Abstract
Neisseria are pathogenic bacteria that cause gonorrhea, septicemia, and meningitis. Like other pathogenic bacteria, Neisseria must acquire iron for survival from their local environment within the human host. Instead of secreting siderophores to scavenge iron, Neisseria steal iron from human iron binding proteins such as hemoglobin, transferrin and lactoferrin for survival. Recently we reported the crystal structures of the N. meningitidis transferrin receptors TbpA and TbpB, as well as the structures of apo and holo human transferrin. We also analyzed these proteins using small angle X-ray scattering and electron microscopy to provide the molecular details explaining how Neisseria are able to interact with and extract iron from transferrin. Here, we utilize the structural reports, as well as the recently reported structure of the N-lobe of LbpB from Moraxella bovis, to assemble improved 3D homology models for the neisserial lactoferrin import receptors LbpA and LbpB, both of which are important vaccine targets against N. meningitidis. We then analyzed these models to gain structural insights into the lactoferrin-iron import system and form a mechanistic model fashioned in parallel to the homologous transferrin-iron import system.
Keywords: Neisseria, meningitidis, gonorrhoeae, lactoferrin, transferrin, iron acquisition, TonB
Introduction
While there are 11 species of Neisseria that colonize humans, the only two that are pathogenic are Neisseria gonorrhoeae, which causes the sexually transmitted disease gonorrhea, and Neisseria meningitidis, which colonizes 10–20% of humans typically without issues but can in rare cases become pathogenic, rapidly leading to septicemia and meningitis (Virji, 2009). Gonococcal infections, with an incidence of over 300,000 cases per year in the United States, are treated using antibiotics and while typically not life threatening, can lead to more serious conditions such as salpingitis, pelvic inflammatory disease, and infertility in women, and prostatitis, epididymitis, and infertility in men (Sparling, 1990). Meningcoccal infections, however, with an incidence of less than 1,000 cases per year, are typically more serious given the prompt onset of infection and potential to progress into septicemia and meningitis (CDC, 2012; Stephens et al., 2007; Virji, 2009). Therefore, rapid treatment with antibiotics is required for meningococcal infections. But even after antibiotic treatment, there is still ~15% mortality rate, with ~20% of those that survive suffering long term consequences such as seizures or stroke, deafness, neurological deficit, and/or limb loss (Tan et al., 2010). While vaccines are available against four of the five serogroups of N. meningitidis, these vaccines have limitations (Deasy and Read, 2011). Moreover, no vaccines are commercially available against N. meningitidis serogroup B or against N. gonorrhoeae. While current antibiotics remain effective against meningococcal infections, an alarming recent report from the CDC (CDC, 2011) has indicated the emergence of gonococcal strains that have developed resistance to all available antibiotics.
To combat these pathogens, current efforts are aimed at producing new and improved vaccines against Neisseria and one approach is to target the iron transport systems that are essential for survival and typically well conserved across strains. Like other pathogenic bacteria, Neisseria must acquire iron from their host for virulence and survival and do so by using a number of surface-exposed transport systems that scavenge iron from the host environment. While Gram-negative bacteria typically secrete siderophores (small compounds that have a very high affinity for iron) into the local environment to scavenge iron for uptake, Neisseria do not produce siderophores and therefore must rely on other methods of iron acquisition. To accomplish this, Neisseria use a number of surface receptors to hijack human host iron transport proteins and to steal iron directly from these proteins, which include hemoglobin, transferrin, and lactoferrin. Two types of receptors are utilized for this iron extraction and transport process: an integral outer membrane transporter that fully spans the outer membrane and a co-receptor lipoprotein that is anchored to the outer leaflet of the outer membrane via an N-terminal lipid modification. A unique set of receptors (transporter and co-receptor) is present for each host iron binding protein.
These transporters have been predicted to contain a TonB-dependent transporter fold, which contains a ~150 residue N-terminal plug domain tucked inside a 22-stranded beta-barrel domain (Noinaj et al., 2010). This was confirmed recently with the report of the crystal structure of the transferrin-iron transporter, TbpA, from N. meningitidis serogroup B (strain K454) in complex with human transferrin (Noinaj et al., 2012b). Here, TbpA was found to bind exclusively to the C-lobe of transferrin. This interaction was mediated by TbpA's very long and extended extracellular loops and an extended loop from the plug domain. Additionally, periplasmic loop 8, which is well conserved across strains, was suggested as a potential site for docking FbpA, the periplasmic iron accepting protein. Further analysis led to the identification of a helix finger within extracellular loop 3 which is postulated to catalyze the release of iron from transferrin by hijacking the pH-sensitive switch mechanism normally used to deliver iron to host cells. Once released, the iron would then transiently interact with the iron-binding motif EIEYE within the plug domain prior to release into the periplasm. Additionally, the crystal structure of the co-receptor, TbpB, from N. meningitidis serogroup B (strain M982) in complex with human transferrin was also recently reported (Calmettes et al., 2011). Here, the N-lobe of TbpB was found to bind exclusively to the C-lobe of transferrin, however, at a unique site not overlapping with that observed for TbpA. It was further proposed that in addition to TbpB's role in specifically binding and concentrating iron-loaded transferrin at the neisserial surface, it may also serve to lock transferrin in the closed iron-bound state until delivery to TbpA for iron extraction and import, thereby increasing the efficiency of the transferrin-iron import mechanism. TbpB has been reported to also participate in both iron extraction from transferrin and the release of apo-transferrin from TbpA following iron transport; however, the exact mechanisms for these roles remain elusive (DeRocco et al., 2009; Siburt et al., 2009).
The lactoferrin-iron import system is the most similar to the transferrin-iron import system with the substrates, transporters, and co-receptors each having significant sequence (and presumably structural) conservation with one another (Adamiak et al., 2012). In addition, the mechanism for iron acquisition in these two systems is also thought to parallel one another, suggesting that the receptors share many of the same structural features. For example, the crystal structures of lactoferrin and transferrin are structurally similar to one another, containing an N-terminal lobe (N-lobe) and a C-terminal lobe (C-lobe), each of which can bind one atom of iron (Baker et al., 1998; Wally and Buchanan, 2007). Each lobe of transferrin and lactoferrin is found in an open conformation in the absence of iron and undergoes a large hinge-like conformational change to a tightly closed state upon iron binding (Noinaj et al., 2012b; Wally and Buchanan, 2007; Wally et al., 2006).
Neisseria have evolved lactoferrin receptors that can circumvent the protective role of lactoferrin and instead, use it as an iron source for survival. While the physiological role of transferrin is to deliver iron to cells within the host, lactoferrin is thought to serve a more protective role by foraging any available iron and safeguarding it from invading bacteria (Sanchez et al., 1992). Interestingly, while lactoferrin binds iron with higher affinity than transferrin, no system has been identified in the host where lactoferrin is utilized as an iron source or delivery system. Further, while iron removal in transferrin is known to be regulated by a di-lysine trigger (N-lobe) and a pH sensing triad (C-lobe) (He et al., 1999; Nurizzo et al., 2001), no mechanism has been described for how iron can be systematically removed from lactoferrin.
Like the transferrin-iron import system, the neisserial lactoferrin receptors specifically recognize only human lactoferrin and not lactoferrins from other species (Schryvers and Morris, 1988; Schryvers and Gonzalez, 1989). Additionally, studies have shown that while LbpB may serve a protective role on the surface of Neisseria, it is not required for viability and does not increase the efficiency of iron uptake as has been observed with TbpB. Only LbpA, which transports iron across the outer membrane, is required for viability when lactoferrin is the sole iron source (Biswas et al., 1999). While the role of LbpB is expected to function similarly, it has not been as extensively studied as TbpB so mechanistic differences could surface with future studies. There are other differences in the transferrin and lactoferrin systems too. Lactoferrin differs from transferrin by containing N-terminal peptides, lactoferricins, which are released upon acidic proteolysis (Bellamy et al., 1992). These cationic peptides can be bactericidal for both Gram-positive (Streptococcus pneumonia) and Gram-negative (N. meningitidis, N. gonorrhoeae) strains (Morgenthau et al., 2012a; Senkovich et al., 2007). In addition to binding lactoferrin, it was recently reported that LbpB may also contribute to meningococcal virulence by neutralizing the bactericidal effects of the host protective peptide lactoferricin (Morgenthau et al., 2012a).
Both the transferrin- and lactoferrin-iron import systems can be found in all clinical isolates of meningococcal infections. While the transferrin-iron import system can be found in all clinical isolates of gonococcal infections as well, only half express the lactoferrin receptors (Biswas and Sparling, 1995; Mickelsen et al., 1982), suggesting that expression of LbpA and LbpB may be deleterious for the gonococcus despite other advantages this iron import system may provide. Although not required for virulence, the lactoferrin-iron import system may serve a secondary role in iron acquisition during infection. Either system can provide the iron essential to survival; substituting the transferrin receptors with their lactoferrin counterparts generated a gonoccocal strain that retained virulence (Anderson et al., 2003). While the lactoferrin receptors may be beneficial for iron transport, there seems to be a selective advantage against maintaining the genes that encode them in the gonococcus. It remains to be determined whether the differences between the neisserial pathogens with respect to the retention of the genes that encode the lactoferrin receptors can be attributed to the different sites of infection or perhaps to the presence of capsule on the surface of N. meningitidis. Conceivably, the meningococcal capsule could provide some level of protection to the outer membrane from the lactoferricin molecule, thereby making lactoferrin a viable iron source for the meningococcus. For the unencapsulated gonococcus, binding lactoferrin too near the surface may be detrimental and thus the genes encoding the binding proteins have been lost. Still, given the conservation of the system, the lactoferrin receptors remain potential vaccine targets against Neisseriae and may be more broadly protective against N. meningitidis.
Having recently reported the crystal structures of TbpA and TbpB from N. meningitidis (Noinaj et al., 2012b), plus apo and holo human transferrin structures (Noinaj et al., 2012b; Wally and Buchanan, 2007), we were in an ideal position to translate these findings to the related neisserial lactoferrin receptors LbpA and LbpB. With no crystal structures currently reported for these receptors, here we present improved homology models of the lactoferrin receptors LbpA and LbpB from N. meningitidis (strain MC58), analyze the models and highlight interesting features that are conserved with the transferrin-iron import system. We predict that the outer membrane proteins TbpA and LbpA are structurally similar, including electrostatic surface charge distributions used for interacting with the C-lobe of Tf or Lf, respectively. TbpB and LbpB show lower sequence conservation but likely adopt the same bi-lobed architecture. Negatively charged regions that may bind cationic lactoferricins (Morgenthau et al., 2012a) are located in the C-lobe of LbpB, whereas binding to Lf is predicted to occur through the LbpB N-lobe. With reference to the transferrin-iron import system (Noinaj et al., 2012b), we also form a mechanism model for how Neisseria may utilize lactoferrin as an iron source.
Materials and Methods
Sequence Alignments
Sequences used in this study for LbpA and LbpB were gathered from Uniprot (uniprot.org) and sequence alignments were performed using ClustalX (Larkin et al., 2007) and organized and prepared using ESPript 2.2 (Gouet et al., 1999). Secondary structure elements were either added automatically using ESPript 2.2 or manually based on DSSP (Kabsch and Sander, 1983) analysis results. Sequence identities and sequence similarities were computed using the Sequence Identity and Similarity (SIAS) server. Final edits and annotations were made using Adobe Illustrator.
Homology modeling
The homology model for LbpA from N. meningitidis strain MC58 (NmLbpA) was formed by first using Chainsaw (CCP4) (Stein, 2008) to thread the sequence of NmLpbA onto the reported NmTbpA crystal structure (PDB code 3V8X), based on the pairwise sequence alignment. This model was further completed within COOT (Emsley and Cowtan, 2004; Emsley et al., 2010), missing side-chains added using Deepview/Swiss-PdbViewer (Guex and Peitsch, 1997) and final model minimization performed using Chiron (Ramachandran et al., 2011). For the homology model of LbpB from N. meningitidis strain MC58 (NmLbpB), the Swiss Model server (Arnold et al., 2006; Kiefer et al., 2009; Peitsch, 1995) was used to create a model for the N-lobe based on the crystal structure of the N-lobe of LbpB from M. bovis (PDB code 3UAQ). A model for the C-lobe was based on the crystal structure of NmTbpB (PDB code 3V8U). The two models were then aligned onto the NmTbpB full length structure and merged to form a model for the full length LbpB structure. Complexes were then modeled by aligning the models for LbpA and LbpB and the crystal structure of diferric hLF (PDB code 1LFG) to the structures of TbpA and TbpB in complex with transferrin (PDB codes 3V8X and 3VE1). Figures were made using PyMOL (Schrödinger) and assembled and annotated using Adobe Illustrator.
Results and Discussion
Homology modeling for NmLbpA and the NmLbpA/hLF complex
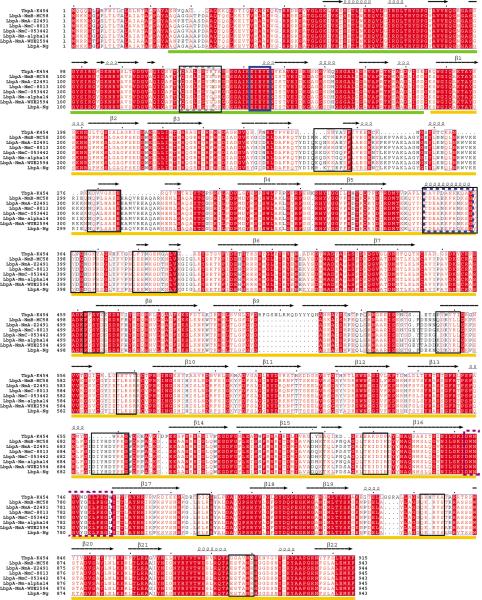
For comparison and alignment, the sequences of LbpAs from seven different neisserial strains were used and compared to TbpA from N. meningitidis serogroup B strain K454 (NmTbpA) (Figure 1). Those include (1) N. meningitidis serogroup B/strain MC58 (Uniprot ID - Q06379), (2) N. meningitidis serogroup A/strain Z2491 (Uniprot ID - Q9JTK4), (3) N. meningitidis serogroup C/strain 8013 (Uniprot ID - C9WYH7), (4) N. meningitidis serogroup C/strain 053442 (Uniprot ID - A9M0Y0), (5) N. meningitidis strain alpha 14 (Uniprot ID -C6S5V8), (6) N. meningitidis serogroup A/strain WUE 2594 (Uniprot ID - E7BIZ2), and (7) N. gonorrhoeae (Uniprot ID - Q50952). These sequences were aligned using ClustalX (Larkin et al., 2007), sequence identities and similarities calculated (Table 1) and the output alignment fed into ESPript 2.2 (Gouet et al., 1999) for final representation.
Figure 1. Sequence alignment of neisserial LbpAs with neisserial TbpA.
Sequence alignment of neisserial LbpAs with TbpA from N. meningitidis serogroup B/strain K454. Fully conserved residues are shaded in red highlight and secondary structure predictions based on the structure of TbpA are indicated above the sequences. The plug domain is indicated by a green bar below the sequence and the beta-domain by a gold bar with beta-strands that form the beta-barrel domain numbered sequentially. The extended plug loop is indicated by a gray dashed box, the conserved EIEYE motif by a blue box, the location of the loop 3 helix finger by a blue dashed box, periplasmic loop 8 by a purple dashed box, and residue ranges predicted to participate in the interaction with hLF indicated by black boxes.
Table 1.
Sequence identity (green) and similarity (blue) analysis for LbpA from different strains of Neisserial compared to TbpA.
| Sequence Similarity (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| TbpA-K454 | LbpA-NmB-MC58 | LbpA-NmA-Z2491 | LbpA-NmC-8013 | LbpA-NmC-053442 | LbpA-Nm-alpha14 | LbpA-NmA-WUE2594 | LbpA-Ng | |
| TbpA-K454 | 100 | 67.42 | 67.52 | 67.42 | 67.21 | 67.21 | 67.11 | 67.73 |
| LbpA-NmB-MC58 | 41.11 | 100 | 97.73 | 97.52 | 96.9 | 97.52 | 97.31 | 98.55 |
| LbpA-NmA-Z2491 | 41.75 | 95.15 | 100 | 98.14 | 98.04 | 97.31 | 98.96 | 97.01 |
| LbpA-NmC-8013 | 41.44 | 95.25 | 95.67 | 100 | 97.73 | 97.73 | 98.24 | 97.21 |
| LbpA-NmC-053442 | 41.75 | 94.43 | 94.84 | 95.05 | 100 | 97.42 | 97.73 | 96.8 |
| LbpA-Nm-alpha14 | 41.75 | 96.08 | 94.22 | 95.77 | 95.87 | 100 | 97.62 | 97.73 |
| LbpA-NmA-WUE2594 | 41.64 | 94.02 | 96.7 | 96.08 | 94.94 | 95.05 | 100 | 96.59 |
| LbpA-Ng | 41.75 | 96.9 | 94.43 | 94.84 | 93.81 | 95.77 | 93.81 | 100 |
| TbpA-K454 | LbpA-NmB-MC58 | LbpA-NmA-Z2491 | LbpA-NmC-8013 | LbpA-NmC-053442 | LbpA-Nm-alpha14 | LbpA-NmA-WUE2594 | LbpA-Ng | |
| Sequence Identity (%) | ||||||||
For homology modeling, we chose to focus on LbpA from N. meningitidis strain MC58 since its genome has been fully sequenced (Tettelin et al., 2000). Attempts to build a model using the Swiss Model server (Arnold et al., 2006; Kiefer et al., 2009; Peitsch, 1995) failed while trying to build insertions within loops. Therefore homology modeling was performed manually using a pairwise sequence alignment of LbpA and TbpA from ClustalX (Larkin et al., 2007) which was fed into Chainsaw (CCP4) (Stein, 2008) to build an initial model. Insertions were then manually built using COOT (Emsley and Cowtan, 2004; Emsley et al., 2010), missing side chains added using Deepview/Swiss-PdbViewer (Guex and Peitsch, 1997), and energy minimization performed using Chiron (Ramachandran et al., 2011) (Supplementary Model S1).
Given that NmLbpB is 41% identical and 67% similar to NmTbpA (Table 1), we were able to produce a model that showed good conservation to NmTbpA, allowing us to identify several shared features. The overall architecture is the same: a 22-stranded beta-barrel encompasses a plug domain with a central 4-stranded beta sheet (Figures 1–3). Mapping the topology of the NmLbpA model onto a 2D diagram (Figure 2A), we were able to clearly see that the plug domain was well conserved, including the iron binding motif EIEYE and the larger plug domain (as compared to other known TBDT structures (Noinaj et al., 2010)) (Figure 1). Additionally, the β–strands of the beta-domain are well conserved with the most divergent features found in the extracellular loops, particularly loops 2, 3 and 5. Loops 1, 4, 7, 9, 10, and 11 are well conserved with loop 11 showing the highest degree of conservation. While conserved disulfides were modeled for both loop 4 and loop 5, LbpA lacks the cysteines that form the disulfide bond in loop 2 in NmTbpA. Periplasmic loop 8, which is postulated to participate in docking apo FbpA, is also well conserved. Interestingly, the loop 3 helix finger, which we suggested to play an important role in catalyzing iron release from transferrin by NmTbpA, was quite divergent from that of NmTbpA, despite retaining a core of basic residues. To verify that this sequence still retained a propensity to form an α-helix, secondary structure prediction was performed on the NmLbpA loop 3 helix finger sequence using CFSSP server (Chou and Fasman, 1974) and compared to the results for the loop 3 helix finger for NmTbpA. For both sequences, an α-helix was predicted, indicating that while the sequences diverge, the propensity to form an a-helix is retained. The conservation of the loop 3 helix in LbpA suggests that the mechanism of iron release from Lf may be similar to the way TbpA catalyzes iron release from Tf (Noinaj et al., 2012b), despite a lack of pH sensitive residues near the iron-binding site in the Lf C-lobe (discussed below).
Figure 3. A model for the structure of neisserial LbpA.
A. A homology model for LbpA with the β-domain shown in gold, the plug domain in green, the putative TonB box as blue spheres, the loop 3 (L3) helix finger in purple, the conserved iron binding motif (EIEYE) as red spheres, and insertions shown in red cartoon (also indicated by red arrows). The location of the extended plug loop (green) is also depicted along with periplasmic loop 8 (periLoop 8, purple), which may serve as a docking site for FbpA. B. View of LbpA from the top to show substrate binding surface and the electrostatic surface properties (−5kT/e to +5kT/e) which are significantly less electropositive than that of TbpA (blue indicates electropositive, red indicates electronegative). The ovals indicate predicted binding sites for the C1 (dashed) and C2 (solid) subdomains of hLF. C. The diferric hLF crystal structure (PDB code 1LFG) shown in two orthogonal views along with respective electrostatic surface potential representations. The ovals indicate the C1 (dashed) and C2 (solid) subdomain surface regions predicted to interact with LbpA, which show nice charge complementation to LbpA.
Figure 2. Topology diagram of NmLbpA based on the homology to NmTbpA.
A. Using a sequence alignment of neisserial LbpA from N. meningitidis strain MC58 (NmLbpA) with TbpA from N. meningitidis serogroup B strain/K454 (NmTbpA) and homology modeling, a 2D topology diagram of NmLbpA was formed. Those residues being fully conserved are shown as blue circles, those have strong conservation in green, those having weak conservation in gray, and those having no conservation are in white. The locations of insertions are indicated by dashed circles, disulfides by short bold black connecting lines, and the iron binding motif (EIEYE) as red boxes. B. Secondary structure prediction for the loop 3 helix finger for NmLbpA showing a propensity for α-helix, similar to what was predicted and observed in the crystal structure for NmTbpA.
While only one large deletion was observed for NmLbpA following β-strand 9, a number of insertions are found which map primarily to extracellular loops 2 and 7 (Figures 1, 2A, and 3A). The electrostatic surface properties of the NmLbpA model were analyzed and found to be electropositive along the top of the beta-barrel domain, although much less so than was observed for the NmTbpA structure (Figure 3B). Additionally, a patch of electronegative charge is present along the inner face of the extended loops, which was not observed in the NmTbpA structure.
Using the reported crystal structure of NmTbpA in complex with human transferrin (PDB codes 3V8X), we modeled hLF in complex with NmLbpA (Figure 4A). Here, we aligned the NmLbpA model and the diferric hLF coordinates with NmTbpA and transferrin, respectively, and then merged the structures into a single complex burying ~2,500 Å2 of surface. This model shows NmLbpA interacting exclusively via the C-lobe of hLF with the loop 3 helix finger positioned along the cleft between the C1 and C2 subdomains of hLF (Figure 4B) and in close proximity to the iron binding site. While diverging in sequence compared to the loop 3 helix finger in NmTbpA, the helix finger in NmLbpA is predicted to retain its helical fold (Figure 2B) and also still contains a number of basic residues (K390, R392, K394) (Figure 4C). However, unlike NmTbpA, the NmLbpA helix finger also contains a core of negatively charged residues (D386, E387, E389, D393). While requiring experimental verification, it is likely that these differences have evolved to specifically catalyze iron release from lactoferrin, which lacks the pH sensing triad contained by transferrin. Additionally, the conserved extended plug loop is ideally positioned to interact with the base of the C1 domain, possibly playing a role in sensing the presence of ligand. Residues Q127, L129, and G131 are completely conserved here (Figures 1 and 4D), indicating they may serve to interact with the base of the C1 domain of hLF, much like was observed for the NmTbpA/transferrin complex. Further evidence in support of our homology modeling comes from an analysis of the electrostatic surface, which shows that the electrostatic properties of lactoferrin along the C1 and C2 subdomains nicely complement the electrostatic properties of NmLbpA (Figure 3B and 3C). Lastly, using this model of the NmLbpA/hLF complex, we were able to map those regions of the NmLbpA structure that are predicted to make direct interactions with hLF (Figure 1).
Figure 4. Homology model for the LbpA-hLF complex based on the TbpA-hTF crystal structure.
A. Homology model for LbpA-hLF complex with LbpA shown in blue (plug domain) and gold (β-domain) and hLF shown in pink (N-lobe) and green (C-lobe) with red spheres indicating the locations of the bound iron atoms. The dashed black line indicates the predicted binding interface which buries ~2,500 Å2 of surface area. B. Zoomed view of the loop 3 helix finger and the plug loop interacting with hLF along the C1 and C2 subdomains. C. Loop 3 helix finger showing the localization of negative charged residues (D386, E387, E389, D393) and positively charged residues (K390, R392, K394). D. The conserved extended plug loop that interacts with the base of the C1 domain of hLF.
Homology modeling for NmLbpB and the NmLbpB/hLF complex
For LbpB, the sequences of LbpBs from six different neisserial strains, as well as the N-lobe sequence of LbpB from M. bovis (Uniprot ID - H9KVH9), for which a crystal structure was recently reported (PDB code 3UAQ) (Arutyunova et al., 2012), were used and compared to TbpB from N. meningitidis serogroup B strain K454 (NmTbpB) (Figure 5). Those include (1) N. meningitidis serogroup B/strain MC58 (Uniprot ID - Q9JYK4), (2) N. meningitidis serogroup C/strain 8013 (Uniprot ID - C9WYH6), (3) N. meningitidis serogroup C/strain 053442 (Uniprot ID - A9M0Y1), (4) N. meningitidis strain alpha 14 (Uniprot ID - C6S5V7), (5) N. meningitidis serogroup A/strain WUE 2594 (Uniprot ID - E7BIZ3), and (6) N. gonorrhoeae (Uniprot ID - Q9Z4N2). These sequences were aligned using ClustalX (Larkin et al., 2007), sequence identities and similarities calculated (Table 2) and the output alignment fed into ESPript 2.2 (Gouet et al., 1999) for final representation.
Figure 5. Sequence alignment of neisserial LbpBs with neisserial TbpB.
Sequence alignment of neisserial LbpBs with TbpB from N. meningitidis serogroup B strain/K454. Fully conserved residues are shaded in red highlight and secondary structure predictions, based on the N-lobe structure of LbpB from M. bovis (PDB code 3UAQ) and the structure of TbpB (PDB code 3V8U), are indicated above the alignment. Those loops predicted to participate in the interaction with hLF are indicated by blue boxes and those loops found disordered in the TbpB crystal structure are indicated by gray boxes. The N-lobe is indicated by the cyan bar below the sequences while the C-lobe is indicated by the dark blue bar.
Table 2.
Sequence identity (green) and similarity (blue) analysis for LbpB from different strains of Neisserial compared to TbpB.
| Sequence Similarity (%) | |||||||
|---|---|---|---|---|---|---|---|
| TbpB-K454 | LbpB-NmB-MC58 | LbpB-NmC-8013 | LbpB-NmC-053442 | LbpB-Nm-alpha14 | LbpB-NmA-WUE2594 | LbpB-Ng | |
| TbpB-K454 | 100 | 55.9 | 53.97 | 56.02 | 53.25 | 55.9 | 55.54 |
| LbpB-NmB-MC58 | 26.74 | 100 | 87.71 | 79.15 | 92.53 | 80.84 | 84.21 |
| LbpB-NmC-8013 | 25.78 | 74.69 | 100 | 77.71 | 83.73 | 78.07 | 80.48 |
| LbpB-NmC-053442 | 26.86 | 67.95 | 65.3 | 100 | 77.83 | 90.60 | 82.65 |
| LbpB-Nm-alpha14 | 23.73 | 87.34 | 72.16 | 65.30 | 100 | 79.87 | 82.28 |
| LbpB-NmA-WUE2594 | 27.22 | 68.55 | 67.46 | 80.96 | 68.43 | 100 | 85.18 |
| LbpB-Ng | 26.5 | 73.61 | 70.84 | 72.16 | 72.16 | 75.54 | 100 |
| TbpB-K454 | LbpB-NmB-MC58 | LbpB-NmC-8013 | LbpB-NmC-053442 | LbpB-Nm-alpha14 | LbpB-NmA-WUE2594 | LbpB-Ng | |
| Sequence Identity (%) | |||||||
For homology modeling, again we chose to focus on LbpB from N. meningitidis strain MC58 (NmLbpB) since its genome has been fully sequenced (Tettelin et al., 2000). The initial homology modeling of NmLbpB was performed using the Swiss Model server (Arnold et al., 2006; Kiefer et al., 2009; Peitsch, 1995) to create the starting models of each lobe, which were then merged to form a final model. For the N-lobe of NmLbpB, the known structure of LbpB from M. bovis (MbLbpB) (PDB code 3UAQ) was used to form the N-lobe model for NmLbpB. For the C-lobe, the structure of TbpB from N. meningitidis serogroup B - strain K454 (NmTbpB) (PDB code 3V8U) was used to form the C-lobe model for NmLbpB. Then, the N-lobe and C-lobe models for NmLbpB were aligned to the full length NmTbpB structure (PDB code 3V8U) and merged into a single model of full length NmLbpB (Supplementary Model S2).
The N-lobe of NmLbpB is 33% identical and 55% similar to the N-lobe of MbLbpB and full length NmLbpB is 27% identical and 56% similar to NmTbpB (Table 2). This allowed us to create the best possible model for the N-lobe of NmLbpB by using the reported structure of the N-lobe of MbLbpB, while forming a model for the C-lobe of NmLbpB based on the reported NmTbpB crystal structure. The resulting model contained two structurally similar lobes, each containing an eight stranded beta-barrel domain flanked on one side by a `handle' domain also largely composed of β–strands (Figure 6A). Four large loops were not observed in the NmTbpB crystal structure, however, they were modeled by the Swiss Model server and are depicted in gray ribbon for reference. Similar to NmTbpB, the predicted binding interactions of NmLbpB are found along the extended loops of the N-lobe. While the exact role of the large disordered loops of the C-lobe remains to be determined, they could participate in a direct interaction with NmLbpA or the membrane surface as has been suggested for NmTbpB (Noinaj et al., 2012a).
Figure 6. A model for LbpB and the LbpB-hLF complex.
A. Homology model for LbpB shown in cyan (N-lobe) and blue (C-lobe). The dashed line indicates the region predicted to interact with hLF. B. A model for the LbpB-hLF complex with LbpB in cyan (N-lobe) and blue (C-lobe) and hLF in pink (N-lobe) and green (C-lobe). The dashed line indicated the binding interface which buries ~1,400 Å2 of surface area. C. An orthogonal view of panel B.
NmLbpB is thought to serve a similar role in the lactoferrin-iron import system as NmTbpB does in the transferrin-iron import system, to sequester and concentrate only iron bound substrate at the neisserial surface and to shuttle this substrate to the transporter for iron extraction and import. Using the known complex structure of NmTbpB with human transferrin (PDB codes 3VE1), we were able to model hLF in complex with NmLbpB. Here, the interface of the interaction was mediated along the distal surface of the N-lobe of NmLbpB with the distal surface of the C-lobe of hLF, bridging both the C1 and C2 subdomains and burying ~1,400 Å2 of surface area (Figures 6B and 6C). Given that hLF has a significantly higher affinity for iron than transferrin, it remains to be determined if NmLbpB also serves to increase the fidelity of iron transport by locking hLF in a closed conformation prior to delivery to the transporter as has been suggested for NmTbpB. NmLbpB would then presumably retain its interaction with hLF until a conformational change in hLF, which would likely be catalyzed by NmLbpA during iron extraction, leads to dissociation of the complex.
In addition to its iron uptake functions (shared with the transferrin system), NmLbpB mediates protection against the bactericidal cationic peptides, lactoferricins, formed from proteolysis of apo hLF (Morgenthau et al., 2012b). While no structural work has been done on an LbpB-lactoferricin complex, a surface protein from S. pneumoniae called PspA also binds and neutralizes lactoferricin. The crystal structure of a PspA-lactoferricin complex shows interactions between the cationic peptide and a cluster of negatively charged residues on PspA (Senkovich et al., 2007). Several clusters of acidic residues are located on the C-lobe of NmLbpB (Morgenthau et al., 2012a). This suggests that the N-lobe of NmLbpB may function in iron import while the NmLbpB C-lobe may confer protection against lactoferricin.
Mechanism for the lactoferrin- iron acquisition system in Neisseria
Based on the sequence, structural and mechanistic conservation with the homologous transferrin-iron import system, we can form a parallel mechanistic model for the lactoferrin-iron acquisition system in Neisseria (Figure 7). Here, NmLbpB would preferentially bind hLF at the cell surface, thereby concentrating the surface with iron-loaded substrate. We predict that the N-lobe of LbpB will bind to the C-lobe of hLF. NmLbpB would then shuttle hLF to NmLbpA where a transient triple complex would be formed with LbpA also binding to the C-lobe of hLF (at a non-overlapping site with LbpB). Formation of the LbpA-LbpB-hLF complex then catalyzes a conformational change in hLF, likely in a TonB-dependent manner. This conformational change would lead to iron release from hLF and subsequent dissociation of NmLbpB. A systematic TonB-dependent conformational change within the entire plug domain would then occur, which would allow the formation of a transient docking site for iron via the iron binding motif EIEYE. Further unfolding of the plug domain would consequently allow the iron to become exposed to the periplasm where it would then bind FbpA, which is thought to dock along the periplasmic face of NmLbpA. FbpA would then shuttle the iron across the periplasm where it would eventually be transported into the cytoplasm by the ABC transporter FbpBC to be used for essential cellular functions.
Figure 7. Stepwise mechanism for the acquisition of iron from lactoferrin by Neisseria.
Based on similarities to the hTF acquisition system, a stepwise mechanism can be postulated. Once within the host, the neisserial co-receptor LbpB would bind soluble hLF and shuttle it to the iron transporter LbpA. Upon the LbpA-hLF complex docking onto LbpA and combined with the Ton system, a conformational change within the plug domain of LbpA would catalyze iron release and import through the β-domain, where it would immediately bind to FbpA and be further shuttled across the periplasm and into the cytoplasm by FbpB/C.
In summary, the crystal structures of NmTbpA and NmTbpB in complex with transferrin, as well as the N-lobe structure for MbLbpB, were recently reported. Using these structures, we have formed and made available improved homology models for the lactoferrin receptors NmLpbA and NmLbpB from Neisseria (Supplementary Models S1 and S2). Our analysis has identified several conserved structural features in NmLbpA such as the extended plug loop, the large extracellular loops, the iron binding motif EIEYE, and the periplasmic loop 8 which may serve as a docking site for FbpA. Interestingly, we also found that while the loop 3 helix finger is structurally conserved, its sequence is not and we postulate that these differences may be necessary and specific for catalyzing the removal of iron from lactoferrin. Furthermore, we use our models to predict specific regions of residues within NmLbpA and NmLbpB which may participate in binding lactoferrin. Both receptors contribute significantly to the pathogenesis of N. meningitidis and are important vaccine candidates. While no crystal structures have been reported for the neisserial lactoferrin receptors, we have provided and analyzed models for both receptors that should assist in directing current functional and therapeutic studies.
Supplementary Material
Acknowledgements
N.N. and S.K.B. are supported by the Intramural Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. Funding was provided to C.N.C. by U.S. Public Health Service grant numbers AI065555 and AI084400 from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health. C.N.C. is also supported by the SE STI Center grant (U19 AI31496) from the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamiak P, Beddek AJ, Pajon R, Schryvers AB. Patterns of sequence variation within the Neisseria meningitidis lactoferrin binding proteins. Biochem Cell Biol. 2012;90:339–350. doi: 10.1139/o11-076. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Hobbs MM, Biswas GD, Sparling PF. Opposing selective forces for expression of the gonococcal lactoferrin receptor. Mol Microbiol. 2003;48:1325–1337. doi: 10.1046/j.1365-2958.2003.03496.x. [DOI] [PubMed] [Google Scholar]
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Arutyunova E, Brooks CL, Beddek A, Mak MW, Schryvers AB, Lemieux MJ. Crystal structure of the N-lobe of lactoferrin binding protein B from Moraxella bovis. Biochem Cell Biol. 2012;90:351–361. doi: 10.1139/o11-078. [DOI] [PubMed] [Google Scholar]
- Baker EN, Anderson BF, Baker HM, MacGillivray RT, Moore SA, Peterson NA, Shewry SC, Tweedie JW. Three-dimensional structure of lactoferrin. Implications for function, including comparisons with transferrin. Adv Exp Med Biol. 1998;443:1–14. [PubMed] [Google Scholar]
- Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K, Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta. 1992;1121:130–136. doi: 10.1016/0167-4838(92)90346-f. [DOI] [PubMed] [Google Scholar]
- Biswas GD, Sparling PF. Characterization of lbpA, the structural gene for a lactoferrin receptor in Neisseria gonorrhoeae. Infect Immun. 1995;63:2958–2967. doi: 10.1128/iai.63.8.2958-2967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas GD, Anderson JE, Chen CJ, Cornelissen CN, Sparling PF. Identification and functional characterization of the Neisseria gonorrhoeae lbpB gene product. Infect Immun. 1999;67:455–459. doi: 10.1128/iai.67.1.455-459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmettes C, Yu RH, Silva LP, Curran D, Schriemer DC, Schryvers AB, Moraes TF. Structural variations within the transferrin binding site on transferrin-binding protein B, TbpB. J Biol Chem. 2011;286:12683–12692. doi: 10.1074/jbc.M110.206102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Cephalosporin Susceptibility Among Neisseria gonorrhoeae Isolates --- United States, 2000--2010. MMWR Morb Mortal Wkly Rep. 2011;60:873–877. [PubMed] [Google Scholar]
- CDC Summary of Notifiable Diseases - United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;59:1–116. [PubMed] [Google Scholar]
- Chou PY, Fasman GD. Prediction of protein conformation. Biochemistry. 1974;13:222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Deasy A, Read RC. Challenges for development of meningococcal vaccines in infants and children. Expert Rev Vaccines. 2011;10:335–343. doi: 10.1586/erv.11.3. [DOI] [PubMed] [Google Scholar]
- DeRocco AJ, Yost-Daljev MK, Kenney CD, Cornelissen CN. Kinetic analysis of ligand interaction with the gonococcal transferrin-iron acquisition system. Biometals. 2009;22:439–451. doi: 10.1007/s10534-008-9179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouet P, Courcelle E, Stuart DI, Metoz F. ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics. 1999;15:305–308. doi: 10.1093/bioinformatics/15.4.305. [DOI] [PubMed] [Google Scholar]
- Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- He QY, Mason AB, Tam BM, MacGillivray RT, Woodworth RC. Dual role of Lys206-Lys296 interaction in human transferrin N-lobe: iron-release trigger and anion-binding site. Biochemistry. 1999;38:9704–9711. doi: 10.1021/bi990134t. [DOI] [PubMed] [Google Scholar]
- Kabsch W, Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Arnold K, Kunzli M, Bordoli L, Schwede T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2009;37:D387–392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Mickelsen PA, Blackman E, Sparling PF. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from lactoferrin. Infect Immun. 1982;35:915–920. doi: 10.1128/iai.35.3.915-920.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthau A, Livingstone M, Adamiak P, Schryvers AB. The role of lactoferrin binding protein B in mediating protection against human lactoferricin. Biochem Cell Biol. 2012a;90:417–423. doi: 10.1139/o11-074. [DOI] [PubMed] [Google Scholar]
- Morgenthau A, Livingstone M, Adamiak P, Schryvers AB. The role of lactoferrin binding protein B in mediating protection against human lactoferricin. Biochem Cell Biol. 2012b;90:417–423. doi: 10.1139/o11-074. [DOI] [PubMed] [Google Scholar]
- Noinaj N, Buchanan SK, Cornelissen CN. The transferrin-iron import system from pathogenic Neisseria species. Molecular Microbiology. 2012a doi: 10.1111/mmi.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noinaj N, Guillier M, Barnard TJ, Buchanan SK. TonB-dependent transporters: regulation, structure, and function. Annu Rev Microbiol. 2010;64:43–60. doi: 10.1146/annurev.micro.112408.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noinaj N, Easley NC, Oke M, Mizuno N, Gumbart J, Boura E, Steere AN, Zak O, Aisen P, Tajkhorshid E, Evans RW, Gorringe AR, Mason AB, Steven AC, Buchanan SK. Structural basis for iron piracy by pathogenic Neisseria. Nature. 2012b;483:53–58. doi: 10.1038/nature10823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurizzo D, Baker HM, He QY, MacGillivray RT, Mason AB, Woodworth RC, Baker EN. Crystal structures and iron release properties of mutants (K206A and K296A) that abolish the dilysine interaction in the N-lobe of human transferrin. Biochemistry. 2001;40:1616–1623. doi: 10.1021/bi002050m. [DOI] [PubMed] [Google Scholar]
- Peitsch MC. Protein modeling by E-mail Bio/Technology. 1995;13:658–660. [Google Scholar]
- Ramachandran S, Kota P, Ding F, Dokholyan NV. Automated minimization of steric clashes in protein structures. Proteins. 2011;79:261–270. doi: 10.1002/prot.22879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez L, Calvo M, Brock JH. Biological role of lactoferrin. Arch Dis Child. 1992;67:657–661. doi: 10.1136/adc.67.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schryvers AB, Morris LJ. Identification and characterization of the human lactoferrin-binding protein from Neisseria meningitidis. Infect Immun. 1988;56:1144–1149. doi: 10.1128/iai.56.5.1144-1149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schryvers AB, Gonzalez GC. Comparison of the abilities of different protein sources of iron to enhance Neisseria meningitidis infection in mice. Infect Immun. 1989;57:2425–2429. doi: 10.1128/iai.57.8.2425-2429.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkovich O, Cook WJ, Mirza S, Hollingshead SK, Protasevich II, Briles DE, Chattopadhyay D. Structure of a complex of human lactoferrin N-lobe with pneumococcal surface protein a provides insight into microbial defense mechanism. J Mol Biol. 2007;370:701–713. doi: 10.1016/j.jmb.2007.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siburt CJ, Roulhac PL, Weaver KD, Noto JM, Mietzner TA, Cornelissen CN, Fitzgerald MC, Crumbliss AL. Hijacking transferrin bound iron: protein-receptor interactions involved in iron transport in N. gonorrhoeae. Metallomics. 2009;1:249–255. doi: 10.1039/b902860a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling PF. Biology of Neisseria gonorhoeae. In: Holmes KK, Mardh P-A, Sparling PF, Weisner PJ, editors. Sexually Transmitted Diseases. McGraw Hill; 1990. pp. 131–138. [Google Scholar]
- Stein N. CHAINSAW: a program for mutating pdb files used as templates in molecular replacement. J. Appl. Cryst. 2008;41:641–643. [Google Scholar]
- Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369:2196–2210. doi: 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- Tan LK, Carlone GM, Borrow R. Advances in the development of vaccines against Neisseria meningitidis. N Engl J Med. 2010;362:1511–1520. doi: 10.1056/NEJMra0906357. [DOI] [PubMed] [Google Scholar]
- Tettelin H, Saunders NJ, Heidelberg J, Jeffries AC, Nelson KE, Eisen JA, Ketchum KA, Hood DW, Peden JF, Dodson RJ, Nelson WC, Gwinn ML, DeBoy R, Peterson JD, Hickey EK, Haft DH, Salzberg SL, White O, Fleischmann RD, Dougherty BA, Mason T, Ciecko A, Parksey DS, Blair E, Cittone H, Clark EB, Cotton MD, Utterback TR, Khouri H, Qin H, Vamathevan J, Gill J, Scarlato V, Masignani V, Pizza M, Grandi G, Sun L, Smith HO, Fraser CM, Moxon ER, Rappuoli R, Venter JC. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science. 2000;287:1809–1815. doi: 10.1126/science.287.5459.1809. [DOI] [PubMed] [Google Scholar]
- Virji M. Pathogenic neisseriae: surface modulation, pathogenesis and infection control. Nat Rev Microbiol. 2009;7:274–286. doi: 10.1038/nrmicro2097. [DOI] [PubMed] [Google Scholar]
- Wally J, Buchanan SK. A structural comparison of human serum transferrin and human lactoferrin. Biometals. 2007;20:249–262. doi: 10.1007/s10534-006-9062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wally J, Halbrooks PJ, Vonrhein C, Rould MA, Everse SJ, Mason AB, Buchanan SK. The crystal structure of iron-free human serum transferrin provides insight into inter-lobe communication and receptor binding. J Biol Chem. 2006;281:24934–24944. doi: 10.1074/jbc.M604592200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.