Abstract
Background
Psychopathic traits are associated with increases in antisocial behaviors such as aggression and are characterized by reduced empathy for others’ distress. This suggests that psychopathic traits may also impair empathic pain sensitivity. However, whether psychopathic traits affect responses to the pain of others versus the self has not been previously assessed.
Method
We used whole-brain functional magnetic resonance imaging (fMRI) scanning to measure neural activation in 14 adolescents with Oppositional Defiant Disorder or Conduct Disorder and psychopathic traits, as well as 21 healthy controls matched on age, sex, and intelligence. Activation in structures associated with empathic pain perception was assessed as adolescents viewed photographs of pain-inducing injuries. Adolescents imagined either that the body in each photograph was their own or that it belonged to another person. Behavioral and neuroimaging data were analyzed using random-effects analysis of variance.
Results
Youths with psychopathic traits showed reduced activity within regions associated with empathic pain as the depicted pain increased. These regions included rostral anterior cingulate cortex, ventral striatum (putamen), and amygdala. Reductions in amygdala activity particularly occurred when the injury was perceived as occurring to another. Empathic pain responses within both amygdala and rostral anterior cingulate cortex were negatively correlated with the severity of psychopathic traits as indexed by PCL:YV scores.
Conclusions
Youths with psychopathic traits show less responsiveness in regions implicated in the affective response to another’s pain as the perceived intensity of this pain increases. Moreover, this reduced responsiveness appears to predict symptom severity.
Keywords: Psychopathy, adolescents, empathy, pain, amygdala, Conduct Disorder
INTRODUCTION
Psychopathic traits, including reduced empathy and guilt, affect a subgroup of youths with the disruptive behavior disorders Conduct Disorder and Oppositional Defiant Disorder (Christian, Frick, Hill, Tyler, & Frazer, 1997). These traits are detectable early in childhood, persist into adulthood, and heighten risk for recurrent antisocial acts and criminal behaviors (Lynam, Loeber, & Stouthamer-Loeber, 2008). Youths with psychopathic traits are characterized clinically by reduced empathy, but whether psychopathy impairs youths’ empathic pain responses is currently unknown.
Viewing or imagining pain typically elicits activation in a distributed network of regions that includes the anterior cingulate cortex, anterior insula, amygdala, striatum, somatosensory cortex, supplementary motor cortex, and periaqueductal gray (see Lamm, Decety, & Singer, 2011). These regions include those mediating both somatosensory (e.g., somatosensory cortex) and affective dimensions of pain processing (e.g., anterior cingulate cortex, insula, striatum, and amygdala) (Singer et al., 2004). In youths, the amygdala may play a particularly strong role in empathy for pain (Decety & Michalska, 2010). If psychopathy impairs pain responding, youths with psychopathic traits should show reduced activation within regions mediating affective pain responses. However, the opposite was reported in a small sample (N=8) of youths with Conduct Disorder, who exhibited elevated responses to accidental pain in insula, anterior cingulate cortex, amygdala and dorsal striatum (Decety, Michalska, Akitsuki, & Lahey, 2009). Increased amygdala responding to aversive images has been previously reported in youths with Conduct Disorder (Herpertz et al., 2009). However, these studies did not examine the moderating influence of psychopathic traits, which affect approximately 30% of youth with Conduct Disorder (Christian et al., 1997). Conduct Disorder without psychopathic traits may be associated with heightened emotional lability and responsiveness (Frick & Dickens, 2006). Thus, we wished to test whether youths with disruptive behavior disorders and psychopathic traits show increased responsiveness to pain stimuli, consistent with the previous study of Conduct Disorder (Decety et al., 2009), or reduced responsiveness, consistent with reports of individuals with psychopathic traits (Birbaumer et al., 2005; Kiehl et al., 2001; Marsh et al., 2008; Jones et al., 2009; Marsh et al., 2011; White et al., 2012).
Given that reduced empathy is an essential clinical component of psychopathy, we also specifically aimed to examine responses to the pain of others. Previous work has established that very similar patterns of activation are observed in the pain matrix during empathic pain and pain experienced by the self (Lamm, Decety, & Singer, 2011). Therefore, following a previously validated procedure, participants viewed pain stimuli while imagining the victim to be themselves (Own Pain condition) or another person (Other’s Pain condition), the latter representing empathic pain perception (Jackson, Meltzoff, & Decety, 2005). This enabled us to assess whether atypical pain responsiveness in youths with psychopathic traits is particularly marked for empathic pain.
METHODS
PARTICIPANTS
Thirty-seven participants recruited through newspaper ads, fliers, and referrals underwent fMRI scanning, including 15 male and female adolescents (ages 10–17) with Oppositional Defiant Disorder or Conduct Disorder and psychopathic traits and 22 control participants (Table 1). Data from 2 participants, one from each group, were excluded due to movement > 4 mm. (These youths were included in behavioral analyses; behavioral responses were not used to create regressors, so this does not affect imaging analyses.) Written informed assent and consent were obtained from participating youths and parents. This study was approved by the Institutional Review Board at the National Institute of Mental Health.
TABLE 1.
Characteristics of Youths With Psychopathic Traits and Healthy Comparison Participants.
| Variable | Healthy Control (N=21) | Psychopathic traits (N=14) | p-value |
|---|---|---|---|
| Age, y (SD) | 14.3 (1.8) | 15.4 (2.3) | n.s. |
| IQ, mean (SD) | 106.9 (12.9) | 100.5 (10.9) | n.s. |
| Male sex, No. (%) | 15 (71%) | 8 (57%) | n.s. |
| DSM-IV diagnoses (current), No. (%) | |||
| Conduct disorder | 0 | 7 | — |
| Oppositional-defiant disorder | 0 | 9 | — |
| Attention deficit hyperactivity disorder | 0 | 8 | — |
| Pediatric psychopathic trait rating scale scores, mean (SD) | |||
| Antisocial Process Screening Device (APSD) | 5.6 (3.8) | 28.4 (4.8) | <.001 |
| Psychopathy Checklist: Youth Version (PCL:YV) | — | 23.9 (3.5) | — |
| PCL:YV Factor 1 | 11.8 (2.2) | — | |
| PCL:YV Factor 2 | 9.9 (2.5) | — | |
Table includes youths included in neuroimaging analyses (excluding two youths with psychopathic traits and one healthy youth with excessive movement during scanning).
Youths and parents were administered the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS-PL; Kaufman et al., 1997) by an experienced clinician trained and supervised by an expert child psychiatrist (D.S. Pine). This clinician was trained to show good inter-rater reliability (kappa >0.75 for all diagnoses). A trained researcher administered the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). Exclusion criteria for all participants included pervasive developmental disorder; substance dependence; Tourette’s syndrome; current or lifetime history of psychosis; depression; bipolar disorder; generalized, social, or separation anxiety disorder; post-traumatic stress disorder; neurologic disorder; history of head trauma; and IQ less than 75. No youths in either group met criteria for substance abuse or dependence. All parents completed the Antisocial Process Screening Device (APSD) (Frick & Hare, 2001; Frick et al., 2000), a 20-item parent-completed instrument assessing psychopathic traits and conduct and impulsivity problems in youths. Youths meeting K-SADS-PL criteria for Conduct Disorder or Oppositional Defiant Disorder returned to complete the Psychopathy Checklist-Youth Version (PCL:YV) (Forth, Kosson, & Hare, 2003). This 20-item instrument assesses adolescent interpersonal/affective deficits and antisocial behavior, which correspond to the two-factor solution originally proposed for this instrument (Forth et al., 2003; Forth, 1995; Harpur, Hare, & Hakstian, 1989). Researchers’ scoring followed separate semi-structured interviews of the participant and a parent or guardian and demonstrated good inter-rater reliability (R=0.91). Youths scoring ≥ 20/40 on both APSD and PCL:YV were included in the psychopathic traits group consistent with prior studies (Marsh et al., 2011; Marsh et al., 2008; Finger et al., 2008). Healthy controls did not meet criteria for any K-SADS-PL diagnosis and scored <20 on the APSD.
STIMULI AND TASK
The task is a validated paradigm in which participants view 90 photographs of hands and feet, 30 featuring severe pain (e.g., toes shut in a door), 30 featuring moderate pain (e.g., toes being stubbed against a door frame), and 30 featuring neutral situations (e.g., a foot on the floor next to a door) (Jackson, Brunet, Meltzoff, & Decety, 2006). Participants completed three runs of the task after reading the following instructions,
“In this task you will be viewing pictures of hands and feet in different situations. Your job will be to evaluate how painful each situation is using the buttons you are holding. Each picture you see will be presented with a rating scale like this [a sample rating scale was shown]. You will use the buttons 1 through 4 that you will holding to evaluate the level of pain. Also, in between each small group of slides, you will see a slide that says either “YOURSELF” or “SOMEONE ELSE.” For all the slides that follow, please imagine that the situation in the picture is happening either to you or to somebody else. So for example, if you saw this series of slides [sample YOURSELF prompt and image slides were shown], you would rate what level of pain you would experience in those situations. And then if you saw this series of slides next [sample YOURSELF prompt and image slides were presented], you would switch to rating what level of pain someone else (meaning a specific but unfamiliar person) would experience in those situations.”
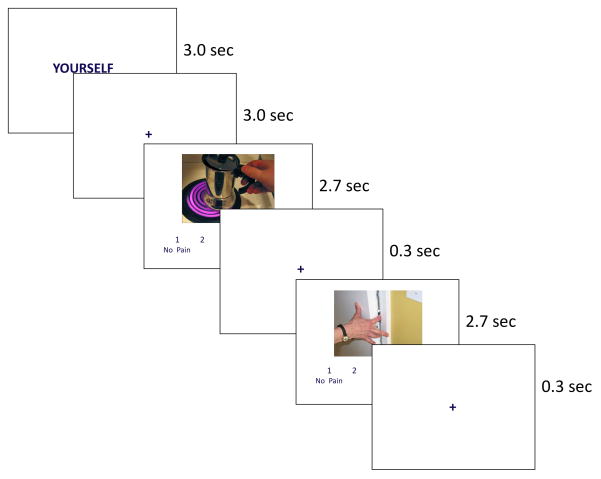
The validity of this paradigm has been supported by previously published work (Jackson et al., 2006; Lamm et al., 2007), which has established that this task results in pain network activation comparable to that resulting from a variety of pain tasks (Lamm et al., 2011; Singer et al., 2004). As noted, participants switched perspectives following “Yourself” and “Someone Else” prompt slides. After each prompt, participants viewed 6 images, during which they rated how painful each image appeared using a 4-point scale anchored by “No pain” and “Worst possible pain” (Figure 1). Prompts comprised the blocked component of the design, and pain level the event-related component. Responses were collected using a 4-button box.
FIGURE 1.
Design of Neuroimaging Task and Sample Images
Participants viewed each stimulus twice: once following a Yourself prompt, and once following a Someone Else prompt. Each prompt was 3 sec and was followed by a 3 sec fixation, then 6 image trials. Each image trial was 3 sec, consisting of an image (2.7 sec) then a fixation (0.3 sec). Response data were collected during the entire trial. Each of 3 task runs contained 10 prompts, 60 image trials, and 30 “jitter” fixation trials (3.0 sec) interspersed between images. The inclusion of jitter trials increases power in event-related fMRI paradigms (Dale, 1999). Each run of the task began and ended with a 15 sec fixation, making each run of the task 5.5 minutes long. Four possible randomized sequences of stimulus presentations were created for the task. Each participant was randomly selected to view one of the four sequences.
SCANNING ACQUISITION
T2* weighted images were collected during fMRI scanning using a 1.5 Tesla GE Signa scanner (GE Medical Systems, Milwaukee, WI) (matrix 64×64; repetition time, 3000 milliseconds; echo time, 30 milliseconds; field of view, 240 mm; voxels, 3.75×3.75×4). Functional images were acquired with a gradient echo-planar imaging (EPI) sequence (axial plane, 31 contiguous axial slices). The task was yoked to the repetition time intervals. High-resolution T1-weighted anatomical images were also acquired (three-dimension Spoiled GRASS with inversion recovery prep pulse; number of 1.5 mm axial slices, 128; field of view, 240 mm; number of acquisitions, 1; repetition time, 8.1 ms, echo time, 1.8 milliseconds; matrix, 256×256).
PRE-PROCESSING OF NEUROIMAGING DATA
Imaging data were pre-processed and analyzed using Analysis of Functional Neuroimages (AFNI). The first 4 trials of each run were discarded, and then functional images from the 3 time series were concatenated, despiked, motion corrected, spatially smoothed using a 6.0 mm full-width half-maximum Gaussian filter, and activation outside the brain was masked. The time series was then normalized such that the resulting regression coefficients represented a percent signal change from the mean. Regressors were created for Own Pain and Other’s Pain trials and weighted according to the intensity of depicted pain (severe, moderate, or none) according to a priori classifications of the images into each category. A regressor of no interest was created for trials in which participants did not provide a response. Fixation trials were modelled implicitly.
All regressors were created by convolving the train of stimulus events with a gamma-variate hemodynamic response function. Linear regression modeling used the full set of regressors to model baseline drift and residual motion artefact. The baseline was modeled by a 1st-order function and motion artefacts were modeled using the 6 estimated rigid-body motion parameters. This produced a beta coefficient and associated t statistic for each voxel and regressor. Participants’ anatomical scans were individually registered to the Talaraich and Tourneoux Atlas, as normalization of adolescent brains does not appear to introduce major distortions during event-related fMRI (Burgund et al., 2002).
DATA ANALYSIS
Behavioral data were analyzed using a 2 (group: psychopathic traits, healthy controls) × 2 (condition: Own Pain, Other’s Pain) × 3 (pain intensity: severe, moderate, none) repeated-measures analysis of variance (ANOVA) on both participants’ responses to the images and on their response times. Main effects and interactions are reported at p<0.05, two-tailed.
Whole-brain group analyses on the event-related blood oxygen level dependent (BOLD) data were conducted in AFNI. To identify the main effect of pain, we conducted a whole-brain single sample contrast comparing regressors weighted according to the intensity of depicted pain to a baseline of zero. Thus, regions of activation would be identified to the extent that increasing activation corresponded to increasing intensity of depicted pain. Again, consistent with prior work incorporating these stimuli, pain intensity in analyses of imaging data was determined according to our a priori classifications, not according to participants’ own ratings (Jackson et al., 2006), and regressors were weighted according to these classifications.
Next, in order to identify whether activation in the hypothesized brain regions is differentially associated with pain perception across groups, we used two analytic strategies. First, to identify whether a true group × emotion interaction existed we conducted a whole-brain analysis of group differences across conditions using a 2 (group: psychopathic traits, healthy controls) × 2 (condition: Own Pain, Other’s Pain) ANOVA. Consistent with prior work featuring analyses of variance (White et al., 2012), initial thresholding was set at a p value of p < 0.005, with an extant threshold of 10 contiguous voxels, a combination that has been demonstrated to produce a desirable balance between Type I and Type II error rates, a critical consideration for omnibus interactions (Lieberman & Cunningham, 2009). Second, to investigate the nature of interaction effects, we performed focused tests of our hypothesis, calculated independently from the ANOVA, by conducting whole-brain contrasts within AFNI For these contrast tests, we applied a threshold that yielded a whole-brain false discovery rate (FDR)<0.05 across contrasts (p<0.0001, uncorrected, with an extent threshold of 10 voxels). Areas of differential activation that survive both analytic strategies are reported. We also examined the main effect of pain intensity in both groups combined to determine responsiveness to pain stimuli across groups. This was achieved using a single sample contrast test.
Additional planned analyses were performed on mean parameter estimates extracted from the functionally defined clusters identified by the ANOVA. Independent samples t tests were performed to specify the nature of main effects and interactions. We also assessed the relationship between PCL:YV scores and patterns of atypical activation in youths with psychopathic traits. These analyses were conducted within the clinical population only, so they are independent from the original ANOVA conducted across groups and do not introduce statistical bias. Two follow-up ANOVAs were also conducted that excluded, respectively, youths with diagnoses of ADHD or reported use of psychotropic medication to rule these variables out as contributing to identified group differences in neural activation.
RESULTS
BEHAVIORAL DATA
Results of the ANOVA revealed no main effects or interaction involving the effects of condition or group (all p>.10). A significant main effect of pain intensity was identified, F(2,70)=202.94, p<.001, such that participants judged severe pain stimuli as the most painful, M=3.19, SD=0.46, followed by moderate pain, M=2.51, SD=0.43, followed by neutral, M=1.57, SD=0.44. Analysis of response times revealed no significant main effects or interactions (all p>.10).
No significant group differences in average movement during scanning were observed in any plane (all p > .10).
IMAGING DATA
As noted above, group differences in responses to the images were analyzed using a 2 (group: psychopathic traits, healthy controls) × 2 (condition: Own Pain, Other’s Pain) × 3 (pain intensity: severe, moderate, none) ANOVA. Main effects and interactions for the ANOVA are presented in Table 2. The regions reported in the table are the only regions implicated in the whole brain analysis at the predetermined threshold.
TABLE 2.
Regions With Significant Group-by-Pain Condition Interaction Effects and Significant Main Effects of Pain Condition and Group
| Region | L/R | BA | X | Y | z | F | Voxels |
|---|---|---|---|---|---|---|---|
| Group (Psychopathic < Controls) | |||||||
| Medial frontal gyrus | L | 10 | −4 | 57 | 25 | 15.43 | 12 |
| Rostral anterior cingulate cortex | L | 32 | −1 | 35 | 24 | 21.94 | 53 |
| R | 32 | 8 | 35 | 27 | 16.18 | 27 | |
| Putamen/lentiform nucleus | R | 20 | 2 | 2 | 13.40 | 10 | |
| Pain Condition (Other < Self) | |||||||
| Middle occipital gyrus | L | 19 | −25 | −84 | −17 | 11.39 | 20 |
| R | 18 | 29 | −88 | −3 | 12.16 | 23 | |
| Group × Pain Condition | |||||||
| Superior frontal gyrus | L | 9 | −22 | 41 | 24 | 16.85 | 32 |
| Rostral anterior cingulate cortex | L/R | 24 | 2 | 33 | 7 | 14.26 | 13 |
| Insula | R | 13 | 32 | 1 | 22 | 12.25 | 10 |
| Amygdala/uncus | L | −16 | 0 | −33 | 14.10 | 18 | |
Regions labeled according to MNI atlas. All regions significant at p<.005 uncorrected with extent threshold of 10 voxels
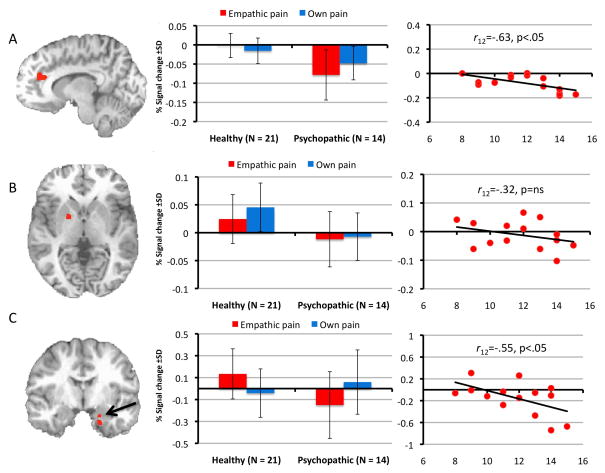
We first determined whether youths with psychopathic traits show reduced or increased responsiveness in regions previously associated with pain responding. The results of the ANOVA, F(2,66)=9.09, p<.005, identified a main effect of group in several of these regions, including left and right rostral anterior cingulate cortex (Figure 2a) and ventral striatum (putamen) (Figure 2b); within all regions, healthy youths showed greater increases as a function of apparent pain intensity relative to youths with psychopathic traits.
FIGURE 2.
Clusters Identified Using a Group-by-Pain Condition Analysis of Variance. The images on the left show the region of left rostral anterior cingulate cortex (A) in which a group effect was observed; and regions of ventral striatum/putamen (B), and amygdala (C) in which a group-by-pain condition interaction was observed. Center graphs summarize activation in these regions of interest across groups and conditions. The scatter plots at right show correlations between activation in these regions and Factor 1 scores on the Psychopathy Checklist: Youth Version.
We next examined whether psychopathy differentially moderates responses to Other’s Pain versus Own Pain. The results of the group-by-condition interaction, revealed significant clusters of differential activation within the left amygdala/uncus (Figure 2c), left superior frontal gyrus, insula and right rostral anterior cingulate cortex. Within all regions (except rostral anterior cingulate cortex) healthy youths showed greater increases in activation than youths with psychopathic traits to Other’s Pain, t(33)=3.15, 3.72, 2.23; p<.005, <.001, <.05 respectively) whereas no group differences were observed to Own Pain (all p >.10). No group differences in rostral anterior cingulate cortex emerged in either condition (p >.10). Planned contrasts conducted in AFNI confirmed that this interaction was driven by greater differentiation between groups during the Other’s Pain condition. A significant cluster in left amygdala/uncus emerged for this contrast, xyz=−13, −3, −23, 11 voxels, as well as a frontal cluster spanning the left superior frontal gyrus, insula, and rostral anterior cingulate cortex, xyz=11, 17, 68, t(33)=3.62 p<.05 (whole-brain FDR corrected for multiple comparisons). No comparable clusters emerged for the Own Pain contrast.
We examined the main effect of pain intensity across groups using a single sample contrast test, which revealed increases in activity as a function of pain intensity within dorsomedial/dorsal anterior cingulate cortex, bilateral anterior insula, supplementary motor cortex, and somatosensory cortex (see Figure 3; Table 3). These significant relationships between pain intensity and increased BOLD responses were seen in both groups.
FIGURE 3.
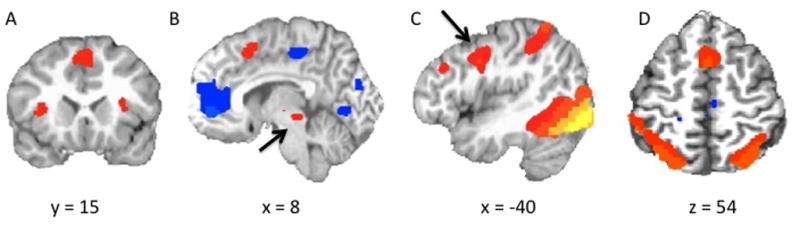
Clusters Identified Using a Single-Sample Contrast Across Groups. The images show regions in which activation increased parametrically with increasing levels of perceived pain including bilateral anterior insula and dorsomedial frontal cortex/dACC (A); periaqueductal gray (B); supplementary motor cortex (C); and somatosensory cortex (D).
TABLE 3.
Regions With Significant Increase in Activation Corresponding to Depicted Pain Intensity
| Region | L/R | BA | x | y | z | F | Voxels |
|---|---|---|---|---|---|---|---|
| (Pain > Baseline) | |||||||
| Dorsomedial frontal cortex / dACC | R | 6 | 2 | 9 | 52 | 9.18 | 110 |
| Middle frontal gyrus / supplementary motor cortex | L | 9 | −49 | 3 | 42 | 7.98 | 126 |
| R | 6 | 57 | 6 | 42 | 6.85 | 65 | |
| R | 9 | 54 | 28 | 33 | 7.50 | 63 | |
| L | 9 | −46 | 35 | 33 | 8.11 | 58 | |
| Insula | R | 13 | 35 | 20 | 7 | 7.54 | 26 |
| L | 13 | −31 | 17 | 10 | 6.64 | 23 | |
| Thalamus / parahippocampal gyrus / periaqueductal gray | R | 11 | −20 | 11 | 11.74 | 29 | |
| L | −13 | −32 | −3 | 8.95 | 41 | ||
| Fusiform gyrus / somatosensory cortex | R | 37 | 47 | −65 | −26 | 14.70 | 2218 |
| L | 19 | −31 | −77 | −24 | 12.26 | 2174 | |
Regions labeled according to MNI atlas. All regions significant at p < .000001 (p < .001 whole-brain FDR corrected) with extent threshold of 10 voxels. This threshold was selected to enable differentiation of clusters.
Among youths with psychopathic traits, we then assessed the relationship between symptom severity as assessed by the PCL:YV and responsiveness in four regions previously linked to pain responding and identified by the ANOVA (Table 4). Within the amygdala, r(12)=-.55, p<.05, and left rostral anterior cingulate cortex, r(12)=-.63, p<.05, increases in activity were inversely correlated with interpersonal and affective features of psychopathy (Factor 1) but not antisocial behavior features (Factor 2) for Other’s Pain only (Figure 2a and 2c). No significant Own Pain correlations were identified. Because of our relatively small sample, we regressed mean parameter estimates for the identified clusters against Factor 1 scores and generated Mahalanobis distance values to identify multivariate outliers and found none for any variable (all ps>.05). Correlations between symptom severity as assessed by the APSD (either total score or CU subscale) and responsiveness in the four regions identified above were not significant.
TABLE 4.
Comparison of Regions in Which Activation Correlated With Psychopathy Checklist:Youth Version Scores in Adolescents with Psychopathic Traits
| PCL:YV Total | Factor 1 | Factor 2 | |
|---|---|---|---|
| Empathic Pain condition | |||
| Left amygdala | −0.29_ | −0.55* | 0.23_ |
| Left rostral anterior cingulate cortex | −0.60* | −0.63* | −0.25_ |
| Right rostral anterior cingulate cortex | −0.33_ | −0.44_ | 0.03_ |
| Right putamen | −0.24_ | −0.32_ | −0.09_ |
| Own Pain condition | |||
| Left amygdala | −0.27_ | −0.35_ | −0.16_ |
| Left rostral anterior cingulate cortex | −0.12_ | −0.14_ | −0.10_ |
| Right rostral anterior cingulate cortex | 0.07_ | 0.26_ | −0.06_ |
| Right putamen | −0.24_ | −0.16_ | 0.03_ |
Correlations labeled with an asterisk (*) are significant at p<.05
CONTROLLING FOR ALTERNATE HYPOTHESES
Psychopathic traits include symptoms consistent with ADHD diagnoses (e.g., impulsive behavior), however, evidence suggests that aberrant patterns of neural activation in ADHD are dissociable from the characteristic deficits seen in youths with psychopathic traits (4). Nonetheless, the preceding analysis was repeated separately without the 8 youths with psychopathic traits who met criteria for ADHD. In this study, the effects of interest were replicated when including only youths without ADHD diagnoses, with similar group-by-condition effects observed within the amygdala/parahippocampal gyrus (xyz=−13, −12, −23) and a region proximal to the original insula cluster (xyz=29, −9, 28) and group effects within the left rostral anterior cingulate (xyz=−7, 32, 23).
To account for possible effects of medication use, the analysis was again repeated excluding 2 youths with psychopathic traits taking psychotropic medication. The effects of interest were again replicated, with similar group-by-condition effects within the amygdala (xyz=−16, −6, −23) and a region proximal to the insula cluster identified in the original analysis (xyz=32, −22, 25), and group effects within the rostral anterior cingulate cortex (xyz=−1, 35, 24).
DISCUSSION
The current study addressed two features of pain perception in youths with psychopathic traits. First, it investigated whether youths with psychopathic traits show heightened or reduced responses within regions associated with pain responding. Second, it assessed responses to Other’s Pain relative to Own Pain. Youths with psychopathic traits showed reduced responsiveness in rostral anterior cingulate cortex and right ventral striatum (putamen/lentiform nucleus) to increases in perceived pain. In addition, within amygdala and insula, these youths showed lack of response to increases in Other’s Pain, but not Own Pain. The severity of interpersonal and affective features of psychopathy was inversely associated with responsiveness to Other’s Pain, but not Own Pain, within amygdala and left rostral anterior cingulate cortex.
Youths with psychopathic traits exhibit reduced amygdala responses to fearful facial expressions (Marsh et al., 2008; Jones et al., 2009; White et al., 2012; Viding et al., 2012) and during affective theory of mind tasks (Sebastian et al., 2012). Reduced amygdala responses to sad facial expressions have also been reported in youths with undifferentiated Conduct Disorder (Passamonti et al., 2010). These deficits may be critical to increased interpersonal violence in psychopathy, as fearful and sad facial expressions may modulate aggressive behavior, and accurate perception of these cues is associated with empathic arousal and the inhibition of violence (Blair, 2005; Marsh, Adams, & Kleck, 2005; Marsh, Kozak, & Ambady, 2007). And, importantly, fear and sadness are states that typically accompany the anticipation of, and response to, infliction of harm or pain. This study extends the existing literature to pain cues themselves, showing psychopathic traits impair empathic pain responses. It also links amygdala dysfunction to deficient empathic responses to pain in psychopathy.
A distinction has been drawn between those regions mediating somatosensory responses to pain and those mediating affective responses (Decety, 2010; Singer et al., 2004). Here, youths with psychopathic traits showed appropriate BOLD responses to pain stimuli as a function of intensity within somatosensory cortex (as well as dorsomedial frontal and supplementary motor cortex), as did healthy youths (see Figure 3 and Table 3). This suggests that psychopathy does not impair somatosensory responses to pain. Moreover, these data importantly imply that putative group differences in imagery could not be the origin of the current results unless we assume that imagery effects are selective for the more emotion-relevant regions of rostral anterior cingulate cortex, amygdala and the striatum.
Neurobiological accounts of psychopathy do not generally implicate somatosensory and supplementary motor cortex (Blair, 2007; Kiehl, 2006). However, participants with psychopathic traits showed dysfunction in regions mediating affective responses to pain. In rostral anterior cingulate cortex and putamen, youths with psychopathic traits showed reduced responsiveness to increasing Other’s and Own pain. In the amygdala, youths with psychopathic traits showed reduced responsiveness to increasing Other’s Pain. Supporting the specific link between psychopathy and impaired empathic pain, a selective inverse relationship was also identified between amygdala and rostral anterior cingulate cortex responses to Other’s Pain and the severity of the affective and interpersonal component (but not the antisocial behavior component) of psychopathy.
That psychopathy is associated with reduced activity within putamen and rostral anterior cingulate cortex is of particular interest, given divergent findings about activation in these regions as a function of psychopathic traits. Increased dorsal striatal volume has been reported in psychopathic adults (Glenn, Raine, Yaralian, & Yang, 2010), and a positive correlation has been found between striatal gray matter volume and callous-unemotional traits in children with Conduct Disorder (Fairchild et al., 2011). Functionally, atypical dorsal striatal responses to reinforcement information have been reported in youths (Finger et al., 2008; Finger et al., 2011) and adults (Buckholtz et al., 2010). Striatal dysfunction has been linked to difficulties in reinforcement learning in this population (Finger et al., 2011). Reduced ventral striatal activity in this study may reflect reduced reinforcement-based learning to pain cues.
Dysfunction in anterior cingulate cortex has also been hypothesized in psychopathy (Kiehl, 2006), but the literature is equivocal. Reduced anterior cingulate cortex activity has been reported in adults with psychopathic traits during fear conditioning (Birbaumer et al., 2005) and affective memory tasks (Kiehl et al., 2001). But two studies in youths with psychopathic traits report intact dorsal anterior cingulate cortex responding to unexpected punishments (Finger et al., 2008) and response incongruency (Marsh et al., 2011). This apparent inconsistency may relate to regional specialization within anterior cingulate cortex. The region of anterior cingulate cortex implicated by Finger and colleagues (Finger et al., 2008), and by Marsh and colleagues (Marsh et al., 2011), is dorsal to the region identified here and in both instances activity was associated with increased response conflict. Mediation of response conflict appears intact or even superior in individuals with psychopathic traits (Hiatt, Schmitt, & Newman, 2004). By contrast, the region of anterior cingulate cortex identified by Birbaumer and Kiehl and in this study is more closely tied to emotional processing and is relatively rostral (Birbaumer et al., 2005; Kiehl et al., 2001). Moreover, the region of ventromedial prefrontal cortex in which impaired reinforcement signaling was previously seen (Finger et al., 2011) extends into rostral anterior cingulate cortex. The current findings, in conjunction with earlier work (Birbaumer et al., 2005; Kiehl et al., 2001; Finger et al., 2011; Marsh et al., 2011), suggest affective processing in more rostral anterior cingulate cortex may be dysfunctional in psychopathy whereas the regions of dorsal anterior cingulate cortex implicated in mediating response conflict may be preserved.
The current results contrast with those previously reported for youths with Conduct Disorder undifferentiated by psychopathic traits (Decety et al., 2009). In that study, youths with Conduct Disorder showed increased amygdala and striatum responses to accidentally incurred pain. However, these data may be reconcilable. In this study, amygdala responding to Other’s Pain was inversely related to interpersonal and affective component of psychopathy, which is linked to empathy. But amygdala responses did not correspond to the impulsive and antisocial behavior factor of psychopathy. Thus, amygdala dysfunction to Other’s Pain is progressively greater as the affective component of psychopathy becomes more pronounced. Even among participants selected for elevated PCL:YV scores, an inverse relationship emerged between the affective factor (also termed callous-unemotional traits) and empathic responses in the amygdala. Youths with Conduct Disorder who do not have callous-unemotional traits, constituting about 70% of the total, may exhibit increased emotional lability (Blair et al., 2006; Frick & Dickens, 2006). Youths with Conduct Disorder in the prior study of pain perception may have been predominantly emotional labile, had low callous-unemotional scores, and, in line with the inverse relationship observed here, shown increased amygdala responses to others’ pain. This pattern would be consistent with findings assessing evoked response potentials in youths with Conduct Disorder who vary in psychopathic traits (Cheng, Hung, & Decety, 2011).
There is considerable agreement that conduct problems can emerge via multiple developmental trajectories (Crowe & Blair, 2008; Frick & White, 2008). Distinctions are drawn between youth with conduct problems and callous-unemotional traits and those with conduct problems and increased anxiety or impulsivity, which have led to suggestions of primary and secondary psychopathy (Kimonis et al 2012). Primary psychopathy (cf. Kimonis) is associated with reduced amygdala responses to fearful expressions and dysfunctional striatal and ventromedial frontal cortex activity during reinforcement based decision making (Marsh et al., 2008; Jones et al., 2009; Finger et al., 2011; Sebastian et al., 2012; White et al., 2012; Viding et al., 2012). Conduct problems associated with secondary psychopathy are associated with increased amygdala responses to threat and heightened sensitivity to threat (Herpertz et al., 2008; Passamonti et al., 2010; Kimonis et al., 2012; Viding et al., 2012); for a review of the putative neurobiological differences between these forms of conduct problems, see Crowe and Blair (2008). The current study focused primarily on youth with primary psychopathy and observed, consistent with previous research (Marsh et al., 2008; Jones, et al., 2009; White et al., 2012), reduced amygdala responses to pain stimuli. This reduction in amygdala activity became more marked as psychopathic traits increased.
LIMITATIONS
Some limitations of this study should be considered. First, our behavioral measures revealed no main effect of group or of target. This is consistent with the results of prior studies that have employed simple evaluation tasks during neuroimaging paradigms (Decety et al., 2009), and may reflect the use of different strategies by psychopathic and non-psychopathic individuals to perform the task. Psychopathy is associated with increased reliance on areas involved in executive functioning and semantic knowledge during socio-affective tasks (Glenn, Raine, Schug, Young, & Hauser, 2009; Marsh & Cardinale, 2012). Similar group differences may have facilitated task performance in psychopathic youths in this task, albeit at subthreshold activation levels. It is not known whether psychopathy results in any generalized effects on imagery, which we did not assess in this paradigm using a questionnaire or other test. Imagery ability is clearly important in the present task, which required study participants to imagine that the limbs depicted in photographs belonged to themselves or another person. However, the fact that we did find extensive activation in pain-relevant somatosensory regions across groups and across conditions of the task is consistent with imagery being spared in psychopathy.
In addition, future research should precisely pinpoint whether the identified group differences reflect disruptive behavior diagnoses or psychopathic traits. Mitigating this concern, however, are the previously discussed correlations between interpersonal and affective psychopathic traits and activation in amygdala and anterior cingulate cortex. These correlations strengthen our conclusion that the activation patterns we observed specifically reflected psychopathic traits.
Potential alternate hypotheses should also be considered. A follow-up ANOVA excluded youths with ADHD diagnoses, which are frequently comorbid with psychopathic traits, and identified similar patterns of activation. Two youths in the present study were also taking psychotropic medications, but a second follow-up ANOVA excluding these children again resulted similar patterns of responding The consistent results of these ANOVAs, which both resulted in a loss of statistical power, support the stability of the patterns we identified. Finally, our sample size was similar to sample sizes use in many previous studies of this population (Decety et al., 2009; Marsh et al., 2008; Marsh et al., 2011; White et al., 2012) but the present findings should be replicated in a larger sample.
CONCLUSION
Adolescents with disruptive behavior disorders and psychopathic traits exhibited less responsiveness to increasing perceived pain intensity within structures typically implicated in affective responses to others’ pain. Given suggestions that the pain of others may trigger empathic distress in the observer, providing a basis for moral development (Hoffman, 1982), we conclude that dysfunction in response to others’ pain may contribute to the behavioral deficits observed in this population.
KEY POINTS.
Psychopathy is a developmental disorder associated with increases in disruptive behavior and with reduced empathy. However, no previous neuroimaging study has assessed empathic pain responding in psychopathy
The results of this neuroimaging study found that adolescents with psychopathic traits and disruptive behavior disorders show reduced activation in areas associated with affective components of empathic pain, including striatum, anterior cingulate cortex, and amygdala.
Reduced responsivity in amygdala and rostral anterior cingulate cortex in response to others’ pain (but not own pain) correlated with the severity of psychopathic traits as measured by PCL:YV Factor 1 scores.
These findings add to accumulating evidence regarding the importance of assessing psychopathic traits to distinguish among adolescents with disruptive behavior disorders. Disruptive adolescents with and without psychopathic traits exhibit contrasting patterns of neural activation to stimuli like distress cues and pain cues, suggesting that distinct neurobiological mechanisms underlie behavioral disturbances in these groups.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH:NIMH. The authors wish to thank Ken Towbin, Andy Speer, and Steven Sinclair for their assistance with this research. Data included in this manuscript were presented at the 2012 meeting of the Social & Affective Neuroscience Society.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, et al. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Archives of General Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Applying a cognitive neuroscience perspective to the disorder of psychopathy. Development and Psychopathology. 2005;17:865–891. doi: 10.1017/S0954579405050418. [DOI] [PubMed] [Google Scholar]
- Blair RJ. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences. 2007;11:387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nature Neuroscience. 2010;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, et al. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Hung A, Decety J. Dissociation between affective sharing and emotion understanding in juvenile psychopaths. Development and Psychopathology. 2011 doi: 10.1017/S095457941200020X. Under review. [DOI] [PubMed] [Google Scholar]
- Christian RE, Frick PJ, Hill NL, Tyler L, Frazer DR. Psychopathy and conduct problems in children: II. Implications for subtyping children with conduct problems. Journal of the American Academy of Child &Adolescent Psychiatry. 1997;36:233–241. doi: 10.1097/00004583-199702000-00014. [DOI] [PubMed] [Google Scholar]
- Crowe S, Blair RJR. The development of antisocial behavior: What can we learn from functional neuroimaging studies? Development & Psychopathology. 2008;20:1145–1159. doi: 10.1017/S0954579408000540. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J. The neurodevelopment of empathy in humans. Developmental Neuroscience. 2010;32:257–267. doi: 10.1159/000317771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Michalska KJ. Neurodevelopmental changes in the circuits underlying empathy and sympathy from childhood to adulthood. Developmental Science. 2010;13:886–899. doi: 10.1111/j.1467-7687.2009.00940.x. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Akitsuki Y. Who caused the pain? An fMRI investigation of empathy and intentionality in children. Neuropsychologia. 2008;46:2607–2614. doi: 10.1016/j.neuropsychologia.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Akitsuki Y, Lahey BB. Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biological Psychology. 2009;80:203–211. doi: 10.1016/j.biopsycho.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, Passamonti L, Hurford G, Hagan CC, von dem Hagen EA, van Goozen SH, et al. Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. American Journal of Psychiatry. 2011;168:624–633. doi: 10.1176/appi.ajp.2010.10081184. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Blair KS, Reid ME, Sims C, Ng P, et al. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. American Journal of Psychiatry. 2011;168:152–162. doi: 10.1176/appi.ajp.2010.10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, et al. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Archives of General Psychiatry. 2008;65:586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forth AE, Kosson DS, Hare RD. The Psychopathy Checklist: Youth Version. Toronto: Multi-Health Systems; 2003. [Google Scholar]
- Forth AE. Psychopathy and young offenders: Prevalence, family background, and violence. Ottawa, ON: Ministry of the Solicitor General of Canada; 1995. [Google Scholar]
- Frick P, Bodin S, Barry C. Psychopathic traits and conduct problems in community and clinic-referred samples of children: Further development of the psychopathy screening device. Psychological Assessment. 2000;12:382–393. [PubMed] [Google Scholar]
- Frick PJ, Hare RD. The Antisocial Process Screening Device. Toronto: Multi-Health Systems; 2001. [Google Scholar]
- Frick PJ, Dickens C. Current perspectives on conduct disorder. Current Psychiatry Reports. 2006;8:59–72. doi: 10.1007/s11920-006-0082-3. [DOI] [PubMed] [Google Scholar]
- Frick P, White S. Research review: The importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. Journal of Child Psychology & Psychiatry. 2008;49:359–375. doi: 10.1111/j.1469-7610.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Schug RA, Young L, Hauser M. Increased DLPFC activity during moral decision-making in psychopathy. Molecular Psychiatry. 2009;14:909–911. doi: 10.1038/mp.2008.104. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Yaralian PS, Yang Y. Increased volume of the striatum in psychopathic individuals. Biol Psychiatry. 2010;67:52–58. doi: 10.1016/j.biopsych.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpur TJ, Hare RD, Hakstian AR. Two-factor conceptualization of psychopathy: Construct validity and assessment implications. Psychological Assessment. 1989;1:6–17. [Google Scholar]
- Herpertz SC, Huebner T, Marx I, Vloet TD, Fink GR, Stoecker T, et al. Emotional processing in male adolescents with childhood-onset conduct disorder. Journal of Child Psychology & Psychiatry. 2009;166:95–102. doi: 10.1111/j.1469-7610.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- Hiatt KD, Schmitt WA, Newman JP. Stroop tasks reveal abnormal selective attention among psychopathic offenders. Neuropsychology. 2004;18:50–59. doi: 10.1037/0894-4105.18.1.50. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Huebner T, Marx I, Vloet TD, Fink GR, Stoecker T, Shah NJ, Konrad K, Herpertz-Dahlmann B. Emotional processing in male adolescents with childhood-onset conduct disorder. Journal of Child Psychology & Psychiatry. 2008;49:781–791. doi: 10.1111/j.1469-7610.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- Hoffman ML. Affect and moral development. New Directions for Child and Adolescent Development. 1982;1982:83–103. [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–761. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. American Journal of Psychiatry. 2009;166:95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Research. 2006;142:107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, et al. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological Psychiatry. 2001;50:677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Kimonis E, Frick P, Cauffman E, Goldweber A, Skeem J. Primary and secondary variants of juvenile psychopathy differ in emotional processing. Development & Psychopathology. 2012;24:1091–1103. doi: 10.1017/S0954579412000557. [DOI] [PubMed] [Google Scholar]
- Lamm C, Batson C, Decety J. The neural substrate of human empathy: Effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience. 2007;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive & Affective Neuroscience. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynam DR, Loeber R, Stouthamer-Loeber M. The stability of psychopathy from adolescence into adulthood. Criminal Justice &Behavior. 2008;35:228. doi: 10.1177/0093854807310153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Adams RB, Jr, Kleck RE. Why do fear and anger look the way they do? Form and social function in facial expressions. Personality & Social Psychology Bulletin. 2005;31:73–86. doi: 10.1177/0146167204271306. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Cardinale EM. When psychopathy impairs moral judgments: Neural responses during judgments about causing fear. Social Cognitive & Affective Neuroscience. 2012 doi: 10.1093/scan/nss097. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger E, Mitchell DGV, Reid ME, Sims C, Kosson DS, et al. Reduced Amygdala Response to Fearful Expressions in Children and Adolescents With Callous-Unemotional Traits and Disruptive Behavior Disorders. American Journal of Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Kozak MN, Ambady N. Accurate identification of fear facial expressions predicts prosocial behavior. Emotion. 2007;7:239–251. doi: 10.1037/1528-3542.7.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, Jurkowitz IT, Schechter JC, Yu HH, et al. Reduced amygdala-orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Research. 2011;194:279–286. doi: 10.1016/j.pscychresns.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Fairchild G, Goodyer IM, Hurford G, Hagan CC, Rowe JB, et al. Neural abnormalities in early-onset and adolescence-onset conduct disorder. Archives of General Psychiatry. 2010;67:729–738. doi: 10.1001/archgenpsychiatry.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C, McCrory E, Cecil C, Lockwood PL, De Brito SA, Fontaine NM, Viding E. Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Archives of General Psychiatry. 2012;69:814–822. doi: 10.1001/archgenpsychiatry.2011.2070. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CA, De Brito SA, McCrory EJ. Amygdala response to preattentive masked fear in children with conduct problems: The role of callous-unemotional traits. American Journal of Psychiatry. 2012;169:1109–1116. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- White SF, Marsh AA, Fowler KA, Schechter JC, Adalio C, Pope K, et al. Reduced amygdala response in youths with disruptive behavior disorders and psychopathic traits: Decreased emotional response versus increased top-down attention to nonemotional features. American Journal of Psychiatry. 2012;169:750–758. doi: 10.1176/appi.ajp.2012.11081270. [DOI] [PMC free article] [PubMed] [Google Scholar]