Abstract
Background
The development of addiction is marked by a pathological associative learning process that imbues incentive salience to stimuli associated with drug use. Recent efforts to treat addiction have targeted this learning process using cue exposure therapy augmented with D-cycloserine (DCS), a glutamatergic agent hypothesized to enhance extinction learning. To better understand the impact of DCS-facilitated extinction on neural reactivity to drug cues, the present study reports fMRI findings from a randomized, double-blind, placebo-controlled trial of DCS-facilitated cue exposure for cocaine dependence.
Methods
Twenty-five participants completed two MRI sessions (before and after intervention), with a cocaine-cue reactivity fMRI task. The intervention consisted of 50mg of DCS or placebo, combined with two sessions of cocaine cue exposure and skills training.
Results
Participants demonstrated cocaine cue activation in a variety of brain regions at baseline. From the pre- to post-study scan, participants experienced decreased activation to cues in a number of regions (e.g., accumbens, caudate, frontal poles). Unexpectedly, placebo participants experienced decreases in activation to cues in the left angular and middle temporal gyri and the lateral occipital cortex, while DCS participants did not.
Conclusions
Three trials of DCS-facilitated cue exposure therapy for cocaine dependence have found that DCS either increases or does not significantly impact response to cocaine cues. The present study adds to this literature by demonstrating that DCS may prevent extinction to cocaine cues in temporal and occipital brain regions. Although consistent with past research, results from the present study should be considered preliminary until replicated in larger samples.
Keywords: D-cycloserine, DCS, cocaine dependence, fMRI, cue reactivity, exposure therapy
1. INTRODUCTION
Recent animal research has demonstrated that repeated drug use leads to the development of increasingly habitual drug-seeking and using behavior that is promoted by the transfer of incentive salience from the drug itself to a wide variety of cues associated with drug reward via associative learning (Robinson and Berridge, 1993). Animal models have shown that this process is marked by shifts in drug cue processing from ventral to more dorsal regions of the striatum (Everitt and Robbins, 2005). The neurobiologic underpinning of this pathological associative learning is believed to be dysfunctional glutamate-mediated long-term potentiation processes (Kalivas, 2009). Because of the central role of associative learning in the development and maintenance of addiction, there have been a number of efforts to evaluate the clinical utility of therapies that target extinction of conditioned responses by exposing individuals to drug cues in the absence of drug reward. Unfortunately, such therapies have not consistently demonstrated high clinical efficacy (Conklin and Tiffany, 2002). Recent efforts to strengthen the efficacy of cue exposure therapies have focused on using D-cycloserine (DCS), a partial glutamate N-methyl-D-aspartate receptor agonist, to enhance extinction learning of conditioned drug-seeking and using behavior (Myers and Carlezon, 2012). The success of these attempts has been mixed; whereas some have found a beneficial effect of DCS on facilitating cue extinction in nicotine dependence (Santa Ana et al., 2009), others have demonstrated that DCS may increase cue-induced craving in cocaine dependence (Price et al., 2009, 2012). These inconsistent findings suggest that DCS may influence extinction differently based on the substance under consideration. One potentially fruitful approach to characterizing the varied effects of DCS on addictive behavior may be to study the underlying neural signature of DCS-facilitated cue exposure treatment using contemporary imaging methods.
The present investigation was a sub-study of a clinical trial investigating the use of DCS to facilitate extinction of responses elicited by cocaine cues. All participants were randomized to receive either DCS or placebo prior to each of two days of cocaine cue (i.e., paraphernalia) exposure. Extinction procedures included skills training designed to reduce reactivity to cocaine cues during the post-cue exposure consolidation period in the hope that DCS would enhance consolidation of reduced craving to cocaine cues learned within cue exposure sessions. Participants of the sub-study (n = 25) additionally completed a cocaine-cue reactivity fMRI paradigm prior to and following the cue extinction sessions. We hypothesized that all participants would experience decreased brain activation to drug cues across MRI scans, but this decrease would be greater in the DCS-treated participants relative to placebo-treated participants.
2. METHODS
2.1. Participants
Twenty-five cocaine-dependent men and women aged 18-65 were recruited from a larger (n = 47) clinical trial of DCS facilitation of cocaine-cue extinction (Santa Ana et al., 2012) through media advertisements and clinical referrals in the local Charleston, SC area. All trial participants were invited to participate in the present fMRI sub-study. Trial participants who did not participate in the fMRI sub-study were excluded for having ferrous metal implants (37%), claustrophobia (5%), left-handedness (5%), or for unknown reasons (53%; primarily lack of interest in participating). Individuals who participated in the parent trial, but not in the fMRI sub-study, were approximately evenly split between DCS (53%) and placebo (47%) treatment groups. All study procedures were performed in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki, with approval from the Medical University of South Carolina (MUSC) Institutional Review Board.
All participants met DSM-IV criteria for Cocaine Dependence within 3 months preceding the study and indicated cocaine as their primary drug of choice. Participants were right-handed. Exclusionary criteria included medications for addiction (e.g., topirimate, naltrexone, suboxone), major medical (e.g., diabetes, HIV) and psychiatric conditions (e.g., affective disorders, posttraumatic stress disorder), pregnancy or nursing, ferrous metal implants or pacemakers, and DSM-IV criteria for non-cocaine substance dependence (except caffeine, nicotine, marijuana, or alcohol) within the past 60 days. Participants were required to maintain at least 72 hours of abstinence from cocaine, alcohol, and all other drugs of abuse as confirmed by breathalyzer, urine drug screen (UDS), and self report, prior to each study appointment; positive UDS for tetrahydrocannabinol (THC) was acceptable as long as subjects denied marijuana use within the preceding 72 hours. This testing strategy ensured that participants were abstinent from cocaine and other drugs of abuse for at least 72 hours preceding their first MRI session through the completion of their second MRI session.
2.2. Procedure
Following a phone or in-person screening, participants were scheduled for a baseline diagnostic visit during which they completed a diagnostic interview for DSM-IV disorders along with a number of self-report measures (see “Measures” below). Once all inclusion and no exclusion criteria were met, participants were scheduled for their first fMRI visit within one-week. Participants with positive breath alcohol or urine drug screens at the first MRI visit were rescheduled; participants with positive screens at any subsequent visit were excluded. One week following their first MRI visit, participants completed a second, identical MRI scan; scans typically occurred on two consecutive Fridays. Between MRI visits, on intervening Mondays and Wednesdays, participants underwent two outpatient cocaine-cue extinction sessions (described fully in Santa Ana et al., 2012), separated by one day. Each cocaine-cue extinction session included 4 brief alternating blocks of pre-recorded cognitive behavioral therapy skills training and in vivo handling of paraphernalia and simulated cocaine. Skills training included guided imagery for craving reduction, urge surfing, coping with automatic thoughts to use cocaine, and distraction techniques (Santa Ana et al., 2010). After learning each skill, participants were encouraged to apply the skill to managing their craving during cue exposures. Participants’ subjective craving and physiological reactivity to cocaine cues were recorded during and following each cue exposure block. Fifteen minutes preceding the first cue exposure block, on each day of the extinction sessions, participants were randomly assigned to receive either 50mg of DCS or matched placebo. Participants received the same medication before both cue extinction session. Medication was compounded and packaged by the Investigational Drugs Services (IDS) at MUSC in identical capsules within blister packs. Both study personnel and participants were blind to group assignment.
2.3. Measures
Substance use disorders (SUD) were assessed using the SUD module of the Structured Clinical Interview for DSM-IV (SCID; First et al., 1994). Other axis I psychiatric disorders were assessed using the Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). Cocaine use in the three months preceding the first visit, as well as throughout the study, was assessed using the Timeline Follow-back method (Sobell and Sobell, 1996). Demographics and cocaine use history (e.g., years of use) were assessed using an in-house questionnaire.
2.4. Cocaine cue-reactivity paradigm
The present investigation utilized a cocaine cue-reactivity fMRI paradigm adapted from an established alcohol-cue reactivity paradigm (George et al., 2001; Myrick et al., 2008). Subjects were shown pictures of cocaine and related objects (e.g., crack pipe), neutral objects (e.g., furniture), and visual control images that lack object recognition over six 90-second epochs. Of the 30 cocaine images, 13 pictured crack cocaine, 14 pictured powder cocaine, and 3 pictured both crack and powder cocaine. Fourteen of the 30 pictures contained both cocaine and cocaine paraphernalia (e.g., lighter, crack pipe, rolled paper money, razor), 12 contained cocaine only, and 4 depicted cocaine use. Each 90-second epoch contains three 24-second blocks (cocaine images, neutral objects, control images), containing five pictures displayed for 4.8 seconds each, and one 18 second rest block (i.e., cross-hair). The image blocks are balanced with respect to luminosity (i.e., brightness). Blocks and stimuli within blocks are presented in pseudorandom order. During the task, participants were asked to rate their craving, from zero (“none”) to four (“severe”), after each block using a handpad. Participants’ cocaine craving scores were computed by taking their average craving rating following cocaine blocks and subtracting from it their average craving rating following neutral object blocks. This cocaine cue-reactivity paradigm was developed for a placebo-controlled trial of N-acetylcysteine (NAC) for cocaine dependence (LaRowe et al., 2005, 2007). A recent examination of the association between motivation/treatment status and brain activation to cocaine cues utilizing the present fMRI cocaine cue paradigm provided further support for the validity of the paradigm (Prisciandaro et al., in press).
2.5. Image acquisition
Participants underwent two identical MRI scans separated by one week. MRI scans were performed in a Siemens 3.0T Trio (Erlangen, Germany) MR scanner with a 12-channel head coil. Following localizer and anatomical scans, the cue reactivity scan was acquired using an echo-planar gradient-echo pulse sequence (TR = 2200 ms, TE = 35 ms, flip angle = 90%). Images were acquired with approximate AC-PC alignment. Each brain volume consisted of 36 transverse slices (64 × 64 matrix, 3.0 mm thickness, no gap). Voxel size was 3.0 × 3.0 × 3.0 mm3.
2.6. Image analysis
fMRI analyses were conducted using Statistical Parametric Mapping software 8 (SPM8, The Wellcome Department of Cognitive Neurology, London). All volumes within the cue reactivity scan were realigned to the first volume. Images were stereotactically normalized into a standard space, with a resolution of 3 × 3 × 3 mm voxels using a Montreal Neurological Institute (MNI) template. Data were smoothed with an isotropic 8 mm Gaussian kernel and were high-pass filtered with a cut off period of 240 s (i.e., twice the task cycle duration). Following preprocessing, fMRI data were analyzed within a general linear model (GLM) mixed effects framework. Within-task data from individual participants were analyzed using fixed-effects GLM, with cocaine-cue activity modeled as a box-car function convolved with the standard canonical hemodynamic response function; six movement parameters (3 rotation values in radian and 3 translation values in mm) were included as covariates to control for the influence of residual head motion. Autocorrelation was statistically controlled using an AR(1) model. Following these intra-individual GLM analyses, cocaine minus neutral image contrast maps were generated and entered into inter-individual random-effects analyses. To identify brain regions that activated significantly more to cocaine cues relative to neutral objects, we performed a one-sample t-test on participants’ cocaine minus neutral image contrast maps from the first MRI session. To examine the relationship between participants’ subjective craving and their brain activation to cocaine versus neutral cues, the above one-sample t-test was repeated with participants’ cocaine craving scores entered as a covariate. To examine the effects of cue-extinction treatment and medication (DCS vs. placebo) on brain activation to cocaine cues, we performed a 2×2 mixed effects ANOVA. The impact of cue-extinction treatment was assessed via the within-subjects main effect of MRI session (pre-scan vs. post-scan) on brain activation to cues, and the effect of medication was assessed via the interaction of MRI session and medication group on brain activation to cues. All group-level statistical maps were thresholded using cluster-level inference in SPM8. For the MRI session × medication group interaction effect, we used a voxelwise threshold of p < 0.01 and a cluster threshold of p < 0.05. For all other analyses, we used a voxelwise threshold of p < 0.001 and a cluster threshold of p < 0.05.
2.7. Behavioral data analysis
Participants’ average subjective craving to cocaine versus neutral images was examined at baseline using a paired samples t-test. To examine the effects of cue-extinction treatment (pre-scan vs. post-scan) and medication (DCS vs. PLA) on subjective craving to cocaine cues versus neutral cues, we performed a 2×2 mixed effects ANOVA on cocaine craving scores (i.e., average craving rating following cocaine blocks minus average craving rating following neutral object blocks).
3. RESULTS
Ten participants randomized to DCS and 15 participants randomized to placebo completed both MRI visits. All participants completed the parent clinical trial. Groups did not significantly differ on any demographic or cocaine use history characteristics (Table 1).
Table 1.
Baseline characteristics by treatment group (n = 25)
| DCS (n = 10) | PLA (n = 15) | P | |
|---|---|---|---|
| Demographics | |||
| Age, M (SD) | 48.8 (11.6) | 43.7 (10.0) | 0.10 |
| Gender, % male | 100.0 | 86.7 | 0.50 |
| Race, % African American | 70.0 | 80.0 | 0.65 |
| Marital status, % married | 10.0 | 26.7 | 0.61 |
| Education, % ≥ high school | 90.0 | 86.7 | 1.00 |
| Smoking status, % smokers | 90.0 | 80.0 | 0.63 |
| Treatment status, % in treatment | 60.0 | 40.0 | 0.43 |
| Cocaine Use History | |||
| Total years of cocaine use, M (SD) | 18.4 (10.3) | 17.6 (5.2) | 1.00 |
| Age of CD onset, M (SD) | 33.5 (10.9) | 27.5 (8.0) | 0.19 |
| % cocaine use days (past 90), M (SD) | 40.1 (23.6) | 46.1 (26.3) | 0.98 |
| $ spent on cocaine (past 90), M (SD) | 2013.0 (1316.9) | 2363.0 (1494.1) | 0.69 |
Note. DCS = D-Cycloserine; PLA = Placebo; CD = Cocaine Dependence.
3.1. Baseline analyses
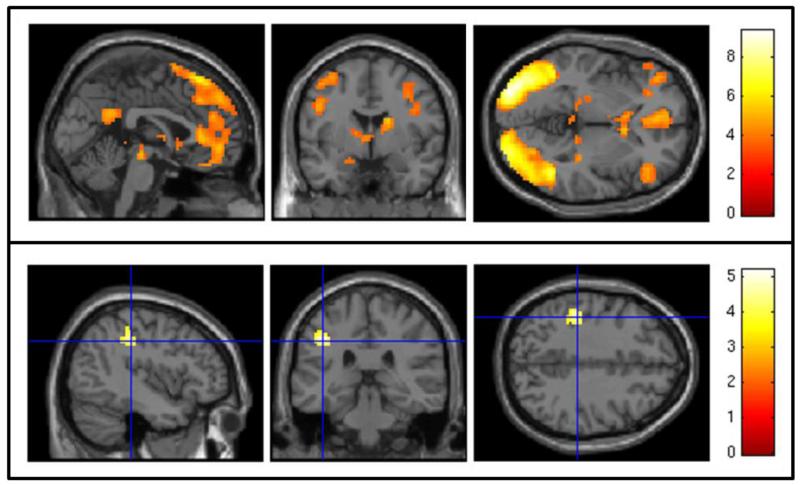
Two participants, both randomized to placebo, were excluded from the baseline MRI scan analysis due to excessive head motion (i.e., ≥ 3 mm/degrees in any direction). Across all participants (n = 23), cocaine cues, relative to neutral objects, were associated with a widespread pattern of activation including bilateral occipital cortices, left frontal regions (e.g., inferior frontal cortex, frontal orbital cortex), right dorsolateral prefrontal cortex (DLPFC), right nucleus accumbens and caudate, left hippocampus, and posterior cingulate gyrus (Table 2; Figure 1, top panel). Participants’ subjective craving to cocaine images was significantly higher than their subjective craving to neutral objects (paired t[20] = 5.52, p < 0.001; Cohen’s d = 1.12). Furthermore, participants’ subjective craving to cocaine versus neutral cues was significantly correlated with their brain activation to cocaine versus neutral cues in a 62-voxel spatial cluster with peak activation in the anterior division of the supramarginal gyrus of the parietal lobe (−42, −31, 38; Figure 1, bottom panel).
Table 2.
Activation to cocaine versus neutral cues across participants
| Contrast | Cluster | Z Max | P | Voxel | MNI (x, y, z) | Anatomy |
|---|---|---|---|---|---|---|
| Cocaine > Neutral |
1 | 5.89 | < .001 | 1700 | −36, −85, −4 | L lateral occipital cortex, inferior (BA 18) |
| 5.81 | −30, −94, −1 | L occipital pole | ||||
| 5.67 | −39, −64, −7 | L cerebral white matter | ||||
| 2 | 5.87 | < .001 | 2219 | −24, 44, 41 | L frontal pole | |
| 5.81 | −9, 62, 23 | L frontal pole | ||||
| 5.17 | −12, 41, 50 | L cerebral cortex | ||||
| 3 | 5.59 | < .001 | 343 | −42, 35, 11 | L inferior frontal gyrus, pars triangularis | |
| 4.55 | −39, 41, 2 | L cerebral white matter | ||||
| 4.52 | −30, 29, −16 | L frontal orbital cortex | ||||
| 4 | 5.56 | < .001 | 1706 | 30, −82, 5 | R cerebral white matter | |
| 5.47 | 33, −94, 5 | R occipital pole | ||||
| 5.43 | 30, −82, 23 | R lateral occipital cortex, superior | ||||
| 5 | 5.11 | < .001 | 699 | 42, 8, 29 | R precentral gyrus (BA 9) | |
| 4.88 | 42, 38, 8 | R frontal pole | ||||
| 4.83 | 57, 14, 35 | R precentral gyrus (BA 9) | ||||
| 6 | 4.56 | < .001 | 222 | 9, 14, −4 | R nucleus accumbens | |
| 4.36 | 9, 11, 5 | R caudate | ||||
| 4.28 | 18, −1, 20 | R caudate | ||||
| 7 | 4.23 | < .001 | 289 | −21, −16, −13 | L hippocampus | |
| 4.11 | −3, −22, −13 | brainstem | ||||
| 3.89 | −12, −31, −7 | brainstem | ||||
| 8 | 4.03 | .013 | 79 | −3, −52, 26 | cingulate gyrus, posterior | |
| 9 | 3.82 | .012 | 80 | 51, −34, 50 | R supramarginal gyrus, anterior |
Note: Analyses (one-sample t-test) completed using cluster thresholding (z > 3.50 and corrected cluster threshold of p < .05). Anatomy = most probable region identified using the Harvard-Oxford cortical and subcortical structural atlases; Brodmann areas (where available) identified by the Talairach-Tournoux Atlas. Z Max = local maximum z-value, MNI (x, y, z) = Montreal Neurological Institute coordinates for the local maximum, BA = Brodmann Area.
Figure 1.
Baseline Analyses. Top Panel: SPM map of blood oxygen level dependent (BOLD) response to cocaine cues minus neutral objects across all participants using a voxelwise threshold of z > 3.50 and a cluster threshold of p < 0.05. Bottom Panel: SPM map of the association between subjective craving and BOLD response to cocaine minus neutral cues using a voxelwise threshold of z > 3.53 and a cluster threshold of p < 0.05. Crosshair is centered on the peak-activated voxel of a cluster in the anterior division of the supramarginal gyrus of the parietal lobe.
3.2. 2×2 mixed-effects ANOVA
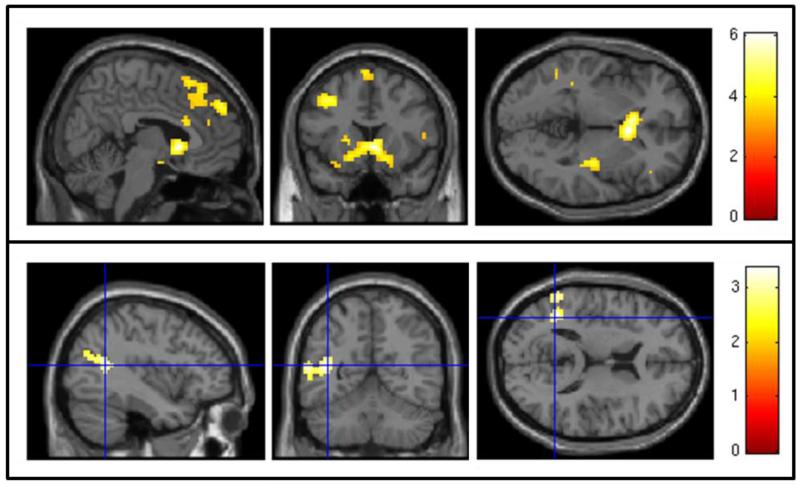
Two additional participants, one placebo and one DCS, were excluded from the 2×2 mixed effects ANOVA due to excessive head motion. Across medication groups, participants experienced significant decreases in brain activation to cocaine cues from pre to post-scan in a variety of anatomical regions, including bilateral nucleus accumbens and caudate, left frontal pole and middle frontal gyrus, right frontal pole, and bilateral temporal gyri (Table 3; Figure 2, top panel). There was no significant change in subjective craving to cocaine versus neutral cues from pre to post-scan across participants (paired t[20] = 1.46, p = 0.16; Cohen’s d = −0.39). No brain regions evidenced significant increases in brain activation to cues from pre to post-scan. There was a significant interaction between medication group and MRI session in a 160-voxel spatial cluster with peak activations in the left angular gyrus, lateral occipital cortex (superior division), and middle temporal gyrus (temporooccipital part; Figure 2, bottom panel). In these brain regions, the placebo group, but not the DCS group, experienced a significant decrease in activation to cocaine cues from pre to post-scan. In terms of subjective craving, there was no significant interaction between medication group and MRI session (p = .13; placebo participants’ craving from pre-scan to post-scan: Cohen’s d = −0.37; DCS participants’ craving from pre-scan to post-scan: Cohen’s d = 0.47).
Table 3.
Activation to cocaine versus neutral cues post-study versus pre-study
| Contrast | Cluster | Z Max | P | Voxel | MNI (x, y, z) | Anatomy |
|---|---|---|---|---|---|---|
| Pre-study > Post-study |
1 | 5.05 | < .001 | 341 | 6, 14, −1 | R nucleus accumbens |
| 4.60 | −6, 17, −1 | L nucleus accumbens | ||||
| 4.18 | −3, 8, −7 | L cerebral cortex (BA 25) | ||||
| 2 | 4.68 | < .001 | 1302 | −24, 41, 41 | L frontal pole | |
| 4.56 | 18, 29, 41 | R cerebral white matter | ||||
| 4.36 | −15, 35, 53 | L superior frontal gyrus | ||||
| 3 | 4.18 | .036 | 70 | −39, 14, 41 | L middle frontal gyrus | |
| 4 | 4.13 | .003 | 163 | 48, −13, −16 | R insular cortex | |
| 4.11 | 33, −22, 5 | R insular cortex | ||||
| 4.06 | 33, −16, −7 | R cerebral white matter | ||||
| 5 | 4.00 | .009 | 120 | 45, 44, 8 | R frontal pole (BA 10) | |
| 3.92 | 54, 23, 5 | R inferior frontal gyrus | ||||
| 3.71 | 57, 26, 14 | R inferior frontal gyrus | ||||
| 6 | 3.85 | .040 | 67 | −45, −40, 5 | L cerebral white matter | |
| 3.42 | −48, −52, −1 | L cerebral white matter | ||||
| 3.40 | −54, −37, 2 | L middle temporal gyrus, posterior |
Note: Analyses (within-subjects main effect of MRI session from 2×2 mixed effects ANOVA) completed using cluster thresholding (z > 3.32 and corrected cluster threshold of p < .05). Anatomy = most probable region identified using the Harvard-Oxford cortical and subcortical structural atlases; Brodmann areas (where available) identified by the Talairach-Tournoux Atlas. Z Max = local maximum z-value, P = cluster-level p value, MNI (x, y, z) = Montreal Neurological Institute coordinates for the local maximum, BA = Brodmann Area.
Figure 2.
2×2 mixed-effects ANOVA results. Top Panel: SPM map of blood oxygen level dependent (BOLD) response to cocaine cues minus neutral objects at pre-scan versus post-scan using a voxelwise threshold of z > 3.32 and a cluster threshold of p < 0.05. Bottom Panel: SPM map of the interaction between medication group (D-cycloserine vs. placebo) and MRI session (pre-scan vs. post-scan) on blood oxygen level dependent (BOLD) response to cocaine cues minus neutral objects using a voxelwise threshold of z > 2.43 and a cluster threshold of p < 0.05. Crosshair is centered on the peak-activated voxel of a cluster including the left angular gyrus, lateral occipital cortex, and middle temporal gyrus.
3.3. Sensitivity analysis
Because our groups contained relatively few participants, we conducted sensitivity analyses to determine the minimum medication group × MRI session interaction effect that would have been detectable in the present study with ≥ 80% power across a variety of substantively meaningful brain regions. Mean contrast values, reflecting activation to cocaine versus neutral images, were extracted from the anterior cingulate cortex, orbitofrontal cortex, and dorsal striatum; these regions were defined anatomically via the Anatomic Automatic Labeling atlas (Tzourio-Mazoyer et al., 2002). The minimum detectable effect size ranged from f = 0.33 to f = 0.53, which can be described as “medium” to “large” effect sizes (Faul et al., 2007). The 2 × 2 mixed effects ANOVA reported above was subsequently estimated in each of the defined brain regions to calculate observed interaction effect sizes in each region. Observed effect sizes ranged from f = 0.00 to 0.20. For comparison, the significant interaction found in the present study had an effect size of f = 0.64. Importantly, inspection of the means revealed that, in all defined brain regions, interaction effects reflected decreases in cocaine cue responding in the placebo condition, but not the DCS condition, across sessions. In sum, the present study was adequately powered to detect medium to large effects; in all defined brain regions, with the exception of the temporooccipital region from which a significant interaction effect was reported, interaction effect sizes were small and consistent in suggesting that placebo participants, but not DCS participants, experienced decreased cue activation across sessions.
4. DISCUSSION
The present investigation was an fMRI sub-study of a placebo-controlled clinical trial exploring the use of DCS in facilitating cocaine-cue extinction. Consistent with a number of fMRI studies in cocaine-dependent individuals (Bonson et al., 2002; Childress et al., 1999; Garavan et al., 2000; Grant et al., 1996; Kilts et al., 2004; Maas et al., 1998; Wang et al., 1999; Wexler et al., 2001), participants demonstrated activation to cocaine versus neutral cues in a wide variety of expected brain regions at baseline (e.g., right DLPFC, nucleus accumbens, caudate, and posterior cingulate). Also consistent with previous studies, subjective craving to cocaine cues was significantly higher than craving to neutral cues, and cocaine craving was correlated with regional brain activation to cocaine cues (Grant et al., 1996; Maas et al., 1998; Wang et al., 1999). There has been little consistency across past studies regarding the region(s) of brain activation that correlates with subjective craving; identified regions have included dorsolateral prefrontal cortex, medial temporal lobe, cerebellum (Grant et al., 1996), anterior cingulate (Maas et al., 1998), and insula (Wang et al., 1999). In the present study, activation to cocaine cues in the parietal lobe, a region most notably involved in sensory integration, was associated with subjective craving. Interestingly, Garavan and colleagues (2000) found that only activation in the parietal lobe was specifically associated with viewing cocaine versus non-drug, appetitive cues. Though this commonality between Garavan et al. (2000) and the present study’s findings is intriguing, further research is needed to better understand the association between regional brain activation to cocaine cues and subjective craving given the lack of cross-study consistency.
From the pre-study to post-study fMRI visit, participants experienced decreased activation to cocaine cues in a variety of brain regions (e.g., bilateral nucleus accumbens, caudate, frontal poles). Only one previous study has examined the impact of cue exposure therapy on brain activation to drug cues (Vollstadt-Klein et al., 2011). Following 9 sessions of cue exposure therapy over the course of 3 weeks, patients with alcohol dependence demonstrated greater decreases in brain activation to alcohol cues in the anterior cingulate, insula, inferior parietal lobule, superior and middle frontal gyri, putamen, and caudate, relative to non-treatment controls. Although the present study did not employ a no-treatment control group, participants experienced decreases in activation to drug cues following cue exposure therapy in very similar brain regions as did participants in Vollstadt-Klein and colleagues’ (2011) study. Together, these findings suggest that cue exposure therapy reduces activation to drug cues in a variety of frontostriatal brain regions that have been heavily implicated in the acquisition and maintenance of addiction (Feil et al., 2010).
Unexpectedly, placebo participants experienced significant decreases in activation to cocaine cues in the left angular gyrus, lateral occipital cortex, and middle temporal gyrus, while DCS participants’ did not. In addition, subjective craving data followed a similar pattern with a decrease in subjective craving in the placebo group and a slight increase in the DCS group, though these findings did not reach statistical significance. Together, these findings suggest that cue exposure decreased craving to cocaine cues, and that administration of DCS prior to cue exposure appears to have retarded the acquisition of the inhibitory learning typically associated with extinction. Although inconsistent with rodent research that has demonstrated that DCS facilitates extinction of cocaine conditioned place preference (Thanos et al., 2010) and impairs reacquisition of cocaine self-administration (Nic Dhonnchadha et al., 2010), these findings are consistent with two earlier studies of DCS in cocaine-dependent human participants conducted by our research group. In our first pilot study of DCS-facilitation of cocaine cue extinction, DCS or placebo was administered 2 hours prior to each of two extinction sessions and a trend towards elevated craving during cue exposure sessions in DCS- versus placebo-treated participants was found (Price et al., 2009). We hypothesized that DCS-treated participants may have experienced elevated craving because DCS was active during cue exposure as opposed to the post-cue-exposure consolidation of memory for extinction learning. As such, in the second study, DCS or placebo was administered 15 minutes prior to cue exposure procedures to ensure that DCS would be active during the consolidation of extinction learning (Price et al., 2012). In spite of these changes, data from this second study also suggested that DCS may increase cue-induced craving for cocaine rather than facilitate extinction learning. As discussed (Price et al. 2012), DCS may increase craving for cocaine by potentiating, via glutamatergic activation, the reconsolidation of memories regarding the original learning of cocaine-cue associations. Interestingly, although a number of studies have demonstrated successful DCS-facilitated extinction in individuals with anxiety disorders (de Kleine et al., 2012; Hofmann et al., 2006; Norberg et al., 2008), including a neuroimaging study in snake phobia (Nave et al., 2012), a recent investigation of DCS facilitated extinction in Posttraumatic Stress Disorder found that DCS was associated with elevated PTSD symptoms relative to placebo (Litz et al., 2012); similar to Price et al.’s suggestion that DCS may have facilitated reconsolidation of memories regarding the original learning of cocaine-cue associations, the authors suggested that DCS may have facilitated reconsolidation of trauma memories. The present study was designed to overcome this limitation by providing skills training during the post-cue exposure consolidation period in the hope that DCS would enhance consolidation of reduced craving to cocaine cues learned within cue exposure sessions to manage cocaine cue-induced craving. Unfortunately, the clinical trial from which the present sub-study was derived did not demonstrate significant differences between DCS and placebo in subjective or physiological response to cocaine cues either within or between extinction sessions (Santa Ana et al., 2012). The findings from the present fMRI study suggest that, consistent with our previous work, DCS may have interfered with extinction learning in spite of the procedures used. In sum, although DCS facilitated extinction has shown promise in the treatment of nicotine dependence in humans (Santa Ana et al., 2009), it cannot be presently recommended for the treatment of cocaine use disorders.
These conclusions should be viewed in light of the limitations of the present study. First, our sample size was small. As a result, our lack of significant medication group differences in most (i.e., non-temporal/occipital) brain regions may have been due to a lack of statistical power. Sensitivity analyses suggested that the present study was adequately powered to detect medium to large interaction effects and that non-significant interaction effect sizes across multiple brain regions were small and consistent in suggesting that placebo participants, but not DCS participants, experienced decreased cue activation across sessions. Second, the obtained pattern of significant medication group differences was not expected a priori. Further research in larger samples will be important to evaluate the generalizability of the findings. Third, we based our study design (e.g., two exposure therapy sessions within one week) on other studies primarily in the clinical anxiety disorders literature (e.g., Ressler et al. 2004). The most optimum length of DCS-facilitated extinction therapy is presently unknown in individuals with drug dependence. It is possible, therefore, that two sessions of DCS-facilitated exposure therapy within one week was insufficient to produce a clinical effect. Fourth, although we have suggested that experimental cue extinction procedures were responsible for observed decreases in brain activation to cocaine cues that occurred between the pre and post-study MRI visits, we did not employ a control group that did not undergo extinction procedures to properly test this hypothesis. Thus, decreases in activation to cocaine cues across MRI visits could have been due to a variety of alternative factors (e.g., the passage of time). Two lines of evidence argue against this interpretation: 1) research has demonstrated that activation to drug cues across repeated MRI visits is remarkably stable (Schacht et al., 2010), 2) investigations of cue-exposure treatments that have employed proper control groups have found between-scan decreases in activation to drug cues in similar brain regions (Vollstadt-Klein et al., 2010).
In summary, in this fMRI study of cocaine cue extinction, neural correlates of extinction were identified that are consistent with previous research demonstrating reduced frontostriatal activation to alcohol cues following cue exposure therapy (Vollstadt-Klein et al., 2010). In addition, the comparison of the data from the DCS versus placebo groups were consistent with findings from earlier investigations of DCS in our lab suggesting that DCS may increase brain activation to cocaine cues in individuals with cocaine dependence. Although consistent with past research, results from the present study should be considered preliminary until replicated in larger samples.
Acknowledgments
Role of Funding Source: Funding for this study was provided by NIH grant R01 DA023188 (Brady); the NIH had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. Dr. Prisciandaro was supported by NIDA F32 DA032250.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: Dr. Brady designed the study and wrote the protocol. Dr. McRae-Clark coordinated the study implementation. Dr. Myrick and Mr. Henderson designed the imaging protocol. Dr. Santa Ana and Dr. Saladin created the psychosocial treatment protocol. Dr. Prisciandaro conducted the analyses and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Author Disclosures
Conflict of Interest: No conflict declared.
Clinical Trials Registration: D-Cycloserine Facilitation of Cocaine-cue Extinction, ClinicalTrials.gov Identifier: NCT00759473 (http://clinicaltrials.gov/ct2/show/NCT00759473).
REFERENCES
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HJ, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am. J. Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- de Kleine RA, Hendriks GJ, Kusters WJC, Broekman TG, van Minnen A. A randomized placebo-controlled trial of D-Cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biol. Psychiatry. 2012;71:962–968. doi: 10.1016/j.biopsych.2012.02.033. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Feil J, Sheppard D, Fitzgerald PB, Yucel M, Lubman DI, Bradshaw JL. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci. Biobehav. Rev. 2010;35:248–275. doi: 10.1016/j.neubiorev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition. New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am. J. Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch. Gen. Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc. Natl. Acad. Sci. USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Pollack MH, Otto MW. Augmentation treatment of psychotherapy for anxiety disorders with D-cycloserine. CNS Drug Rev. 2006;12:208–217. doi: 10.1111/j.1527-3458.2006.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KPG. The neural correlates of cue-induced craving in cocaine-dependent women. Am. J. Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, Brady K, Kalivas PW, Malcolm R. Is cocaine desire reduced by N-Acetylcysteine? Am. J. Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Myrick H, Henderson S, Kalivas PW, Malcolm R. Cue Reactivity and Neuroimaging in Cocaine Dependent Subjects: A Double-blind Placebo-controlled Pilot Study Involving N-Acetylcysteine; Poster presented at the 35th annual meeting of the Society for Neuroscience; Washington, D. C.. 2005. [Google Scholar]
- Litz BT, Sallers-Pedneault K, Steenkamp MM, Hermos JA, Bryant RA, Otto MW, Hofmann SG. A randomized placebo-controlled trial of D-cycloserine and exposure therapy for posttraumatic stress disorder. J. Psychiatr. Res. 2012;46:1184–1190. doi: 10.1016/j.jpsychires.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RG, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am. J. Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA., Jr D-cycloserine effects on extinction of conditioned responses to drug-related cues. Psychopharmacology. 2012;71:947–955. doi: 10.1016/j.biopsych.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch. Gen. Psychiatry. 2008;65:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Szalay JJ, Achat-Mendes C, Platt DM, Otto MW, Spealman RD, Kantak M. D-cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacology. 2010;35:357–367. doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave AM, Tolin DF, Stevens MC. Exposure therapy, D-cycloserine, and functional magnetic resonance imaging in patients with snake phobia: a randomized pilot study. J. Clin. Psychiatry. 2012;73:1179–1186. doi: 10.4088/JCP.11m07564. [DOI] [PubMed] [Google Scholar]
- Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol. Psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Price KL, Baker NL, McRae-Clark AL, Saladin ME, DeSantis SM, Santa Ana EJ, Brady KT. A randomized, placebo-controlled laboratory study of the effects of D-cycloserine on craving in cocaine-dependent individuals. Psychopharmacology. 2012 doi: 10.1007/s00213-011-2592-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KL, McRae-Clark AL, Saladin ME, Moran-Santa Maria MM, DeSantis SM, Back SE, Brady KT. D-cycloserine and cocaine cue reactivity: preliminary findings. Am. J. Drug Alcohol Abuse. 2009;35:434–438. doi: 10.3109/00952990903384332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, McRae-Clark AL, Myrick H, Henderson S, Brady KT. Brain activation to cocaine cues and motivation/treatment status. Addict. Biol. 2012 doi: 10.1111/j.1369-1600.2012.00446.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch. Gen. Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain. Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Santa Ana EJ, Rounsaville BJ, Frankforter T, Nich C, Babuscio T, Poling J, Gonsai K, Hill KP, Carroll KM. D-cycloserine attenuates reactivity to smoking cues in nicotine dependent smokers: a pilot investigation. Drug Alcohol Depend. 2009;104:220–227. doi: 10.1016/j.drugalcdep.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa Ana EJ, Prisciandaro JJ, Shaftman SR, Saladin ME, McRae-Clark AL, Brady KT. Effect of D-cycloserine and cue exposure therapy on physiological and subjective craving in cocaine dependence. 2012. Unpublished Manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa Ana EJ, Saladin ME, Price KL. A cocaine cue exposure and response prevention (C-CERP) manual for cocaine dependent participants. 2010. Unpublished Treatment Manual. [Google Scholar]
- Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict. Biol. 2012 doi: 10.1111/j.1369-1600.2012.00464.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavas J, Weiller E, Hergueta T, Baker R, Dunbar GC. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Followback User’s Guide: A Calendar Method for Assessing Alcohol and Drug Use. Addiction Research Foundation; Toronto: 1996. [Google Scholar]
- Thanos PK, Bermeo C, Wang GJ, Volkow ND. D-Cycloserine accelerates the extinction of cocaine-induced conditioned place preference in C57bL/c mice. Behav. Brain Res. 2009;199:345–349. doi: 10.1016/j.bbr.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer B, Landeau D, Papathanassiou F, Crivello O, Etard N, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Buhler M, von der Goltz C, Hermann D, Mann K, Kiefer F. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biol. Psychiatry. 2011;69:1060–1066. doi: 10.1016/j.biopsych.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hilzemann RJ, Pappas NR, Wong CT, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. Am. J. Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]