Abstract
While extinction has been used as a treatment to reduce the power of drug cues, a better method is needed. Research with traditional reinforcers has shown that counterconditioning – pairing an appetitive cue with an aversive stimulus – can suppress cue-controlled behavior. The present experiment compared the counterconditioning and extinction of cocaine cues. Male rats were first trained to self-administer cocaine during a light cue. In the second phase, the light was paired with footshock in the Counterconditioning group. The Extincton group was treated similarly, except light presentations did not end in footshock. Counterconditioning suppressed cocaine seeking to a greater extent than extinction while the counterconditioning treatment was actively administered. On a subsequent stimulus compounding test where footshock was discontinued and the light was presented simultaneously with an untreated cocaine cue (a tone), suppressive effects of counterconditioning were evident during the early portion of the test, but not during later trials. Overall, results of the present experiment suggest that counterconditioning produces only temporarily suppressive effects on cue-controlled cocaine seeking. Methods for directly weakening the cue-drug association (e.g., “deepened extinction”) may prove to be more useful potential drug cue treatments.
Drug cues, such as people, places, or things (e.g., paraphernalia) associated with drug use, play an important role in driving drug abuse and addiction. For example, drug cues elicit craving for the drug (Childress et al., 1999; Volkow et al., 2006), activate the same brain reward circuitry that is activated by the drug itself (Volkow et al., 2006, 2008), and contribute to relapse after abstinence (Grusser et al., 2004; Kosten et al., 2006; Sinha & Li, 2007). An intervention that reduces the power of drug cues could help to improve treatment outcomes.
Extinction has been used in such an effort. Extinction is the presentation of a conditioned cue without the reinforcer (or unconditioned stimulus; US) with which the cue was previously paired. For example, cue-exposure therapy is an extinction-based treatment where drug users are repeatedly exposed to drug cues without the drug (Drummond, Tiffany, Glautier, & Remington, 1995). Experiencing the cues in the absence of the drug should theoretically weaken or inhibit the cue-drug association and thereby reduce the ability of the drug cues to drive drug-related behavioral or neurological responses. While there have been a handful of cue-exposure studies reporting promising results (Loeber, Croissant, Heinz, Mann, & Flor, 2006; Rohsenow et al., 2001), the outcomes of most cue-exposure studies have been disappointing (e.g., Marissen, Blanken, Franken, van den Brink, & Hendriks, 2007; for review, see Conklin & Tiffany, 2002). This suggests that a treatment that is more effective than extinction is needed.
Counterconditioning is an alternative method for suppressing the effects of conditioned cues. In counterconditioning, a formerly appetitive cue (e.g., a stimulus associated with food) is paired with an aversive stimulus such as electric shock instead of its original appetitive US (Lovibond & Dickinson, 1982). After several such pairings, the formerly appetitive cue no longer controls the appetitively motivated behavior it once did. With non-drug reinforcers or USs, counterconditioning an appetitive cue by pairing it with an aversive US has been shown to be an effective means of suppressing the originally conditioned effects of the cue in both animals (Lovibond & Dickinson, 1982) and humans (Kerkhof, Vansteenwegen, Baeyens, & Hermans, 2011; Van Gucht, Baeyens, Vansteenwegen, Hermans, & Beckers, 2010). Some studies with non-drug cues have found counterconditioning to be more effective than extinction (e.g., Kerkhof et al., 2011).
There have been attempts to investigate the effectiveness of counterconditioning as a method for treating drug cues in humans. For example, in studies of aversion therapy, drug-related cues (e.g., a cocaine-like powder) have been paired with injections of emetic drugs or with mild electric shock applied to the wrist (Bordnick, Elkins, Orr, Walters, & Thyer, 2004; Frawley & Smith, 1990, 1992; Smith & Frawley, 1993). It is hard to interpret the results of these studies because either they did not include control groups at all (Frawley & Smith, 1990, 1992; Smith & Frawley, 1993) or they did not include control groups exposed to the cues without the aversive US, thus making it impossible to separate the effects of extinction from counterconditioning (Bordnick et al., 2004). Despite this lack of conclusive experimental evidence for its effectiveness, counterconditioning is used as a treatment for cocaine craving in some drug rehabilitation clinics (e.g., Schick Shadel Hospital, Seattle, WA).
The present study investigated within an animal model whether counterconditioning was a more effective treatment than extinction for reducing the power of a cocaine cue. Rats were first trained to self-administer cocaine whenever a light or a tone was present, but not when these cues were absent. After these were established as cues controlling cocaine-seeking behavior, rats were divided into two groups prior to beginning a second phase where the light was the cue targeted for treatment (the tone was not presented). The Counterconditioning (CC) group received presentations of the light stimulus followed by a brief footshock. Cocaine self-administration was discontinued during this phase. The Extinction (Ext) group was treated similarly except light presentations did not end with footshock. After Phase 2, cocaine seeking in the two groups was evaluated with a stimulus compounding test, a sensitive assay of the associative properties conditioned to cues (Weiss, 1978). On this test, the light and the tone were presented simultaneously for the first time. Usually, compounding two appetitive cues, including cocaine cues, results in more responding to the compound than to either stimulus alone (e.g., Kearns, Weiss, Schindler, & Panlilio, 2005; Panlilio, Weiss, & Schindler, 1996). However, if the formerly cocaine-paired light were converted into an aversive cue in the CC group, no additive effects would be expected based on the results of previous studies with non-drug reinforcers showing that compounding an aversive cue with an appetitive cue does not result in an enhancement of responding (Weiss, Thomas, & Weissman, 1996; Weiss & Schindler, 1989).
Methods
Subjects
Twenty adult male Sprague-Dawley rats began the experiment. Subjects previously participated in a short-term morphine self-administration experiment in different chambers, with different levers, and stimuli distinct from those used in the present experiment. Rats were individually housed in plastic cages with wood-chip bedding and metal wire tops. They were maintained at 85% of their free-feeding weights (approximately 330–400 g) throughout the experiment by feeding them approximately 15 g of rat chow following training sessions. Rats had unlimited access to water in their home cages. The colony room where the rats were housed had a 12-h light:dark cycle with lights on at 08:00 h. Training sessions were conducted 5–7 days per week during the light phase of the light:dark cycle. Throughout the experiment, rats were treated in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, 1996) and all procedures were approved by American University’s Institutional Animal Care and Use Committee (IACUC).
Apparatus
Training took place in six operant chambers. Each chamber was 20 cm high, 23 cm long, and 18 cm wide and had aluminum front and rear walls, white translucent plastic side walls, a clear plexiglass ceiling, and a grid floor. A response lever and food trough were located on the front wall of the chamber. A tone stimulus (approximately 4500 Hz and 75 dB) was delivered by a model SC628HR Sonalert (Mallory Sonalerts, Indianapolis, IN) that was mounted on top of the chamber. A light stimulus was provided by two 15-cm, 25-W, 120-VAC tubular light bulbs located 10 cm outside each of the side walls of the chamber. These light bulbs were operated at 90 VAC. Footshock was produced by a Lehigh Valley Electronics model 113-04 constant-current shocker, a BRS/LVE model SGS-004 solid state shocker/scrambler, a Lehigh Valley Electronics model 113-33 shocker/scrambler, or a Med-Associates model ENV-414 shocker/scrambler, depending on the particular chamber. Each chamber was housed inside a sound attenuation chest (Weiss, 1970) that had a continuously operating ventilation fan. A shielded houselight mounted near the ceiling and rear wall of the sound attenuation chest provided a low level of continuous illumination during all sessions. Experimental procedures were controlled by Med-Associates software (Med-PC, St. Albans, VT) running on a PC located in an adjacent room.
Cocaine (provided by the National Institute on Drug Abuse) in a saline solution at a concentration of 2.56 mg/ml was infused at a rate of 3.19 ml/min by 10-ml syringes driven by Harvard Apparatus (South Natick, MA) or Med-Associates (St. Albans, VT) syringe pumps located outside of the sound attenuation chests. Tygon tubing extended from the 10-ml syringes to a 22-gauge rodent single-channel fluid swivel and tether apparatus (Alice King Chatham Medical Arts, Hawthorne, CA) that descended through the ceiling of the chamber. Cocaine was delivered to the subject through Tygon tubing that passed through the metal spring of the tether apparatus. This metal spring was attached to a plastic screw cemented to the rat’s head to reduce tension on the catheter.
Procedure
Surgery
Rats had been surgically prepared with chronic indwelling jugular vein catheters in the prior study, using a modification of the procedure originally developed by Weeks (1962). In brief, under ketamine (60 mg/kg) and xylazine (10 mg/kg) anesthesia, approximately 3 cm of Silastic tubing (0.044mm i.d., 0.814mm o.d.) was inserted into the right jugular vein. This Silastic tubing was connected to 8 cm of vinyl tubing (Dural Plastics; 0.5mm i.d., 1.0mm o.d.) that was passed under the skin around the shoulder and exited the back at the level of the shoulder blades. The vinyl tubing was threaded through a section of Tygon tubing (10 mm long, 4 mm diameter) that served as a subcutaneous anchor. Six stainless steel jeweler’s screws were implanted in the skull, to which a 20-mm plastic screw was cemented with dental acrylic. Catheters were flushed daily with 0.1 ml of a saline solution containing 1.25 U/ml heparin and 0.08 mg/ml gentamycin.
Phase 1: Cocaine self-administration
Rats were first trained to self-administer cocaine on a 3-component multiple schedule. During tone or light discriminative stimulus (SD) components which lasted 180 s on average (range: 150–210 s), 0.5 mg/kg cocaine infusions were available for lever pressing according to a variable-interval (VI) 60-s schedule. These SD components alternated with SΔ components lasting 60 s on average (range: 45–75 s) where all stimuli were absent and lever pressing did not result in cocaine. Each of the types of SD component (tone or light) were equally likely to follow an SΔ component, with the restriction that there were no more than two consecutive SD components of the same type. A 30-s response correction contingency operated during the final 30 s of each SΔ component. According to this contingency, a 30-sec SΔ-termination clock was reset to zero by a response. This contingency was designed to reduce response rates during SΔ components. Sessions lasted for 150 min. Rats were trained with the schedule parameters described above for a minimum of 5 sessions and until they self-administered at least 20 infusions in two consecutive sessions.
Then, the length of SD components was decreased to a mean of 60 s (range: 50 to 70 s) and the mean programmed duration of SΔ components was increased to 120 s (range: 90 to 150 s). Rats were trained on this terminal baseline procedure until (1) response rates during both tone and light SD components were at least 3 times higher than in SΔ components for two consecutive sessions, and (2) response rates in the tone and the light were within 50% of each other when averaged over 2 sessions.
Phase 2: Counterconditioning or Extinction
Rats were then assigned to either the CC group or the Ext group. For the CC group, self-administration was followed by 6 sessions of counterconditioning of the light by pairing it with footshock. During each such session, the light stimulus was presented 10 times. Each light component lasted 60 s and ended with the presentation of a 0.5 s, 0.5 mA footshock, a commonly used shock intensity and duration in aversive conditioning studies (e.g., Rescorla, 2006). Light components were separated by light-off components lasting an average of 180 s (range: 150 to 210 s). The lever remained in the chamber and rats could press it during either component type. Lever pressing did not produce cocaine at any time and the response correction contingency in SΔ was discontinued. The tone was not presented during this phase. The Ext group was treated similarly except that footshock was not presented at the end of light components. On the day following the final extinction or counterconditioning session, all rats were placed in the chamber for 60 min with all stimuli remaining off. The purpose of this context habituation session was to reduce fear which may have been conditioned to the context in the CC group so that this potential contextual fear would not interfere with responding on the stimulus compounding test.
Stimulus Compounding Test
This test consisted of 60-s stimulus components that alternated with 120-s periods where all stimuli were off. There were 18 stimulus presentations, 6 each of tone alone (T), light alone (L), and the tone-plus-light (TL) compound. The test stimuli were presented in the following order: T-L-TL-L-TL-T-TL-T-L-TL-L-T-L-T-TL-T-TL-L. Lever pressing did not produce cocaine during the test and no shock was presented at any time for either group.
At the end of the self-administration phase, rats’ catheters were tested for patency by by aspirating blood through the catheter. If patency could not be confirmed by drawing blood back, 0.1 ml of a saline solution containing 0.3 mg ketamine and 0.4 mg xylazine was infused into the catheter at the end of the study and patency was assumed if rapid ataxia (loss of motor control within 10 s) was observed. Subjects discovered to have non-patent catheters were excluded from the study (see below).
Data analysis
For all statistical tests, α = 0.05. Response rate (responses/min) was the primary measure of interest. Repeated measures analyses of variance (ANOVAs), followed by posthoc t-tests where appropriate, were performed on the response rate data. The Benjamini-Hochberg (1995) procedure was used to keep α ≤ 0.05 for collections of multiple t-tests.
Results
One rat (subject S8) was dropped from the experiment before being assigned to a group due to failure to acquire cocaine self-administration. A rat from the CC group (subject S13) was found to have a non-functional catheter and was therefore excluded from the study. This subject had the highest self-administration baseline response rates of all subjects in either group. Excluding this rat from the CC group caused there to be a relatively large (but not statistically significant) difference in baseline rates between the groups, which were intended to be matched in terms of baseline rate. To correct this, the rat (subject S1) with the highest baseline rate in the Ext group was also excluded from analyses and figures. This made baseline rates in the two groups more comparable and did not importantly change any conclusions or statistical results. After the rats described above were excluded, the numbers of subjects in the Ext and CC groups were 9 and 8, respectively.
Phase 1: Self-administration
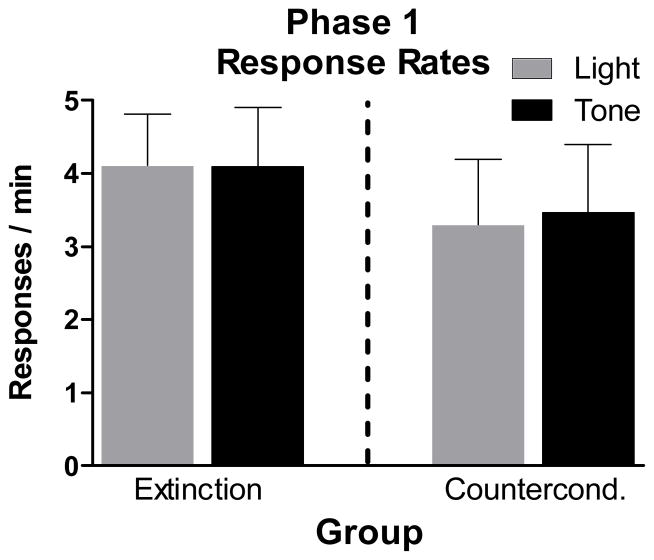
The mean numbers of total self-administration sessions for rats in the Ext and CC Groups were 12.7 (± 1.1 SEM) and 12.8 (± 0.9), respectively. This included 6.8 (± 0.7) and 6.6 (± 0.7) sessions on the Phase 1 terminal baseline schedule for the Ext and CC Groups, respectively. Figure 1 presents mean response rates during light components and during tone components on the final self-administration session. A 2 × 2 (Stimulus X Group) repeated measures ANOVA performed on these rates indicated that there were no significant effects of Stimulus or Group or their interaction (all Fs < 1, ps > 0.5). Rats in both groups made on average less than 0.75 responses/min during the absence of tone and light periods. On the final self-administration session, the mean numbers of cocaine infusions self-administered by the Ext and CC Groups were 35.4 (± 1.6) and 31.9 (± 2.9), respectively.
Figure 1.
Mean (± SEM) response rates to tone (black bars) and to light (gray bars) on the final Phase 1 cocaine self-administration session for the Extinction and Counterconditioning Groups.
Phase 2: Counterconditioning or Extinction
Figure 2 presents mean response rates to the light over two-trial blocks for each of the 6 sessions of Phase 2 for the CC and Ext groups. Counterconditioning was more effective than extinction in suppressing responding occasioned by the light during this phase. A 6 × 2 (Session X Group) repeated measures ANOVA performed on the whole-session response rates during light components confirmed that there was a significant effect of Group (F[1,13] = 18.2, p < 0.005) and Session (F[5,65] = 4.3, p < 0.005), but there was no significant interaction (F[5,65] = 1.7, p > 0.14). Mean response rates during light-off periods (not presented in figure) remained low throughout this phase and both groups made less than 0.1 responses/min on the final treatment session.
Figure 2.
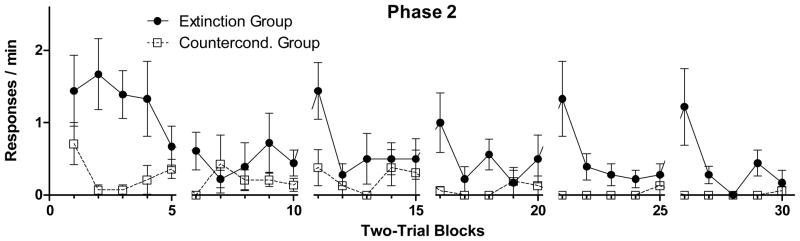
Mean (± SEM) response rates to light on two-trial blocks over the 6 sessions of Phase 2 for the Extinction group (filled circles) and the Counterconditioning group (open squares). There were 10 trials (5 two-trial blocks) per session.
On inspecting within-session patterns of responding, it was noted that the Ext Group, but not the CC Group, developed a tendency to display spontaneous recovery of responding to the light early in a session. This tendency was most pronounced during the final 2 sessions of the phase, where response rates in the Ext group were over 1 response per min on the first block of each session before quickly declining, while response rates in the CC group were essentially zero throughout each session. Ad-hoc 5 × 2 (Block X Group) repeated measures ANOVAs conducted on the block-by-block response rates within sessions 5 and 6 confirmed these impressions by revealing that there were significant Block X Group interaction effects for both sessions (F[4,60]s > 2.6, ps < 0.05).
Phase 3: Stimulus Compounding Test
Figure 3 presents mean (± SEM) response rates over two-trial blocks on the stimulus compounding test. On the first test block, the Ext group made approximately 10 times as many responses to TL as did the CC group. However, on the final block, this order reversed, with the CC group making approximately 3 times as many TL responses as the Ext group. A similar pattern was observed for the light, though on a smaller scale in terms of total number of responses. Over the course of the test, the CC group responded more to the tone than did the Ext group.
Figure 3.
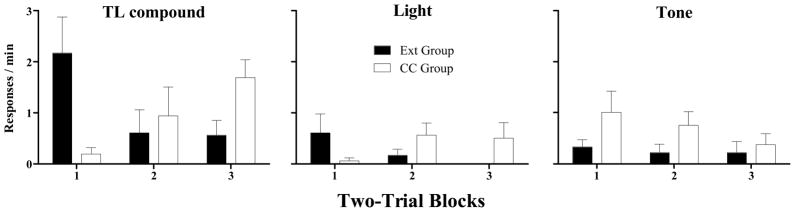
Mean (± SEM) response rates to the tone-plus-light compound (TL; left panel), light alone (center panel), and tone alone (right panel) on two-trial blocks of the stimulus compounding test for the Extinction Group (black bars) and the Counterconditioning Group (white bars).
Differences between the groups are also apparent if the distributions of responses over test stimuli are compared within each block. A large summative effect of compounding the tone and the light was observed for the Ext group in the first block, with TL response rates approximately 4–5 times those of either the light or the tone alone. In contrast, no summative effect was observed for the CC group on the first block. On the third block, however, the CC group showed a summative effect, with responding to TL being 3–4 times higher than that in either light or tone alone. The Ext group also responded more to TL than to either tone or light alone on this block.
Statistical analyses confirmed the impressions described above. A 3 × 3 × 2 (Stimulus X Block X Group) repeated-measures ANOVA performed on the test response rates confirmed that there was a significant main effect of Stimulus (F[2,30] = 12.5, p < 0.005), a significant Block X Group interaction (F[2,30] = 4.0, p < 0.05), and a significant Stimulus X Block X Group interaction (F[4,60] = 4.3, p < 0.005). There were no other significant main effects or interactions (Fs < 2.3, ps > 0.1).
Given the significant Stimulus X Block X Group interaction, individual 2 × 3 (Group X Block) repeated measures ANOVAs were performed separately for each stimulus to determine more specifically how the groups differed. For TL, there was a significant Group X Block interaction (F[2,30] = 5.3, p < 0.05), but no significant main effects of Group or Block (Fs < 1). Subsequent t-tests performed separately for each block indicated that the Ext group responded significantly more than the CC group on the first block (t[15] = 2.6, p < 0.25), but the CC group responded significantly more than the Ext group on the last block (t[15] = −2.5, p = 0.025). For the light, there was a marginally significant Group X Block interaction (F[2,30] = 3.3, p = 0.049), but no main effects of Group or Block (Fs < 1). The interaction for the light parallels that observed for TL. On subsequent t-tests, there were no significant differences in responding to light alone between the groups on any individual block (t[15]s < 1.7, ps > 0.1). For the tone, there was a significant main effect of Group (F[1,15] = 5.5, p < 0.05), but no significant effect of Block (F < 1) or Group X Block interaction (F = 1.0).
To further analyze how the groups differed, individual 2 × 3 (Group X Stimulus) repeated measures ANOVAs were performed separately for each block. For the first block, there was a significant main effect of Stimulus (F[2,30] = 3.5, p < 0.05) and a significant Stimulus X Group interaction (F[2,30] = 8.5, p < 0.005), but no main effect of Group (F[1,15] = 2.2, p > 0.15). Subsequent paired-samples t-tests performed separately for each group indicated that, in the Ext group, TL rates were higher than tone or light alone rates (t[8]s > 2.8, ps < 0.025), but there was no difference between tone alone and light alone (t[8] = 0.9, p > 0.35). For the CC group, there were no significant differences among test stimuli on this block (t[7]s < 2.2, ps > 0.05). On the second block, the Group X Stimulus repeated measures ANOVA indicated there were no significant effects of Stimulus, Group, or their interaction (Fs < 2.1, ps > 0.15). On the third block, there was a significant main effect of Stimulus (F[2,30] = 8.5, p < 0.005) and Group (F[1,15] = 6.6, p < 0.05), but no significant interaction (F[2,30] = 2.2, p > 0.13). Subsequent paired-samples t-tests revealed that, collapsed over groups, responding to TL on the third block was significantly higher than that to either tone or light alone (t[16]s > 2.6, ps < 0.02), but tone alone and light alone did not differ from each other (t[16] = 0.3, p > 0.75).
Discussion
The present study found that counterconditioning produced temporarily suppressive effects on cue-controlled cocaine seeking. During Phase 2, while the counterconditioning treatment was actively administered, the CC group displayed less cocaine seeking during the light than the Ext group, which only received extinction of the light. The temporarily suppressive effects of counterconditioning were also observed on the stimulus compounding test when light-shock pairings were first discontinued. This was especially apparent in responding to the tone-plus-light compound (TL). Initially, the CC group made significantly fewer responses to TL than did the Ext group. But late in the test, the CC group actually responded more than the Ext group to TL. Relatedly, a robust summative effect of compounding the tone and the light was observed on the first block of the test in the Ext group only, where response rates to TL were over 3 times those in tone or light alone. This additive summation in the Ext group replicates the outcome of a recent study from this lab (Kearns & Weiss, 2012) showing that simultaneously presenting an extinguished cocaine cue, which might only control a low response rate on its own, with a still-active (i.e., non-extinguished) one results in a substantial enhancement of cocaine seeking. In contrast, no additive summation was observed in the CC group on the first block of the test, suggesting that, at least initially, the light functioned as an aversive stimulus capable of suppressing cocaine seeking (for similar results see Kearns, Weiss, & Panlilio, 2002; Vanderschuren & Everitt, 2004).
The effects of counterconditioning very quickly dissipated when the treatment was discountinued. After just 2 shock-free presentations of each stimulus (i.e., after the first block of the test), the CC group no longer displayed any evidence of conditioned fear or aversion to the light. By the third block of the test, the CC group showed a summative effect of compounding the tone and the light (as did the Ext group), which is what would be expected when compounding two appetitive cocaine cues. The pattern of behavior displayed by the CC group on the stimulus compounding test suggests that counterconditioning did not counteract or reverse the cocaine-related associative properties originally conditioned to the light. Instead, counterconditioning seems to have only overlaid an additional cue-shock association on top of the original cue-drug association. When this cue-shock association was weakened by non-shock-paired presentations of the light, the original cue-drug association revealed itself again. This outcome is consistent with previous studies with non-drug cues showing that the original association conditioned to a cue survives counterconditioning. For example, Bouton and associates have shown reinstatement (Brooks, Hale, Nelson & Bouton, 1995), spontaneous recovery (Bouton & Peck, 1992), and renewal (Peck & Bouton, 1990) of the originally trained response after counterconditioning.
A stronger shock intensity might have been expected to produce a more lasting effect, though this likely would have also produced more generally suppressive effects (e.g., freezing) on behavior rather than cue-specific effects (Annau & Kamin, 1961). Perhaps conducting the counterconditioning treatment in a way that light presentations were more unpredictably followed by shock would have produced a more persistent effect. For example, having the light followed by shock on a randomly selected 25% of trials during the counterconditioning treatment may have made it more difficult for rats to learn that the light was no longer paired with shock when shock presentations were discontinued. However, even with a reduced probability of shock pairings or a stronger shock intensity, rats would be expected to eventually learn that the light was no longer paired with shock. Thus, such changes would likely have only postponed the reappearance of cocaine seeking to the counterconditioned cue, rather than qualitatively change the associative properties of the cue.
The treatment that the CC group received in the present study may be thought of as a counterconditioning-plus-extinction treatment, since this group (like the Ext group) could make non-reinforced lever presses in the presence of the light during Phase 2. The treatment that the CC group in the present experiment received was modeled on that used in human counterconditioning treatment, where drug cues are paired with an aversive stimulus and the drug-taking response undergoes extinction (e.g., subjects snort a white powder, but do not experience the effects of cocaine). A potential limitation of the current study is the absence of a third comparison group that received the counterconditioning treatment without access to the lever during phase 2. Such a group could potentially provide information on the contribution of counterconditioning alone (i.e., without the influence of extinction) to reduced drug seeking and should be investigated in future research with this design.
An interesting and unanticipated result observed on the stimulus compounding test was that the CC group responded during tone components significantly more than did the Ext group. It is possible that counterconditioning produced a displacement of cocaine-seeking responses from the counterconditioned stimulus (the light) to the untreated stimulus (the tone). This is reminiscent of early studies on behavioral contrast showing that when responding during one component of a two-component schedule is punished, response rates in the unpunished component increase (Brethower & Reynolds, 1962). This suggests that counterconditioning resulted merely in a redistribution of cocaine-seeking responses, rather than a net decrease in cocaine seeking as compared to the effects of extinction. This does not bode well for counterconditioning as a potential therapeutic intervention, unless it could be applied to multiple possible drug cues, or perhaps to all drug cues. Otherwise, a reduction of drug seeking in the presence of a few treated cues could be associated with an increase in drug seeking in the presence of other, untreated cues.
Alternatively, it is possible that the significantly lower responding to tone in the Ext group (as compared to the CC group on) the stimulus compounding test was due to “secondary extinction” (Vurbic & Bouton, 2011), where the effects of extinction of the light during Phase 2 generalized to the tone. Some support for this idea comes from the fact that rats in the Ext group responded to light alone and tone alone at comparable rates during the test, despite the fact that only the light was subjected to extinction during Phase 2. A limitation that prevents more definitely concluding that secondary extinction occurred here is the lack of a no-treatment control group not exposed to any cues during Phase 2. Future research with this paradigm including such a group could help in deciding whether the difference between the groups in tone response rates during the test was due more to reduced tone responding in the Ext group vs. heightened tone responding in the CC group.
In summary, counterconditioning resulted in a brief, stimulus-specific reduction in drug seeking that only lasted while the treated cue was actively paired with an aversive stimulus. More promising methods of treating drug cues could be those that directly weaken, or even eliminate, the cue-drug association. Very recent drug-cue extinction research suggests ways that this might be accomplished. For example, two studies (Janak, Bowers, & Corbit, 2012; Kearns, Tunstall, & Weiss, 2012) have found that applying a deepened extinction treatment – where the targeted cue is extinguished in compound with another drug cue – significantly enhances the effects of extinction by weakening the cue-drug association to a greater extent than standard extinction. Another recent study has shown that retrieving the cue-drug association (by briefly presenting the cue) during a sensitive window prior to an extinction session can virtually eliminate traces of the association (Xue et al., 2012). Interventions that incorporate these association-weakening methods may prove to be able to more permanently neutralize the power of drug cues.
References
- Annau Z, Kamin LJ. The conditioned emotional response as a function of intensity of the US. Journal of Comparative and Physiological Psychology. 1961;54:428–32. doi: 10.1037/h0042199. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57B:289–300. [Google Scholar]
- Bordnick PS, Elkins RL, Orr TE, Walters P, Thyer BA. Evaluating the relative effectiveness of three aversion therapies designed to reduce craving among cocaine abusers. Behavioral Interventions. 2004;19:1–24. [Google Scholar]
- Bouton ME, Peck CA. Spontaneous recovery in cross-motivational transfer (counterconditioning) Animal Learning & Behavior. 1992;20:313–321. [Google Scholar]
- Brethower DM, Reynolds GS. A facilitative effect of punishment on unpunished behavior. Journal of the Experimental Analysis of Behavior. 1962;5:191–199. doi: 10.1901/jeab.1962.5-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DC, Hale B, Nelson JB, Bouton ME. Reinstatement after counterconditioning. Animal Learning & Behavior. 1995;23:383–390. [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Drummond DS, Tiffany ST, Glautier S, Remington B. Addictive Behaviour: Cue Exposure Theory and Practice. Wiley and Sons; West Sussex, England: 1995. [Google Scholar]
- Frawley PJ, Smith JW. Chemical aversion therapy in the treatment of cocaine dependence as part of a multimodal treatment program: treatment outcome. Journal of Substance Abuse Treatment. 1990;7:21–29. doi: 10.1016/0740-5472(90)90033-m. [DOI] [PubMed] [Google Scholar]
- Frawley PJ, Smith JW. One-year follow-up after multimodal inpatient treatment for cocaine and methamphetamine dependencies. Journal of Substance Abuse Treatment. 1992;9:271–286. doi: 10.1016/0740-5472(92)90020-o. [DOI] [PubMed] [Google Scholar]
- Grusser S, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Janak PH, Bowers MS, Corbit LH. Compound stimulus presentation and the norepinephrine reuptake inhibitor atomoxetine enhance long-term extinction of cocaine-seeking behavior. Neuropsychopharmacology. 2012;37:975–985. doi: 10.1038/npp.2011.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns DN, Tunstall BJ, Weiss SJ. Deepened extinction of cocaine cues. Drug and Alcohol Dependence. 2012;124:283–287. doi: 10.1016/j.drugalcdep.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns DN, Weiss SJ, Panlilio LV. Conditioned suppression of behavior maintained by cocaine self-administration. Drug & Alcohol Dependence. 2002;65:253–261. doi: 10.1016/s0376-8716(01)00167-3. [DOI] [PubMed] [Google Scholar]
- Kearns DN, Weiss SJ. Extinguished cocaine cues increase drug seeking when presented simultaneously with a non-extinguished cocaine cue. Drug Alcohol Depend. 2012;121:140–147. doi: 10.1016/j.drugalcdep.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns DN, Weiss SJ, Schindler CW, Panlilio LV. Conditioned inhibition of cocaine seeking in rats. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31:247–253. doi: 10.1037/0097-7403.31.2.247. [DOI] [PubMed] [Google Scholar]
- Kerkhof I, Vansteenwegen D, Baeyens F, Hermans D. Counterconditioning: an effective technique for changing conditioned preferences. Experimental Psychology. 2011;58:31–38. doi: 10.1027/1618-3169/a000063. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent subjects. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Loeber S, Croissant B, Heinz A, Mann K, Flor H. Cue exposure in the treatment of alcohol dependence: Effects on drinking outcome, craving and self-efficacy. British Journal of Clinical Psychology. 2007;45:515–529. doi: 10.1348/014466505X82586. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Dickinson A. Counterconditioning of appetitive and defensive CRs in rabbits. Quarterly Journal of Experimental Psychology. 1982;34B:115–126. doi: 10.1080/14640748208400880. [DOI] [PubMed] [Google Scholar]
- Marissen MA, Franken IH, Blanken P, van den Brink W, Hendriks VM. Cue exposure therapy for the treatment of opiate addiction: results of a randomized controlled clinical trial. Psychotherapy and Psychosomatics. 2007;76:97–105. doi: 10.1159/000097968. [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Panlilio LV, Weiss SJ, Schindler CW. Cocaine selfadministration increased by compounding discriminative stimuli. Psychopharmacology. 1996;125:202–208. doi: 10.1007/BF02247329. [DOI] [PubMed] [Google Scholar]
- Peck CA, Bouton ME. Context and performance in aversive-to-appetitive and appetitive-to-aversive transfer. Learning and Motivation. 1990;21:1–31. [Google Scholar]
- Rescorla RA. Deepened extinction from compound stimulus presentation. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:135–144. doi: 10.1037/0097-7403.32.2.135. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Gulliver SB, Colby SM, Binkoff JA, Abrams DB. Cue exposure with coping skills training and communication skills training for alcohol dependence: 6- and 12-month outcomes. Addiction. 2001;96:1161–1174. doi: 10.1046/j.1360-0443.2001.96811619.x. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CSR. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug and Alcohol Review. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Smith JW, Frawley PJ. Treatment outcome of 600 chemically dependent patients treated in a multimodal inpatient program including aversion therapy and pentothal interviews. Journal of Substance Abuse Treatment. 1993;10:359–369. doi: 10.1016/0740-5472(93)90021-s. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Van Gucht D, Baeyens F, Vansteenwegen D, Hermans D, Beckers T. Counterconditioning reduces cue-induced craving and actual cue-elicited consumption. Emotion. 2010;10:688–695. doi: 10.1037/a0019463. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. Journal of Neuroscience. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vurbic D, Bouton ME. Secondary extinction in Pavlovian fear conditioning. Learning & Behavior. 2011;39:202–211. doi: 10.3758/s13420-011-0017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JR. Experimental morphine addiction: method for automatic intravenous injections in unrestrained rats. Science. 1962;138:143–144. doi: 10.1126/science.138.3537.143. [DOI] [PubMed] [Google Scholar]
- Weiss SJ. An effective and economical sound attenuation chamber. Journal of the Experimental Analysis of Behavior. 1970;13:37–39. doi: 10.1901/jeab.1970.13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SJ. Discriminated response and incentive processes in operant conditioning: a two-factor model of stimulus control. Journal of the Experimental Analysis of Behavior. 1978;30:361–381. doi: 10.1901/jeab.1978.30-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SJ, Schindler CW. Integrating control generated by positive and negative reinforcement on an operant baseline: Appetitive-aversive interactions. Learning & Behavior. 1989;17:433–446. [Google Scholar]
- Weiss SJ, Thomas DA, Weissman RD. Combining operant-baseline-derived conditioned excitors and inhibitors from the same and different incentive classes: an investigation of appetitive-aversive interactions. Quarterly Journal of Experimental Psychology. 1996;49B:357–381. doi: 10.1080/713932635. [DOI] [PubMed] [Google Scholar]
- Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, Zhu WL, Ding ZB, Bao YP, Shi J, Epstein DH, Shaham Y, Lu L. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336:241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]