Abstract
Conversion of thyroxine (T4) to 3,5,3′-triiodothyronine (T3) in rat brain has recently been shown in in vivo studies. This process contributes a substantial fraction of endogenous nuclear T3 in the rat cerebral cortex and cerebellum. Production of T4 metabolites besides T3 in the brain has also been suggested. To determine the nature of these reactions, we studied metabolism of 0.2-1.0 nM [125I]T4 and 0.1-0.3 nM [131I]T3 in whole homogenates and subcellular fractions of rat cerebral cortex and cerebellum. Dithiothreitol (DTT) was required for detectable metabolic reactions: 100 mM DTT was routinely used. Ethanol extracts of incubation mixtures were analyzed by paper chromatography in t-amyl alcohol:hexane:ammonia and in 1-butanol:acetic acid. Rates of production of iodothyronines from T4 and T3 were greater at pH 7.5 than at 6.4 or 8.6 and greater at 37°C than at 22° or 4°C. Lowering the pH, reducing the protein or DTT concentrations, and preheating homogenates to 100°C all increased excess I− production but reduced iodothyronine production.
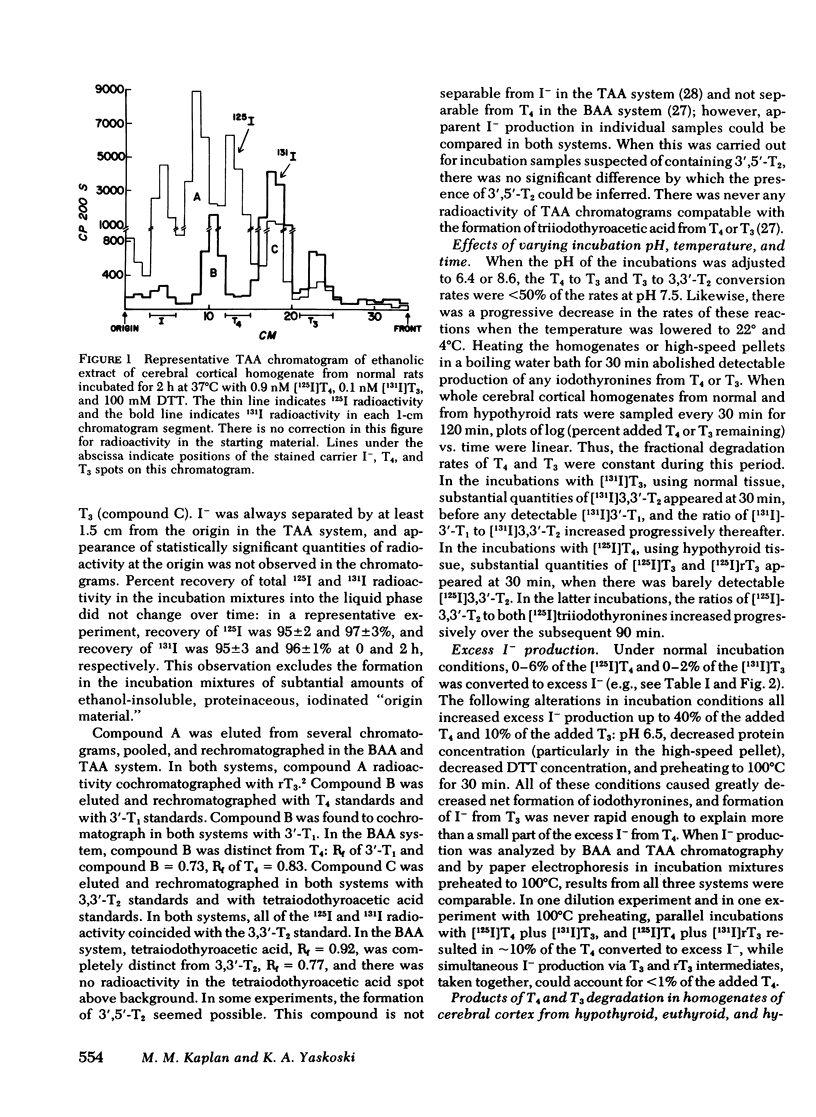
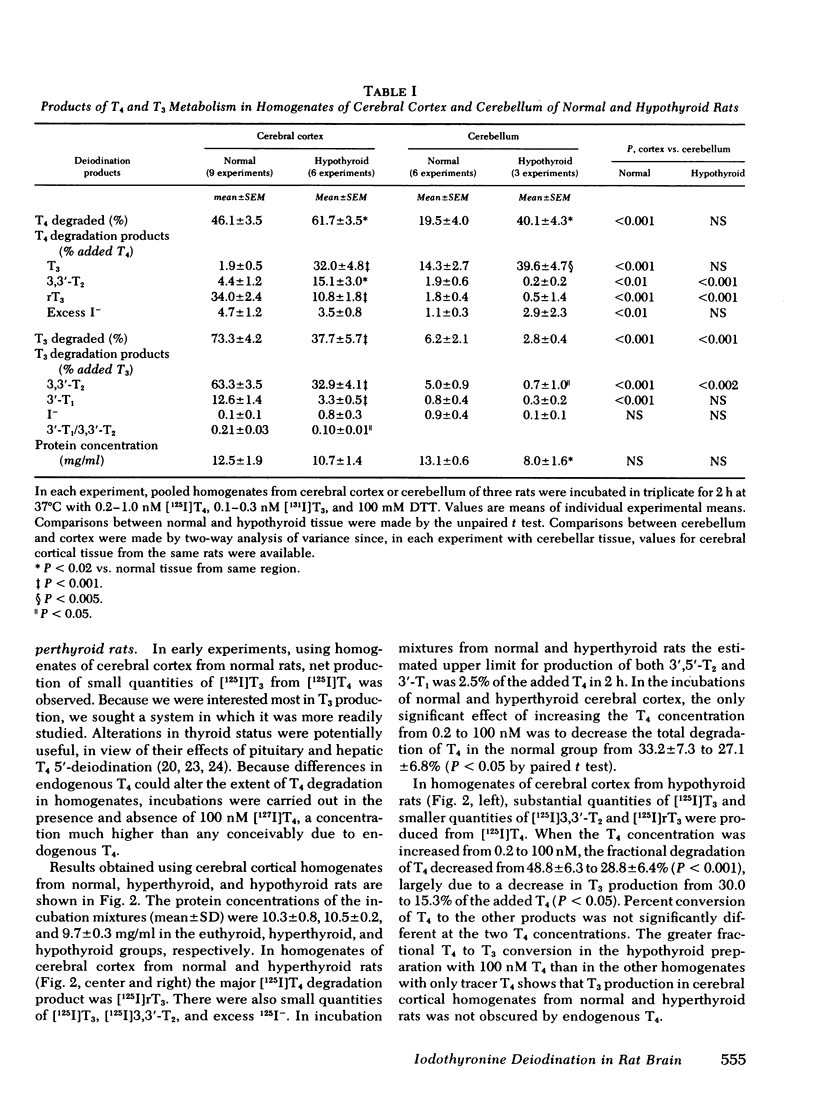
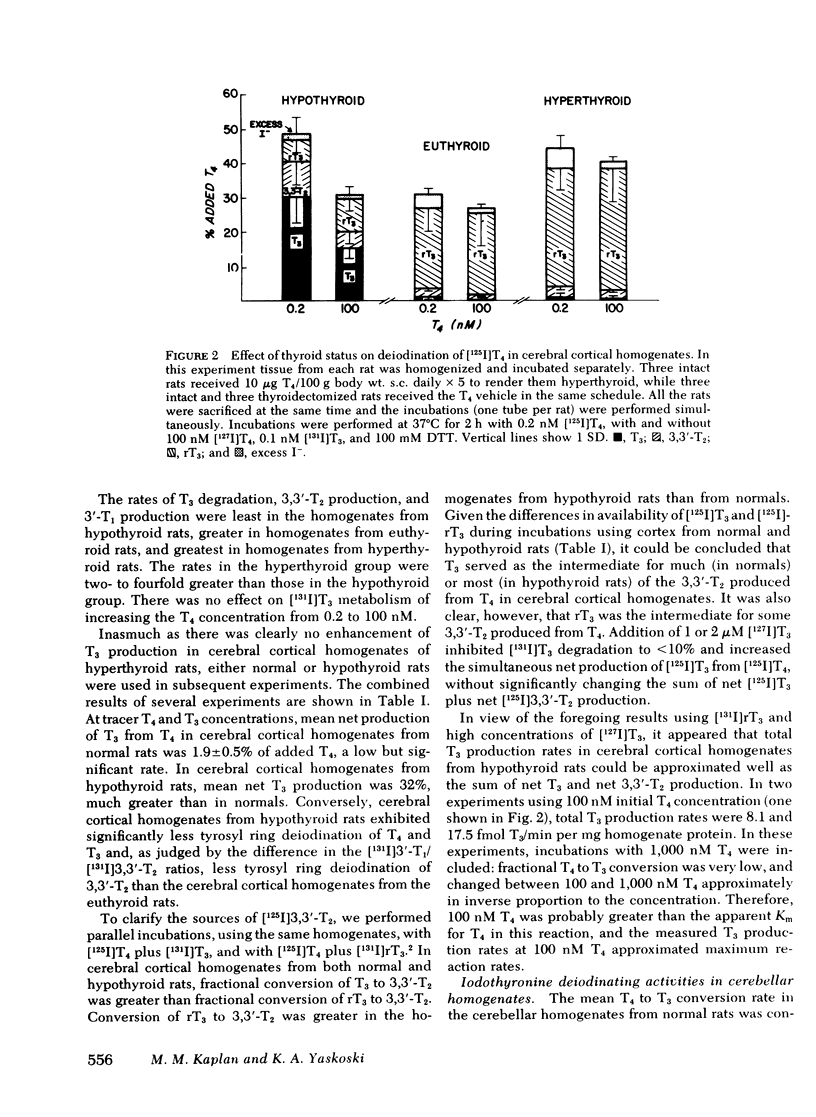
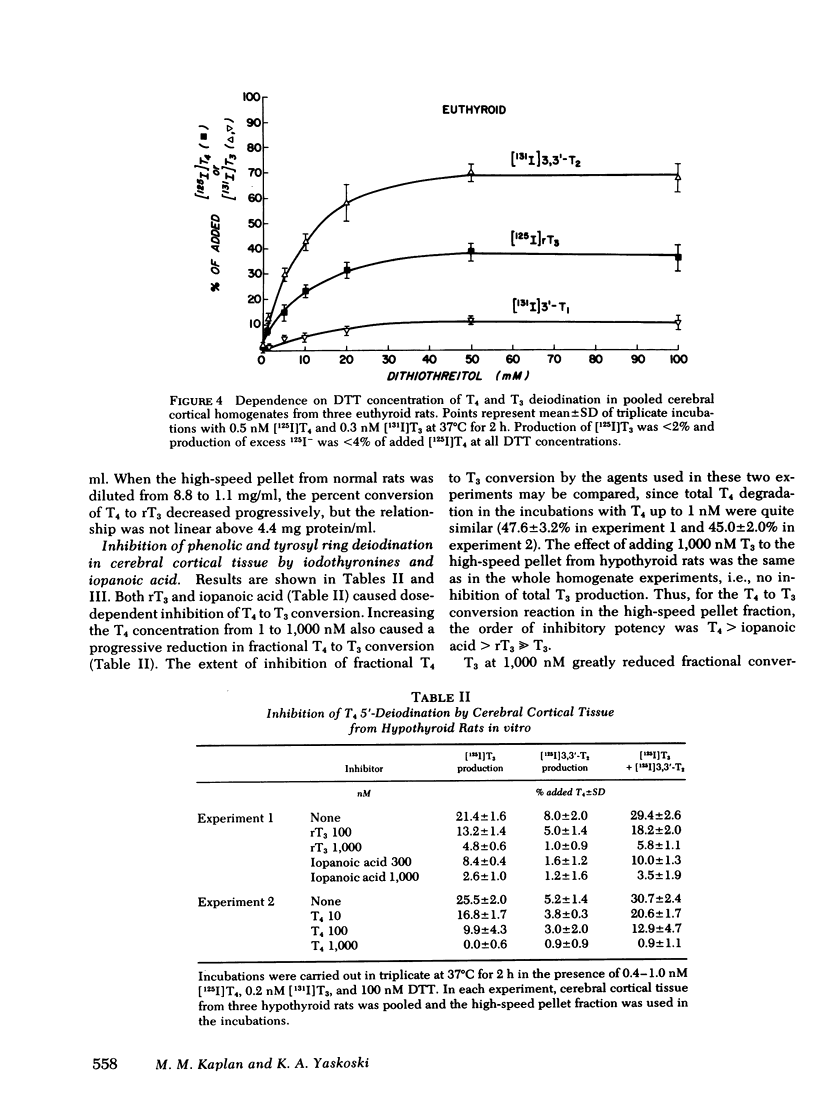
In cerebral cortical homogenates from normal rats, products of T4 degradation were as follows (percent added T4±SEM in nine experiments): T3, 1.9±0.5%; 3,3′,5′-triiodothyronine (rT3), 34.0±2.4%; 3,3′-diiodothyronine (3,3′-T2), 5.8±1.6%; 3′-iodothyronine (3′-T1), ≤2.5%; and excess I−, 4.7±1.2%. In the same experiments, products of T3 degradation were 3,3′-T2, 63.3±5.5%, and 3′-T1, 12.6±1.4%. Cerebral cortical homogenates from hyperthyroid rats and normals were similar in regard to T4 to T3 deiodination. In contrast, in cerebral cortical homogenates from hypothyroid rats, phenolic ring deiodination rates were increased and tyrosyl ring deiodination rates were decreased compared with normals.
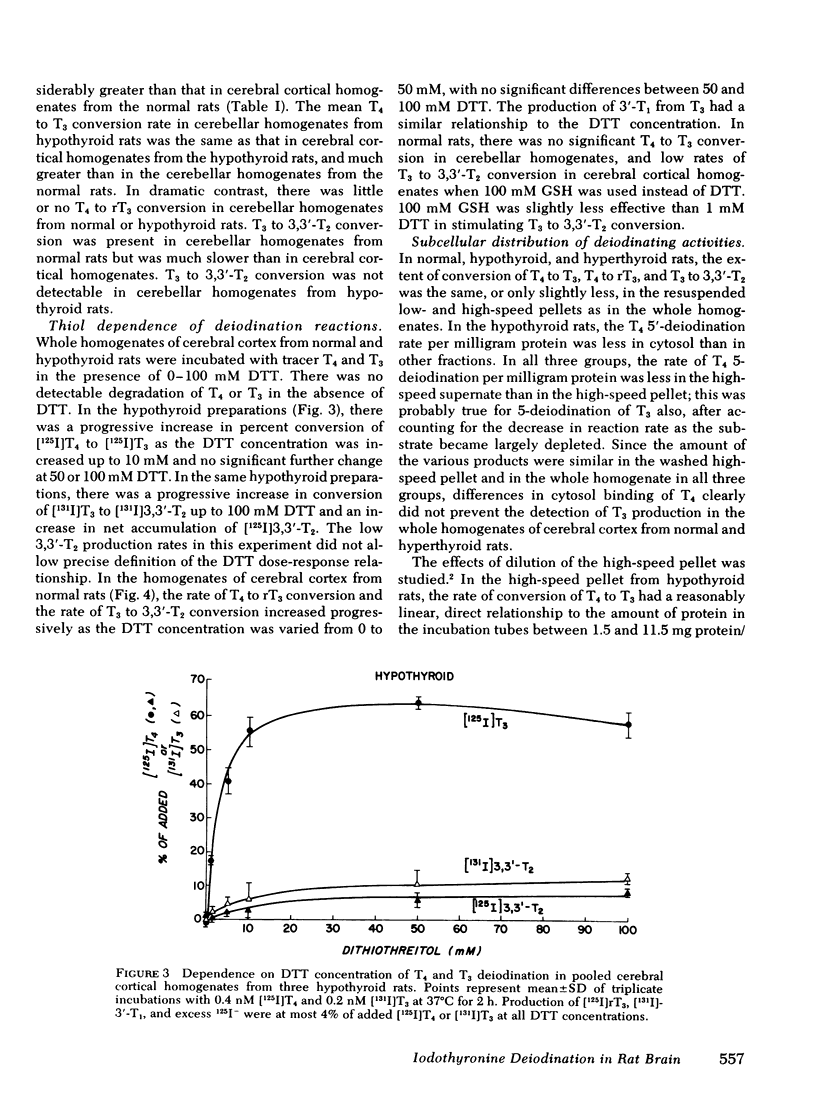
T4 to T3 conversion rates in cerebellar homogenates were greater than rates in cerebral cortical homogenates from the same normal rats and less than rates in cerebellar homogenates from hypothyroid rats. T4 and T3 tyrosyl ring deiodination rates were greatly diminished in cerebellar homogenates compared with cerebral cortical homogenates in normal and hypothyroid rats. High-speed (1,000-160,000 g) pellets from cerebral cortical homogenates were enriched in phenolic and tyrosyl ring deiodinating activities relative to cytosol. Fractional conversion of T4 to T3 was inhibited by T4, iopanoic acid, and rT3, but not by T3. Tyrosyl ring deiodination reactions were inhibited by T3, T4, and iopanoic acid, but not by rT3.
These studies demonstrate separate phenolic and tyrosyl ring iodothyronine deiodinase enzymes in rat brain. The brain phenolic ring deiodinase serves in vivo as a T4 5′-deiodinase and closely resembles anterior pituitary T4 5′-deiodinase in physiological and biochemical characteristics. The physiological significance of the tyrosyl ring iodothyronine deiodinase enzyme is unclear; it shares several properties with rat hepatic T4 5-deiodinase.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHER R. Myxoedematous madness. Br Med J. 1949 Sep 10;2(4627):555–562. doi: 10.1136/bmj.2.4627.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsam A., Sexton F., Ingbar S. H. The effect of thyroidectomy, hypophysectomy, and hormone replacement on the formation of triiodothyronine from thyroxine in rat liver and kidney. Endocrinology. 1978 Nov;103(5):1759–1767. doi: 10.1210/endo-103-5-1759. [DOI] [PubMed] [Google Scholar]
- Balázs R. Influence of metabolic factors on brain development. Br Med Bull. 1974 May;30(2):126–134. doi: 10.1093/oxfordjournals.bmb.a071182. [DOI] [PubMed] [Google Scholar]
- Bellabarba D., Peterson R. E., Sterling K. An improved method for chromatography of iodothyronines. J Clin Endocrinol Metab. 1968 Feb;28(2):305–307. doi: 10.1210/jcem-28-2-305. [DOI] [PubMed] [Google Scholar]
- Cavalieri R. R., Gavin L. A., Bui F., McMahon F., Hammond M. Conversion of thyroxine to 3,3',5'-triiodothyronine (reverse-T3) by a soluble enzyme system of rat liver. Biochem Biophys Res Commun. 1977 Dec 7;79(3):897–902. doi: 10.1016/0006-291x(77)91195-0. [DOI] [PubMed] [Google Scholar]
- Cheron R. G., Kaplan M. M., Larsen P. R. Physiological and pharmacological influences on thyroxine to 3,5,3'-triiodothyronine conversion and nuclear 3,5,3'-triiodothyronine binding in rat anterior pituitary. J Clin Invest. 1979 Nov;64(5):1402–1414. doi: 10.1172/JCI109598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiraseveenuprapund P., Buergi U., Goswami A., Rosenberg I. N. Conversion of L-thyroxine to triiodothyronine in rat kidney homogenate. Endocrinology. 1978 Feb;102(2):612–622. doi: 10.1210/endo-102-2-612. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. A study of extrathyroidal conversion of thyroxine (T4) to 3,3',5-triiodothyronine (T3) in vitro. Endocrinology. 1977 Aug;101(2):453–463. doi: 10.1210/endo-101-2-453. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Wu S. Y., Nakamura Y., Solomon D. H. Monodeiodination of 3,5,3'-triiodothyronine and 3,3',5'-triiodothyronine to 3,3'-diiodothyronine in vitro. Endocrinology. 1978 Apr;102(4):1099–1106. doi: 10.1210/endo-102-4-1099. [DOI] [PubMed] [Google Scholar]
- Crantz F. R., Larsen P. R. Rapid thyroxine to 3,5,3'-triiodothyronine conversion and nuclear 3,5,3'-triiodothyronine binding in rat cerebral cortex and cerebellum. J Clin Invest. 1980 Apr;65(4):935–938. doi: 10.1172/JCI109749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. J., Rappeport J. R., Lutz J. H., Gregerman R. I. Three thyrotoxic criminals. Ann Intern Med. 1971 May;74(5):743–745. doi: 10.7326/0003-4819-74-5-743. [DOI] [PubMed] [Google Scholar]
- Dozin-van Roye B., de Nayer P. Triiodothyronine binding to brain cytosol receptors during maturation. FEBS Lett. 1978 Dec 1;96(1):152–154. doi: 10.1016/0014-5793(78)81081-3. [DOI] [PubMed] [Google Scholar]
- Dratman M. B., Crutchfield F. L., Axelrod J., Colburn R. W., Thoa N. Localization of triiodothyronine in nerve ending fractions of rat brain. Proc Natl Acad Sci U S A. 1976 Mar;73(3):941–944. doi: 10.1073/pnas.73.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dratman M. B., Crutchfield F. L. Synaptosomal [125I]triiodothyronine after intravenous [125I]thyroxine. Am J Physiol. 1978 Dec;235(6):E638–E647. doi: 10.1152/ajpendo.1978.235.6.E638. [DOI] [PubMed] [Google Scholar]
- GRINBERG R., VOLPERT E. M., WERNER S. C. In vivo deiodination of labeled L-thyroxine to L-3,5,3'-triiodothyronine in mouse and human pituitaries. J Clin Endocrinol Metab. 1963 Feb;23:140–142. doi: 10.1210/jcem-23-2-140. [DOI] [PubMed] [Google Scholar]
- Grussendorf M., Hüfner M. Induction of the thyroxine (T4) to triiodothyronine (T3) converting enzyme in rat liver by thyroid hormones and analogs. Clin Chim Acta. 1977 Oct 1;80(1):61–66. doi: 10.1016/0009-8981(77)90264-9. [DOI] [PubMed] [Google Scholar]
- Harris A. R., Fang S. L., Vagenakis A. G., Braverman L. E. Effect of starvation, nutriment replacement, and hypothyroidism on in vitro hepatic T4 to T3 conversion in the rat. Metabolism. 1978 Nov;27(11):1680–1690. doi: 10.1016/0026-0495(78)90290-1. [DOI] [PubMed] [Google Scholar]
- Hesch R. D., Brunner G., Söling H. D. Conversion of thyroxine (T4) and triiodothyronine (T3) and the subcellular localisation of the converting enzyme. Clin Chim Acta. 1975 Mar 10;59(2):209–213. doi: 10.1016/0009-8981(75)90031-5. [DOI] [PubMed] [Google Scholar]
- Höffken B., Ködding R., Von Zur Mühlen A., Hehrmann T., Jüppner H., Hesch R. D. Regulation of thyroid hormone metabolism in rat liver fractions. Biochim Biophys Acta. 1978 Feb 13;539(1):114–124. doi: 10.1016/0304-4165(78)90126-5. [DOI] [PubMed] [Google Scholar]
- Hüfner M., Grussendorf M. Investigations on the deiodination of thyroxine (T4) to 3,3'-diiodothyronine (3,3'-T2) in rat liver homogenate. Clin Chim Acta. 1978 May 2;85(3):243–251. doi: 10.1016/0009-8981(78)90301-7. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M. Changes in the particulate subcellular component of hepatic thyroxine-5'-monodeiodinase in hyperthyroid and hypothyroid rats. Endocrinology. 1979 Aug;105(2):548–554. doi: 10.1210/endo-105-2-548. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Tatro J. B., Breitbart R., Larsen P. R. Comparison of thyroxine and 3,3',5'-triiodothyronine metabolism in rat kidney and liver homogenates. Metabolism. 1979 Nov;28(11):1139–1146. doi: 10.1016/0026-0495(79)90153-7. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M. Thyroxine 5'-monodeiodination in rat anterior pituitary homogenates. Endocrinology. 1980 Feb;106(2):567–576. doi: 10.1210/endo-106-2-567. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Utiger R. D. Iodothyronine metabolism in liver and kidney homogenates from hyperthyroid and hypothyroid rats. Endocrinology. 1978 Jul;103(1):156–161. doi: 10.1210/endo-103-1-156. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Utiger R. D. Iodothyronine metabolism in rat liver homogenates. J Clin Invest. 1978 Feb;61(2):459–471. doi: 10.1172/JCI108957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I., Yamada T., Shichijo K. Nonenzymatic deiodination of thyroxine in vitro. Metabolism. 1966 Dec;15(12):1140–1148. doi: 10.1016/0026-0495(66)90104-1. [DOI] [PubMed] [Google Scholar]
- LARSON F. C., TOMITA K., ALBRIGHT E. C. The deiodination of thyroxine to triiodothyronine by kidney slices of rats with varying thyroid function. Endocrinology. 1955 Sep;57(3):338–344. doi: 10.1210/endo-57-3-338. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsen P. R., Dick T. E., Markovitz B. P., Kaplan M. M., Gard T. G. Inhibition of intrapituitary thyroxine to 3.5.3'-triiodothyronine conversion prevents the acute suppression of thyrotropin release by thyroxine in hypothyroid rats. J Clin Invest. 1979 Jul;64(1):117–128. doi: 10.1172/JCI109430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J. L., Rosenberg I. N. Subcellular distribution of thyroxine 5'-deiodinase in the rat kidney: a plasma membrane location. Endocrinology. 1978 Jul;103(1):274–280. doi: 10.1210/endo-103-1-274. [DOI] [PubMed] [Google Scholar]
- MORREALE DE ESCOBAR G., ESCOBAR DEL REY F., LLORENTE RODRIGUEZ P. Activation by inert proteins of the flavin-induced photodependent deiodination of thyroxine. J Biol Chem. 1962 Jun;237:2041–2043. [PubMed] [Google Scholar]
- Maciel R. M., Ozawa Y., Chopra I. J. Subcellular localization of thyroxine and reverse triiodothyronine outer ring monodeiodinating activities. Endocrinology. 1979 Feb;104(2):365–371. doi: 10.1210/endo-104-2-365. [DOI] [PubMed] [Google Scholar]
- Obregon M. J., Morreale de Escobar G., Escobar del Rey F. Concentrations of triiodo-L-thyronine in the plasma and tissues of normal rats, as determined by radioimmunoassay: comparison with results obtained by an isotopic equilibrium technique. Endocrinology. 1978 Dec;103(6):2145–2153. doi: 10.1210/endo-103-6-2145. [DOI] [PubMed] [Google Scholar]
- Obregon M. J., Roelfsema F., Morreale de Escobar G., Escobar del Rey F., Querido A. Exchange of triiodothyronine derived from thyroxine with circulating triiodothyronine as studied in the rat. Clin Endocrinol (Oxf) 1979 Mar;10(3):305–315. doi: 10.1111/j.1365-2265.1979.tb02085.x. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974 Sep;95(3):897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- RALL J. E., RAWSON R. W., TATA J. R. Metabolism of L-thyroxine and L-3:5:3'-triiodothyronine by brain tissue preparations. Endocrinology. 1957 Jan;60(1):83–98. doi: 10.1210/endo-60-1-83. [DOI] [PubMed] [Google Scholar]
- Reichlin S., Volpert E. M., Werner S. C. Hypothalamic influence of thyroxine monodeiodination by rat anterior pituitary gland. Endocrinology. 1966 Feb;78(2):302–306. doi: 10.1210/endo-78-2-302. [DOI] [PubMed] [Google Scholar]
- Ruiz-Marcos A., Sanchez-Toscano F., Escobar del Rey F., Morreale de Escobar G. Severe hypothyroidism and the maturation of the rat cerebral cortex. Brain Res. 1979 Feb 23;162(2):315–329. doi: 10.1016/0006-8993(79)90292-0. [DOI] [PubMed] [Google Scholar]
- Schwartz H. L., Oppenheimer J. H. Nuclear triiodothyronine receptor sites in brain: probable identity with hepatic receptors and regional distribution. Endocrinology. 1978 Jul;103(1):267–273. doi: 10.1210/endo-103-1-267. [DOI] [PubMed] [Google Scholar]
- Schwartz H. L., Oppenheimer J. H. Ontogenesis of 3,5,3'-triiodothyronine receptors in neonatal rat brain: dissociation between receptor concentration and stimulation of oxygen consumption by 3,5,3'-triiodothyronine. Endocrinology. 1978 Sep;103(3):943–948. doi: 10.1210/endo-103-3-943. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Dick T. E., Larsen P. R. The contribution of local tissue thyroxine monodeiodination to the nuclear 3,5,3'-triiodothyronine in pituitary, liver, and kidney of euthyroid rats. Endocrinology. 1978 Oct;103(4):1196–1207. doi: 10.1210/endo-103-4-1196. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Contributions of plasma triiodothyronine and local thyroxine monodeiodination to triiodothyronine to nuclear triiodothyronine receptor saturation in pituitary, liver, and kidney of hypothyroid rats. Further evidence relating saturation of pituitary nuclear triiodothyronine receptors and the acute inhibition of thyroid-stimulating hormone release. J Clin Invest. 1978 May;61(5):1247–1259. doi: 10.1172/JCI109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorimachi K., Robbins J. Metabolism of thyroid hormones by cultured monkey hepatocarcinoma cells. Nonphenolic ring dieodination and sulfation. J Biol Chem. 1977 Jul 10;252(13):4458–4463. [PubMed] [Google Scholar]
- Valcana T., Timiras P. S. Nuclear triiodothyronine receptors in the developing rat brain. Mol Cell Endocrinol. 1978 Jun;11(1):31–41. doi: 10.1016/0303-7207(78)90030-8. [DOI] [PubMed] [Google Scholar]
- Vigouroux E., Clos J., Legrand J. Uptake and metabolism of exogenous and endogenous thyroxine in the brain of young rats. Horm Metab Res. 1979 Mar;11(3):228–232. doi: 10.1055/s-0028-1092714. [DOI] [PubMed] [Google Scholar]
- Visser T. J., Does-Tobé I., Docter R., Hennemann G. Subcellular localization of a rat liver enzyme converting thyroxine into tri-iodothyronine and possible involvement of essential thiol groups. Biochem J. 1976 Aug 1;157(2):479–482. doi: 10.1042/bj1570479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser T. J., Fekkes D., Docter R., Hennemann G. Sequential deiodination of thyroxine in rat liver homogenate. Biochem J. 1978 Jul 15;174(1):221–229. doi: 10.1042/bj1740221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke J., Orskov H. Synthesis of 125I monolabelled 3, 5, 3'-triiodothyronine and thyroxine of maximum specific activity for radioimmunoassay. Scand J Clin Lab Invest. 1973 Dec;32(4):357–360. doi: 10.3109/00365517309084359. [DOI] [PubMed] [Google Scholar]


