Abstract
Objective
Col2a1 gene mutations cause premature degeneration of knee articular cartilage in disproportionate micromelia (Dmm) and spondyloepiphesial dysplasia congenita (sedc) mice. The present study analyzes the temporomandibular joint (TMJ) in Col2a1 mutant mice in order to provide an animal model of TMJ osteoarthritis (OA) that may offer better understanding of the progression of this disease in humans.
Design
Dmm/+ mice and controls were compared at two, six, nine and 12 months. Craniums were fixed, processed to paraffin sections, stained with Safranin-O/Fast Green, and analyzed with light microscopy. OA was quantified using a Mankin scoring procedure. Unfolded protein response (UPR) assay was performed and immunohistochemistry (IHC) was used to assay for known OA biomarkers.
Results
Dmm/+ TMJs showed fissuring of condylar cartilage as early as 6 months of age. Chondrocytes were clustered, leaving acellular regions in the matrix. Significant staining of HtrA1, Ddr2 and Mmp-13 was observed in Dmm/+ mice (p< 0.01). We detected upregulation of the UPR in knee but not TMJ.
Conclusions
Dmm/+ mice are subject to early-onset OA in the TMJ. We observed upregulation of biomarkers and condylar cartilage degradation concomitant with OA. An upregulated UPR may exacerbate the onset of OA. The Dmm/+ mouse TMJ is a viable model for the study of the progression of OA in humans.
Keywords: Disproportionate micromelia (Dmm), Temporomandibular joint, osteoarthritis, Col2a1, Ddr2
Introduction
Temporomandibular joint (TMJ) growth occurs through regulated synthesis and degradation of various components in the extra cellular matrix (ECM) (1). Osteoarthritis is reported as the most common disease associated with the TMJ (2) and occurs when articular cartilage remodeling is disturbed. We and others have shown that OA in the knee follows a pathway of articular cartilage degeneration involving HtrA1, Ddr2, and Mmp13 (3, 4).
The Disproportionate micromelia (Dmm) mouse has a mutation that causes mild dwarfism in the heterozygote. Dmm mice have a three-nucleotide deletion of the Col2a1 gene (human homologue is COL2A1) (5). These genes code for type II collagen, the most abundant protein in hyaline cartilage (6, 7). It has been reported that in Dmm/+ cartilage the ECM has a significant reduction in type II collagen. (8).
Dmm/+ mice have decreased proteoglycan in the interterritorial matrix (ITM), yielding early - onset OA of the knee joint (7) and decreasing the ability of chondrocytes to maintain articular cartilage integrity. Mechanical forces applied to damaged joints lead to pain and dysfunction (9). We recently reported a mechanism of OA in another Col2a1 deficient mouse model, sedc, with progressive expression of HtrA1, Ddr2 and Mmp13 (4).
Research on TMJ OA involving animal models is still developing (2, 10–14). Currently, TMJ disorder diagnoses are based on non-specific symptoms. Treatments cover these symptoms rather than reversing degradation (9, 15–17).
An OA animal model is an important tool in understanding TMJ disease (2, 10, 15, 18). Human studies can only describe TMJ OA, whereas animal models may elucidate a cause and effect relationship that might allow for TMJ disease reversal and a universal understanding of TMJ OA pathobiochemistry that is not currently available (14).
Xu et al. have shown such a relationship in a Col11a1 mutant chondrodysplasia (cho) mouse TMJ OA (19). The degradation pathway includes increased proteoglycan production to compensate for destruction, proteoglycan loss, fibrillation, and eventual degradation of cartilage (20–25). Chondrocyte clustering and increased proteoglycan production in the pericellular matrix (PCM) have also been identified as early OA indicators (7). Furthermore, articular cartilage degradation associated with knee OA in genetic and mechanical models has also been reported (3, 13).
Important mechanistic insight into OA pathogenesis has been provided by published data linking increased expression of HtrA1, Ddr2 and Mmp13 and knee OA in both genetic and mechanical models (3, 4). The unfolded protein stress response (UPR) has also been implicated in OA progression (26). We have reported that Dmm mice exhibit distended ER and Golgi, consistent with the misfolded type II collagen being trapped in the ER rather than secreted to the ECM (7). Although OA degradation is absent in newborn Dmm/+ mice, we suspected that intracellular accumulation of misfolded type II collagen might trigger perpetual UPR. If present, it could predispose mice to early-onset OA. The present study suggests that these pathways can initiate OA in the TMJ as well as knee joint.
Materials and Methods
Facilities, Animals and Genotyping
Mice were maintained in the animal care facility of Brigham Young University under an approved IACUC protocol. They were provided Harlan Teklad rodent diet 8604, a standard nutritional, grained-based diet, and kept in paper-bedded 5.7 L cages in a room with 12-hr-light/12-hr-dark cycles.
Heterozygous mice (Dmm/+) of a C3H strain were crossed to produce wildtype (+/+) and Dmm/+ mice. Homozygotes (Dmm/Dmm) die immediately after birth due to dwarfism that inhibits the ability to respire (6, 7). Approximately 21 days postpartum pups were isolated from dams and numbered using earmark identification. Tail samples were obtained for genotyping.
DNA was isolated from tail samples and polymerase chain reaction (PCR) was performed according to previously published methods (5) (7).
Tissue Processing
TMJs from Dmm/+ mice and controls ages two months (n= three each genotype), six months (n=three each genotype), nine months (n= four Dmm/+ and three +/+), and 12 months (n=three each genotype) were collected. Mice were asphyxiated with CO2 and heads were removed from bodies. Brain and skin were removed and heads were fixed in 4% paraformaldehyde. Heads were decalcified as previously described (4) and dissected, leaving only a section containing the right TMJ.
Samples were processed and embedded in paraffin wax, sectioned and placed on slides as previously described (4).
Every fifth slide was stained with Safranin-O and Fast Green (SO&FG). Stained sections were viewed under an Olympus BX51 light microscope and photographed by a computer-operated Olympus DP72 digital camera.
Determination of OA Severity
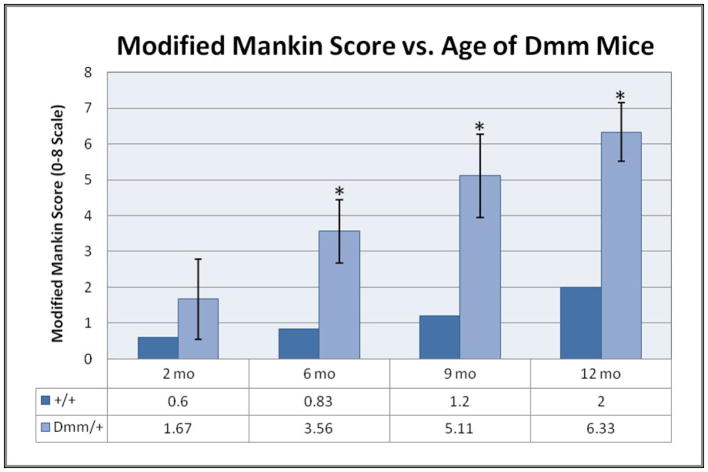
SO&FG-stained tissues were used to assess the osteoarthritic state of condylar cartilage using a modified Mankin scoring system (9, 20, 21, 27) see also Table 1 in supplemental material. The modified Mankin scoring system is based on three criteria: interterritorial matrix staining (IMS) of de-calcified cartilage with SO, pericellular matrix (PCM) staining (PMS) with SO, and spatial arrangement of chondrocytes (SAC) (See Fig. 1). Scores from the three criteria were summed providing an overall score. Higher scores indicate more severe OA. Each joint provided five scores, and values were averaged with scores of mice of similar age and genotype, providing an average score for each age and genotype.
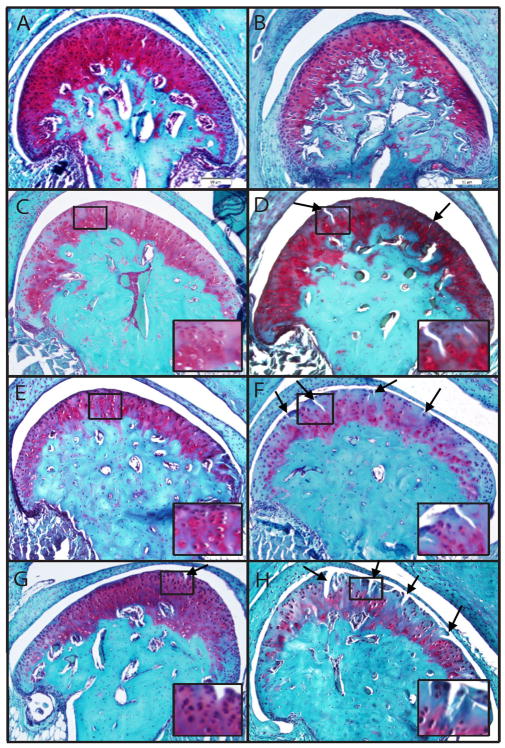
Figure 1.
Proteoglycan prevalence observed by SO staining was diminished in Dmm/+ mice compared to wild type. Proteoglycan prevalence was observed in Dmm/+ mice at 2, 9, and 12 months (B, F, H) compared to wild type mice (A, E, G); however, an increase in proteoglycan staining was observed in Dmm/+ mice at 6 months (D) compared to wildtype controls (C). In addition, common fissuring of articular cartilage as a result of the loss of columnar arrangement of chondrocytes was observed in 6, 9, and 12 month old Dmm/+ mice (D, F, H, arrows) but not until 12 months in wildtype animals (G, arrow). Images are representative and insets indicate areas of irregular SAC and degradation in Dmm/+ mice or normal cartilage in wildtype.
Immunohistochemical Analysis
Immunohistochemistry was performed on sections from every sixth slide from three wildtype and three Dmm/+ animals at six months. Slides were stained with antibodies against HtrA1, Ddr2, and Mmp13 as previously described (4). Differences in staining intensity were measured qualitatively with comparison to wild type control. The qualitative results of the immunohistochemical staining for the Dmm/+ and wild type samples were analyzed statistically using Chi-Squared tests to evaluate differences between the two samples in staining patterns for key OA biomarkers.
UPR Analysis
Articular cartilage was isolated from knee joints of 13 +/+ and 17 Dmm/+ newborn (0–4 days) mice. TMJ fibrocartilage disc tissue was isolated from 1 +/+ and 3 Dmm/+ 2-month-old mice. Individual samples were homogenized in RIPA buffer (50 mM Tris-HCl, pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, and Thermo Scientific Protease Inhibitor Cocktail (product #78410). Protein concentration was measured by Bradford assay, and 10 μg protein per lane were resolved on polyacrylamide gels and analyzed by western blot.
Statistical Analysis
A two-way analysis of variance was used to compare the means of Dmm/+ data (i.e., scores in relation to age and in comparison with the means of +/+ data). The data were blocked to account for multiple measurements obtained from each mouse. T-tests were used to compare IMS sub-scores, PMS sub-scores, and SAC sub-scores from Dmm/+ and age-matched +/+ cartilage. P-values less than 0.01 were considered statistically significant for this study. The UPR results were analyzed using an unpaired Student’s t-test. P-values less than 0.05 were considered significant.
Results
Increased Mankin Scoring in Dmm/+ Mice
We observed an increase in proteoglycan staining and fissuring of condylar cartilage at 6 months in Dmm/+ mice compared to wildtype (Fig. 1). At six, nine and 12 months, Dmm/+ mice exhibited significantly elevated modified Mankin scores compared to wildtype (Fig. 2). Dmm/+ mice exhibited significantly more erosion than +/+ (data not shown). The +/+ mice of all ages showed normal staining of the ITM and PCM. Dmm/+ cartilage at nine and twelve months displayed a lack of columnar arrangement of chondrocytes with multiple areas of hypocellularity caused by clustering. Areas of the ITM showed little or no staining and the PCM showed enhanced staining. Two month Dmm/+ mice showed little deviation from wildtype, but older mice show greater sub-scores for each of the criteria (IMS, PMS, SAC) at all age groups when compared to wildtype.
Figure 2.
The TMJ in the Dmm/+ mice displayed premature articular cartilage erosion and higher Mankin scores due to decreased cellularity, decreased IMS staining and increased PMS staining. Dmm/+ mice had significantly greater defects in chondrocyte arrangement and proteoglycan production and distribution, evidenced by significantly greater Mankin scores for the 6, 9 and 12 month Dmm/+ mice. (P < .01)
IMS sub-scores stayed consistent for +/+ mice at all ages. Dmm/+ cartilage showed proteoglycan deficiency in areas of fissuring as early as 6 months, which increased throughout the condyle with age until no staining was observed at nine and twelve months. PMS sub-scores showed that +/+ cartilage had enhanced proteoglycan staining starting at around nine months. Dmm/+ mice showed this kind of staining as early as six months, increasing with age. Sub-scores for the SAC show normal cartilage in +/+ mice, while Dmm/+ scores show cartilage irregularities at as early as two months. +/+ mice showed only slight hypercellularity and minimal points of hypocellularity in older mice, whereas Dmm/+ mice became intensely hypocellular throughout the articular cartilage.
Known biomarkers of OA present in TMJ
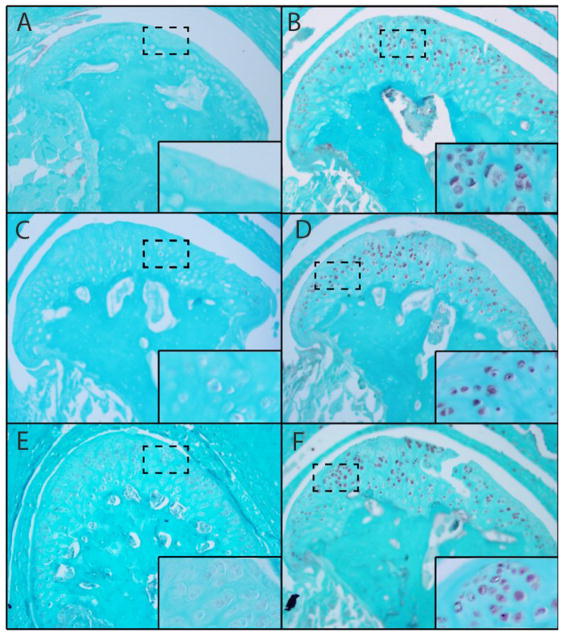
Immunohistochemical staining of HtrA1, Ddr2 and Mmp13 was virtually undetectable in TMJs from six month old wild type mice. Conversely, we observed significant expression of these biomarkers in age matched Dmm/+ mice (See Fig. 3) (p< 0.01).
Figure 3.
Significantly increased antibody staining of Ddr2, Mmp13, HtrA1 in Dmm/+ mice. Significant staining of OA biomarkers HtrA1 (B,A); Ddr2 (D,C) and Mmp13 (F,E) was observed in Dmm/+ compared to six month, aged matched, wildtype mice respectively (p< 0.01). This suggests that the Ddr2 degradative pathway plays an active role in the articular cartilage of Dmm/+ mice. Insets show virtually no staining in WT mice and greatly increased staining of biomarkers in Dmm/+.
UPR is activated in articular cartilage of newborn Dmm/+ mice
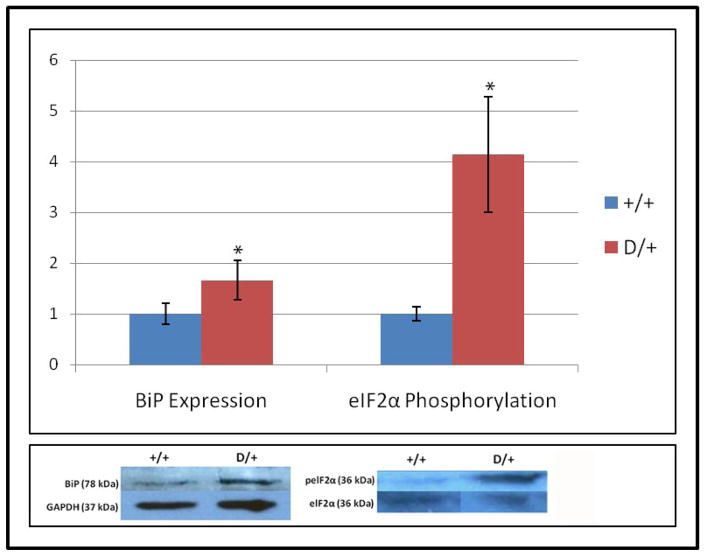
Initial experiments with TMJ from 2-month-old mice revealed that dissections primarily yielded TMJ disc fibrocartilage with very little condylar cartilage. Fibrocartilage, however, expresses only low levels of Col2a1 and is therefore less likely than condylar cartilage to exhibit accumulation of misfolded type II collagen in the ER. Indeed, neither BiP (an ER chaperone protein that increases during early UPR) nor phospho-eIF2α (a translation initiation factor that is required for synthesis of several UPR proteins) were elevated in TMJ disc fibrocartilage (data not shown). BiP expression in Dmm/+ newborn articular cartilage showed a 1.66-fold increase compared to wildtype (p=0.01) (see Fig. 4). In addition, the ratio of phosphorylated to unphosphorylated eIF2α was increased 4.3-fold in Dmm/+ mice (n=6) compared to wildtype (n=5) (p<0.05) (see Fig. 4).
Figure 4.
UPR is activated in Dmm/+ animals. BiP levels were elevated 1.66-fold in Dmm/+ (n=11) compared to wildtype (n=8) newborn knee articular cartilage (p=0.01). In addition, the ratio of phosphorylated to unphosphorylated eIF2α was increased 4.3-fold in Dmm/+ mice knee articular cartilage (n=6) compared to wildtype (n=5) (p<0.05). The lower panel shows representative western blots for each antibody.
Discussion
A large number of human chondrodysplasias are caused by mutations in COL2A1(28–33). Most of these mutations, however, are in the triple helical region of the gene. Since such mutations lead to the synthesis of truncated chains that do not participate in trimer assembly due to the absence of the C-propeptide, their molecular consequences are different from the mutation described here. One common human chondrodysplasia described (34) as a C-propeptide mutation in COL2A1 in a Stickler Syndrome family may represent the closest human counterpart to the Dmm mutation. In this human case, a single nucleotide deletion in exon 50 of COL2A1 causes a frameshift in translation and a premature stop codon in exon 51. The mutant C propeptide consists of only the amino-terminal 30% of the normal sequence. This region contains five of the eight cysteines in the type II C-propeptide (including the four residues involved in interchain disulfide bonds) but is lacking the region that contains the Dmm mutation. Heterozygous Dmm that we used in the present study may therefore represent a good murine model for this condition and is therefore useful for further studies of pathogenesis and testing of novel therapeutic approaches.
It is of interest to note that at least one of the five reported cases of COL2A1C propeptide mutation resulted in distended RER (35). This human mutation caused shortened limbs, shortened trunk, and midface hypoplasia reminiscent of the Dmm phenotype we describe in the present study. The heterozygous Dmm mouse thus is a relevant model for human COL2A1 C-propeptide mutations that result in congestion of the RER (discuss distended RER below with regard to UPR and reference distended RER in previous Dmm papers), with abnormal pro-α1(II) chains that cannot assemble into normal trimers.
We have demonstrated that Dmm/+ mice, which harbor a Col2a1 C-propeptide mutation, show condylar cartilage abnormalities in the TMJ at early ages. The disorganization of chondrocytes and abnormalities in proteoglycan development appear to alter articular cartilage integrity and cause degradation. This is consistent with our previous report that aggrecan levels are lower in Dmm/+ mice as compared to WT controls (36). Thus, specific components of the ECM are increased and decreased in this mouse model of OA. Scoring these characteristics confirmed that Dmm/+ cartilage has qualities of early-onset OA, with six, nine, and twelve month mice showing statistically significant differences compared to controls.
Our previous ultrastructural analyses of articular cartilage from Dmm/+ knee joints showed distended ER and Golgi in chondrocytes (36). Likewise, the ECM of knee articular cartilage from Dmm/+ mice showed a significant reduction in type II collagen (8). These data are consistent with the intracellular accumulation rather than secretion of misfolded type II collagen. Such an accumulation would be expected to trigger the UPR stress response, which causes a global reduction in protein synthesis while selectively upregulating proteins that aid in protein folding (37). Under ordinary circumstances, a temporary reduction in new protein synthesis allows clearance of the unfolded protein backlog. In the case of Dmm/+ mice, however, the Col2a1 mutation together with the constant demand for type II collagen in cartilage would ensure that new mutant protein was continually synthesized and UPR was perpetually activated. This would likely result in chondrocytes that were functionally compromised due to perpetual suppression of protein synthesis. In the most severe cases of UPR, apoptosis would be triggered. It seems likely that perpetual activation of the UPR pathway in articular and condylar chondrocytes would compromise articular and condylar structure, contributing to earlier onset OA.
In the present study we found that the TMJ disc fibrocartilage, which expresses low levels of type II collagen, does not show activation of the UPR, but knee articular cartilage from newborn Dmm/+ mice does. This result is interesting given that the UPR is detectable in newborns even though histological evidence of OA does not appear in the Dmm/+ knee joint until after 3 months of age, consistent with UPR contributing to the earlier onset of OA. TMJ condylar cartilage is more like knee articular cartilage as far as type II collagen expression levels, suggesting that OA in the TMJ of Dmm/+ mice might also be exacerbated by UPR.
We observed a significant decrease in proteoglycan shown by decreased SO staining throughout condylar cartilage in Dmm/+ mice. Dmm/+ joints showed many areas lacking stain, diminished stain intensity, and a disorganization of chondrocytes. Another sign of OA includes proteoglycan upregulation to combat breakdown caused by inflammatory cytokines. Hypercellularity and cell clustering also occur in the knee of Dmm/+ mice (7). Our data are consistent with this in that the Dmm/+ TMJ PCM had areas of SO-positive staining, while the ECM had areas void of both cells and staining.
Fibrillation in six-month Dmm/+ mice TMJs was also noted. By nine and twelve months, degradation was so severe that parts of the condylar cartilage were completely severed, while wildtype mice maintained healthy cartilage. SO-positive staining was less intense by this stage because damaged chondrocytes stopped producing proteoglycans (38).
Our present work demonstrates that OA progression in the Dmm TMJ follows the same pathway as cho TMJs and Dmm knees, i.e. presence of key biomarkers of OA, consistent with what has only been demonstrated in knee joints (3, 4, 14, 16, 17). The Dmm/+ TMJ model also mimics human TMJ dysfunction associated with OA, and is an acceptable model for the pathogenesis of this disorder. We also confirm the involvement of HtrA1, Ddr2, and Mmp13 in its progression. Because a degenerative signaling axis that involves HtrA1, Ddr2, and Mmp13 may be universal to OA, elucidation of these biomarkers in OA of the TMJ may lead to notable studies of future clinical significance. In fact, despite increasing emphasis on the burden of TMJ-related disease, the discovery that Dmm/+ mice model TMJ pathology makes possible additional study into targets potentially useful in the treatment or attenuation of TMJ OA.
Supplementary Material
Acknowledgments
The authors thank Dr. Dennis Eggett (Department of Statistics, Brigham Young University) for conducting statistical analyses, and William Hale, Michael Henderson, Shaela Avery, Joshua Lloyd, David Robinson, Karen Xue, Alexandra Wink and Christopher Stockdale for procedural help.
Footnotes
Authors’ Contributions
RES, LCB and DLK wrote the grants that supported this work. They and PRR oversaw experimental design, execution and interpretation of data. MLR, DWH and DJF also assisted in experimental design, performed experimental procedures, and interpreted data. All authors contributed to writing the manuscript. JTF, MR, JC, TW and BAN conducted key experiments and assisted in the interpretation of results.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gepstein A, Arbel G, Blumenfeld I, Peled M, Livne E. Association of metalloproteinases, tissue inhibitors of matrix metalloproteinases, and proteoglycans with development, aging, and osteoarthritis processes in mouse temporomandibular joint. Histochemistry and cell biology. 2003;120(1):23–32. doi: 10.1007/s00418-003-0544-1. Epub 2003/06/27. [DOI] [PubMed] [Google Scholar]
- 2.El-Hakim IE, Elyamani AO. Preliminary evaluation of histological changes found in a mechanical arthropatic temporomandibular joint (TMJ) exposed to an intra-articular Hyaluronic acid (HA) injection, in a rat model. J Craniomaxillofac Surg. 2011;39(8):610–4. doi: 10.1016/j.jcms.2010.12.001. Epub 2011/01/11. [DOI] [PubMed] [Google Scholar]
- 3.Polur I, Lee PL, Servais JM, Xu L, Li Y. Role of HTRA1, a serine protease, in the progression of articular cartilage degeneration. Histol Histopathol. 2010;25(5):599–608. doi: 10.14670/hh-25.599. Epub 2010/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holt DW, Henderson ML, Stockdale CE, Farrell JT, Kooyman DL, Bridgewater LC, et al. Osteoarthritis-like changes in the heterozygous sedc mouse associated with the HtrA1-Ddr2-Mmp-13 degradative pathway: a new model of osteoarthritis. Osteoarthritis Cartilage. 2011 doi: 10.1016/j.joca.2011.11.008. Epub 2011/12/14. [DOI] [PubMed] [Google Scholar]
- 5.Pace JM, Li Y, Seegmiller RE, Teuscher C, Taylor BA, Olsen BR. Disproportionate micromelia (Dmm) in mice caused by a mutation in the C- propeptide coding region of Col2a1. Dev Dyn. 1997;208(1):25–33. doi: 10.1002/(SICI)1097-0177(199701)208:1<25::AID-AJA3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Brown KS, Cranley RE, Greene R, Kleinman HK, Pennypacker JP. Disproportionate micromelia (Dmm): an incomplete dominant mouse dwarfism with abnormal cartilage matrix. J Embryol Exp Morphol. 1981;62:165–82. [PubMed] [Google Scholar]
- 7.Bomsta BD, Bridgewater LC, Seegmiller RE. Premature osteoarthritis in the Disproportionate micromelia (Dmm) mouse. Osteoarthritis Cartilage. 2006;14(5):477–85. doi: 10.1016/j.joca.2005.11.011. Epub 2006/01/25. [DOI] [PubMed] [Google Scholar]
- 8.Fernandes RJ, Seegmiller RE, Nelson WR, Eyre DR. Protein consequences of the Col2a1 C-propeptide mutation in the chondrodysplastic Dmm mouse. Matrix Biol. 2003;22(5):449–53. doi: 10.1016/s0945-053x(03)00077-5. [DOI] [PubMed] [Google Scholar]
- 9.Bertram S, Rudisch A, Innerhofer K, Pumpel E, Grubwieser G, Emshoff R. Diagnosing TMJ internal derangement and osteoarthritis with magnetic resonance imaging. J Am Dent Assoc. 2001;132(6):753–61. doi: 10.14219/jada.archive.2001.0272. Epub 2001/07/04. [DOI] [PubMed] [Google Scholar]
- 10.Ghassemi-Nejad S, Kobezda T, Rauch TA, Matesz C, Glant TT, Mikecz K. Osteoarthritis-like damage of cartilage in the temporomandibular joints in mice with autoimmune inflammatory arthritis. Osteoarthritis Cartilage. 2011;19(4):458–65. doi: 10.1016/j.joca.2011.01.012. Epub 2011/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai N, Tanaka E, Langenbach GE, van Wessel T, Sano R, van Eijden TM, et al. Jaw-muscle activity changes after the induction of osteoarthrosis in the temporomandibular joint by mechanical loading. J Orofac Pain. 2008;22(2):153–62. Epub 2008/06/14. [PubMed] [Google Scholar]
- 12.de Grandmont P. Osteoarthrosis/osteoathritis in the temporomandibular joints. Int J Prosthodont. 2007;20(4):357–8. Epub 2007/08/19. [PubMed] [Google Scholar]
- 13.Xu L, Polur I, Lim C, Servais JM, Dobeck J, Li Y, et al. Early-onset osteoarthritis of mouse temporomandibular joint induced by partial discectomy. Osteoarthritis Cartilage. 2009;17(7):917–22. doi: 10.1016/j.joca.2009.01.002. Epub 2009/02/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez Blanco M, Bagan JV, Fons A, Poveda Roda R. Osteoarthrosis of the temporomandibular joint. A clinical and radiological study of 16 patients. Med Oral. 2004;9(2):110–15. 06–10. Epub 2004/03/03. [PubMed] [Google Scholar]
- 15.Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 2008;87(4):296–307. doi: 10.1177/154405910808700406. Epub 2008/03/26. [DOI] [PubMed] [Google Scholar]
- 16.Haskin CL, Milam SB, Cameron IL. Pathogenesis of degenerative joint disease in the human temporomandibular joint. Crit Rev Oral Biol Med. 1995;6(3):248–77. doi: 10.1177/10454411950060030601. Epub 1995/01/01. [DOI] [PubMed] [Google Scholar]
- 17.Bermejo-Fenoll A, Saez-Yuguero R. Differential diagnosis of temporomandibular joint disorders (TMD) Med Oral Patol Oral Cir Bucal. 2005;10(5):468–9. Epub 2005/11/03. [PubMed] [Google Scholar]
- 18.Puzas JE, Landeau JM, Tallents R, Albright J, Schwarz EM, Landesberg R. Degradative pathways in tissues of the temporomandibular joint. Use of in vitro and in vivo models to characterize matrix metalloproteinase and cytokine activity. Cells Tissues Organs. 2001;169(3):248–56. doi: 10.1159/000047888. Epub 2001/07/17. [DOI] [PubMed] [Google Scholar]
- 19.Xu L, Flahiff CM, Waldman BA, Wu D, Olsen BR, Setton LA, et al. Osteoarthritis-like changes and decreased mechanical function of articular cartilage in the joints of mice with the chondrodysplasia gene (cho) Arthritis Rheum. 2003;48(9):2509–18. doi: 10.1002/art.11233. Epub 2003/09/18. [DOI] [PubMed] [Google Scholar]
- 20.Buckwalter JA, Mankin HJ. Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect. 1998;47:477–86. [PubMed] [Google Scholar]
- 21.Sunk IG, Bobacz K, Hofstaetter JG, Amoyo L, Soleiman A, Smolen J, et al. Increased expression of discoidin domain receptor 2 is linked to the degree of cartilage damage in human knee joints: a potential role in osteoarthritis pathogenesis. Arthritis Rheum. 2007;56(11):3685–92. doi: 10.1002/art.22970. Epub 2007/10/31. [DOI] [PubMed] [Google Scholar]
- 22.Arner EC. Aggrecanase-mediated cartilage degradation. Curr Opin Pharmacol. 2002;2(3):322–9. doi: 10.1016/s1471-4892(02)00148-0. Epub 2002/05/22. [DOI] [PubMed] [Google Scholar]
- 23.Ehrlich MG, Armstrong AL, Treadwell BV, Mankin HJ. The role of proteases in the pathogenesis of osteoarthritis. J Rheumatol. 1987;14(Spec No):30–2. Epub 1987/05/01. [PubMed] [Google Scholar]
- 24.Flannelly J, Chambers MG, Dudhia J, Hembry RM, Murphy G, Mason RM, et al. Metalloproteinase and tissue inhibitor of metalloproteinase expression in the murine STR/ort model of osteoarthritis. Osteoarthritis Cartilage. 2002;10(9):722–33. doi: 10.1053/joca.2002.0818. Epub 2002/08/31. [DOI] [PubMed] [Google Scholar]
- 25.Kostoulas G, Lang A, Nagase H, Baici A. Stimulation of angiogenesis through cathepsin B inactivation of the tissue inhibitors of matrix metalloproteinases [published erratum appears in FEBS Lett 2000 Jan 28;466(2–3):394] FEBS Lett. 1999;455(3):286–90. doi: 10.1016/s0014-5793(99)00897-2. [DOI] [PubMed] [Google Scholar]
- 26.Colbert RA, DeLay ML, Layh-Schmitt G, Sowders DP. HLA-B27 misfolding and spondyloarthropathies. Adv Exp Med Biol. 2009;649:217–34. doi: 10.1007/978-1-4419-0298-6_16. Epub 2009/09/08. [DOI] [PubMed] [Google Scholar]
- 27.Lippiello L, Hall D, Mankin HJ. Collagen synthesis in normal and osteoarthritic human cartilage. J Clin Invest. 1977;59(4):593–600. doi: 10.1172/JCI108676. Epub 1977/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee B, Vissing H, Ramirez F, Rogers D, Rimoin D. Identification of the molecular defect in a family with spondyloepiphyseal dysplasia. Science. 1989;244(4907):978–80. doi: 10.1126/science.2543071. Epub 1989/05/26. [DOI] [PubMed] [Google Scholar]
- 29.Ala-Kokko L, Baldwin CT, Moskowitz RW, Prockop DJ. Single base mutation in the type II procollagen gene (COL2A1) as a cause of primary osteoarthritis associated with a mild chondrodysplasia. Proc Natl Acad Sci U S A. 1990;87(17):6565–8. doi: 10.1073/pnas.87.17.6565. Epub 1990/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmad NN, Ala-Kokko L, Knowlton RG, Jimenez SA, Weaver EJ, Maguire JI, et al. Stop codon in the procollagen II gene (COL2A1) in a family with the Stickler syndrome (arthro-ophthalmopathy) Proc Natl Acad Sci U S A. 1991;88(15):6624–7. doi: 10.1073/pnas.88.15.6624. Epub 1991/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown DM, Nichols BE, Weingeist TA, Sheffield VC, Kimura AE, Stone EM. Procollagen II gene mutation in Stickler syndrome. Archives of ophthalmology. 1992;110(11):1589–93. doi: 10.1001/archopht.1992.01080230089027. Epub 1992/11/01. [DOI] [PubMed] [Google Scholar]
- 32.Ritvaniemi P, Hyland J, Ignatius J, Kivirikko KI, Prockop DJ, Ala-Kokko L. A fourth example suggests that premature termination codons in the COL2A1 gene are a common cause of the Stickler syndrome: analysis of the COL2A1 gene by denaturing gradient gel electrophoresis. Genomics. 1993;17(1):218–21. doi: 10.1006/geno.1993.1306. Epub 1993/07/01. [DOI] [PubMed] [Google Scholar]
- 33.Winterpacht A, Hilbert M, Schwarze U, Mundlos S, Spranger J, Zabel BU. Kniest and Stickler dysplasia phenotypes caused by collagen type II gene (COL2A1) defect. Nat Genet. 1993;3(4):323–6. doi: 10.1038/ng0493-323. Epub 1993/04/01. [DOI] [PubMed] [Google Scholar]
- 34.Ahmad NN, Dimascio J, Knowlton RG, Tasman WS. Stickler syndrome. A mutation in the nonhelical 3′ end of type II procollagen gene. Archives of ophthalmology. 1995;113(11):1454–7. doi: 10.1001/archopht.1995.01100110114034. Epub 1995/11/01. [DOI] [PubMed] [Google Scholar]
- 35.Zabel B, Hilbert K, Stoss H, SupertiFurga A, Spranger J, Winterpacht A. A specific collagen type II gene (COL2A1) mutation presenting as spondyloperipheral dysplasia. Am J Med Genet. 1996;63(1):123–8. doi: 10.1002/(SICI)1096-8628(19960503)63:1<123::AID-AJMG22>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 36.Seegmiller RE, Bomsta BD, Bridgewater LC, Niederhauser CM, Montano C, Sudweeks S, et al. The heterozygous disproportionate micromelia (dmm) mouse: morphological changes in fetal cartilage precede postnatal dwarfism and compared with lethal homozygotes can explain the mild phenotype. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2008;56(11):1003–11. doi: 10.1369/jhc.2008.951673. Epub 2008/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454(7203):455–62. doi: 10.1038/nature07203. Epub 2008/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tasab M, Batten MR, Bulleid NJ. Hsp47: a molecular chaperone that interacts with and stabilizes correctly-folded procollagen. EMBO J. 2000;19(10):2204–11. doi: 10.1093/emboj/19.10.2204. Epub 2000/05/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.