Abstract
Purpose
Ezrin is a cytoskeletal protein involved in tumor growth and invasion. However its prognostic value for survival in patients with solid tumor remains controversial.
Methods
Several databases were searched, including Pubmed, Embase and Cochrane databases. The endpoints were overall survival (OS), progression-free survival (PFS). The pooled hazard ratio (HR) or odds ratio (OR), and 95% confidence intervals (CI) were calculated employing fixed- or random-effects models depending on the heterogeneity of the included trials.
Results
Twenty-seven eligible trials involving 4693 patients were ultimately identified. A summary hazard ratio (HR) of all studies and sub-group hazard ratios were calculated. The combined HR suggested that a positive Ezrin expression had an impact on overall survival (OS) [1.95, 95% confidence interval (CI) 1.60–2.39; P<0.001] in all eligible studies and progress free survival (PFS): (2.30 95% CI 1.0–3.61; P = 0.001). Similar results were also observed in subgroup analysis, according to tumor types, regions, patients' number and publication year.
Conclusions
Our findings suggested that Ezrin protein expression might be a factor for a poor prognosis in patients with solid tumor. So large well-designed prospective studies are now needed to confirm the clinical utility of Ezrin as an independent prognostic marker.
Introduction
Ezrin, a member of the ezrin/radixin/moesin (ERM) family, is an important molecule linking the cytoskeleton to the membrane [1]. Ezrin is essential for many fundamental cellular processes, including determination of the cell shape, polarity, surface structure, cell adhesion, motility, cytokinesis, phagocytosis, and integration of membrane transport through signaling pathways [1], [2], [3], all of which are expected to promote tumor progression. Indeed, recent studies have revealed that ezrin may have an important role in tumorigenesis, development, invasion, and metastasis, probably through regulation of adhesion molecules, participation in cell signal transduction, and signaling to other cell membrane channels in the tumor [4], [5]. For long a large number of studies have been focused on identifying the prognostic value of Ezrin in solid tumors and most studies suggest that Ezrin is beneficial for tumor growth and, therefore, associated with poor prognosis including carcinomas of the breast [6], soft tissue sarcoma [7], ovary cancer [8], Gastrointestinal stromal tumors [9],colorectal cancer [10] and non-small cell lung cancer [11]. In this study, we sought to conduct a meta-analysis to estimate the prognostic importance of Ezrin level for overall survival (OS) and disease-free survival (DFS) among patients with solid tumors, aiming to gain insights into whether Ezrin could provide useful guidance in the biological understanding and treatment of solid tumors.
Materials and Methods
Literature search
We conducted a comprehensive search in the Pubmed and Embase to include in the present meta-analysis. We combined search terms for Ezrin expression and solid tumors: (“solid tumor” or “solid cancer”) or “Ezrin” or “prognosis”. And the last search was updated on 31 Dec 2012. We also reviewed the Cochrane Library for relevant articles. The references cited in those included studies were also reviewed to complete the search.
Study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [12].
Inclusion and exclusion criteria
Inclusion criteria for this study were as follows: (1) proven diagnosis of solid tumor, (2) Ezrin evaluation using immunohistochemical method, (3) association of Ezrin with overall survival (OS), and/or disease-free survival (DFS). Reviews, letters to the editors, and articles published in a book were excluded. We avoided duplication of data by examining the names of all authors and medical centers involved for each article. Authors that published multiple reports on the same sample were included once. We did not weight each study by a quality score because no such score had received general agreement for meta-analysis of observational studies [13].
Data extraction
Two independent reviewers (HK and QWX) read titles and abstracts of all candidate articles. Articles that could not be categorized based on title and abstract alone were retrieved for full-text review. Articles were independently read and checked for inclusion criteria of articles in this study. Any disagreement in quality assessment and data collection was discussed and solved together. The following data were collected: (1) article data including publication date, first author's name and country; (2) demographic data regarding inclusion criteria, age, regions, number of patients and number of Ezrin positive; (3) tumor data of Underlying malignancies; (4) survival data including OS, DFS and follow-up period; (5) method of Ezrin measurement, cut-off used for assessing Ezrin positivity. Any differences in the data extraction were resolved together by two authors.
Statistical analysis
Hazard ratios (HRs) and its 95% confidence intervals (CIs) were used to estimate the association between Ezrin and patients' prognosis. For those HRs that were not given directly in the published articles, the published data including the number of patients at risk in each groups, the total number of events and figures from original articles were used to estimate the HR according to the methods described by Parmar et al [14]. If the only exploitable survival data were in the form of figures, we read Kaplan-Meier curves by Engauge Digitizer version 4.1 (free software down-loaded from http://sourceforge.net) and extracted survival rate from them to reconstruct the HR and its standard error (SE). All the data analyses were performed with Stata version 11.0 (Stata Corporation, College Station, TX, USA) and we used Q-tests and P-values to estimate the heterogeneity. If P-value was greater than 0.05 which indicated a lack of heterogeneity among studies, a fixed-effects model was used to calculate the HR and its 95%CI according to the method of Mantel and Haenszel [15]. Otherwise, a random-effects model (the DerSimonian-Laird method) was used. By convention, an observed HR>1 implied a worse prognosis in the Ezrin positive group. The impact of Ezrin on survival was considered to be statistically significant if the 95%CI for the HR did not overlap 1.
Results
Study selection and characteristics
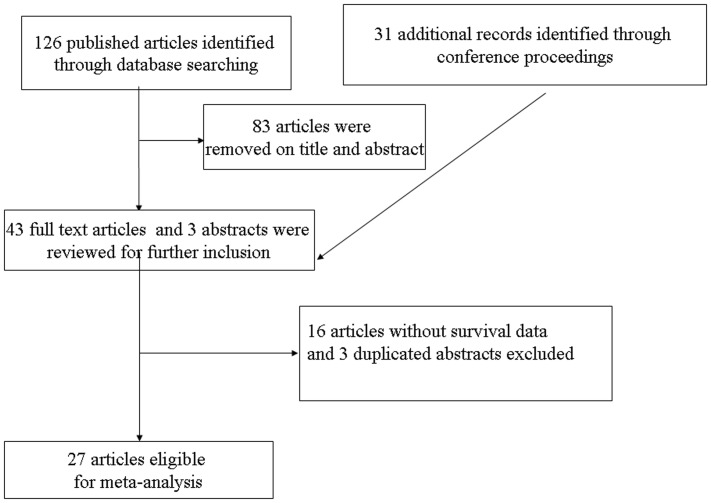
A total of 126 potentially relevant studies were retrieved electronically, 99 of which were excluded for the reasons shown in figure 1. Full-text copies of the remaining 43 citations were obtained and were evaluated in more detail. Finally, a total of 27 trials with 4693 patients were available for the meta-analysis.
Figure 1. Methodological Flow Chart of the Systematic.
The main features of the eligible studies for Ezrin were summarized in Table 1. The total number of patients included for meta-analysis was 4693, ranging from 40 to 487 per study. In total, 22 studies had data on OS [6], [7], [8], [10], [11], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], and 7 study have data on DFS [6], [9], [23], [33], [34], [35], [36]. 13 reports originated from Asia, 14 from Non Asia. Number of positive patients ranged from 12 to 240 in the included 27 studies.
Table 1. Main Characteristics of the Eligible Studies.
| Author | Year | Region | No of patients | Underlying malignancies | Technology | Positive (%) | Survival analysis | HR estimation | HR (95%) | Cut-off For Ezrin + |
| Makitie | 2001 | Finland | 130 | Uveal Malignant Melanoma | IHC | 83 | OS | HR | 2.52 (1.4–4.51) | at least positive |
| Moilanen | 2003 | Finland | 440 | Ovarian carcinoma | IHC | 318 | OS | K-M | 1.34 (1.06–1.62) | ≥10% |
| Weng | 2005 | Sweden | 50 | soft tissue sarcomas | IHC | 25 | OS | K-M | 1.48 (0.67–3.28) | >1% |
| Yeh | 2005 | Taiwan | 84 | pancreatic cancer | IHC | 49 | OS | K-M | 1.36 (0.98–1.89) | at least moderate |
| Kobel | 2006 | Germany | 105 | ovarian carcinoma | IHC | 51 | OS | K-M | 2.16 (1.31–3.55) | at least moderate |
| Kobel | 2006 | Germany | 164 | endometrioid carcinomas | IHC | 83 | OS | K-M | 1.46 (0.52–4.14) | at least moderate |
| Madan | 2006 | USA | 40 | HNSCC | IHC | 19 | OS | HR | 1.82 (1.0–3.2) | ≥10% |
| Mhawech-Fauceglia | 2007 | Switzerland | 108 | HNSCC | IHC | 93 | DFS | HR | 0.266 (0.63–1.111) | at least moderate |
| Elzagheid | 2008 | Finland | 74 | Colorectal cancer | IHC | 61 | OS | K-M | 1.88 (0.81–4.36) | at least moderate |
| Gao | 2009 | China | 193 | esophageal carcinoma | IHC | 90 | OS | HR | 1.46 (0.99–2.15) | ≥50% |
| Palou | 2009 | Spain | 92 | bladder tumours | IHC | 12 | OS | K-M | 7.21 (1.43–36.36) | >20% |
| IHC | DFS | K-M | 4.01 (1.10–14.62) | |||||||
| Wei | 2009 | Taiwan | 347 | GIST | IHC | 229 | DFS | HR | 2.363 (1.254–4.454) | ≥50% |
| Huang | 2010 | Taiwan | 78 | myxofibrosarcomas | IHC | 38 | OS | K-M | 3.13 (1.34–7.28) | at least moderate |
| Kang | 2010 | Korea | 100 | hepatocellular carcinoma | IHC | 28 | DFS | K-M | 2.25 (1.41–3.57) | >10% |
| Aishima | 2011 | Japan | 41 | intrahepatic cholangiocarcinoma | IHC | 20 | OS | K-M | 1.81 (0.92–3.55) | >11% |
| Carneiro | 2011 | Sweden | 227 | soft tissue sarcomas | IHC | 109 | DFS | HR | 1.8 (1.1–2.8) | at least moderate |
| Korkeila | 2011 | Finland | 176 | Rectal cancer | IHC | 15 | DFS | K-M | 3.26 (1.09–9.72) | at least moderate |
| Lam | 2011 | HongKong | 150 | Gastric cancer | IHC | 117 | OS | HR | 2.016 (1.099–2.933) | at least moderate |
| Li | 2011 | China | 436 | Gastric carcinoma | IHC | 236 | OS | K-M | 2.07 (1.35–3.19) | at least moderate |
| Patara | 2011 | Brazil | 250 | Colorectal cancer | IHC | 21 | OS | K-M | 1.62 (0.75–3.49) | at least moderate |
| Wang | 2011 | China | 200 | nasopharyngeal carcinoma | IHC | 134 | OS | K-M | 1.96 1.091–2.828) | at least moderate |
| Wang | 2011 | China | 75 | Salivary gland adenoid cystic carcinoma (SACC) | IHC | 23 | OS | HR | 2.23 (1.02–4.9) | at least intense |
| Xie | 2011 | China | 307 | Esophageal | IHC | 240 | OS | K-M | 1.47 (1.08–2.01) | at least moderate |
| Jorgren | 2012 | Sweden | 104 | Rectal cancer | IHC | 86 | OS | K-M | 1.89 (1.16–3.1) | at least moderate |
| Lee | 2012 | Korea | 112 | NSCLCs | IHC | 33 | OS | HR | 1.853 (1.053–3.623) | at least positive |
| Schlecht | 2012 | USA | 123 | HNSCC | IHC | 34 | OS | HR | 3.11 (1.35–7.15) | ≥10% |
| Ma | 2013 | China | 487 | Breast cancer | IHC | 74 | OS | HR | 3.711 (3.112–4.371) | ≥75% |
| DFS | HR | 3.805 (3.002–4.386) |
HR hazard ratio, K-M Kaplan Meier, OS overall survival, DFS disease free survival, IHC immunohistochemical.
Publication bias
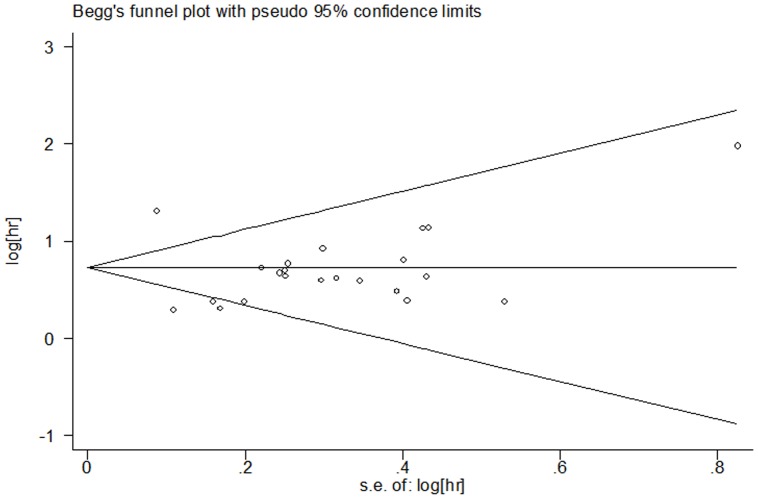
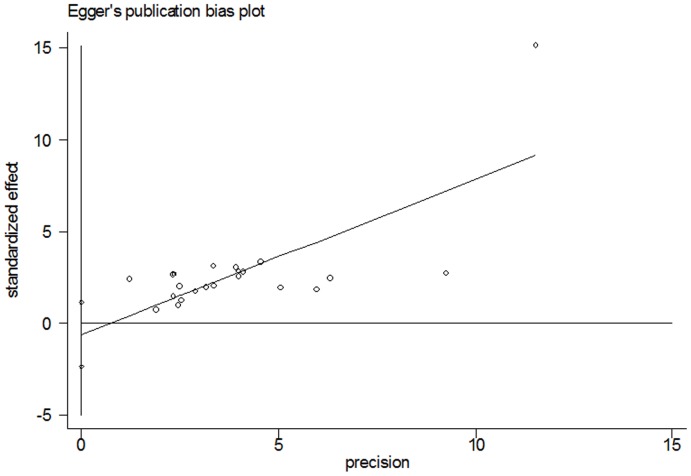
NoevidenceofpublicationbiaswasdetectedfortheHR of OS and PFS in this studybyeitherBeggorEgger'stest(HRofOS:Begg'stestp = 0.085,Egger'stestp = 0.455;HR of PFS: Begg'stestp = 0.293,Egger'stestp = 0.764) (Fig. 4 and Fig. 5).
Figure 4. Begg's test result of OS.
Figure 5. Egger's test result of OS.
Meta-analysis
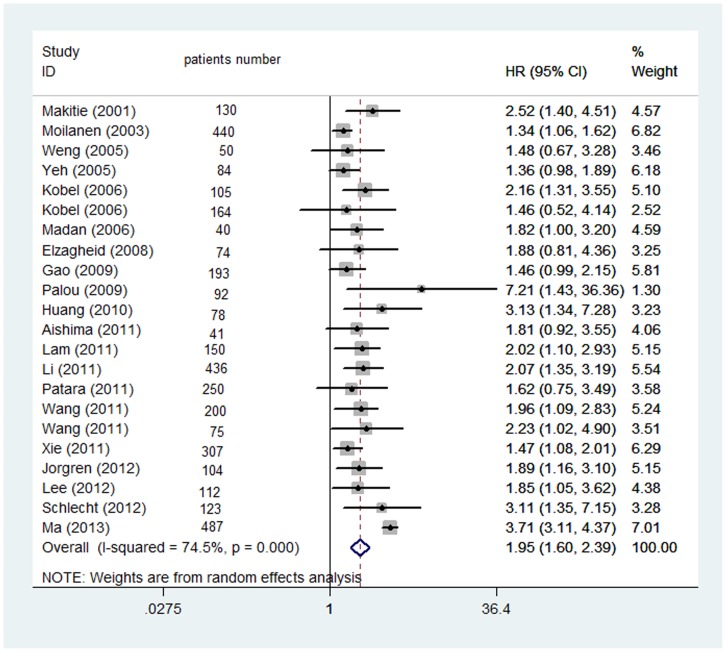
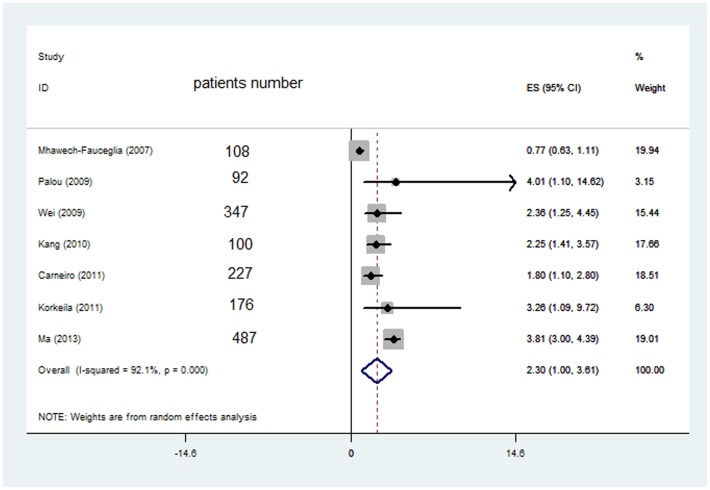
The results of the meta-analysis were shown in Fig. 2 and Fig. 3. The combined HR for 22 studies evaluating Ezrin overexpression on OS was 1.95, (95% CI: 1.60–2.39), suggesting that Ezrin overexpression was an indicator of poor prognosis for solid tumor. Significant heterogeneity was observed among the studies. (Q = 55.4, I2 = 62.1%, P<0.001). When grouped according to geographic settings of individual studies, the combined HRs of Asian studies and non-Asian studies were 2.006 (95% CI: 1.483–2.529) and 1.498 (95%CI: 1.260–1.735) respectively. Subgroupanalysiscouldhelpusdiscoverpotentialinformation of what the clinicians were interested in. Therefore, we studied some factors that might be related with survival. The studies from the tumor types, regions, patients' number and publication year were considered as the subgroup analysis factors. Finally, all subgroup analyses favored Ezrin overexpression be associated with poor OS (Table 2). 7 studies evaluating Ezrin overexpression on PFS was 2.30, (95% CI: 1.00–3.61), indicate that Ezrin overexpression was an indicator of poor prognosis for solid tumor using random effect model(Q = 96.05, I2 = 92.1%, P<0.001).
Figure 2. Ezrin expression and OS.
Figure 3. Ezrin expression and PFS.
Table 2. Stratified analysis of pooled hazard ratios of cancer patients with Ezrin expression.
| Stratified analysis | No. of studies | No. of patients | Pooled HR (95%CI) | Heterogeneity | |
| I2% | p-value | ||||
| Tumor type | |||||
| Head and neck cancer | 5 | 568 | 2.070(1.488–2.652) | 0% | 0.894 |
| Digestive cancer | 9 | 1639 | 1.565(1.325–1.806) | 0% | 0.871 |
| Other types | 8 | 1478 | 2.255(1.131–3.379) | 87.3 | <0.001 |
| Region | |||||
| Asian | 11 | 2163 | 2.006(1.483–2.529) | 77.1 | <0.001 |
| Non Asian | 11 | 1572 | 1.498(1.260–1.735) | 0% | 0.726 |
| No. of patients | |||||
| ≥150 | 9 | 2627 | 1.518(1.320–1.717) | 33.5 | 0.150 |
| <150 | 13 | 1108 | 1.694(1.386–2.002) | 0% | 0.874 |
| Publication year | |||||
| <2009 | 10 | 1372 | 1.437(1.232–1.642) | 0% | 0.819 |
| ≥2010 | 12 | 2363 | 2.150(1.611–2.688) | 67.9 | <0.001 |
Discussion
Ezrin is a member of the ERM (Ezrin, Radixin and Moesin) family, which was first described as linkers between membrane proteins and actin filaments. It has been implicated in the determination of cell shape, membrane organization, cell polarization, migration, division and they participate in various signaling pathways [5], [37], [38]. Alterations of ezrin expression can mediate many changes in the metastasis-associated cell surface signals and intra-cellular signaling cascade that confer the metastatic capability in tumor cells. Therefore, it is conceivable that ezrin overexpression and/or deregulation could contribute to the metastatic behaviors of tumors. Evidence from both animal models and prospective human studies show correlations between ezrin expression levels and tumor progression [37], [39], consistent with a crucial role for ezrin in tumor dissemination.
Meta-analysis is useful to integrate results from independent studies for a specified outcome. Pooled results from the combining relevant studies are statistical powerful, and make it possible to detecting effects that may be missed by individual studies.To date, no meta-analysis has been undertaken for any studies that evaluate Ezrin as a prognostic marker in solid tumor. In this meta-analysis, 27 eligible studies that compared the survival of solid tumor according to Ezrin expression level of the primary tumor met the enrollment criteria. The data were organized according to disease-free and overall survival; then combined results demonstrated that Ezrin overexpression was associated with a poor OS (HR, 1.95; 95%CI, 1.60–2.39; P<0.001.) and PFS (HR, 2.30; 95%CI, 1.00–3.61; P = 0.001.) in solid tumor using a random effect. Due to significant heterogeneity among included studies, we then perform a subgroup analysis according to tumor types, regions, patients' number and publication year. Allsubgroup analysesfavoredEzrin overexpression be associated with poorOS. In all our data helped to clarify the results of individual studies and to identify patients at high risk for whom specific- or adjuvant-therapy might be necessary since Ezrin overexpression is a prognostic factor for solid tumor.
There is significant heterogeneity among included studies in this systematic review, although we used random-effects models during pooling data of subgroup. The heterogeneity in these studies could be explained by different characteristics of included patients, or differences in the techniques used to detect alterations in Ezrin expression, including antigen retrieval methods, choice of Ezrin antibody, dilutions of the antibodies, and revelation protocols. What's more, different sample types including tissue microarray (TMA) and the whole section might also contribute to the heterogeneity because it is possible that more false-negative cases are obtained in TMA than the whole section. Finally, the differences of methodology among included studies also were sources of heterogeneity and caused selection biases potentially [40].
Several important limitations need to be considered when interpreting our analysis. First of all, the number of included studies was relatively small with only about 4693 cases. Patients had received different treatments; preoperative TNM category and histologic types were various. Whereas, we were unable to assess these potential confounders present in individual studies. Second, although we tried to identify all relevant data, potential publication bias was unavoidable and some data could still be missing. Third, although immunohistochemistry was the most commonly applied method for detecting Ezrin in situ, RT-PCR method had also been used for the evaluation of the levels of Ezrin gene or mRNA expression in tumor tissue. Studies measuring Ezrin gene or mRNA level by RT-PCR was not yet included in this meta-analysis. Moreover the cutoff value was defined differently (1%, 10%, 20%, 50%, 75%) in these studies, leading to between-study heterogeneity. Thus we had adopted random effect model and subgroup sensitivity analyses to adjust for the shortcomings.
Finally,this study was constrained to studies published in English language .Although we detected no evidence of publication bias using the graphical method, it was difficult to completely rule out this possibility.
In summary, this present study shows a significant correlation between Ezrin expression and OS as well as DFS rate in solid tumor patients. Ezrin may have prognostic significance for patients with solid tumor based on currently obtained data.However,one should be cautious when interrupting these results due to the limitations of our studies.Further high-quality studies are still needed to confirm these results.
Acknowledgments
We thank all authors whose publications could be included in our meta-analysis.
Funding Statement
No current external funding sources for this study.
References
- 1. Bretscher A, Edwards K, Fehon RG (2002) ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3: 586–599. [DOI] [PubMed] [Google Scholar]
- 2. Wu KL, Khan S, Lakhe-Reddy S, Jarad G, Mukherjee A, et al. (2004) The NHE1 Na+/H+ exchanger recruits ezrin/radixin/moesin proteins to regulate Akt-dependent cell survival. J Biol Chem 279: 26280–26286. [DOI] [PubMed] [Google Scholar]
- 3. Ng T, Parsons M, Hughes WE, Monypenny J, Zicha D, et al. (2001) Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. EMBO J 20: 2723–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McClatchey AI (2003) Merlin and ERM proteins: unappreciated roles in cancer development? Nat Rev Cancer 3: 877–883. [DOI] [PubMed] [Google Scholar]
- 5. Elliott BE, Meens JA, SenGupta SK, Louvard D, Arpin M (2005) The membrane cytoskeletal crosslinker ezrin is required for metastasis of breast carcinoma cells. Breast Cancer Res 7: R365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma L, Liu YP, Zhang XH, Geng CZ, Li ZH (2013) Relationship of RhoA signaling activity with ezrin expression and its significance in the prognosis for breast cancer patients. Chin Med J (Engl) 126: 242–247. [PubMed] [Google Scholar]
- 7. Weng WH, Ahlen J, Astrom K, Lui WO, Larsson C (2005) Prognostic impact of immunohistochemical expression of ezrin in highly malignant soft tissue sarcomas. Clin Cancer Res 11: 6198–6204. [DOI] [PubMed] [Google Scholar]
- 8. Moilanen J, Lassus H, Leminen A, Vaheri A, Butzow R, et al. (2003) Ezrin immunoreactivity in relation to survival in serous ovarian carcinoma patients. Gynecol Oncol 90: 273–281. [DOI] [PubMed] [Google Scholar]
- 9. Wei YC, Li CF, Yu SC, Chou FF, Fang FM, et al. (2009) Ezrin overexpression in gastrointestinal stromal tumors: an independent adverse prognosticator associated with the non-gastric location. Mod Pathol 22: 1351–1360. [DOI] [PubMed] [Google Scholar]
- 10. Patara M, Santos EM, Coudry Rde A, Soares FA, Ferreira FO, et al. (2011) Ezrin expression as a prognostic marker in colorectal adenocarcinoma. Pathol Oncol Res 17: 827–833. [DOI] [PubMed] [Google Scholar]
- 11. Lee HW, Kim EH, Oh MH (2012) Clinicopathologic implication of ezrin expression in non-small cell lung cancer. Korean J Pathol 46: 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Altman DG (2001) Systematic reviews of evaluations of prognostic variables. BMJ 323: 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 15. Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22: 719–748. [PubMed] [Google Scholar]
- 16. Makitie T, Carpen O, Vaheri A, Kivela T (2001) Ezrin as a prognostic indicator and its relationship to tumor characteristics in uveal malignant melanoma. Invest Ophthalmol Vis Sci 42: 2442–2449. [PubMed] [Google Scholar]
- 17. Yeh TS, Tseng JH, Liu NJ, Chen TC, Jan YY, et al. (2005) Significance of cellular distribution of ezrin in pancreatic cystic neoplasms and ductal adenocarcinoma. Arch Surg 140: 1184–1190. [DOI] [PubMed] [Google Scholar]
- 18. Kobel M, Gradhand E, Zeng K, Schmitt WD, Kriese K, et al. (2006) Ezrin promotes ovarian carcinoma cell invasion and its retained expression predicts poor prognosis in ovarian carcinoma. Int J Gynecol Pathol 25: 121–130. [DOI] [PubMed] [Google Scholar]
- 19. Kobel M, Langhammer T, Huttelmaier S, Schmitt WD, Kriese K, et al. (2006) Ezrin expression is related to poor prognosis in FIGO stage I endometrioid carcinomas. Mod Pathol 19: 581–587. [DOI] [PubMed] [Google Scholar]
- 20. Madan R, Brandwein-Gensler M, Schlecht NF, Elias K, Gorbovitsky E, et al. (2006) Differential tissue and subcellular expressionof ERM proteins in normal and malignant tissues: cytoplasmic ezrin expression has prognostic signficance for head and neck squamous cell carcinoma. Head Neck 28: 1018–1027. [DOI] [PubMed] [Google Scholar]
- 21. Elzagheid A, Korkeila E, Bendardaf R, Buhmeida A, Heikkila S, et al. (2008) Intense cytoplasmic ezrin immunoreactivity predicts poor survival in colorectal cancer. Hum Pathol 39: 1737–1743. [DOI] [PubMed] [Google Scholar]
- 22. Gao SY, Li EM, Cui L, Lu XF, Meng LY, et al. (2009) Sp1 and AP-1 regulate expression of the human gene VIL2 in esophageal carcinoma cells. J Biol Chem 284: 7995–8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palou J, Algaba F, Vera I, Rodriguez O, Villavicencio H, et al. (2009) Protein expression patterns of ezrin are predictors of progression in T1G3 bladder tumours treated with nonmaintenance bacillus Calmette-Guerin. Eur Urol 56: 829–836. [DOI] [PubMed] [Google Scholar]
- 24. Huang HY, Li CF, Fang FM, Tsai JW, Li SH, et al. (2010) Prognostic implication of ezrin overexpression in myxofibrosarcomas. Ann Surg Oncol 17: 3212–3219. [DOI] [PubMed] [Google Scholar]
- 25. Aishima S, Fujita N, Mano Y, Kubo Y, Tanaka Y, et al. (2011) Different roles of S100P overexpression in intrahepatic cholangiocarcinoma: carcinogenesis of perihilar type and aggressive behavior of peripheral type. Am J Surg Pathol 35: 590–598. [DOI] [PubMed] [Google Scholar]
- 26. Lam EK, Wang X, Shin VY, Zhang S, Morrison H, et al. (2011) A microRNA contribution to aberrant Ras activation in gastric cancer. Am J Transl Res 3: 209–218. [PMC free article] [PubMed] [Google Scholar]
- 27. Li L, Wang YY, Zhao ZS, Ma J (2011) Ezrin is associated with gastric cancer progression and prognosis. Pathol Oncol Res 17: 909–915. [DOI] [PubMed] [Google Scholar]
- 28. Wang L, Lin GN, Jiang XL, Lu Y (2011) Expression of ezrin correlates with poor prognosis of nasopharyngeal carcinoma. Tumour Biol 32: 707–712. [DOI] [PubMed] [Google Scholar]
- 29. Wang YY, Chen WL, Huang ZQ, Yang ZH, Zhang B, et al. (2011) Expression of the membrane-cytoskeletal linker Ezrin in salivary gland adenoid cystic carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 112: 96–104. [DOI] [PubMed] [Google Scholar]
- 30. Xie JJ, Xu LY, Wu ZY, Zhao Q, Xu XE, et al. (2011) Prognostic implication of ezrin expression in esophageal squamous cell carcinoma. J Surg Oncol 104: 538–543. [DOI] [PubMed] [Google Scholar]
- 31. Jorgren F, Nilbert M, Rambech E, Bendahl PO, Lindmark G (2012) Ezrin expression in rectal cancer predicts time to development of local recurrence. Int J Colorectal Dis 27: 893–899. [DOI] [PubMed] [Google Scholar]
- 32. Schlecht NF, Brandwein-Gensler M, Smith RV, Kawachi N, Broughel D, et al. (2012) Cytoplasmic ezrin and moesin correlate with poor survival in head and neck squamous cell carcinoma. Head Neck Pathol 6: 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mhawech-Fauceglia P, Dulguerov P, Beck A, Bonet M, Allal AS (2007) Value of ezrin, maspin and nm23-H1 protein expressions in predicting outcome of patients with head and neck squamous-cell carcinoma treated with radical radiotherapy. J Clin Pathol 60: 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kang YK, Hong SW, Lee H, Kim WH (2010) Prognostic implications of ezrin expression in human hepatocellular carcinoma. Mol Carcinog 49: 798–804. [DOI] [PubMed] [Google Scholar]
- 35. Carneiro A, Bendahl PO, Akerman M, Domanski HA, Rydholm A, et al. (2011) Ezrin expression predicts local recurrence and development of metastases in soft tissue sarcomas. J Clin Pathol 64: 689–694. [DOI] [PubMed] [Google Scholar]
- 36. Korkeila EA, Syrjanen K, Bendardaf R, Laulajainen M, Carpen O, et al. (2011) Preoperative radiotherapy modulates ezrin expression and its value as a predictive marker in patients with rectal cancer. Hum Pathol 42: 384–392. [DOI] [PubMed] [Google Scholar]
- 37. Khanna C, Wan X, Bose S, Cassaday R, Olomu O, et al. (2004) The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med 10: 182–186. [DOI] [PubMed] [Google Scholar]
- 38. Curto M, McClatchey AI (2004) Ezrin...a metastatic detERMinant? Cancer Cell 5: 113–114. [DOI] [PubMed] [Google Scholar]
- 39. Yu Y, Khan J, Khanna C, Helman L, Meltzer PS, et al. (2004) Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med 10: 175–181. [DOI] [PubMed] [Google Scholar]
- 40. Garcia VM, Batlle JF, Casado E, Burgos E, de Castro J, et al. (2011) Immunohistochemical analysis of tumour regression grade for rectal cancer after neoadjuvant chemoradiotherapy. Colorectal Dis 13: 989–998. [DOI] [PubMed] [Google Scholar]