Abstract
During pregnancy, females undergo several physiologically driven changes that facilitate adaptive behaviours and prepare the mother to care for her developing offspring. The nonapeptide hormone oxytocin is best recognised for its involvement in mammalian pregnancy and has been tightly associated with maternal care, in addition to its roles in pregnancy, parturition, and lactation. A closely related nonapeptide hormone, arganine vasopressin, has received considerably less attention for its role in pregnancy but has recently been implicated in modulating maternal care and aggression. Here, we examine the expression patterns of receptors for oxytocin (OTR) and vasopressin (V1aR) over the course of pregnancy, ranging from non-mated virgin to immediately postpartum female prairie voles (Microtus ochrogaster). Surprisingly, we found that OTR was highly stable in all measured structures in the forebrain. V1aR was also stable throughout most of the brain. Two exceptions to this were found in the ventral pallidum (VPall) and the paraventricular nucleus of the hypothalamus (PVN); both significantly correlated with the length of time females were pregnant. Changes in the PVN may reflect functional feedback in vasopressin release, or preparatory changes for ensuing maternal behaviour. The results also indicate an unappreciated role for VPall V1aR in pregnancy, which may relate to the function of the VPall in hedonic ‘liking’ and motivational ‘wanting.’ Taken together, our data indicate that with a few compelling exceptions, nonapeptide dynamics during prairie vole pregnancy are largely limited to changes in the synthesis and release of oxytocin and vasopressin, not the receptors to which they bind.
Keywords: oxytocin, vasopressin, Microtus ochrogaster, paraventricular nucleus of the hypothalamus, pregnancy, ventral pallidum
Introduction
It is well recognised that female physiology undergoes dynamic changes throughout the course of pregnancy [1]. In mammals, many of these changes are governed by a combination of endocrine and neuroendocrine mechanisms (including actions of sex steroids, luteinising hormone, and nonapeptides) and environmental cues (such as the presence of young), which induce and coordinate the expression of maternal behaviour [2, 3].
The neuromodulatory nonapeptide oxytocin (OT) asserts perhaps one of the more profound influences on the maternal brain [4]. OT has engendered broad interest due to its involvement in a wide variety of social behaviours including sexual behaviour, pairbonding, social memory, trust, and social cognition (for example see [5]). However OT is classically recognised for its impact on maternal physiology (i.e., induction of parturition and lactation) and its control over maternal care and aggression. In addition to being a prime driver of labour and milk ejection [6, 7], OT synthesis and release just prior to and following birth is responsible for the onset of maternal defensive aggression and maternal care (i.e., pup retrieval, licking and grooming offspring, and adopting postures that enable successful nursing and thermoregulation of young) [8]. Although it has received relatively less attention for its role in maternal behaviour, the hypothesis that arganine vasopressin (AVP) is important for the expression of maternal behaviour is beginning to garner support. Indeed, central AVP acting on V1a receptors, is an important regulator of arched back nursing, pup retrieval, and contact with offspring [9]. Because OT and AVP have such profound effects on social behaviour, it is not surprising that they are so deeply involved in among the most social of behaviours – offspring defense and care.
Our understanding of the hormonal coordination of maternal behaviour and the perinatal neuroendocrine changes that govern them has been primarily built on studies in rats employing diverse approaches (including microdialysis, immunolabeling, administration of pharmacological agents, etc.) to provide a relatively clear understanding of the neuroendocrine adaptations that prepare mothers to deliver and care for offspring (e.g., [1, 10–12]). For example, secretion of OT from the paraventricular nucleus and supraoptic nucleus of the hypothalamus regulates neuronal activity important for the peripheral pulsatile secretion of OT from the neurohypophysis [13–15], and operates to initiate and coordinate maternal care [16–22]. AVP works with OT to produce maternal behaviour in similar ways [9, 23–30].
The majority of what we have learned about the nonapeptide control of maternal behaviour over the past half century has primarily focused on the synthesis, release, and action of central and peripheral OT and AVP (refs Op cit.). What is striking is that only a few studies have attempted to describe the changes that the receptors for these neuropeptides undergo. Of these studies, two important themes have emerged: 1) oxytocin and vasopressin receptor densities are relatively stable, with the possible exception of hypothalamic structures such as the paraventricular nucleus, and 2) most of the dynamic changes occur postpartum and into lactation. Focusing on the former point, OTRs in the paraventricular hypothalamus (PVN), and possibly in the supraoptic nucleus (SON), bed nucleus of the stria terminalis (BST) and medial preoptic area (mPOA), appear to only upregulate in the latest stages of pregnancy [31–34]. Strangely, despite the clearest results for OTR upregulation in the PVN, levels of OTR mRNA in the PVN do not change over pregnancy [35, 36]. On the other hand, AVP mRNA increases in the PVN and SON during the peripartum period [18, 19, 29]. Taken together, these data have implicated an important role in maternal priming for OTR- and V1aR-mediated sensitivity to nonapeptides in a select few – primarily hypothalamic – neural structures.
Although studies such as those described above are invaluable toward understanding the neuroendocrine control of and physiological adaptations for maternal behaviour, it is crucial to consider the extent to which such studies may be generalised [37]. Rats are social breeders, and demonstrate the social organisation and promiscuous/polygynous mating system seen in most mammalian species. On the other hand, mammalian social monogamy is closely associated with exclusive bonds with mating partners and biparental care [38]. Although uncommon among mammals, social monogamy has been identified in several taxa, including some aquatic species of mammal, bats, carnivores, ungulates, rodents, and primates, including humans [39]. Due in large part to their size, adaptability to the lab, and similarity to established rodent models, none of these species are more easily studied than prairie voles (Microtus ochrogaster). Indeed, prairie voles have provided deep insight into the neurobiology of social monogamy and bi-parental care [40–42], and may therefore represent a great opportunity to learn more about the neuroendocrine dynamics that occur during pregnancy. To broaden our understanding of the changing maternal brain, we provide a description of the receptor dynamics in a bi-parental and socially monogamous rodent model. To this extent, our study, is among the first to investigate receptor densities as a function of female pregnancy, and represents the first within a socially monogamous mammal.
Materials and Methods
Animals
All animals used in this study were from the F1 generation originating from wild caught animals trapped near Champagne, Illinois. Field-caught animals were transported to Oklahoma State University where they were housed and bred in polycarbonate cages (29 × 18 × 13 cm) under a 14:10 light:dark cycle. At 21 days, we weaned offspring, and separated them into same sex litters. All animals had ad libitum access to rodent chow (Harland Teklad, Madison, WI, U.S.A) and water. Temperature was maintained at 21 ± 2°C.
Study design
We created 20 pairs of sexually naive adult female and male prairie voles of similar age and weight. Age ranged from 62 to 114 d. Immediately following introduction, each pair was video recorded from the date of pairing until mating was confirmed; all but one pair mated (see results below). Gestation lasts approximately 21 days in prairie voles. From the 20 pairs, we planned four equally sized groups of animals to encompass the first, second, and third, weeks of pregnancy and post-parturition. To this end, we sacrificed females with CO2 gas at 4, 11, 18, days after first observed mating, or on the day of parturition. In the latter case, females were sacrificed within 2 hours of giving birth. Our animal procedures were in accordance with the guidelines set and approved by the Institutional Animal Care and Use Committee of Oklahoma State University.
Our design was intended to produce four groups of females spanning the course of early-stage pregnancy to immediately post-partum. Unfortunately, first mating did not translate into fertilisation (see results), and the degree to which females were pregnant was discordant with our original planed comparisons. Therefore, rather than treating our animals as four discrete categories, we grouped all females together and analysed the data as correlations (see Analyses below) between nonapeptide receptor expression in the brain and the length of time females were pregnant.
Determination of pregnancy and time since fertilisation
To estimate the length of time females were pregnant, we assessed the crown-rump (C-R) length of embryos. C-R length has been suggested as a reliable indicator of time since fertilisation where 1 mm is approximately 1 day since fertilisation [43].
To harvest embryos, we extracted each foetus from the mothers’ uterine horns immediately following brain extraction (see below). Each embryo was placed on a clean surface, we removed the embryonic sac and placenta, and we took three measures of the C-R length. We then calculated the mean embryo C-R length per litter to estimate the approximate time since fertilisation for each breeding pair [43].
Tissue extraction, and cryosectioning
Upon sacrifice, we immediately extracted brains, snap froze them on powdered dry ice, and stored them in −80°C. Later, we coronally cryosectioned brains at 20μm and mounted sections at 100μm intervals on Superfrost Plus slides (Fisher Scientific). Each of four sets was then stored at−80°C until they were used to visualise receptor density using autoradiography.
Autoradiography
On two of the four sets of brains, we used 125I radioligands to visualise either oxytocin receptor (ornithine vasotocin analog, ([125I]-OVTA); NEX 254, PerkinElmer; Waltham, MA) or vasopressin 1a receptor (vasopressin (Linear), V-1A antagonist (Phenylacetyl1, 0-Me-D-Tyr2, [125I-Arg6]-); NEX 310, PerkinElmer; Waltham, MA). To process tissues, we lightly fixed sections in 0.1% paraformaldehyde for 2 min, washed them two times in 1X Tris for 10 min, incubated them either with 40pM [125I]-OVTA or with 50pM 125I-labled vasopressin (Linear), V-1A antagonist for 90 min. Next, we washed slides in a series of 5 min baths of 1X Tris with MgCl2 followed by a final wash in 1X Tris with Mgcl2 for 30 min, and then rapidly air-dried them.
We exposed radiolabeled tissue to film (GE Healthcare, Buckinghamshire, UK) for three (V1aR) or four (OTR) days; different exposure lengths compensated for differing degrees of ligand radioactive decay at the time of use. The relative density of ligand binding was assessed by inferring that receptor density relates to the optical density of exposed film [44], and in this way optical density measurements serve as a proxy for receptor density. We used 125I labelled radiographic standards (American Radiolabeled Chemicals; St Louis, MO) to allow for conversion of optical density to receptor density. We digitised films on a Microtek ArtixScan M1 (Microtek, Santa Fe Springs, CA) and measured optical densities using NIH ImageJ Software. We calculated receptor density by first converting optical density to disintegrations per minute (dpm), adjusted for tissue equivalence (TE; for 1 mg in the rat brain), by using a log function to fit curves generated by radiographic standards.
We measured optical density for each structure of interest three times (once on a series of three brain sections, bilaterally). We also measured nonspecific binding on each section by measuring the background levels of cortex (bilaterally) in areas that do not express either peptide receptor on each of the same sections measured. The values for each structure were averaged, converted to dpm/mg TE, and adjusted to represent specific binding by subtracting nonspecific binding from total binding for each area.
We measured OTR in the prefrontal cortex (PFC), anterior insular cortex (ICa), nucleus accumbens (NAcc), septohippocampal nucleus (SHi), dorsal and ventral component of the lateral septum (LSd and LSv), caudate-putamen (striatum; CPu), medial insular cortex (ICm), central amygdala (CeA), basolateral amygdala (BLA), hippocampus (Hi), posterior insular cortex (ICp), and the intermedial dorsal, centromedial, and centrolateral thalamic nuclei (IMD, CM, and CL, respectively).
We measured V1aR in the main and accessory olfactory bulbs (MOB and AOB, respectively), ventral pallidum (VPall), lateral septum (LS), medial, lateral and ventral portions of the bed nucleus of the stria terminalis (BSTm, BSTl, BSTv, respectively), anterior hypothalamus (AH), paraventricular nucleus (PVN), superchiasmatic nucleus (SCN), posterior cingulate/retrosplenial cortex (RSC), lateral dorsal thalamic nucleus (LDTh), medial dorsal thalamic nucleus (MDTh), ventroposterior thalamic nucleus (VPTh), central amygdala (CeA), medial amygdala (MeA), and the ventral medial nucleus of the hypothalamus (VMH).
Unfortunately, we were unable to quantify OTR or V1aR in some structures (primarily hypothalamic) that are known to influence maternal behaviours in other rodents. These included the PVN, mPOA, SON, and BST for OTR and the mPOA for V1aR. Receptor expression has not been described in other studies using prairie voles and is indistinguishable from background in these structures [44–47], but see [48].
Analyses
We used a multivariate general linear model with average litter crown-rump length as a covariate to compare receptor expression across the course of pregnancy for multiple neural structures expressing either OTR or V1aR. This analysis enabled us to evaluate correlations between pup C-R length (i.e., how long females were pregnant) and receptor expression in each structure listed above, while controlling for issues of inflated power associated with multiple comparisons.
Ultimately, our primary interest was to determine whether receptor expression differed as a function of pregnancy. Given our inability to control when fertilisation occurred (see above), our primary analyses treated our sample of 20 females as a continuous set of data beginning with females that had not yet mated, through females that had mated but were not pregnant, to females in various stages of pregnancy, and ending with females that had just given birth. Although the number of females at any given stage of this continuum varied, treating the data as a spectrum of stages of pregnancy can reveal the general pattern of receptor dynamics during this crucial phase of life.
Results
Latency to first mating, average litter size, and average crown-rump length
Because female prairie voles are induced ovulators [49, 50], pairs usually require 2 to 3 days of co-habitation before mating occurs. The majority of our animals demonstrated this pattern, with 15 of 20 pairs mating within the first 72 hours of pairing. Of the remaining five pairs, two mated on the fourth day, one mated on day 11, one on day 18, and one pair never mated.
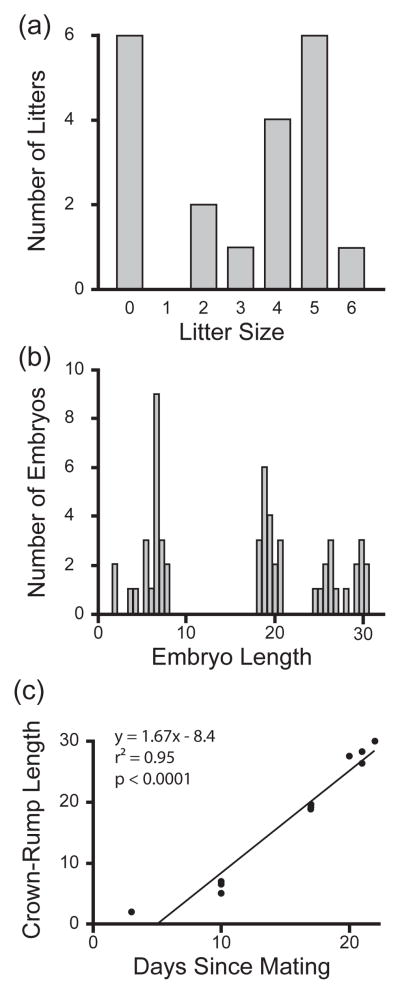
Litter sizes ranged from 0 to 6 embryos (Figure 1a); mean (± SE) litter size was 3.0 ± 0.50 pups per litter. Of the total 56 embryos, the average C-R length was 16.5 mm (±1.24), ranging from 2 to 30.5 mm (Figure 1b). Six pairs never produced offspring. This highlights an important point: although many of our pairs mated more or less according to our intended design, first mating did not guarantee fertilisation. Therefore brain sampling occurred across a spectrum of time points during the course of pregnancy. Ultimately the range of how long females had been pregnant (estimated by average litter C-R length) at the time brains were extracted ranged from an un-mated virgin female (N = 1), to mated females that were not fertilised (N = 5), to females that had been pregnant for less than 5 days (N = 2, x̄ length < 3.0 mm), to females that had been pregnant for approximately 6–7 days (N = 4 x̄ length = 6.36 mm), to females that were approximately pregnant for 16–20 days (N = 4 x̄ length = 19.31 mm), to post-partum females (N = 4 x̄ length = 27.83 mm). The range of embryo sizes presented in figure 1b provides an estimation of the extent to which females were pregnant.
Figure 1.
Panels a and b are frequency plots for the litter sizes across breeding pairs (a), and embryo length (measured from crown to rump; mm) for each embryo (b). Panel c shows the relationship between time since mating was first observed and embryo size (measured by crown-rump length in mm).
Wolff et al. [43] have argued that C-R length can be used to predict the length of time female prairie voles have been pregnant. Figure 1c demonstrates the significant relationship between the time since first observed mating (excluding non-pregnant females and one female who became pregnant approximately 17 days after the first observed mating) and average embryo C-R length (Pearson’s correlation; r2 = 0.95, N = 13, p < 0.0001). Notice that the slope of this trend-line was 1.67 (95% CI ± 1.4 – 1.9), which indicates that 1.5 mm of C-R length is a better estimate of each day of postzygotic growth than the 1 mm proposed by Wolff et al. [43]. Nevertheless, this robust relationship strongly indicates that C-R length is a reliable indicator for the degree of female pregnancy.
Forebrain nonapeptide expression
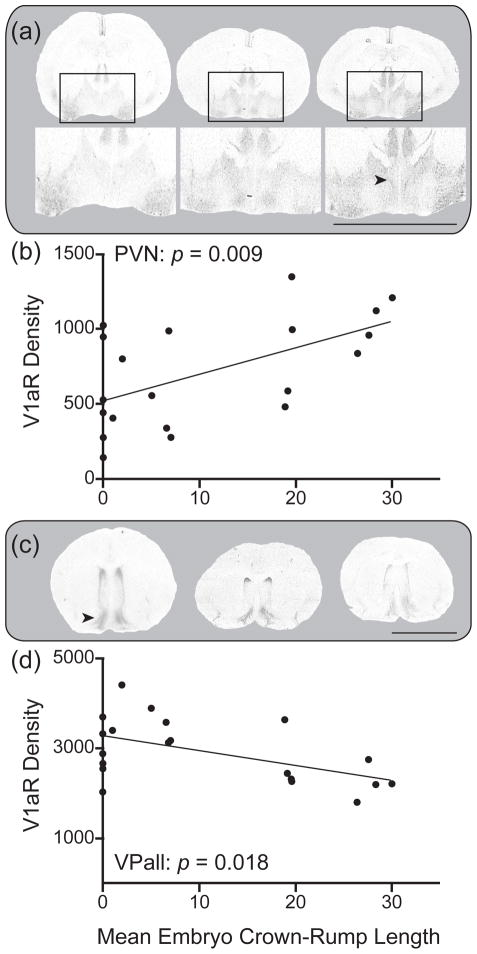
Crown-rump length did not predict the binding density for OTR in any forebrain structure (Wilks’ lambda = 0.375, F(15, 4) = 0.44, p = 0.89; Table 1). In contrast, V1aR binding density significantly changed over the course of pregnancy (Wilks’ lambda = 0.006, F(17, 2) = 18.63, p = 0.05; Table 2). This effect was primarily driven by the fact that C-R length predicted V1aR density in two areas of significant interest. First, C-R length was significantly and positively correlated with V1aR binding density in the PVN (r2 = 0.32, N = 20, F(1) = 8.58, p = 0.009; Figure 2a). In contrast, C-R length was negatively correlated with V1aR density in the VPall (r2 = 0.27, N = 20, F(1) = 6.72, p = 0.018; Figure 2b). Although no other V1aR expressing areas correlated with the degree of female pregnancy, some notable trends included the superchiasmatic nucleus (which showed a similar pattern to the PVN), and the lateral septum (which showed a similar pattern to the VPall) (see Table 2).
Table 1.
Results for OTR expression (125I-Ornathine Vasotocin specific binding, dpm/mg TE) throughout the forebrain for female prairie voles across pregnancy. Abbreviations are defined in text. Pearson’s correlation coefficients (r) and 95% confidence intervals (CI) are provided for reference.
| F(1) | p | r | 95% CI | ||
|---|---|---|---|---|---|
| PFC | 2.60 | 0.12 | 0.35 | −0.10 | 0.69 |
| ICa | 1.20 | 0.29 | 0.25 | −0.22 | 0.62 |
| NAcc | 0.04 | 0.85 | 0.04 | −0.41 | 0.48 |
| SHi | 0.16 | 0.70 | 0.09 | −0.36 | 0.51 |
| LSd | 0.33 | 0.57 | 0.14 | −0.33 | 0.55 |
| LSv | 0.69 | 0.42 | 0.19 | −0.27 | 0.59 |
| CPu | 0.34 | 0.57 | 0.14 | −0.33 | 0.55 |
| ICm | 1.33 | 0.26 | 0.26 | −0.20 | 0.63 |
| CeA | 0.93 | 0.35 | 0.22 | −0.24 | 0.61 |
| BLA | 0.98 | 0.34 | 0.23 | −0.24 | 0.61 |
| Hi | 0.24 | 0.63 | 0.11 | −0.35 | 0.53 |
| ICp | 1.16 | 0.30 | 0.25 | −0.22 | 0.62 |
| IMD | 1.41 | 0.25 | 0.27 | −0.20 | 0.64 |
| CM | 0.01 | 0.94 | 0.02 | −0.43 | 0.46 |
| CL | 0.02 | 0.89 | 0.03 | −0.42 | 0.47 |
Table 2.
Results for V1aR expression (125I-labeled V1aR linear-antagonist specific binding, dpm/mg TE) throughout the forebrain for female prairie voles across pregnancy. Abbreviations are defined in text. Pearson’s correlation coefficients (r) and 95% confidence intervals (CI) are provided for reference. Significant p-values are in bold.
| F(1) | p | r | 95% CI | ||
|---|---|---|---|---|---|
| MOB | 0.47 | 0.50 | 0.16 | −0.31 | 0.56 |
| AOB | 0.72 | 0.41 | 0.20 | −0.27 | 0.59 |
| VPall | 6.72 | 0.02 | −0.52 | −0.78 | −0.10 |
| LS | 2.42 | 0.14 | −0.34 | −0.68 | 0.12 |
| BSTm | 1.20 | 0.29 | −0.25 | −0.62 | 0.22 |
| BSTl | 1.06 | 0.32 | −0.24 | −0.61 | 0.23 |
| BSTv | 0.02 | 0.89 | −0.03 | −0.47 | 0.42 |
| AH | 0.66 | 0.43 | 0.19 | −0.28 | 0.58 |
| PVN | 8.58 | 0.01 | 0.57 | 0.17 | 0.81 |
| SCN | 2.62 | 0.12 | 0.36 | −0.10 | 0.69 |
| RSC | 0.26 | 0.62 | −0.12 | −0.53 | 0.34 |
| LDTh | 1.83 | 0.19 | 0.30 | −0.16 | 0.66 |
| MDTh | 0.04 | 0.85 | 0.04 | −0.41 | 0.48 |
| VPTh | 0.75 | 0.40 | 0.20 | −0.27 | 0.59 |
| CeA | 1.05 | 0.32 | −0.24 | −0.61 | 0.23 |
| MeA | 0.03 | 0.86 | −0.04 | −0.48 | 0.41 |
| VMH | 0.02 | 0.89 | −0.03 | −0.47 | 0.42 |
Figure 2.
The duration of pregnancy (measured as mean litter crown-rump length [mm]) predicts V1aR expression (dpm/mg TE) in the paraventricular nucleus of the hypothalamus (b; PVN) and ventral pallidum (d; VPall). Autoradiograms for the PVN (a) and VPall (c) are presented for females in early, middle, and late stages of pregnancy (respectively from left to right). The scale bar in panel a indicates 5 mm for the expanded autoradiograms (bottom of panel a) showing the PVN; the scale bar in panel c indicates 5 mm for the whole brain sections (for both panels a and c).
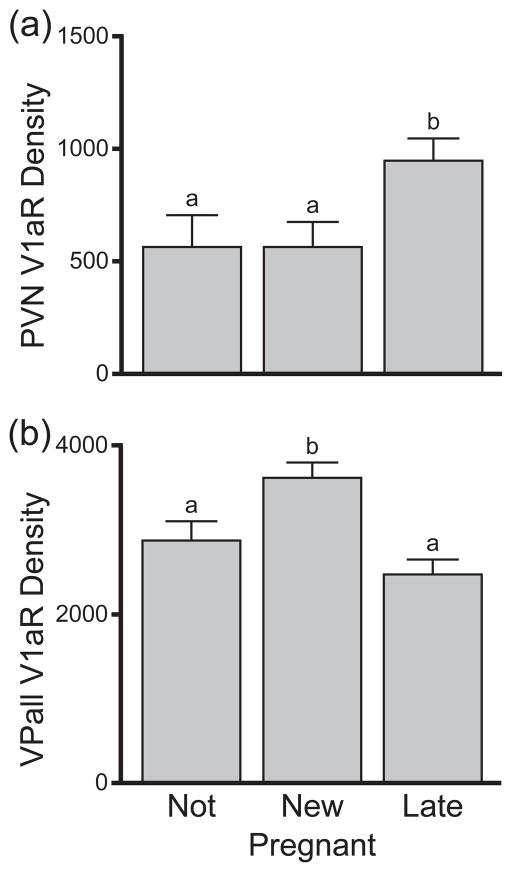
Including non-pregnant (mated or virgin) females might bias the correlation between C-R length and female brain expression because, although they were attributed a C-R measurement of 0, unfertilised females have no embryos to measure. We therefore removed all females that were not pregnant to provide a second measure of the relationship between V1aR expression in the VPall and PVN with C-R length. Removing these individuals did not affect the strength of the relationship in the PVN (r2 = 0.38, N = 14, F(1, 12) = 7.45, p = 0.02) and it strengthened the relationship in the VPall (r2 = 0.66, N = 14, F(1,12) = 22.96, p < 0.001). The correlations across all other brain structures for OTR or V1aR were not affected by removing the non-pregnant females (all r2 ≤ |0.43|, N = 14, p ≥ 0.124). We also pooled animals into three groups: non-pregnant females (N = 6), newly pregnant females (C-R length between 1 and 15 mm; N = 6), and late pregnancy females (C-R length greater than 15 mm; N = 8). V1aR expression in the PVN significantly differed between these three groups (ANOVA: F(2, 19) = 3.60, p < 0.05). Post hoc t-tests revealed that non-pregnant and newly pregnant females demonstrated similar V1aR expression in the PVN (p = 0.99), but each of these groups expressed significantly less V1aR than late pregnancy females (p < 0.05, p = 0.03 respectively; see Figure 3a). V1aR expression in the VPall was also significantly different across these three groups of females (F(2, 19) = 7.63, p < 0.01). However, unlike in the PVN, pallidal V1aR differed between newly pregnant and late pregnancy females (p = 0.03) and between newly pregnant and non-pregnant females (p = 0.04) but not between non-pregnant and late pregnancy females (p = 0.64; see Figure 3b). These data largely correspond with the correlational data (see Figure 2) that indicate an increase of V1aR in the PVN as parturition approaches, and what appears to be a transient increase in pallidal V1aR shortly after fertilisation.
Figure 3.
Mean (±SE) V1aR expression (dpm/mg TE) in the paraventricular nucleus of the hypothalamus (a; PVN) and ventral pallidum (b; VPall) for females that were not pregnant (Not), newly pregnant (New), or in late pregnancy (Late). Different letters (a, b) indicate which groups significantly differed (all p’s < 0.05, otherwise p’s ≥ 0.64).
Discussion
The mammalian nonapeptide hormones oxytocin and vasopressin are profoundly involved in a breadth of social behaviours, including those associated with offspring care [51–53]. Whereas oxytocin has been closely associated with female pregnancy, parturition, lactation, and several aspects of maternal behaviour [1, 8, 10, 54], only recently has the action of vasopressin been linked to female maternal behaviour [8, 9, 32, 55]. Somewhat surprisingly, although a great emphasis has been placed on describing how oxytocin and vasopressin change in the brain during pregnancy, relatively little attention has been given to dynamics of the receptors to which they bind over this course, and none have investigated such changes in socially monogamous mammals.
OTR & V1aR Stability
Above all, our results demonstrate the highly stable nature of forebrain OTR and V1aR during pregnancy. These results are fairly consistent with reports in rats. Indeed, in recent studies that have investigated OTR and V1aR expression during the perinatal period, the overwhelming majority of neural areas studied showed no changes in density of either receptor type [32, 33, 35], but see [36]. Despite the stability of OTR and V1aR during pregnancy, recent studies have demonstrated that there are profound post-partum changes in receptor density in rats. Indeed, the most dramatic differences can often be seen between parturition and in the early to mid lactation period (e.g., [32]). In a series of elegant studies (e.g., [9]) Bosch, Neumann and colleagues have provided compelling evidence that OT/OTR and AVP/V1aR dynamics near and following parturition reflect changes in maternal care and maternal aggression (see [8] and references therein). Furthermore, a rich literature exists on the influence of how the synthesis and release of oxytocin changes over the course of pregnancy and into lactation, accounting for many of the behavioural changes that females demonstrate (e.g., [16, 18, 19, 23–26, 28–30, 56]). Taking these neuromodulatory dynamics together with the relative pre-partum stability of OTR and V1aR, the mounting evidence strongly supports the argument that maternal behaviour is primed by nonapeptide release before birth and driven by adaptively transient sensitivity to release after birth and during lactation. These structural changes at the end of pregnancy and when neonates are most vulnerable potentially reflect an evolved physiological response [57] to increase female sensitivity to hormonal changes, which presumably enable females to behave adaptively to a changing social environment (i.e., the transition toward motherhood). Our data in prairie voles, which are limited to characterising the receptor dynamics throughout the forebrain, indicate that pregnant voles may be similar in most respects to pregnant rats in that receptor expression is highly stable. Any changes that follow pregnancy into lactation have not yet been described, though it is likely that female prairie voles undergo a similar receptor-mediated preparatory transition to motherhood. The form that any such transition takes could be similar to that seen in rats, or might differ from rats – potentially reflecting the rather uncommon mating system that prairie voles demonstrate (e.g., see below).
What behavioural event might lead to a transient change in brain phenotype?
Our goal was to describe how or if OTR and V1aR density changed over the course of pregnancy. We focused on a relatively narrow window of time, immediately following pairing to immediately after birth, because prairie voles show the most pronounced changes in affiliative and territorial behaviour during this period (e.g., [58]). Although our results demonstrate that OTR and V1aR expression is largely stable across pregnancy, we found that V1aR dynamically changes during pregnancy in two particularly compelling structures: the ventral pallidum and the paraventricular hypothalamic nucleus (each discussed in more detail below). We demonstrated that changes in the PVN were most striking in the later stages (second half) of pregnancy (Figure 3a), whereas V1aR in the VPall exhibited a rise after fertilisation and fall as parturition approached (Figure 3b). These different patterns of receptor dynamics raise an interesting question: what was the cause for the apparent shift in brain phenotype within the PVN and VPall? The changes in V1aR in the PVN and/or VPall could represent a transient shift in neural phenotype resulting from pregnancy, or from any number of behaviours that preceded pregnancy (such as mating, pairbonding, etc.). Although it is difficult to disambiguate any effects attributable to pairbonding, mating, and fertilisation because these events are highly coincided, the fact that all but one of the females in our study were mated reduces the likelihood that mating is responsible for the neural changes in either the PVN or VPall.
V1aR in the PVN of non-pregnant females was comparable to newly pregnant females, but later-stage pregnancy females expressed more V1aR in the PVN than either of these two groups (Figure 3a). This pattern of late-pregnancy up-regulation implicates the onset of parturition, lactation, or physiological preparation for offspring arrival as a driver for PVN V1aR dynamics. Results from Zheng et al. [47] are generally supportive of this overall pattern of V1aR up-regulation in the PVN; pregnant females living freely in natural outdoor enclosures tended to express more V1aR in the PVN than non-pregnant females (p = 0.07, unpublished data). Stepping back, the V1aR changes seen in the PVN indicate that hypothalamic sensitivity to vasopressin as parturition (and/or possibly post-parturient lactation) approaches could be particularly important for female prairie voles (see below).
Based on the current results, V1aR in the VPall appears to rise soon after fertilisation, and then fall as pregnancy progresses (Figure 3b). As we stated just above, because all but one female was mated, these results make it tempting to speculate that females transiently up-regulate VPall V1aR immediately after fertilisation (or possibly pairbonding) and then return to a ‘baseline’ following these events. On the other hand, Zheng et al. [47] showed that V1aR density in the VPall was significantly lower in pregnant females than in non-pregnant females. Although Zheng et al. [47] were unable to determine if un-pregnant females had previously mated, their results would suggest that pallidal V1aR has a naturally high starting point for non-pregnant females, followed by a progressive down-regulation only after fertilisation has occurred, a conclusion that is supported by our overall pattern of pallidal V1aR down-regulation (Figure 2d), but not supported by the fact that, on average, non-pregnant females in the current study demonstrated less pallidal V1aR than newly pregnant females (Figure 3b). Given these conflicting sets of data, it is difficult to determine if non-pregnant females and newly pregnant females are similar or different from each other, however it does appear to be the case that behaviours that follow mating (e.g., fertilisation or pairbonding) may serve to initiate V1aR plasticity in the VPall.
V1aR dynamics in the paraventricular hypothalamic nucleus
V1aR density in the PVN increased as pregnancy progressed. While this result has not been characterised in other species, it is worth noting the striking parallelism between the results from our study with those reported for PVN OTR in rats. Specifically, among pregnant rats, only the PVN differs (increases) in OTR expression between early and late stages of pregnancy [32]. V1aR density in the PVN of rats, on the other hand, does not change during or just after pregnancy [32].
In the latest stages of pregnancy and during the postpartum transition toward lactation, several notable changes take hold. Both oxytocin and vasopressin gene expression is stimulated just before parturition and during lactation in rats [29, 30] and OT and AVP immunoreactivity are elevated in the PVN [25], whereas oxytocin – but not vasopressin – is naturally released in late stages of pregnancy and into lactation in the PVN of both sheep and rats [19, 26]. Note that oxytocin in the PVN inhibits local AVP release under stressful conditions [28] and may therefore act to reduce the release of AVP during maternal stress. In any case, the concomitant increase in local OT release to the PVN with the up-regulation of OTR at parturition and into lactation suggests that PVN OT/OTR is particularly important in regulating both partum and postpartum behaviours, a suggestion that is largely supported by behavioural measures in rats and sheep [16, 19, 26, 32, 35].
The influences of oxytocin and vasopressin on behavioural changes during and just after birth – to our knowledge – have not been studied in female prairie voles. However, the up-regulation of PVN V1aR over pregnancy is likely to provide feedback on the synthesis and release of nonapeptides considering that that the PVN is a hypothalamic source of both vasopressin and oxytocin [59]. Although it is probable that oxytocin and vasopressin are released during pregnancy and into lactation in a similar manner to rats, initiation of prairie vole maternal behaviour is relatively independent from the direct control of these and other hormones and requires vaginal-cervical stimulation to express [60]. It is plausible that increased V1aR in the PVN functions to increase the vasopressinergic responsiveness of the PVN to vaginal-cervical stimulation, which may in turn contribute toward modulating the induction of maternal behaviour of prairie voles – perhaps in a similar manner to the role that OT influences rat and sheep maternal behaviour. Species differences such as this are tempting to attribute to differences in social mating systems, however more work in this area would be needed before this hypothesis could be substantiated. Nevertheless, the changes in V1aR density of the PVN during pregnancy in prairie voles probably increase sensitivity to the release of vasopressin over this period. How (or the degree to which) this influences maternal behaviour remains an open question.
V1aR dynamics in the ventral pallidum
V1aR density in the ventral pallidum decreased as a function of pregnancy (Figure 2d). More specifically, pallidal V1aR appears to increase soon after fertilisation, but decrease as females approach parturition (Figure 3b). This is particularly interesting given the strong evidence that V1aR in the VPall is necessary and sufficient to produce pairbonds in male prairie voles, but evidently does not have this effect in females [61, 62], but see [63]. This has largely been interpreted to mean that pallidal V1aR does not play a central role in female bonding [42, 64]. However, this view has recently been called into question [47]. How the ventral pallidum influences female social attachment remains unclear, however its functional role in reward provides promising insight.
The ventral pallidum is the primary output for the ventral striatum and was historically thought to function in motor output [65, 66]. Amore modern view of this structure is that it functions as a point of convergence for limbic reward signals, governing motivational ‘wanting’ and hedonic ‘liking’ [67]. From this perspective, the ventral pallidum appears to alter incentive value of cues and hedonic value of reward. For example, damage to the ventral pallidum decreases the salience of, and drive to seek, reward [68–74]. We postulate that the neuromodulatory effects of AVP on V1aR in the ventral pallidum may suppress ‘wanting’ (and possibly ‘liking’) the reward associated with social interaction (c.f., [67]). In this case, high V1aR would increase sensitivity to naturally released AVP, resulting in reduced mate searching (i.e., monogamy). This hypothesis is supported by comparisons between monogamous and promiscuous species of rodents in which monogamous rodents have more pallidal V1aR than promiscuous species [44, 46, 75]. Moreover, pairbonded female prairie voles living under natural conditions have significantly more V1aR expression in the ventral pallidum than single females [47]. Increasing the expression of V1aR in promiscuous species induces monogamous-like partner preferences, and infusion of AVP in the ventral pallidum of male prairie voles facilitates social bonding [62, 76, 77]. Even more supportive of this idea are the recent results by Barrett et al. [78], which found that experimentally decreased pallidal V1aR in male prairie voles increased promiscuous behaviour. All of these results are consistent with the hypothesis that high pallidal V1aR eliminates the motivation for (or ‘wanting’ of) multiple mating partners and social promiscuity.
So if high pallidal V1aR provides a mechanism to suppress social promiscuity, why might expression patterns change over the course of pregnancy? One possible explanation is that females undergo a transient shift away from ‘wanting’ sexual partners once they have become fertilised. This hypothesis would predict the probability of multiple-male mating should be lowest (and thus female fidelity should be highest) during early pregnancy. If true, then one might expect V1aR manipulations (such as antagonist work or V1aR knockdown) in females should be most likely to disrupt pairbonds in females following pregnancy, and might explain why some V1aR antagonist studies have failed to alter female pairbonding (e.g., [61]). Similarly, the observation that female aggression toward unfamiliar males increases after mating [58], supports this hypothesis that female ‘wanting’ for social interaction early in pregnancy should be reduced.
If the results from the current study are generally representative of the pattern of changes in pallidal V1aR among female prairie voles, then a reduction in VPall V1aR in late pregnancy could represent a shift toward the need to engage in pro-social behaviour with newborn offspring, the approaching need for post-partum fertilisation, or both. In support of the former case, disinhibition of the ventral pallidum under GABAergic afferents from the nucleus accumbens alters the incentive value of retrieving pups facilitating maternal care [79]. AVP acts at V1a receptors to excite GABAergic neurons [80], such that decreased V1aR in this context would enable the disinhibition of the VPall to facilitate maternal care. In support of the later case, it is important to consider that female prairie voles experience postpartum oestrus [81, 82]. Indeed females are most sexually receptive while giving birth, and in some cases engage in mating between the birth of each pup (Ophir, personal observation). Thus, the reduction in V1aR in the ventral pallidum near parturition could contribute to the increased (potentially non-selective) mating drive that postpartum female prairie voles experience. While both hypotheses are plausible, we are unaware of any study that has directly tested either.
Conclusion
Whether due to mating, fertilisation, or something else, there is substantial evidence to suggest that V1aR density within the PVN and VPall is dynamic over the course of pregnancy, and merits further study. Such studies would be greatly enhanced by broadening the scope of female life history to encompass more than pairing through parturition, and include singly-housed virgin females and/or lactating females (post-parturition for more than one day). Such studies would provide a robust understanding of the patterns of change that OTR and V1aR undergo in the brains of this socially monogamous rodent. The potential role of induced ovulation in V1aR dynamics makes future studies even more appealing. Nevertheless, the fact that we have found that nonapeptide receptors across the forebrain of female prairie voles are transient in only a couple of key neural structures provides deep insight into the dynamics underway within the brains of at least one socially monogamous species.
We have demonstrated that nonapeptide receptors are highly stable over the course of pregnancy. The two exceptions to this observation appear to both have ties to maternal care. In the case of the paraventricular nucleus of the hypothalamus, V1aR expression increases as pregnancy progresses; the opposite was true in the ventral pallidum. Although the PVN was not a surprising site to find such changes, the fact that it was V1aR (not OTR) marks a departure from previous reports in rats and may indicate an interesting difference between species, potentially attributable to differences in social organisation and mating system. The changes we observed in pallidal V1aR in pregnant females indicate an important point: that pallidal V1aR is very likely important for female prairie vole social behaviour, bonding, and maternal care. Taken together, our data emphasise the need to consider the phenotypic expression (and functional nature) of receptors in changing social environments, particularly for receptors known to be important in social behaviour.
Acknowledgments
We thank David Zheng for helpful discussions and assistance conducting V1aR autoradiography, and three anonymous reviewers for their valuable and constructive comments. This work was supported by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD065604–01).
References
- 1.Russell JA, Douglas AJ, Ingram CD. Brain preparations for maternity - adaptive changes in behavioral and neuroendocrine systems during pregnancy and lactation. An overview. In: Russell JA, Douglas AJ, Windle RJ, Ingram CD, editors. Progress in Brain Research. Elsevier; 2001. pp. 1–38. [DOI] [PubMed] [Google Scholar]
- 2.Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–4. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- 3.Rosenblatt JS, Mayer AD, Giordano AL. Hormonal basis during pregnancy for the onset of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:29–46. doi: 10.1016/0306-4530(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 4.Leng G, Meddle SL, Douglas AJ. Oxytocin and the maternal brain. Current Opinion in Pharmacology. 2008;8:731–4. doi: 10.1016/j.coph.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Young LJ, Flanagan-Cato LM. Oxytocin, vasopressin and social behavior. Hormones and Behavior. 2012;61:227–9. doi: 10.1016/j.yhbeh.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowley WR, Armstrong WE. Neurochemical regulation of oxytocin secretion in lactation. Endocrine Review. 1992;13:33–65. doi: 10.1210/edrv-13-1-33. [DOI] [PubMed] [Google Scholar]
- 7.Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiological Reviews. 2001;81:629–83. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 8.Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: From central release to sites of action. Hormones and Behavior. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Bosch OJ, Neumann ID. Brain vasopressin in an important regulator of maternal behavior independent of dams’ trait anxiety. Proceedings of the National Academy of Sciences, USA. 2008;105:17139–44. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter CS, Altemus M, Chrousos GP. Neuroendocrine and emotional changes in the post-partum period. In: Russell JA, Douglas AJ, Windle RJ, Ingram CD, editors. Progress in Brain Research. Elsevier; 2001. pp. 241–9. [DOI] [PubMed] [Google Scholar]
- 11.Kinsley CH. Developmental psycobiological influences on rodent parental behavior. Neuroscience and Biobehavioral Reviews. 1994;18:269–80. doi: 10.1016/0149-7634(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 12.Kinsley CH, Lambert KG. The maternal brain. Scientific American. 2006;294:72–9. doi: 10.1038/scientificamerican0106-72. [DOI] [PubMed] [Google Scholar]
- 13.Moos F, Freund-Mercier MJ, Guerne Y, Guerne JM, Stoeckel ME, Richard P. Release of oxytocin and vasopressin by magnocellular nuclei in vitro: Specific facilitory effect of oxytocin on its own release. Journal of Endocrionology. 1984;102:63–72. doi: 10.1677/joe.0.1020063. [DOI] [PubMed] [Google Scholar]
- 14.Moos F, Ingram CD, Wakerley JB, Guerne Y, Freund-Mercier MJ, Richard P. Oxytocin in the ved nucleus of the stria terminalis and lateral septum facilitates burstin of hypothalamic oxytocin neurons in suckled rats. Journal of Neuroendocrinology. 1991;3:163–71. doi: 10.1111/j.1365-2826.1991.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 15.Neumann ID, Koehler E, Landgraf R, Summy-Long J. An oxytocin receptor antagonist infused into the supraoptic nucleus attenuates intranuclear and peripheral release of oxytocin during suckling in conscious rats. Endocrinology. 1994;134:141–8. doi: 10.1210/endo.134.1.8275928. [DOI] [PubMed] [Google Scholar]
- 16.Bosch OJ, Kromer SA, Brunton PJ, Neumann ID. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders druing maternal defense. Neuroscience. 2004;124:439–48. doi: 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 17.Fahrbach SE, Morrell JI, Pfaff DW. Possible role for endogenous oxytocin in estrogen-facilitated maternal behavior in rats. Neuroendocrinology. 1985;40:526–32. doi: 10.1159/000124125. [DOI] [PubMed] [Google Scholar]
- 18.Landgraf R, Neumann ID, Russell JA, Pittman QJ. Push-pull perfusion and microdialysis studies of central oxytocin and vasopressin release in freely moving rats during pregnancy, parturition, and lactation. Annals of the New York Academy of Sciences. 1992;652:326–39. doi: 10.1111/j.1749-6632.1992.tb34364.x. [DOI] [PubMed] [Google Scholar]
- 19.Neumann ID, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient, and lactating rats: A Microdialysis study. Neuroscience. 1993;53:65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen CA, Caldwell JD, Johnson MF, Fort SA, Prange AJ., Jr Oxytocin antiserum delays onset of ovarian steroid-induced maternal behavior. Neuropeptides. 1985;6:175–82. doi: 10.1016/0143-4179(85)90108-8. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen CA, Prange AJ., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proceedings of the National Academy of Sciences, USA. 1979;76:6661–5. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Leengoed E, Kerker E, Swanson HH. Inhibition of post-partum meternal behavior in the rat by injecting an oxytocin antagonist into the cerebral ventricles. Journal of Endocrionology. 1987;112:275–82. doi: 10.1677/joe.0.1120275. [DOI] [PubMed] [Google Scholar]
- 23.Bosch OJ, Neumann ID. Vasopressin released within the central amygdala promotes maternal aggression. European Journal of Neuroscience. 2010;31:883–91. doi: 10.1111/j.1460-9568.2010.07115.x. [DOI] [PubMed] [Google Scholar]
- 24.Bosch OJ, Pfortsch J, Beiderbeck DI, Landgraf R, Neumann ID. Maternal behaviour is associated with vasopressin release in the medial preoptic area and bed nucleus of the stria terminalis in the rat. Journal of Neuroendocrinology. 2010;22:420–9. doi: 10.1111/j.1365-2826.2010.01984.x. [DOI] [PubMed] [Google Scholar]
- 25.Caldwell JD, Greer ER, Johnson MF, Prange AJ, Jr, Pedersen CA. Oxytocin and vasopressin immunoreactivity in hypothalamic and extrahypothalamic sites in late pregnant and postpartum rats. Neuroendocrinology. 1987;46:39–47. doi: 10.1159/000124794. [DOI] [PubMed] [Google Scholar]
- 26.Da Costa APC, Guevara-Guzman RG, Ohkura S, Goode JA, Kendrick KM. The role of oxytocin release in paraventricular nucleus in the control of maternal behavior in the sheep. Journal of Neuroendocrinology. 1996;8:163–77. doi: 10.1046/j.1365-2826.1996.04411.x. [DOI] [PubMed] [Google Scholar]
- 27.Mahata SK, Mahata M, Hortnagl H, Fischer-Colbrie R, Steinter H-J, Dietze SO, et al. Concomitant changes of messernger ribonucleic acid levels of secretogranin II, VGF, vasopressin and oxytocin in the paraventricular nucleus of rats after adrenalectomy and during lactation. Journal of Neuroendocrinology. 1993;5:323–30. doi: 10.1111/j.1365-2826.1993.tb00489.x. [DOI] [PubMed] [Google Scholar]
- 28.Neumann ID, Torner L, Nicola T, Veenema AH. Oxytocin actions within the supraoptic and paraventricular nuclei: Differential effects on peripheral and intranuclear vasopressin release. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2006;291:R29–R36. doi: 10.1152/ajpregu.00763.2005. [DOI] [PubMed] [Google Scholar]
- 29.Van Tol HHM, Bolwerk ELM, Liu B, Burbach JPH. Oxytocin and vasopressin gene expression in the hypothalamo-neurohypophyseal system of the rat during the estrous cycle, pregnancy, and lactation. Endocrinology. 1988;122:945–51. doi: 10.1210/endo-122-3-945. [DOI] [PubMed] [Google Scholar]
- 30.Zingg HH, Lefebvre DL. Oxytocin and vasopressin gene expression during gestation and lactation. Molecular Brain Research. 1988;4:1–6. doi: 10.1016/0169-328x(88)90011-3. [DOI] [PubMed] [Google Scholar]
- 31.Bealer SL, Lipschitz DL, Ramoz G, Crowley WR. Oxytocin receptor binding in the hypothalamus during gestation in rats. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2006;291:R53–R8. doi: 10.1152/ajpregu.00766.2005. [DOI] [PubMed] [Google Scholar]
- 32.Caughey SD, Klampfl SM, Bishop VR, Pfoertsch J, Neumann ID, Bosch OJ, et al. Changes in the intensity of maternal aggression and central oxytocin and vasopressin V1a receptors across the peripartum period in the rat. Journal of Neuroendocrinology. 2011;23:1113–24. doi: 10.1111/j.1365-2826.2011.02224.x. [DOI] [PubMed] [Google Scholar]
- 33.Clerget-Froidevaux M-S, Pittman QJ. AVP V1a-R expression in the rat hypothalamus around parturition: Relevance to antipyresis at term. Experimental Neurology. 2003;183:338–45. doi: 10.1016/s0014-4886(03)00114-6. [DOI] [PubMed] [Google Scholar]
- 34.Insel TR. Regional changes in brain oxytocin receptors post-partum: Time-course and relationship to maternal behavior. Journal of Neuroendocrinology. 1990;2:539–45. doi: 10.1111/j.1365-2826.1990.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 35.Meddle SL, Bishop VR, Gkoumassi E, van Leeuwen FW, Douglas AJ. Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology. 2007;148:5095–104. doi: 10.1210/en.2007-0615. [DOI] [PubMed] [Google Scholar]
- 36.Young LJ, Muns S, Wang Z, Insel TR. Changes in oxytocin receptor mRNA in rat brain during pregnancy and the effects of estrogen and interleukin-6. Journal of Neuroendocrinology. 1997;9:859–65. doi: 10.1046/j.1365-2826.1997.00654.x. [DOI] [PubMed] [Google Scholar]
- 37.Beach FA. The snark was a boojum. American Psychologist. 1950;5:115–24. [Google Scholar]
- 38.Brotherton PNB, Komers PE. Mate guarding and the evolution of soical monogamy in mammals. In: Reichard UH, Boesch C, editors. Monogmay: Mating strategies and partnerships in birds, humans and other mammals. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- 39.Kleiman DG. Monogamy in mammals. Quarterly Review of Biology. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- 40.Carter CS, Getz LL. Monogamy and the prairie vole. Scientific American. 1993;268:100–6. doi: 10.1038/scientificamerican0693-100. [DOI] [PubMed] [Google Scholar]
- 41.Insel TR, Young LJ. The neurobiology of attachment. Nature Reviews: Neuroscience. 2001;2:129–36. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 42.Young LJ, Wang ZX. The neurobiology of pair bonding. Nature Neuroscience. 2004;7:1048–54. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 43.Wolff JO, Ophir AG, Phelps SM. Asynchronous breeding in the socially monogamous prairie vole. Canadian Journal of Zoology. 2008;86:339–43. [Google Scholar]
- 44.Young LJ, Winslow JT, Nilsen R, Insel TR. Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: Behavioral consequences. Behavioral Neuroscience. 1997;111:599–605. doi: 10.1037//0735-7044.111.3.599. [DOI] [PubMed] [Google Scholar]
- 45.Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proceedings of the National Academy of Sciences, USA. 1992;89:5981–5. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. Journal of Neuroscience. 1994;14:5381–92. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng D-Z, Larsson B, Phelps SM, Ophir AG. Female alternative mating tactics, reproductive success and nonapeptide receptor expression in the social decision-making network. Behavioral Brain Research. 2013;246:139–47. doi: 10.1016/j.bbr.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, et al. Neonatal oxytocin manipulations have long-lasting sexually dimorphic effects on vasopressin receptors. Neuroscience. 2007;144:38–45. doi: 10.1016/j.neuroscience.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dluzen DE, Ramirez VD, Carter CS, Getz LL. Male vole urine changes luteinizing-hormone-releasing hormone and norepinephrine in female olfactory-bulb. Science. 1981;212:573–5. doi: 10.1126/science.7010608. [DOI] [PubMed] [Google Scholar]
- 50.Richmond ME, Stehn RA. Olfaction and reproductive behavior in microtine rodents. In: Doty RL, editor. Mammalian olfaction, reproductive processess and behavior. New York: Academic Press; 1976. pp. 197–217. [Google Scholar]
- 51.Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. In: Neumann ID, Landgraf R, editors. Progress in Brain Research. Elsevier; 2008. pp. 331–6. [DOI] [PubMed] [Google Scholar]
- 52.Goodson JL. Nonapeptides and the evolutionary patterning of sociality. Progress in Brain Research. 2008;170:3–15. doi: 10.1016/S0079-6123(08)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goodson JL, Thompson RR. Nonapeptide mechanisms of social cognition, behavior, and species-specific soical systems. Current Opinion in Neurobiology. 2010;20:784–94. doi: 10.1016/j.conb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 54.Young LJ, Winslow JT, Wang Z, Gingrich B, Guo Q, Matzuk MM, et al. Gene targeting approaches to neuroendocrinology: Oxytocin, maternal behavior, and affiliation. Hormones and Behavior. 1997;31:221–31. doi: 10.1006/hbeh.1997.1377. [DOI] [PubMed] [Google Scholar]
- 55.Zhang L, Hernandez VS, Liu B, Medina MP, Nava-Kopp AT, Irles C, et al. Hypothalamic vasopressin system regulation by maternal seperation: Its impact on anxiety in rats. Neuroscience. 2012;215:135–48. doi: 10.1016/j.neuroscience.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 56.Jirikowski GF, Caldwell JD, Pilgrim C, Stumpf WE, Pedersen CA. Changes in immunostaining for oxytocin in the forebrain of the female rat during late pregnancy, parturition and early lactation. Cell Tissue Research. 1989;256:411–7. doi: 10.1007/BF00218899. [DOI] [PubMed] [Google Scholar]
- 57.Ketterson ED, Nolan V. Hormones and life history: An integrative approach. American Naturalist. 1992;140 doi: 10.1086/285396. Supplement: Behavioral mechanisms in evolutionary ecology S33–S62. [DOI] [PubMed] [Google Scholar]
- 58.Getz LL, Carter CS, Gavish L. The mating system by the prairie vole, Microtus ochrogaster: Field and laboratory evidence for pair-bonding. Behavioral Ecology and Sociobiology. 1981;8:189–94. [Google Scholar]
- 59.McEwen B. The roles of vasopressin and oxytocin in memory processing. San Diego: Elsevier Academic Press; 2004. [DOI] [PubMed] [Google Scholar]
- 60.Hayes UL, DeVries GJ. Role of pregnancy and parturition in induction of maternal behavior in prairie voles (Microtus ochrogaster) Hormones and Behavior. 2007;51:265–72. doi: 10.1016/j.yhbeh.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behavioral Neuroscience. 1995;109:782–9. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- 62.Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–8. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- 63.Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 1999;113:1071–9. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- 64.Insel TR, Young LJ. Neuropeptides and the evolution of social behavior. Current Opinion in Neurobiology. 2000;10:784–9. doi: 10.1016/s0959-4388(00)00146-x. [DOI] [PubMed] [Google Scholar]
- 65.Heimer L, Switzer RD, Van Hoesen GW. Ventral striatum and ventral pallidum: Components of the motor system? Trends in Neurosciences. 1982;5:83–7. [Google Scholar]
- 66.Mogenson GJ, Jones DL, Yim CY. From motivation to action: Functional interface between limbic system and the motor system. Progress in Neurobiology. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 67.Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behavioural Brain Research. 2009;196:155–67. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bourdelais A, Kalivas PW. Amphetamine lowers extracellualr GABA concentration in the ventral pallidum. Brain Research. 1990;516:132–6. doi: 10.1016/0006-8993(90)90907-s. [DOI] [PubMed] [Google Scholar]
- 69.Dallimore JE, Mickiewicz AL, Napier TC. Intra-ventral pallidal glutimate antagonists block expression of morphine-induced place preference. Behavioral Neuroscience. 2006;120:1103–14. doi: 10.1037/0735-7044.120.5.1103. [DOI] [PubMed] [Google Scholar]
- 70.Farrar AM, Font L, Pereira M, Mingote S, Bunce JG, Charobak JJ, et al. Forebrain circuitry involved in effort-related choice: Injections of the GABAA agonist muscimol into ventral pallidum alter response allocation in food-seeking behavior. Neuroscience. 2008;152:321–30. doi: 10.1016/j.neuroscience.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harvey SC, Foster KL, McKay PF, Carroll MR, Seyoum R, Woods JE, 2nd, et al. The GABA(A) receptor alpha(1) subtype in the ventral pallidum regulates alcohol-seeking behaviors. Journal of Neuroscience. 2002;22:3765–75. doi: 10.1523/JNEUROSCI.22-09-03765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hiroi N, White NM. The ventral pallidum area is invovled in the acquisition but not expression of the amphetamine conditioned place preference. Neuroscience Letters. 1993;156:9–12. doi: 10.1016/0304-3940(93)90426-l. [DOI] [PubMed] [Google Scholar]
- 73.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. Journal of Neuroscience. 2001;21:8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Waraczynski M, Demco C. Lidocaine inactivation of the ventral pallidum affects responding for brain stimulation reward more than it affects the stimulation’s reward value. Behavioral Brain Research. 2006;173:288–98. doi: 10.1016/j.bbr.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 75.Insel TR, Gelhard R, Shapiro LE. The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neuroscience. 1991;43:623–30. doi: 10.1016/0306-4522(91)90321-e. [DOI] [PubMed] [Google Scholar]
- 76.Lim MM, Wang ZX, Olazabal DE, Ren XH, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–7. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- 77.Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature. 1999;400:766–8. doi: 10.1038/23475. [DOI] [PubMed] [Google Scholar]
- 78.Barrett CE, Keebaugh AC, Ahern TA, Bass CA, Terwilliger EF, Young LJ. Variation in vasopressin receptor (Avpr1a) expression creates diversity in behaviors related to monogamy in prairie voles. Homones and Behavior. 2013;63:518–26. doi: 10.1016/j.yhbeh.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Numan M, Numan MJ, Schwarz JM, Neuner CM, Flood TF, Smith CD. Medial preoptic area interactions with the nucleus accumbens-ventral pallidum circuit and maternal behavior in rats. Behavioral Brain Research. 2005;158:53–68. doi: 10.1016/j.bbr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 80.Hermes MLHJ, Ruijter JM, Klop A, Buijs RM, Renaud LP. Vasopressin increases GABAergic inhibition of rat hypothalamic paraventricular nucleus neurons in vitro. Journal of Neurophysiology. 2000;83:705–11. doi: 10.1152/jn.2000.83.2.705. [DOI] [PubMed] [Google Scholar]
- 81.Witt DW, Carter CS, Carlstead K, Read LD. Sexual and social interactions preceding and during male-induced oestrus in prairie voles, Microtus ochrogaster. Animal Behaviour. 1988;36:1465–71. [Google Scholar]
- 82.Witt DW, Carter CS, Chayer R, Adams K. Patterns of behaviour during postpartum oestrus in prairie voles, Microtus ochrogaster. Animal Behaviour. 1990;39:528–34. [Google Scholar]