Abstract
The P2X7 receptor/channel responds to extracellular ATP and is associated with neuronal death and neuroinflammation in spinal cord injury and amyotrophic lateral sclerosis (ALS). Whether activation of P2X7 directly causes motor neuron death is unknown. We found that cultured motor neurons isolated from embryonic rat spinal cord express P2X7 and underwent caspase-dependent apoptosis when exposed to exceptionally low concentrations of the P2X7 agonist 3′-O-(4-benzoyl)-ATP (BzATP). The P2X7 inhibitors BBG, oATP and KN-62 prevented BzATP-induced motor neuron death. The endogenous P2X7 agonist ATP induced motor neuron death at low concentrations (1-100 μM). High concentrations of ATP (1 mM) paradoxically became protective due to degradation in the culture media to produce adenosine and activate adenosine receptors. P2X7-induced motor neuron death was dependent on neuronal nitric oxide synthase-mediated production of peroxynitrite, p38 activation and autocrine FAS signaling. Taken together, our results indicate that motor neurons are highly sensitive to P2X7 activation, which triggers apoptosis by activation of the well-established peroxynitrite/FAS death pathway in motor neurons.
Keywords: motor neuron disease, amyotrophic lateral sclerosis, purinergic, extracellular ATP, P2X, CD95, p38 kinase, adenosine, nitric oxide, apoptosis
Introduction
Neurologic disorders like trauma, ischemia and inflammation cause a marked increase in extracellular adenosine-5′-triphosphate (ATP) levels (Wang et al. 2004, Phillis et al. 1993, Melani et al. 2005, Piccini et al. 2008). The P2X7 receptor is a non-desensitizing ATP-gated cation channel that can induce pro-inflammatory responses in glia and immune cells that can indirectly initiate neuronal death (Apolloni et al. 2009, Burnstock 2008). P2X7 can also directly induce neuronal death (Burnstock 2008). Inhibition of P2X7 is neuroprotective in animal models of traumatic spinal cord injury, experimental autoimmune encephalomyelitis and Alzheimer’s and Huntington’s disease (Ryu & McLarnon 2008, Matute et al. 2007, Diaz-Hernandez et al. 2009, Jun et al. 2007).
Motor neurons are the most vulnerable neurons after traumatic spinal cord injury and in motor neuron diseases like amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy. A role for P2X7 in inducing motor neuron death during traumatic spinal cord injury has been uncovered in a rat model, where ATP was dramatically increased in the peripheral regions of the injury (Wang et al. 2004). Activation of P2X7 in spinal cord motor neurons caused an irreversible increase in intracellular calcium (Wang et al. 2004), while systemic inhibition of P2X7 protected motor neurons, promoted functional recovery, decreased microglia and astrocyte activation and limited neutrophil infiltration into the spinal cord (Peng et al. 2009).
In ALS patients as well as in the ALS animal models expressing mutant Cu,Zn superoxide dismutase (SOD1G93A), growing evidence indicates that P2X7 could cause motor neuron death acting through astrocytes or microglia. Spinal cord microglia display increased immunoreactivity for P2X7 during the disease (Yiangou et al. 2006, Casanovas et al. 2008). Furthermore, cultured SOD1G93A microglia display an increased sensitivity to ATP and P2X7 activation drives their pro-inflammatory activation (D’Ambrosi et al. 2009).
We have previously shown that P2X7 activation in astrocytes triggers a phenotype change and causes astrocytes to induce death of motor neurons in a co-culture model (Gandelman et al. 2010). In astrocytes cultured from SOD1G93A rats, P2X7 is basally activated in an autocrine manner that maintains their neurotoxic phenotype towards motor neurons (Gandelman et al. 2010). These results indicate that part of the dysfunction of SODG93A astrocytes involves the release of enough ATP to activate P2X7 in vivo. Here, we investigated whether ATP might also activate P2X7 in motor neurons and result in motor neuron death.
Methods
Chemicals and reagents
Cell culture, PCR reagents and secondary antibody were from Life Technologies. FAS:FC and cleaved caspase 3 antibodies were from Cell Signaling Technologies (Danvers, MA). An antibody directed against the intracellular C-terminal domain of P2X7 was from Alomone Labs (Jerusalem, Israel). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Animals
Timed pregnant Sprague-Dawley rats were purchased from Harlan Laboratories (Livermore, CA). Procedures using laboratory animals were in accordance with the international guidelines for the use of live animals and were approved by the Institutional Animal Care Organization of the School of Medicine, Universidad de la República (Montevideo, Uruguay) and by the Oregon State University IACUC.
Primary motor neuron cultures
Motor neurons were prepared from embryonic day 15 rat spinal cords as previously described (Henderson et al. 1995, Gandelman et al. 2010). Briefly, the ventral horns of spinal cords were dissected and incubated in 0.05% trypsin for 15 minutes at 37°C, followed by mechanical dissociation. Motor neurons were then purified by centrifugation on an Optiprep cushion, followed by isolation of p75NTR expressing motor neurons by immunoaffinity selection with the IgG-192 monoclonal antibody. Approximately 1500 motor neurons were plated in each well of a 24-well plate coated with poly-L-ornithine and laminin. Motor neurons were cultured in Neurobasal media supplemented with 2% horse serum, 2% B-27 supplement, 25 μM L-glutamate, 25 μM 2-mercaptoethanol and 500 μM L-glutamine (Henderson et al. 1995). Survival was maintained by the addition of GDNF (1ng/ml). Unless otherwise stated, motor neurons were treated 2 hours after seeding, after they had attached and started differentiating. Motor neuron survival was assessed after culturing for 48 hours by counting all cells displaying intact neurites longer than 4 cell diameters in 2 diameters of the well. Motor neurons were plated to yield 130 motor neurons per well under optimal growth conditions with each condition replicated in triplicate and then repeated with at least three separate motor neuron preparations. This method of measuring survival has been extensively used and validated for measuring motor neuron survival (Henderson et al. 1995, Estevez et al. 1998, Estevez et al. 1999).
RT-PCR
Motor neurons were plated in 35mm dishes and after 18 hours RNA was extracted using Trizol according to the manufacturer’s instructions. RT-PCR was performed using SuperScript® III One-Step RT-PCR System by adding specific primers (P2X7 forward AAGGGAAAGAAGCCCCACGG, P2X7 reverse CCGCTTTTCCATGCCATTTT, Actin forward GAGCAATGATCTTGATCTTCATGGTG, Actin reverse CCTTCCTTCCTGGGTATGGAATCC).
Immunofluorescence
Motor neurons were fixed with ice-cold 4% paraformaldehyde and 0.1% glutaraldehyde in PBS for 15 minutes. Cultures were permeabilized with 0.1% Triton X-100 in PBS for 15 min and blocked for 1 hour with 10% goat serum, 2% bovine serum albumin, and 0.1% Triton X-100 in PBS. Anti-cleaved caspase 3 or P2X7 monoclonal antibody diluted in blocking solution (1:100) was incubated overnight at 4°C. After washing, cultures were incubated for 1 hour at room temperature with Alexa Fluor 488 or 568-conjugated goat anti-mouse antibody (1:500). Nuclei were stained with DAPI (1 μg/mL).
Statistics
Each experiment was repeated at least three times with separate motor neuron preparations and data are reported as mean ± SEM. Statistical analysis was performed by one-way analysis of variance, followed by a Student–Newman–Keuls test. Differences were declared statistically significant if p < 0.05. Statistics were performed using GraphPad Prism 4.
Results
Activation of P2X7 in motor neurons leads to cell death
P2X7 was expressed in spinal motor neurons in culture (Fig 1A). The presence of the mRNA for P2X7 receptor was evidenced by RT-PCR. In addition, the soma and neurites of all motor neurons showed intense immunoreactivity for the receptor (Fig. 1A and B). To investigate the effect of P2X7 activation on motor neurons, increasing concentrations of the P2X7 agonist BzATP were added to the cultures two hours after isolation and survival was assessed after 48 hours. Concentrations of BzATP from 0.1 to 100 μM decreased motor neuron survival similarly, ranging from 74±3% to 69±5% (Fig. 2A). Only 0.01 μM BzATP had no observable effect on motor neuron survival. We saw no difference in death induced by BzATP whether motor neurons were exposed to BzATP immediately after plating or if treatment was delayed overnight to allow motor neurons to attach and extend neurites (not shown). Thus, BzATP decreased survival independently of motor neuron attachment and subsequent differentiation.
Fig. 1. Motor neurons in primary culture express P2X7.
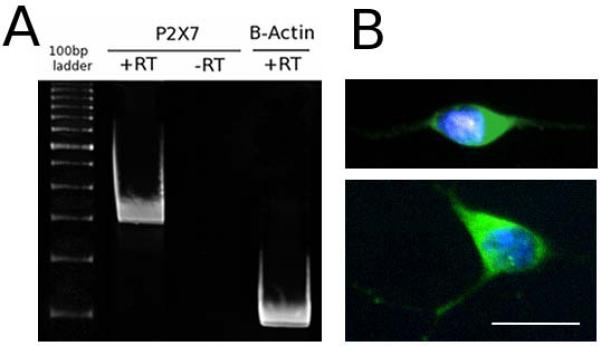
(A) Total RNA was extracted from cultured motor neurons 18 hours after isolation and RT-PCR was performed to reveal the presence of P2X7 mRNA. (B) Motor neurons cultured for 18 hours display immunoreactivity for P2X7 (green) in the soma and neurites. Nuclei were stained with DAPI (blue). The scale bar represents 20 μm.
Fig. 2. Cultured motor neurons are highly sensitive to BzATP.
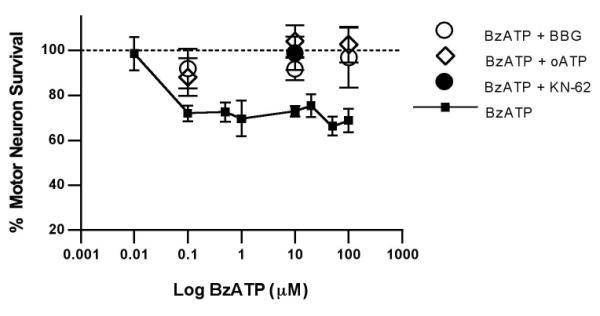
Motor neuron survival in response to 0.1 to 100μM BzATP (squares). The P2X7 inhibitors BBG (1 μM, triangles), oATP (1 μM, inverted triangles) and KN-62 (1 μM, diamonds) prevented motor neuron death induced by 0.1, 10 and 100 μM BzATP. Dotted line indicates survival in absence of BzATP (100%). All BzATP treatments at or above 0.1 μM resulted in significantly decreased survival compared to control cultures (p<0.05). Survival after all of the antagonist treatments was not statistically different from the control cultures.
Inhibiting the P2X7 receptor with BBG, oATP and KN-62 (1 μM) prevented motor neuron death caused by BzATP (at concentrations of 0.1, 10 and 100 μM), which strongly suggests the specific involvement of P2X7 in the induction of motor neuron death by BzATP (Fig. 2A). To investigate the effects of P2X7 activation in motor neurons in the rest of this study, we used treatments with 10 μM BzATP, which causes death of 32±2% motor neurons and is generally recognized to activate P2X7 according to the typical pharmacological profile of this receptor (Donnelly-Roberts et al. 2009).
P2X7 activation triggers apoptosis by activation of the Fas/p38/NO pathway
An increase in Fas-L production followed by autocrine activation of the Fas death receptor results in caspase-dependent apoptosis in motor neurons deprived of trophic factors or expressing ALS-associated mutant SOD1. In this death pathway, Fas signals to p38, leading to NO production that causes motor neuron apoptosis (Raoul et al. 2006, Raoul et al. 2002, Raoul et al. 1999). To test whether this death pathway was activated following P2X7 stimulation, we blocked activation of Fas and found that motor neuron survival after BzATP exposure was restored (92±7% survival). In addition, the p38 inhibitor SB203580 (1 μM) was able to inhibit motor neuron death (96±4%, Fig. 3A). Motor neuron death was also prevented by the general NOS inhibitor L-NAME (100 μM) or the specific nNOS inhibitor TRIM (10μM, Handy et al, 1995). The effect of both inhibitors was reversed by the simultaneous addition of DETA-NONOate (10 μM, Fig. 3B). This concentration of DETA-NONOate will maintain a flow of 40 nM nitric oxide over a 24 h period and does not induce motor neuron death by itself (Estevez et al. 1998).
Fig. 3. BzATP triggered autocrine FAS/p38/nNOS signaling.
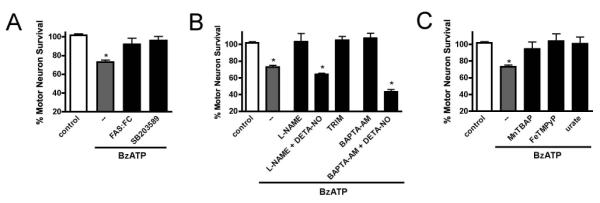
(A) Motor neuron death triggered by BzATP (10 μM) was prevented by SB203580 (10 μM) and FAS:FC. (B) L-NAME (100 μM), TRIM (1 μM) and BAPTA-AM (1 μM) prevented motor neuron death initiated by BzATP, while addition of DETA-NO (10 μM) reinstated death. (C) Motor neuron death caused by BzATP was prevented by addition of MnTBAP (10 μM), FeTMPyP (10 μM) or urate (200 μM). * p<0.05, significantly different from control.
Because P2X7 is known for elevating intracellular calcium levels and NO production by nNOS is activated by calcium, we investigated whether chelation could prevent motor neuron death. Pre-incubation of the cultures with BAPTA-AM (1 μM) prevented motor neuron death induced by BzATP (Fig. 3B) and this protective effect was reversed by addition of DETA NONOate (Fig. 3B). These results indicate a key role for calcium regulation of nNOS in motor neuron death initiated by BzATP. The regulation of intracellular calcium likely also involves additional modulation by endoplasmic reticulum and mitochondrial transporters in addition to P2X7.
NO itself is not toxic to motor neurons, but its diffusion-limited reaction with superoxide to form peroxynitrite is ultimately responsible for motor neuron death (Estevez et al. 1998). Because P2X7 activation is known to initiate production of superoxide in numerous immune and non-immune cell types (Hewinson & Mackenzie 2007), we tested whether superoxide and peroxynitrite played a role in BzATP-induced motor neuron death. The superoxide scavenger MnTBAP (10 μM) and the peroxynitrite scavenger FeTMPyP (10 μM) completely rescued motor neurons from death after P2X7 activation (Fig. 3C). In addition, urate (200 μM), which scavenges peroxynitrite-derived radicals and thereby inhibits tyrosine nitration of proteins (Santos et al. 1999, Robinson et al. 2004), was also able to prevent motor neuron death caused by P2X7 activation (Fig. 3C).
P2X7 activation triggered caspase-dependent death in motor neurons, as it was prevented by the general caspase inhibitor zVAD-fmk (10 μM) and the caspase 3 inhibitor DEVD-fmk (10 μM, Fig. 4A). In addition, extensive immunoreactivity for activated caspase 3 is evidenced in motor neurons 24 hours after treatment with BzATP, in coincidence with fragmented nucleus, known hallmark of apoptosis (Fig. 4B). This agrees with previous reports providing evidence that Fas activates caspase-dependent apoptosis in motor neurons (Raoul, Henderson et al. 1999).
Fig. 4. BzATP triggered caspase-dependent apoptosis.
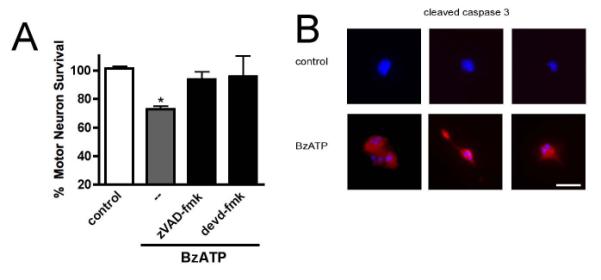
(A) Caspase inhibitors zVAD-fmk and devd-fmk (10 μM) prevented motor neuron death triggered by BzATP (10 μM). (B) Immunofluorescence of cultured motor neurons for cleaved caspase 3 (red), apparent only in motor neurons treated with BzATP. Nuclear morphology was evident with DAPI staining (blue). * p<0.05, significantly different from control. The scale bar represents 20 μm.
ATP degradation to adenosine prevents P2X7-mediated motor neuron death
To more closely resemble conditions during injury or disease in the nervous system, we exposed motor neurons to ATP, the endogenous P2X7 agonist. Addition of 100 μM ATP induced death of 27±7% of motor neurons while 1mM ATP did not cause any significant death (8±4%, Fig. 5A). Because ATP quickly degrades to adenosine, which can increase motor neuron survival by trans-activating Trk receptors (Wiese, Jablonka et al. 2007), we hypothesized that degradation of 1mM ATP could produce enough adenosine to prevent P2X7-mediated death. Indeed, we found that with caffeine (0.5 μM; an inhibitor of P1 receptors), addition of 1mM ATP caused death of 26±7% motor neurons. In addition, high concentrations of adenosine (200 μM) prevented motor neuron death initiated by BzATP (Fig. 5B), while lower doses (1 μM) had no effect, explaining why this antagonizing effect was not seen in motor neurons exposed to lower concentrations of ATP. In agreement with these results, exogenous addition of high doses of adenosine (100 and 200 μM) caused a small but significant increase in basal survival (115±4 and 116±6% respectively) while 1 μM adenosine had no effect (Fig. 5C).
Fig. 5. High concentrations of adenosine produced by ATP degradation can prevent BzATP-induced motor neuron death.
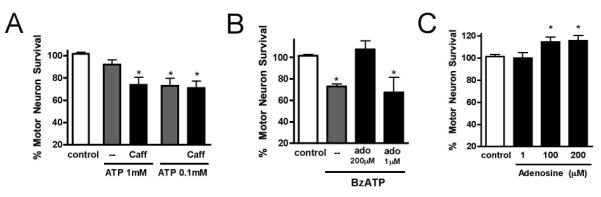
(A) Motor neuron survival in response to the indicated concentrations of ATP (1mM and 100 μM) in presence or absence of caffeine (0.5 μM). (B) Adenosine at a concentration of 200 μM but not at 1 μM prevented motor neuron survival induced by BzATP (10 μM) (C) Basal motor neuron survival increased in response to 1, 100 and 200 μM adenosine. * p<0.05, significantly different from control.
Discussion
Accumulating evidence indicates that P2X7 mediates multiple detrimental effects in many nervous system diseases (Koles et al. 2005, Sperlagh et al. 2006). One of the earliest events after spinal cord trauma is ATP release in areas surrounding experimental spinal cord injury that is also associated with a P2X7 dependent irreversible increase in intracellular calcium (Wang et al, 2004). Surprisingly, the role of P2X7 has received limited investigation in ALS. Increased immunoreactivity for P2X7 in microglia has been observed in ALS tissues (Yiangou et al. 2006). We have recently shown that P2X7 activation caused astrocytes to become neurotoxic to motor neurons (Gandelman et al. 2010). Furthermore, P2X7 was basally activated in SOD1G93A expressing astrocytes and was needed to maintain their neurotoxic phenotype. Here, we found motor neurons themselves can be a direct target of elevated extracellular ATP through activation of P2X7.
P2X7 activation in motor neurons triggered a peroxynitrite-fueled apoptotic cascade, previously described as a distinctive death pathway extensively characterized in the death of motor neurons from trophic factor deprivation, activation of the p75 receptor and expression of mutant SODs (Raoul et al. 2006, Raoul et al. 1999, Raoul et al. 2002, Pehar et al. 2005, Pehar et al. 2007, Pehar et al. 2006). The downstream signaling triggered by P2X7 involves activation of p38 MAPK that in turn activates an autocrine loop involving neuronal NOS activation and Fas signaling. It involves cycles of amplification of increasing nitric oxide synthesis by neuronal NOS, phosphorylation of p38 through DAXX/ASK1, and further FAS-L release. Calcium entry due to P2X7 activation could increase nitric oxide synthesis by stimulating neuronal NOS activity. Supporting this concept, we found that the protection afforded by intracellular calcium chelation from P2X7 activation was reversed by a low steady state concentration of 40 nM nitric oxide.
We have previously shown that nitric oxide is not directly toxic to motor neurons via this pathway, but rather reacts with superoxide to form the oxidant peroxynitrite (Sahawneh et al. 2010). Here, motor neuron death triggered by P2X7 was prevented by two different peroxynitrite scavengers as well as by urate, a radical scavenger that strongly inhibits tyrosine nitration. Thus, the transformation of nitric oxide to peroxynitrite can serve as a switch controlling the survival versus death of stressed motor neurons. Peroxynitrite scavengers have also been shown to be protective in mutant SOD1 transgenic mouse models of ALS (Soon et al. 2011, Wu et al. 2003). Additionally, nitration of a single tyrosine near the ATP binding pocket of HSP90 has recently been shown to be sufficient to activate motor neuron death through a mechanism that involves P2X7 and the release of FAS (Franco et al. 2013).
Fas is strongly implicated in controlling peroxynitrite-induced apoptosis of motor neurons in ALS (Sahawneh et al. 2010), and involved in spinal ischemia, axotomy of the facial nerve, sciatic nerve avulsion and spinal cord injury (Matsushita et al., 2000, Sakurai et al., 1998, Ugolini et al., 2003, Martin et al, 2005, Casha et al, 2005, Demjen et al, 2004). Silencing Fas in the spinal cord of animal models of ALS models improves motor function and extends survival (Locatelli et al, 2007). In spinal cord injury, Fas inhibition specifically spares neurons and axons in the areas surrounding the injury (Casha et al, 2005, Demjen et al, 2004). Here, we found evidence that P2X7 activates Fas-induced death of cultured motor neurons via peroxynitrite, but whether P2X7 triggers this death pathway in vivo remains to be determined. Activation of P2X7 has recently been shown to be involved in FAS-induced death of Jurket cells (Aguirre et al. 2013).
The sensitivity of motor neurons to ATP in vitro was modulated by cleavage to adenosine, which can be catalyzed by extracellular nucleotidases such as CD39 as well as apyrase in culture serum. Adenosine has well established roles as anti-inflammatory and neuroprotective agent (Jacobson & Gao 2006). In our assays, direct addition of high doses of adenosine prevented motor neuron death triggered by BzATP while lower doses had no protective effect. This likely explains how high concentrations of ATP (1 mM) paradoxically protected motor neurons while lower concentrations activated apoptosis via P2X7. Adenosine signaling through the A2A receptor leads to transactivation of the neurotrophin TrkB receptor and prevents motor neuron death in vivo after facial nerve axotomy and in vitro caused by trophic factor deprivation (Wiese et al. 2007). In addition, A2A activation reduces neuronal apoptosis and neurological deficit after spinal cord trauma (Genovese et al. 2010). Both P2X7 antagonists and A2A agonists protect motor neurons from spinal cord trauma (Genovese et al. 2010, Wang et al. 2004, Peng et al. 2009). Thus, ATP-degrading enzymes in the microenvironment surrounding motor neurons might modulate disease progression by limiting the activation of P2X7 while stimulating adenosine receptors. Taken together our results reveal a new regulatory mechanism of motor neuron survival by being a target for elevated extracellular ATP released during the disease. Further knowledge of the interactions between these two pathways could help optimize the design of new highly targeted therapies to prevent motor neuron death in ALS and spinal cord injury.
Acknowledgements
We thank Alvaro G. Estevez (University of Central Florida) for his insightful discussions project and the critical reading of this manuscript and Nathan Lopez (OSU) for invaluable technical support. We acknowledge funding from the National Institute for Environmental and Health Sciences (NIEHS P30ES000210), the National Institutes of Neurological Disorders and Stroke (NINDS R01NS058628A), the National Center for Complementary and Alternative Medicine (NCCAM P01AT002034), and the ALS Association.
Abbreviations
- ALS
Amyotrophic lateral sclerosis
- ASK1
Apoptosis signal-regulating kinase 1
- ATP
adenosine-5′-triphosphate
- A2A
Adenosine receptor
- A2a BBG
Brilliant Blue G
- BzATP
2′(3′)-O-(4-Benzoylbenzoyl) -ATP
- DAXX
Death-associated protein 6
- DEVD-fmk
Benzyloxycarbonyl-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-fluoromethylketone
- DETA-NONOate
(Z)-1-[N- (2-aminoethyl)- N- (2-ammonioethyl)amino]diazen-1-ium-1, 2 diolate
- FeTMPyP
5,10,15,20-Tetrakis(N-methyl-4′-pyridyl)porphinato Iron (III)
- Chloride GDNF
Glial cell-derived neurotrophic factor
- KN-62
4-[(2S)-2-[(5-isoquinolinylsulfonyl)methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl)propyl] phenyl isoquinolinesulfonic acid ester
- L-NAME
NG-Nitro-L-arginine methyl ester
- MnTBAP
Mn(III)tetrakis(4-benzoic acid)porphyrin chloride
- nNOS
neuronal nitric oxide syntahse
- oATP
oxidized ATP
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- RT-PCR
reverse transcription polymerase chain reaction
- SEM
standard error of the mean
- SOD1
Cu/Zn superoxide dismutase-1 SOD1G93A Cu/Zn superoxide dismutase-1 Glycine 93 to Alanine mutation
- TRIM
-(2-Trifluoromethylphenyl) imidazole
- Trk
tropomyosin-receptor-kinase zVAD-fmk - carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone
Footnotes
The authors declare no conflicts of interest.
References
- Aguirre A, Shoji KF, Saez JC, Henriquez M, Quest AF. FasL-triggered death of Jurkat cells requires caspase 8-induced, ATP-dependent cross-talk between Fas and the purinergic receptor P2X(7) J. Cell. Phys. 2013;228:485–493. doi: 10.1002/jcp.24159. [DOI] [PubMed] [Google Scholar]
- Apolloni S, Montilli C, Finocchi P, Amadio S. Membrane compartments and purinergic signalling: P2X receptors in neurodegenerative and neuroinflammatory events. FEBS J. 2009;276:354–364. doi: 10.1111/j.1742-4658.2008.06796.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- Casanovas A, Hernandez S, Tarabal O, Rossello J, Esquerda JE. Strong P2X4 purinergic receptor-like immunoreactivity is selectively associated with degenerating neurons in transgenic rodent models of amyotrophic lateral sclerosis. J Comp Neurol. 2008;506:75–92. doi: 10.1002/cne.21527. [DOI] [PubMed] [Google Scholar]
- D’Ambrosi N, Finocchi P, Apolloni S, Cozzolino M, Ferri A, Padovano V, Pietrini G, Carri MT, Volonte C. The proinflammatory action of microglial P2 receptors is enhanced in SOD1 models for amyotrophic lateral sclerosis. J Immunol. 2009;183:4648–4656. doi: 10.4049/jimmunol.0901212. [DOI] [PubMed] [Google Scholar]
- Diaz-Hernandez M, Diez-Zaera M, Sanchez-Nogueiro J, Gomez-Villafuertes R, Canals JM, Alberch J, Miras-Portugal MT, Lucas JJ. Altered P2X7-receptor level and function in mouse models of Huntington’s disease and therapeutic efficacy of antagonist administration. FASEB J. 2009;23:1893–1906. doi: 10.1096/fj.08-122275. [DOI] [PubMed] [Google Scholar]
- Donnelly-Roberts DL, Namovic MT, Han P, Jarvis MF. Mammalian P2X7 receptor pharmacology: comparison of recombinant mouse, rat and human P2X7 receptors. Br J Pharmacol. 2009;157:1203–1214. doi: 10.1111/j.1476-5381.2009.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez AG, Crow JP, Sampson JB, Reiter C, Zhuang Y, Richardson GJ, Tarpey MM, Barbeito L, Beckman JS. Induction of nitric oxide-dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science. 1999;286:2498–2500. doi: 10.1126/science.286.5449.2498. [DOI] [PubMed] [Google Scholar]
- Estevez AG, Spear N, Manuel SM, Barbeito L, Radi R, Beckman JS. Role of endogenous nitric oxide and peroxynitrite formation in the survival and death of motor neurons in culture. Prog Brain Res. 1998;118:269–280. doi: 10.1016/s0079-6123(08)63214-8. [DOI] [PubMed] [Google Scholar]
- Franco MC, Ye Y, Refakis CA, et al. Nitration of Hsp90 induces cell death. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1215177110. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandelman M, Peluffo H, Beckman JS, Cassina P, Barbeito L. Extracellular ATP and the P2X7 receptor in astrocyte-mediated motor neuron death: implications for amyotrophic lateral sclerosis. J Neuroinflammation. 2010;7:33. doi: 10.1186/1742-2094-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese T, Melani A, Esposito E, et al. Selective adenosine A(2a) receptor agonists reduce the apoptosis in an experimental model of spinal cord trauma. J Biol Regul Homeost Agents. 2010;24:73–86. [PubMed] [Google Scholar]
- Henderson CE, Bloch-Gallego E, Camu W. Purification and culture of embryonic motor neurons: Neural cell culture: a practical approach. IRL Press; Oxford: 1995. [Google Scholar]
- Hewinson J, Mackenzie AB. P2X(7) receptor-mediated reactive oxygen and nitrogen species formation: from receptor to generators. Biochem Soc Trans. 2007;35:1168–1170. doi: 10.1042/BST0351168. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun DJ, Kim J, Jung SY, et al. Extracellular ATP mediates necrotic cell swelling in SN4741 dopaminergic neurons through P2X7 receptors. J Biol Chem. 2007;282:37350–37358. doi: 10.1074/jbc.M707915200. [DOI] [PubMed] [Google Scholar]
- Koles L, Furst S, Illes P. P2X and P2Y receptors as possible targets of therapeutic manipulations in CNS illnesses. Drug News Perspect. 2005;18:85–101. doi: 10.1358/dnp.2005.18.2.886479. [DOI] [PubMed] [Google Scholar]
- Matute C, Torre I, Perez-Cerda F, et al. P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J Neurosci. 2007;27:9525–9533. doi: 10.1523/JNEUROSCI.0579-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melani A, Turchi D, Vannucchi MG, Cipriani S, Gianfriddo M, Pedata F. ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem Int. 2005;47:442–448. doi: 10.1016/j.neuint.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Pehar M, Vargas MR, Cassina P, Barbeito AG, Beckman JS, Barbeito L. Complexity of astrocyte-motor neuron interactions in amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:139–146. doi: 10.1159/000089619. [DOI] [PubMed] [Google Scholar]
- Pehar M, Vargas MR, Robinson KM, Cassina P, Diaz-Amarilla PJ, Hagen TM, Radi R, Barbeito L, Beckman JS. Mitochondrial superoxide production and nuclear factor erythroid 2-related factor 2 activation in p75 neurotrophin receptor-induced motor neuron apoptosis. J Neurosci. 2007;27:7777–7785. doi: 10.1523/JNEUROSCI.0823-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehar M, Vargas MR, Robinson KM, Cassina P, England P, Beckman JS, Alzari PM, Barbeito L. Peroxynitrite transforms nerve growth factor into an apoptotic factor for motor neurons. Free Radic Biol Med. 2006;41:1632–1644. doi: 10.1016/j.freeradbiomed.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Peng W, Cotrina ML, Han X, et al. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2009;106:12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis JW, O’Regan MH, Perkins LM. Adenosine 5′-triphosphate release from the normoxic and hypoxic in vivo rat cerebral cortex. Neurosci Lett. 1993;151:94–96. doi: 10.1016/0304-3940(93)90054-o. [DOI] [PubMed] [Google Scholar]
- Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoul C, Buhler E, Sadeghi C, Jacquier A, Aebischer P, Pettmann B, Henderson CE, Haase G. Chronic activation in presymptomatic amyotrophic lateral sclerosis (ALS) mice of a feedback loop involving Fas, Daxx, and FasL. Proc Natl Acad Sci U S A. 2006;103:6007–6012. doi: 10.1073/pnas.0508774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoul C, Estevez AG, Nishimune H, Cleveland DW, deLapeyriere O, Henderson CE, Haase G, Pettmann B. Motoneuron death triggered by a specific pathway downstream of Fas. potentiation by ALS-linked SOD1 mutations. Neuron. 2002;35:1067–1083. doi: 10.1016/s0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]
- Raoul C, Henderson CE, Pettmann B. Programmed cell death of embryonic motoneurons triggered through the Fas death receptor. J Cell Biol. 1999;147:1049–1062. doi: 10.1083/jcb.147.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KM, Morre JT, Beckman JS. Triuret: a novel product of peroxynitrite-mediated oxidation of urate. Arch Biochem Biophys. 2004;423:213–217. doi: 10.1016/j.abb.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Ryu JK, McLarnon JG. Block of purinergic P2X(7) receptor is neuroprotective in an animal model of Alzheimer’s disease. Neuroreport. 2008;19:1715–1719. doi: 10.1097/WNR.0b013e3283179333. [DOI] [PubMed] [Google Scholar]
- Sahawneh MA, Ricart KC, Roberts BR, et al. Cu,Zn-superoxide dismutase increases toxicity of mutant and zinc-deficient superoxide dismutase by enhancing protein stability. J Biol Chem. 2010;285:33885–33897. doi: 10.1074/jbc.M110.118901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos CX, Anjos EI, Augusto O. Uric acid oxidation by peroxynitrite: multiple reactions, free radical formation, and amplification of lipid oxidation. Arch Biochem Biophys. 1999;372:285–294. doi: 10.1006/abbi.1999.1491. [DOI] [PubMed] [Google Scholar]
- Soon CP, Donnelly PS, Turner BJ, et al. Diacetylbis(N(4)-methylthiosemicarbazonato) copper(II) (CuII(atsm)) protects against peroxynitrite-induced nitrosative damage and prolongs survival in amyotrophic lateral sclerosis mouse model. J Biol Chem. 2011;286:44035–44044. doi: 10.1074/jbc.M111.274407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperlagh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Prog Neurobiol. 2006;78:327–346. doi: 10.1016/j.pneurobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Wang X, Arcuino G, Takano T, et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- Wiese S, Jablonka S, Holtmann B, Orel N, Rajagopal R, Chao MV, Sendtner M. Adenosine receptor A2A-R contributes to motoneuron survival by transactivating the tyrosine kinase receptor TrkB. Proc Natl Acad Sci U S A. 2007;104:17210–17215. doi: 10.1073/pnas.0705267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AS, Kiaei M, Aguirre N, Crow JP, Calingasan NY, Browne SE, Beal MF. Iron porphyrin treatment extends survival in a transgenic animal model of amyotrophic lateral sclerosis. J Neurochem. 2003;85:142–150. doi: 10.1046/j.1471-4159.2003.01639.x. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, Banati RR, Anand P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


