetate. J Cell Biol. 1975 Sep;66(3):647–652. doi: 10.1083/jcb.66.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Horn J. K., Kaplan H. B., Weissmann G. Calcium-induced lysozyme secretion from human polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1974 Sep 23;60(2):807–812. doi: 10.1016/0006-291x(74)90312-x. [DOI] [PubMed] [Google Scholar]
- Hladky S. B., Rink T. J. Potential difference and the distribution of ions across the human red blood cell membrane; a study of the mechanism by which the fluorescent cation, diS-C3-(5) reports membrane potential. J Physiol. 1976 Dec;263(2):287–319. doi: 10.1113/jphysiol.1976.sp011632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn D. C., Lehrer R. I. NADPH oxidase deficiency in X-linked chronic granulomatous disease. J Clin Invest. 1975 Apr;55(4):707–713. doi: 10.1172/JCI107980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Quie P. G., Windhorst D. B., Good R. A. Fatal granulomatous disease of childhood. An inborn abnormality of phagocytic function. Lancet. 1966 Jun 4;1(7449):1225–1228. doi: 10.1016/s0140-6736(66)90238-8. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Dinarello C. A., Henderson W. R., Gallin J. I. Stimulation of neutrophil oxygen-dependent metabolism by human leukocytic pyrogen. J Clin Invest. 1979 Oct;64(4):996–1002. doi: 10.1172/JCI109566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak H. M., Weissmann G. Changes in membrane potential of human granulocytes antecede the metabolic responses to surface stimulation. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3818–3822. doi: 10.1073/pnas.75.8.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDING B. H., SHIRKEY H. S. A syndrome of recurrent infection and infiltration of viscera by pigmented lipid histiocytes. Pediatrics. 1957 Sep;20(3):431–438. [PubMed] [Google Scholar]
- McPhail L. C., DeChatelet L. R., Johnston R. B., Jr Generation of chemiluminescence by a particulate fraction isolated from human neutrophils. Analysis of molecular events. J Clin Invest. 1979 Apr;63(4):648–655. doi: 10.1172/JCI109347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail L. C., DeChatelet L. R., Shirley P. S. Further characterization of NADPH oxidase activity of human polymorphonuclear leukocytes. J Clin Invest. 1976 Oct;58(4):774–780. doi: 10.1172/JCI108528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail L. C., DeChatelet L. R., Shirley P. S., Wilfert C., Johnston R. B., Jr, McCall C. E. Deficiency of NADPH oxidase activity in chronic granulomatous disease. J Pediatr. 1977 Feb;90(2):213–217. doi: 10.1016/s0022-3476(77)80632-x. [DOI] [PubMed] [Google Scholar]
- Moulder J. E., Lo P. S., Fischer J. J. Effect of the radioprotective drugs MEA, DMSO, and WR-2721 on tumor control and skin tolerance in the rat. Cancer Treat Rep. 1977 Aug;61(5):825–833. [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Transport of sodium, potassium, and calcium across rabbit polymorphonuclear leukocyte membranes. Effect of chemotactic factor. J Cell Biol. 1977 May;73(2):428–444. doi: 10.1083/jcb.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarca P., Dri P., Kakinuma K., Tedesco F., Rossi F. Studies on the mechanism of metabolic stimulation in polymorphonuclear leucocytes during phagocytosis. I. Evidence for superoxide anion involvement in the oxidation of NADPH2. Biochim Biophys Acta. 1975 Apr 7;385(2):380–386. doi: 10.1016/0304-4165(75)90367-0. [DOI] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Rosenthal A. S., Balestra D. J. Abnormal bactericidal, metabolic, and lysosomal functions of Chediak-Higashi Syndrome leukocytes. J Clin Invest. 1972 Mar;51(3):649–665. doi: 10.1172/JCI106854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligmann B. E., Gallin E. K., Martin D. L., Shain W., Gallin J. I. Interaction of chemotactic factors with human polymorphonuclear leukocytes: studies using a membrane potential-sensitive cyanine dye. J Membr Biol. 1980;52(3):257–272. doi: 10.1007/BF01869194. [DOI] [PubMed] [Google Scholar]
- Seligmann B., Gallin J. I. Secretagogue modulation of the response of human neutrophils to chemoattractants: studies with a membrane potential sensitive cyanine dye. Mol Immunol. 1980 Feb;17(2):191–200. doi: 10.1016/0161-5890(80)90071-1. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Chemotactic factor-induced generation of superoxide radicals by human neutrophils: evidence for the role of sodium. J Immunol. 1979 Nov;123(5):2428–2435. [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Generation of superoxide radicals by human peripheral neutrophils activated by chemotactic factor. Evidence for the role of calcium. J Lab Clin Med. 1979 Apr;93(4):583–593. [PubMed] [Google Scholar]
- Thoene J. G., Oshima R. G., Crawhall J. C., Olson D. L., Schneider J. A. Cystinosis. Intracellular cystine depletion by aminothiols in vitro and in vivo. J Clin Invest. 1976 Jul;58(1):180–189. doi: 10.1172/JCI108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Schlegel R. J. Impaired leucotactic responsiveness in a child with recurrent infections. Lancet. 1969 Aug 16;2(7616):344–347. doi: 10.1016/s0140-6736(69)92699-3. [DOI] [PubMed] [Google Scholar]
- Whitin J. C., Chapman C. E., Simons E. R., Chovaniec M. E., Cohen H. J. Correlation between membrane potential changes and superoxide production in human granulocytes stimulated by phorbol myristate acetate. Evidence for defective activation in chronic granulomatous disease. J Biol Chem. 1980 Mar 10;255(5):1874–1878. [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. Secretory responses of human neutrophils: exocytosis of specific (secondary) granules by human neutrophils during adherence in vitro and during exudation in vivo. J Immunol. 1979 Jul;123(1):285–294. [PubMed] [Google Scholar]
- Wright D. G., Kirkpatrick C. H., Gallin J. I. Effects of levamisole on normal and abnormal leukocyte locomotion. J Clin Invest. 1977 May;59(5):941–950. doi: 10.1172/JCI108716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]



