Abstract
Nonhuman primates, particularly rhesus macaques (Macaca mulatta), provide important model systems for studying human reproductive infectious diseases such as human immunodeficiency virus, human papillomavirus, and Chlamydia spp. An understanding of the spectrum of spontaneous cervical disease provides essential context for interpreting experimental disease outcomes in the female reproductive tract. This retrospective study characterizes the incidence of inflammatory and/or proliferative cervicovaginal lesions seen over a 14-year period in a multispecies nonhuman primate colony, focusing on rhesus macaques. The most common observations included a spectrum of lymphocytic accumulation from within normal limits to lymphoplasmacytic cervicitis, and suppurative inflammation with occasional squamous metaplasia or polyp formation. These inflammatory spectra frequently occurred in the context of immunosuppression following experimental simian immunodeficiency virus (SIV) infection. Cervical neoplasias were uncommon and included leiomyomas and carcinomas. Cervical sections from 13 representative cases, with an emphasis on proliferative and dysplastic lesions, were surveyed for leukocyte infiltration, abnormal epithelial proliferation, and the presence of papillomavirus antigens. Proliferative lesions showed sporadic evidence of spontaneous papillomavirus infection and variable immune cell responses. These results under-score the importance of pre screening potential experimental animals for the presence of preexisting reproductive tract disease, and the consideration of normal variability within cycling reproductive tracts in interpretation of cervical lesions.
Keywords: animal models, female reproduction, primate pathology, immunohistochemistry, histopathology
Introduction
The female reproductive tract is a complex immunologic environment that must tolerate foreign antigens in sperm to allow pregnancy but at the same time defend against frequent exposure to a wide variety of pathogens. Like other mucosal surfaces in the body, the vagina and cervix contain a variety of defense mechanisms to protect against infection, with some specialization at different locations along the female tract (Wira et al. 2005). These defenses include thick cervical mucus, an acidic vaginal pH, commensal bacteria, antimicrobial peptide secretion, and active surveillance by innate and adaptive immune cells (Hickey et al. 2011). In particular, the endocervical–ectocervical junction, located at the transition area between the stratified squamous epithelium of the ectocervix and the simple columnar glandular epithelium of the endocervix (transformation zone), contains prominent submucosal lymphoid aggregates (mucosa associated lymphoid tissue, MALT) that are initial sites of pathogen interaction with antigen presenting cells (Edwards and Morris 1985). In addition, the immune environment of the vagina and cervix is dynamic, changing over time based on age, systemic reproductive hormone levels, pregnancy, and/or stage within the follicular cycle. For example, following ovulation in mice, neutrophils are seen in higher numbers within the vaginal mucosa (Sonoda et al. 1998). However, in rhesus macaques, minimal variation in resident immune cell populations examined across the menstrual cycle has been reported despite normal cyclic changes in the thickness of the vaginal epithelium and variablity in immune cell distribution along the length of the tract (Ma et al. 2001; Poonia et al. 2006).
Despite the wide variety of antimicrobial defenses, the thin cervical and vaginal mucosae are still vulnerable sites for many infectious diseases (Oakley et al. 2008; Holmes et al. 1999). These diseases may be localized and self-limiting (e.g., bacterial vaginosis) or may spread systemically with catastrophic effect (e.g., human immunodeficiency virus resulting in acquired immunodeficiency syndrome [AIDS]; Levy 2007; Martin 2012). In addition, evidence links some infectious diseases to the development of reproductive tract neoplasms. For example, cervical epithelial tumors in humans are strongly correlated with human papillomavirus infection, and leiomyomas have been associated with Epstein-Barr virus. In addition, benign polyps may arise spontaneously in response to chronic inflammation from various causes such as bacterial cervicitis (Bennett et al. 2009; McClain et al. 1995).
This study characterizes the diversity of spontaneous cervicovaginal histopathology in nonhuman primates over a 14-year period at the New England Primate Research Center (NEPRC), primarily rhesus macaques. Based on physiologic similarity to women, female rhesus macaques are a common model system used to investigate HIV pathogenesis and to test potential prophylactics and therapeutics (Baroncelli et al. 2008; Ambrose et al. 2007; Van Esch et al. 2008). In addition, the rhesus macaque is currently being explored as a model for human papillomavirus infection, based on the natural occurrence of a rhesus papillomavirus (MmPV-1), a close homologue to human papillomavirus type 16, the major cause of cervical cancers in women (Roberts et al. 2011; Wood et al. 2007). The goal of this study was to document the spectrum of spontaneous cervicovaginal disease within the NEPRC colony and to evaluate the leukocyte responses associated with different types of inflammatory and proliferative lesions. The contribution of natural papillomavirus infection to the development of spontaneous proliferative lesions in the case series was also investigated.
Method
Animal Cohort Selection
A database of necropsy and biopsy case records at the NEPRC from 1997 to 2011 was examined, including 2,531 Indian origin rhesus macaques (Macaca mulatta) of which 331 were females over 1 year of age with vaginal and/or cervical tissues collected and examined histologically. In addition, other species were surveyed for cervical pathology including 36 female common marmosets (Callithrix jacchus), 25 cotton top tamarins (Saguinus oedipus), 6 common squirrel monkeys (Saimiri sciureus), 11 long-tailed macaques (Macaca fascicularis), and 3 pig-tailed macaques (Macaca nemestrina) where cervicovaginal tissues were collected. All cases were reviewed by board certified veterinary pathologists at NEPRC. All animal procedures including euthanasia were performed in accordance with guidelines and recommendations of The Guide for the Care and Use of Animals and the standards of the Harvard Medical School Standing Committee on Animals and The Association for the Assessment and Accreditation of Laboratory Animal Care.
Histology and Immunohistochemistry
All animals were necropsied within 24 hr of death and representative sections of tissues were collected, fixed in 10% neutral buffered formalin (NBF), and embedded in paraffin. In all cases, 5-μm histologic sections were stained with hematoxylin & eosin (HE) and in some cases additional vaginal and/or cervical sections were prepared for immunohistochemistry. Routine avidin-biotin-horseradish peroxidase immunohistochemistry was used on these sections to analyze the expression patterns of different leukocyte markers, cellular proliferation, and papillomavirus viral E6 protein (Table 1). The subset of cases chosen for immunohistochemical analysis was selected to evaluate a representative cross section of common lesions (see Tables 2 and 3). The chromagen diaminobenzidine (DAB; Dako, Carpinteria, CA), or in some cases VectorNova RED (Vector Laboratories, Burlingame, CA), was used to develop the sections prior to counterstaining with Mayer’s hematoxylin, dehydration with xylene, mounting, and coverslipping. Negative controls consisted of adjacent sections stained with irrelevant species-, isotype-, and concentration-matched antibodies.
Table 1.
Antibodies used in immunohistochemical analysis of cervicovaginal tissues.
| Primary cell target | Antibody target | Source | Clone | Concentration (mg/ml) |
|---|---|---|---|---|
| T lymphocytes | CD3 | Dako A0452 | Pc | 1.0 |
| T lymphocytes | CD4 | Vector VP-C318 | 1F6 | ND (1:40) |
| T lymphocytes | CD8 | Vector VP-C325 | 1A5 | ND (1:50) |
| B lymphocytes | CD20 | Dako M0755 | L26 | 1.0 |
| Neutrophils | Myeloperoxidase | NeoMarker RB-373 | Rabbit polyclonal | ND (1:200) |
| Dendritic cells | CD207 | Abcam ab49730 | 12D6 | 10.0 |
| Natural killer cells | CD56 | Invitrogen 07-5603 | 12363 | ND (1:500) |
| Macrophages | CD68 | Dako M0814 | KP1 | 1.0 |
| Antigen presenting cells | HLA-DR | Novocastra NCL-LN3 | LN3 | 0.25 |
| Proliferating cells | Ki-67 | Dako M7240 | MIB1 | 1.0 |
| Papillomavirus-infected cells | HPV16 E6 | Santa Cruz SC-460 | C1P5 | 4.0 |
ND (ratio) = Concentration of antibody not specified by the manufacturer; antibody dilution is indicated.
Table 2.
Summary of cervicovaginal histopathology in the 331 female rhesus macaques, of which 215 (65.0%) were SIV positive.
| Cervicovaginal histopathology | Total/% | # SIV+ | # With gross lesions/total | Selected representative cases |
|---|---|---|---|---|
| Increased lymphoid aggregates | 89/27% | 68 | 2/89 | Case 13 |
| Suppurative cervicitis and/or vaginitis | 38/12% | 24 | 14/38 | Cases 5, 6 |
| Dilated/cystic mucosal glands | 12/4% | 5 | 6/12 | |
| Metaplasia, hyperplasia, or dysplasia | 7/2% | 2 | 1/7 | Case 1 |
| Vascular abnormalities | 6/2% | 4 | 0/6 | |
| Vaginal/cervical polyp | 3/0.9% | 1 | 3/3 | Cases 2-4 |
| Leiomyoma | 2/0.6% | 0 | 2/2 | |
| Carcinoma | 1 | 1 | 1/1 | Case 7 |
| Serosal granulosa cell tumor | 1 | 0 | 1/1 | |
| Serosal endometriosis | 1 | 0 | 1/1 | |
| Myonecrosis and regeneration | 1 | 1 | 0/1 | |
| Pericervical abscess | 1 | 1 | 1/1 | |
| Erosive vaginitis | 1 | 1 | 0/1 | |
| Vaginal cryptosporidiosis | 1 | 1 | 0/1 | |
| Vaginal foreign body | 1 | 1 | 1/1 | |
| No significant findings | 165/50% | 105 | 0/165 | Cases 9-2 |
Table 3.
Summary of cases examined by immunohistochemical staining for immune cell subsets and papillomavirus induced lesions.
| Case # | Description | Age (years) | Species | Source | SIV | Ki-67 | E6 |
|---|---|---|---|---|---|---|---|
| 1 | SQMP and dysplasia | 25.0 | Mm | OC | − | +++ | + |
| 2 | Polyp | 14.0 | Mm | NEPRC | − | + | − |
| 3 | Cervicitis with polyp | 7.6 | Mm | NEPRC | SHIV+ | + | − |
| 4 | Cervicitis with dysplastic polyp | 23.3 | Mm | NEPRC | − | +++ | + |
| 5 | Suppurative cervicitis/vaginitis with SQMP | 3.9 | Mm | NEPRC | − | + | − |
| 6 | Suppurative cervicitis/vaginitis with SQMP | 15.1 | Mm | NEPRC | − | + | − |
| 7 | Anaplastic carcinoma | 12.7 | Mm | NEPRC | SIV+ | ++ | + |
| 8 | Papillary carcinoma | 14.9 | Sgo | NEPRC | − | + | + |
| 9 | WNL | ND | Mm | OC | − | − | − |
| 10 | WNL | 2.8 | Mm | NEPRC | − | − | − |
| 11 | WNL | 19.4 | Mm | OC | − | − | − |
| 12 | WNL | 6.6 | Mm | OC | − | − | − |
| 13 | Lymphoid aggregates, WNL | 11.6 | Mm | NEPRC | − | − | − |
Note. SQMP = squamous metaplasia; WNL = within normal limits; NEPRC = New England Primate Research Center; OC = other colony; Mm = Macaca mulatta; Sgo = Saguinus oedipus; SIV = Simian Immunodeficiency Virus; SHIV = Simian–human Immunodeficiency Virus; ND = not determined.
The amount of epithelial Ki-67 staining above the normal basal epithelial layers and the presence of papillomavirus E6 protein within the cervicovaginal epithelium is indicated.
Serology
Antibody titer screening was performed on stored rhesus serum from 13 selected cases, including 5 controls with normal cervicovaginal histology and case history (Table 3), by standard enzyme-linked immunosorbent assay (ELISA) techniques against MmPV-1 L1 protein empty virus-like particles (generously provided by R. Desrosiers, unpublished data) and HPV 16 virus–like particles (HyTest Ltd, Turku, Finland). Controls included a nonspecific anti-mouse IgG (negative) and a mouse anti-HPV L1 antibody (positive; BD Biosciences, San Jose, CA), used at 2 μg/ml. Serum samples were analyzed on pre- and post-SIV inoculation samples when available. Titers from animals with positive papillomavirus immunohistochemistry results were compared to those from control animals by two-tailed Student’s T test.
Results
Case Selection
Cervical and/or vaginal tissues from 331 female intact rhesus macaques between the ages of 1 and 32 years (M = 10.2 years, SD 6.3 years) were examined for the presence of gross and histologic lesions. Of these, 215 (65.0%) animals were experimentally infected with various strains of simian immunodeficiency virus (SIV), and 13 of the animals were infected with other agents including measles virus and lymphocryptovirus. Normal menses were noted in 16 cases, and one animal was recently pregnant (euthanized following dystocia). In addition, records from other species within the colony (common marmosets, cotton top tamarins, squirrel monkeys, long-tailed macaques, pig-tailed macaques) were surveyed for dysplastic/neoplastic lesions of the cervix suggestive of classic papillomavirus-induced lesions.
Incidence of Gross Lesions within the Lower Reproductive Tract of Female Rhesus Macaques
Gross abnormalities were present in 31 of the 331 (9.6%) rhesus cervicovaginal tissues examined in this study, with the remainder trimmed in as part of complete routine tissue collection. The most common gross findings observed at necropsy were abnormal vaginal discharge (16/31, 51.6%), proliferative/neoplastic lesions with mass effect (8/31, 25.8%), and the presence of wood shavings or foreign material in the vagina with or without associated hemorrhage (7/31, 22.5%).
Of cases diagnosed histologically as suppurative vaginitis/cervicitis, gross lesions were associated with 14/38 (36.8%) and included abnormal or abundant discharge with (4/38) or without (9/38) foreign material. In addition, three additional cases of grossly visible polyps were identified, one of which was associated with abnormal discharge. Vaginal discharge was more commonly observed in cases with suppurative cervicitis than in cases with no significant findings (0/165) or those with prominent lymphoid aggregates/lymphocytic cervicitis (2/89; p < .05 in both cases). In addition, active menses was present in five cases of suppurative cervicitis. Grossly visible mucosal abnormalities and/or discharge were also commonly noted in 6/12 (50.0%) of the cases with cysts within the vagina or cervical mucosa. All five cases of neoplasia/endometriosis were appreciated grossly.
Histologic Lesions in Cervicovaginal Tissues
The most frequent histologic observations compared to animals where no histopathologic findings were noted (Figure 1A) were prominent lymphocyte accumulation within the lamina propria, either in a nodular pattern or as a diffuse band of lymphocytes immediately subjacent to the epithelium, and/or lymphocyte infiltration between epithelial layers of the vaginal/ectocervical mucosa (89/331, 26.9%; Figure 1B). These observations were more common in SIV-infected animals (68/215, 31.6%) compared to uninfected animals or those infected with other agents (20/116, 17.2%; p = .01). In most cases, while these mucosal lymphocytic aggregates/infiltrates were noted in the histologic description, they were considered to be within the limits of variability expected for MALT undergoing normal nonspecific antigenic stimulation; However, 13 of the 89 were diagnosed with equivocal chronic, lymphocytic, lymphohistiocytic, or lymphoplasmacytic vaginitis/cervicitis. Luminal bacteria were observed histologically in 2 of the 13 cases within this subset, but no vaginal swabs from these cases were obtained for culture or further analysis.
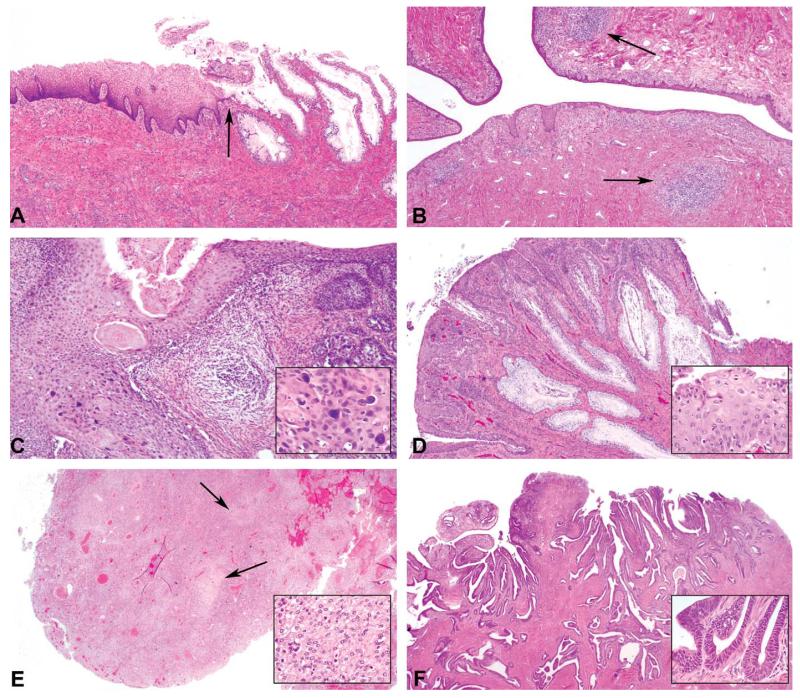
Figure 1.
Representative cervical histology, hematoxylin & eosin. (A) Case 12: Normal rhesus cervix, 4×. The junction between the endo- and ectocervix is indicated (arrow). (B) Case 13: Rhesus ectocervix containing prominent submucosal lymphoid aggregates (arrows), 4×. (C) Case 1: Cervical squamous metaplasia and dysplasia, 4×. Dysplastic keratinocytes are large and bizarre (inset). (D) Case 4: Suppurative cervicitis with dysplastic polyp, 4×. The polyp contains dysplastic, stratified epithelial cells (inset) supported by a fibrovascular core. Small aggregates of neutrophils are present in the cervical lumen. (E) Case 7: Anaplastic carcinoma, 2×. The poorly demarcated, unencapsulated neoplasm is comprised of sheets of pleiomorphic to polygonal cells (inset) with islands of necrosis and hemorrhage (arrows). (F) Case 8: Papillary carcinoma in a cotton top tamarin, 2×. Neoplastic epithelial cells (inset) are arranged in papillary fronds.
Suppurative cervicitis and/or vaginitis were observed in 38/331 cases (11.5%). These findings were characterized by degenerate and nondegenerate neutrophils within the vaginal lumen (frequently entrapped in luminal mucus) and infiltrating the mucosal layers with occasional ulceration (8/38, 21.0%), intralesional colonies of cocci or coccobacilli (7/38, 18.4%), an eosinophilic inflammatory component (4/38, 10.5%), intraepithelial or glandular microabscesses (4/38, 10.5%), squamous metaplasia (2/38, 5.3%), or stromal mineralization (1/38, 2.6%).
Additional histologic findings included cystic dilation of cervical glands (12/331, 3.6%) or vaginal mucosa (three cases with two visible grossly). Six cases of vascular lesions included arteritis (two cases), SIV-associated arteriopathy in experimentally infected cases (three cases), and atherosclerosis (one case). Epithelial lesions included squamous metaplasia and/or dysplasia of endocervical columnar epithelium in three cases (Figure 1C); abnormalities of the vaginal mucosal including dysplasia, hyperplasia, dyskeratosis, and ballooning degeneration (one case each); as well as other miscellaneous lesions in single cases (see Table 2). Seven cases of neoplasms or polyps included inflammatory polyps with suppurative (two cases; Figure 1D) or histiocytic (one case) infiltrate, two leiomyomas, one anaplastic carcinoma (Figure 1E), and one metastatic serosal granulosa cell tumor. One case of serosal endometriosis was identified.
A targeted search for proliferative cervical lesions in several other nonhuman primate species within the database identified one case of a cotton top tamarin with cervical papillary carcinoma (Figure 1F), and one case of adenomatous hyperplasia also in a cotton top tamarin (data not shown). Other findings in these species included a similar range of lesions as those found in rhesus macaques, with sporadic cases of prominent lymphoid aggregates and vaginitis/cervicitis most commonly identified. Suppurative cervicitis/vaginitis was more commonly found in common marmosets (9/36, 25%) than in rhesus macaques (p = .03), and included three cases with a prominent eosinophilic component to the infiltrate. For other lesions, sample sizes from these species were too small to allow for comparison of prevalence with rhesus macaques.
Evaluation of Leukocyte Infiltrates in Cervicovaginal Lesions
A subset of vaginal and cervical sections encompassing the spectrum of common findings with a focus on proliferative and dysplastic lesions was evaluated for inflammation and immune cell infiltration by immunohistochemical staining (See Table 3). The selected rhesus macaque cases included one case of cervical squamous metaplasia and dysplasia (Case 1), three cases of polypoid cervicitis (Cases 2–4), two cases of suppurative cervicitis/vaginitis with squamous metaplasia but without polyp formation (Cases 5 and 6), one anaplastic carcinoma (Case 7), as well as the cervical papillary carcinoma identified in a cotton top tamarin (Case 8). Four rhesus cervicovaginal sections with no significant findings (Cases 9–12) and one with prominent lymphoid aggregates (Case 13), considered within normal limits, were also included in the study set for comparison.
Lymphocytes were the most abundant immune cell subtype in both normal controls and cases with histopathologic lesions. CD20-positive B cells were seen scattered throughout the lamina propria of the endo- and ectocervix, with occasional loose lymphoid aggregates associated with MALT structures that were most frequently observed near the transitional zone of the ectocervix (Figure 2A). Mild to markedly increased numbers of B cells within lymphoid aggregates were seen in the lamina propria of the endo- and/or ectocervix of suppurative cervicitis cases (Cases 2–6), the case of squamous metaplasia with dysplasia (Case 1), and the normal case with prominent lymphoid aggregates noted on routine hematoxylin and eosin histopathology (Case 13; Figure 2B). In contrast, B cells were not frequently observed associated with either of the two neoplasias (Cases 7 and 8).
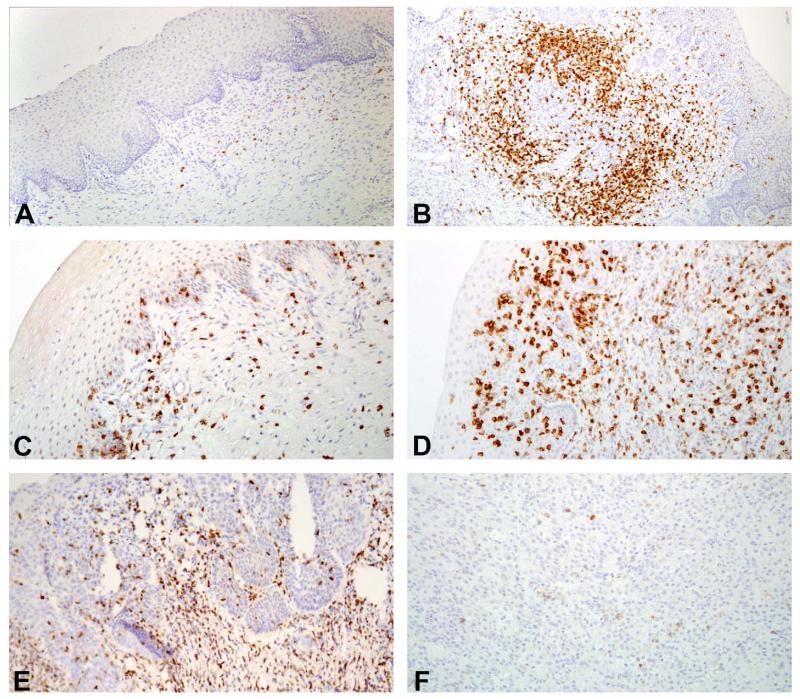
Figure 2.
Immunohistochemical characterization of lymphocytes within the lower female reproductive tract. (A) Normal animal, 10×. CD20+ B lymphocytes are sparsely distributed throughout the lamina propria of the lower female reproductive tract. (B) Case 13: Prominent lymphoid aggregates, 10×: Variable aggregates of CD20+ B cells are a common finding in both SIV-infected and uninfected animals. (C) Normal animal. 20×. CD3+ T lymphocytes are present within the epithelial layers as well as in the lamina propria in a band below the basal lamina and within lymphoid aggregates. (D) Case 13: Prominent lymphoid aggregates, 20×. Abundant T lymphocytes (CD8+ T cells shown) are present intermingled with B cells in lymphoid aggregates as well as diffusely scattered throughout the mucosa and submucosa. (E) Case 1: Cervical squamous metaplasia and dysplasia, 20×. Increased CD3+ T cells are present subjacent to areas with dysplastic epithelium. (F) Case 7: Anaplastic carcinoma, 20×. Occasional T cells, predominantly CD8+ T cells (shown) are scattered throughout the neoplasm.
CD3-positive T lymphocytes were abundant in a band on both sides of the normal endocervical and vaginal basal lamina, both as scattered isolated cells and within MALT aggregates (Case 1, Figure 2C). CD8-positive cytotoxic T cells were predominantly intraepithelial, consistent with previous reports (Pudney, Quayle, and Anderson 2005). In the endocervix, individual CD8 (and CD3) positive T cells were less numerous but still common immediately subjacent to the mucosa and in the deeper layers of the lamina propria and submucosa. Cases with prominent lymphocyte infiltrates contained a similar pattern of T cells as in control tissues, but in mild to markedly increased numbers (Figure 2D). The case with cervical squamous metaplasia and dysplasia (Case 1) contained large numbers of infiltrating T cells in the lamina propria and submucosa subjacent to the areas of ectocervical dysplasia (Figure 2E). Similar increases were not appreciated in the three cases of polyp formation (Case 2–4), and only mild subjective increases were seen in the lamina propria of uncomplicated cervicitis cases (Cases 5 and 6). Interestingly, CD3 and CD8-positive T cells were scattered in low numbers throughout the parenchyma of the anaplastic carcinoma (Case 7; Figure 2F) but were uncommon in the papillary carcinoma (Case 8).
As expected with suppurative cervicitis (Cases 2–6), abundant myeloperoxidase (MPO) positive neutrophils were commonly found entrapped within mucus in the cervical/vaginal lumen and variably infiltrated the superficial mucosa and lamina propria, with fewer numbers in the case of squamous metaplasia and dysplasia (Case 1, data not shown). Normal control tissues also contained scattered small aggregates of neutrophils in the cervical and vaginal lumen associated with cervical secretions or sloughed vaginal keratinocytes, and also had occasional MPO-positive cells in the endocervical lamina propria. In addition, microabscesses were identified within one polyp (Case 3), within an area of necrosis in the anaplastic carcinoma (Case 7), and neutrophils were also abundant adjacent to and infiltrating throughout the papillary carcinoma (Case 8, data not shown).
Human leukocyte antigen (HLA)-DR expression in control cases was primarily observed within scattered cells in the lamina propria morphologically consistent with activated lymphocytes, and within large cells with elongated processes within the epithelial layers morphologically consistent with resident Langerhans cells (Figure 3A). The highest concentrations in some normal sections occurred in the lamina propria and submucosa of the proximal vagina, associated with lymphoid aggregates (MALT). Numbers of HLA-DR-positive cells in the suppurative or polypoid cervicitis cases (Cases 2–6) were not appreciably globally increased over the range of variability seen in controls; however, there were mild multifocal aggregates within the endocervical lamina propria in two of these (Cases 4 and 5). In the case of squamous metaplasia and dysplasia (Case 1), HLA-DR expression was multifocally detected in hyperplastic and dysplastic keratinocytes (Figure 3B). Also multifocally throughout the carcinoma (Case 7), both individual cells and cell aggregates stained positively with HLA-DR (data not shown).
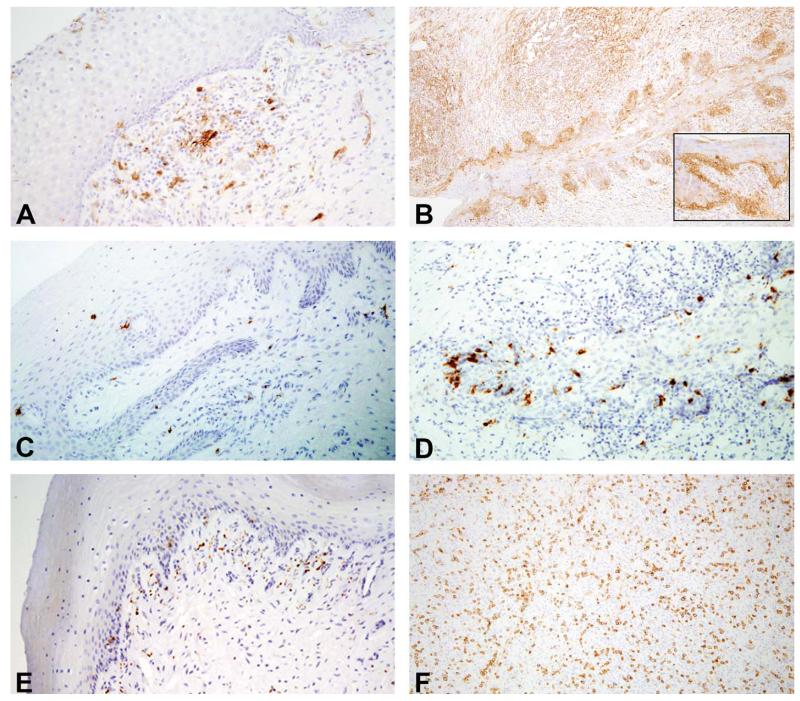
Figure 3.
Immunohistochemical characterization of immune cell components within the lower female reproductive tract. (A) Normal animal, 20×. HLA-DR+ antigen presenting cells are present within loose aggregates in the lamina propria and also scattered sparsely throughout the mucosal epithelial layers. (B) Case 1: Cervical squamous metaplasia and dysplasia, 10×. Hyperplastic and dysplastic keratinocytes display increased HLA-DR expression. (C) Normal animal, 20×. CD207+ Langerhans cells are scattered occasionally throughout as within the lamina propria well as the epithelial layers. (D) Case 1: Cervical squamous metaplasia and dysplasia, 20×. CD207+ langerhans cells are prominent within the areas of epithelial hyperplasia and dysplasia. (E) Normal animal, 20×. CD56 cells are common in the lamina propria of the vagina and ectocervix. (F) Case 7: Anaplastic carcinoma, 20×. CD56+ cells, likely natural killer cells, are abundant throughout the neoplasm.
CD207-positive Langerhans cells were sparsely distributed through the epithelial layers of the normal rhesus ectocervix and vagina, with occasional cells in the superficial lamina propria (Figure 3C). CD207-positive cells were generally absent from the normal endocervix but cells were abundant in areas adjacent to the dysplastic epithelium in Case 1 (Figure 3D). In all other cases of cervicitis and neoplasia, CD207-positive cells were occasionally observed subjacent to the endocervical mucosa, but otherwise the ectocervix and vagina within these sections had a similar pattern and number of positive-staining cells as control tissues.
Control tissues sections had highly variable numbers of individual CD56-positive cells scattered throughout the lamina propria of all portions of the lower female reproductive tract, with low to moderate numbers also in the basal epithelial layers of the ectocervix and vagina (Figure 3E). CD56 reactivity was present in a perivascular band at the level of blood vessel basal lamina, as well as around neurons and Schwann cells within peripheral nerves. All cases of cervicitis had CD56-positive cells present in numbers similar to that seen in control tissues. Notably, a large number of CD56-positive cells were scattered throughout the anaplastic carcinoma (Case 7; Figure 3F) in higher numbers than the CD3- or CD8-positive T cells (Figure 2F), suggesting that these cells are predominantly natural killer and not NKT cells.
Evaluation of Papillomavirus Infection in the Development of Proliferative Lesions
Cervicovaginal tissues from the subset of 13 representative cases listed above were also examined for the presence of abnormal epithelial proliferation by Ki-67 immunohistochemistry. In control cases, Ki-67 expression was limited to the basal epithelial layers of the ectocervix and reserve cells beneath the columnar epithelium of the endocervix (Figure 4A). In cases of dysplastic epithelium with or without polyp formation (Cases 1 and 4), Ki-67 expression in keratinocytes was expanded up to the entire thickness of the epithelial layers (Figure 4B). The two other polyps and non-polypoid cervicitis cases (Cases 2, 3, 5, and 6) contained small multifocal areas of active cellular proliferation within regions of squamous metaplasia (data not shown). The two neoplasms contained only occasional isolated Ki-67 expressing cells (Figure 4C).
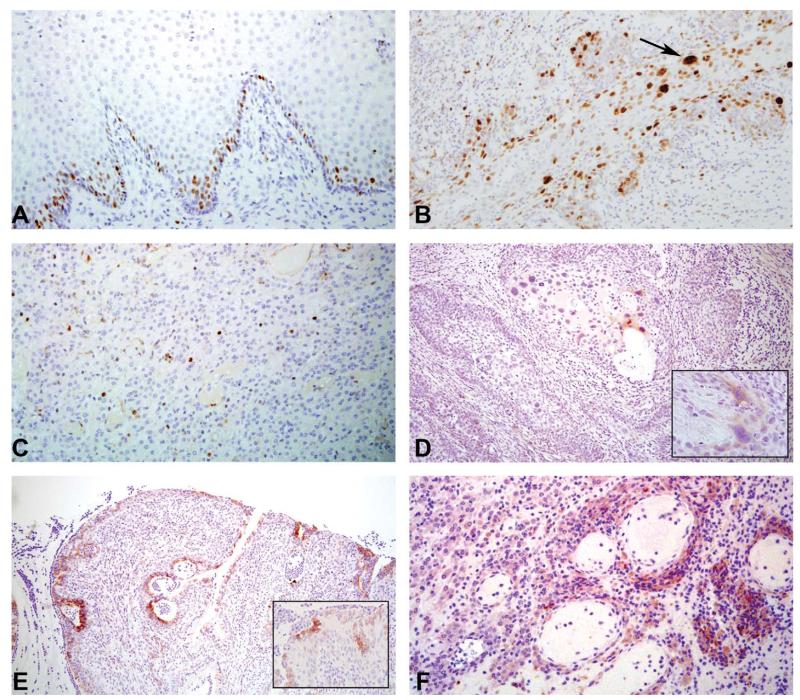
Figure 4.
Immunohistochemical detection of cellular proliferation (Ki-67) (A–C), and papillomavirus antigen (HPV E6) (D–F) in cervicovaginal tissues. (A) Normal animal, 20×. Ki-67+ proliferating cells are normally restricted to the basal cell layers of the ectocervix and vagina, as well as reserve cells beneath the endocervical mucosa (not shown). (B) Case 1: Cervical squamous metaplasia and dysplasia, 20×. Expanded cellular proliferation is seen throughout the epithelial layers. Arrow indicates large, bizarre nucleii of dysplastic keratinocytes. (C) Case 7: Anaplastic carcinoma, 20×. Occasional Ki-67+ proliferating cells are observed within the neoplasm. (D) Case 1: Cervical squamous metaplasia and dysplasia, 10×. Papillomavirus E6 oncoprotein is identified within the cytoplasm and nucleus of dysplastic keratinocytes. (E) Case 4: Suppurative cervicitis with dysplastic polyp, 10×. Papillomavirus E6 oncoprotein expressed within dysplastic epithelium of polyp. (F) Case 7: Anaplastic carcinoma, 20×. E6-positive cells are found in aggregates within the neoplasm. E6+ cells were also detected in the papillary carcinoma identified in a cotton top tamarin (data not shown).
The contribution of papillomavirus infection to the development of dysplastic and proliferative lesions was examined by immunohistochemical staining for the papillomaviral E6 protein expression and serology. Several of the proliferative/dysplastic lesions, specifically the case of cervical epithelial dysplasia (Case 1), dysplastic polyp (Case 4), and both cases of epithelial neoplasia (Cases 7 and 8) stained positively for E6 antigen (Figure 4D–F). In total, 4 of the 6 (66.6%) cases with proliferative cervicovaginal lesions expressed papillomavirus E6 protein compared to none of the remaining 7 cases.
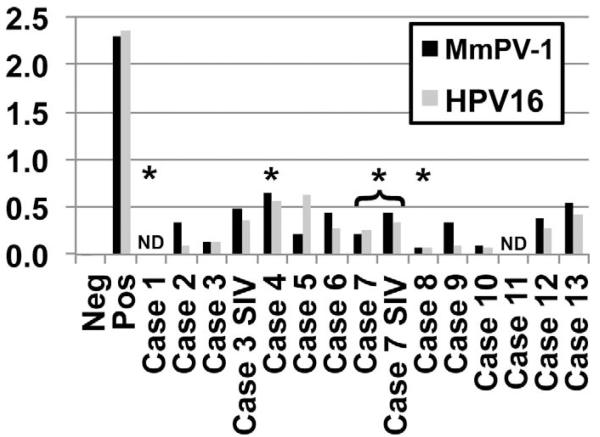
Eleven of the 13 cases (those where serum was available) were examined serologically by ELISA for reactivity against rhesus papillomavirus and human papillomavirus 16 virus-like particles (Figure 5). Surprisingly, titers in all cases were low and were comparable between those cases with positive papillomavirus E6 antigen staining in the cervicovaginal mucosa (Cases 3, 4, 7, 8) and controls (Cases 9–12, p > .05). Interestingly, titers increased slightly but significantly in the two experimentally SIV infected animals (Cases 3 and 7) compared to pre-inoculation levels (p = .03). There was a trend toward higher titers with increasing age, although it did not reach significance with the number of sera available for analysis (data not shown).
Figure 5.
Papillomavirus serologic screening by ELISA of the 13 cases examined within this study. Titers against both rhesus papillomavirus 1 (MmPV-1, black bars) and human papillomavirus 16 (HPV 16, gray bars) were low in all cases and were comparable to controls (Cases 9–13). Paired pre- and postinfection sera from experimentally infected SIV+ animals (Cases 3 and 7) are shown. Asterisks indicate animals with positive papillomavirus E6 antigen staining by immunohistochemistry. ND: not determined; serum unavailable for analysis. Controls include a nonspecific mouse IgG (negative) and an HPV 16 L1 anti-capsid antibody (positive).
Discussion
The most common spontaneous background histologic findings in this study were vaginitis and cervicitis, with gross evidence of inflammation or discharge present in less than 40% of these cases. The most common immune cell infiltrates included accumulations of lymphocytes in the submucosa, lamina propria, and/or epithelium of the cervix and vagina, and neutrophils in the cervical and vaginal lumen with variable mucosal infiltration. However, variable numbers of all immune cell subsets are found within normal cycling reproductive tracts, and the threshold between normal and inflamed is often subjective (Cline et al. 2008). For example, wide variation between even normal individuals and between different regions of the reproductive tract within the same animal is reported for several immune cell types including natural killer cells, lymphocytes, and dendritic cells, which could potentially affect the final histologic diagnoses in animals used for experimental purposes (Ma et al. 2001; Hickey et al. 2011). In this study, the presence of prominent lymphoid aggregates was more frequently observed in cases with SIV infection, likely as part of systemic lymphoproliferative dysfunction, but similar large lymphoid aggregates were also occasionally observed in control animals. In lieu of identifying an infectious agent or physical irritant such as vaginal foreign body in SIV-negative animals, this finding must simply be considered part of a localized or systemic response to unidentified antigen stimulation. In addition, squamous metaplasia of cervical glands is considered an incidental finding that may or may not be related to other concurrent pathologies (Wood 2008). However, given that the majority (61.6%) of cases with histologic evidence of inflammation did not have any corresponding gross lesions, these background lesions have the potential to confound infection challenge or toxicology studies, for example, where part of the data collection would include looking for cytotoxic responses to treatments or an immune cell response to experimental infection. Physical examination prior to study initiation is recommended to rule out grossly visible evidence of inflammation such as vaginal discharge, inflammatory polyps, and intravaginal foreign bodies.
Roles of Immune Cell Subsets in the Lower Female Reproductive Tract
While the number of B and T lymphocytes and Langerhans cells present in the rhesus reproductive tract has been reported to remain constant throughout the ovarian follicular cycle, these studies confirm previous reports that their distribution varies between animals by factors such as age and exposure to antigen stimulation during breeding, and within a single animal by specific anatomic location along the lower reproductive tract (Stevceva et al. 2002; Ma et al. 2001; Di Fabio et al. 2004; Parr et al. 1991; Sharkey et al. 2012). B cells are found most commonly within lymphoid aggregates in the submucosa, especially near the transformation zone, while T cells are most abundantly distributed in a band on either side of the epithelial basal lamina including cells infiltrating the epithelial layers. CD8 T cells are reportedly more common than CD4 T cells in the female reproductive tract, in ratios reported from 1.1:1 to nearly 2:1, with the majority of intraepithelial T cells displaying CD8 positivity (Ma et al. 2001; Pudney, Quayle, and Anderson 2005). Furthermore, in HIV patients, the numbers of CD8 T cells are increased, and they may form aggregates not seen in uninfected patients (Stevceva et al. 2002). However, in one report of HIV-positive patients, T cell cytotoxic function was impaired, and thus control of HPV and other infections of the female genital tract were poorly controlled despite increased numbers of infiltrating immune cells (Kobayashi et al. 2004). In addition, lymphocyte functionality varies throughout the menstrual cycle. B cells have been reported to increase local and systemic secretion of antibodies in the periovulatory period, likely resulting from the effects of low progesterone on T cell function and not altered lymphocyte recruitment to mucosal surfaces (Lu et al. 2002).
HLA-DR is a major histocompatibility complex type II antigen found on antigen presenting cells such as intraepithelial Langerhans cells, B cells, activated T cells, and monocytes. Thus, the number of mucosal HLA-DR-positive cells would be expected to increase with local inflammation and white blood cell infiltration, as reflected by the multifocal aggregates of HLA-DR-positive cells seen in 2 cases in the current study. However, the number of these cells was widely variable even in control animals, likely due to the dynamic spatiotemporal nature of ongoing antigenic stimulation, for example, anatomic or behavioral differences between animals, and the flux of some antigen presenting cell types that migrate between the mucosa and draining lymph nodes. In addition, while human endocervical glandular epithelium constitutively expresses HLA-DR, ectocervical and vaginal keratinocytes express HLA-DR only in response to IFNγ production, for example, with papillomavirus infection as seen in this and other studies (Bjercke et al. 1983; Raju, Teh, and Wee 1994; Fais et al. 1991; Poppe et al. 1998). Interestingly, normal rhesus endocervical epithelium did not stain with HLA-DR, either due to species-specific variation in expression level or reactivity with the specific antibody used.
CD207 is a surface marker specific to Langerhans cells, an important surveillance and antigen presenting dendritic cell type in mucosal surfaces and often considered the first line in recognition of foreign antigens (Hu, Gardner, and Miller 2000). Within the female reproductive tract, the highest concentrations of dendritic cells are found in the basal layers of ectocervical epithelium and at the transformation zone (Poppe et al. 1998; Pudney, Quayle, and Anderson 2005; Parr et al. 1991). With disease states such as papillomavirus-induced cervical intraepithelial neoplasia (CIN) lesions, their numbers have been reported to locally increase in areas of dysplasia but decrease in adjacent areas due to cellular migration, and this pattern was observed in the case of squamous metaplasia and dysplasia examined here (Case 1; Campaner et al. 2007). With progression to cervical cancer, their overall numbers within the reproductive tract may also decrease, and consistent with these reports, essentially no CD207-positive cells were seen associated with the neoplasias in Cases 7 and 8 (Giannini et al. 2002; Poppe et al. 1998; Drijkoningen et al. 1988). Reported numbers of intraepithelial dendritic cells in cases of vaginitis vary from case to case, based on competing factors of increased recruitment to the site of inflammation versus the rapid migration to lymphoid tissues which occurs following antigen recognition (Pudney, Quayle, and Anderson 2005; Stockwin et al. 2000).
CD56 stains a relatively diverse range of tissue types, and within the immune system is a marker of natural killer cells and a subset of activated CD4 or CD8 T cells, known as NKT cells. Within the normal lower female reproductive tract, these cells are generally sparse in uninflamed ecto- and endocervical tissues, do not change with menstrual stage (unlike in the endometrium), decrease in overall number in older animals, and may express a different complement of surface markers than in the endometrium and peripheral blood (Mselle et al. 2007; Pudney, Quayle, and Anderson 2005). Thus, natural killer cell infiltration as detected by different immunohistochemical stains may vary between animals. For example, in one study, NDG2D-positive natural killer cells were decreased with HPV-induced neoplasia and CIN, while in another the number of NK cells as marked by HNK-1 was unchanged in these types of lesions (Syrjanen et al. 1986; Patel and Chiplunkar 2009). In the current study, CD56-positive cells were abundant in the anaplastic carcinoma, consistent with a reported role for NK cells in the immune response to neoplastic cells (Zamai et al. 2007). In other cases within the study set, the number and distribution of NK cells as examined by CD56 staining were not substantially different than in control tissues.
Papillomavirus Contribution to Spontaneous Cervical Lesions in Rhesus Macaques
In the current study, several proliferative epithelial lesions including mucosal squamous metaplasia with dysplasia characterized by nuclear atypia (Case 1), a polyp with dysplastic stratified squamous epithelium (Case 4), and two cases of cervical neoplasia (Cases 7 and 8) were identified by expanded Ki-67 staining. Ki-67 is a marker of proliferating cells that is normally restricted to the basal cell layer of the stratified squamous vaginal and ectocervical mucosa, and to reserve stem cells located beneath the columnar epithelium of the endocervix, that maintain normal epithelial turnover. Two of the three cervical polyps were lined by abundant and convoluted but normal appearing columnar epithelium (Cases 2 and 3), but had mildly increased Ki-67 staining in small multifocal areas of squamous metaplasia. Similar areas were identified multifocally in non-polypoid cases of suppurative cervicitis (Cases 5 and 6).
Papillomaviruses cause spontaneous, experimentally reproducible infections in rhesus macaques leading to cervical dysplasia and neoplasia, similar to the progression of CIN lesions seen in humans (Wood et al. 2007; Ostrow et al. 1990). Many nonhuman primates, including rhesus and cynomolgus macaques, have their own relatively species-specific papillomavirus types (Chen et al. 2009). These infections can affect experimental outcomes, as in one study of baboons where spontaneous infection by a previously unidentified circulating strain precluded evaluation of experimental chlamydia infection (Bergin et al. 2012). Within the current study, positive immunohistochemistry for papillomavirus E6 viral oncoprotein implicated spontaneous infection in several cases of proliferative and dysplastic lesions. Both cervical carcinomas had E6 reactivity that, given the slow progression to tumorigenesis in humans and other species, likely indicates long-standing infection. Similarly, the positive staining in the cases of polypoid cervicitis and epithelial dysplasia likely indicates earlier stages in the potential progression to cancer mediated by papillomaviral infection.
Immunohistochemistry for the E6 protein alone may underestimate the prevalence of papillomaviruses within the colony because in the early stages of infection the expression level of this protein is tightly maintained at low levels by other viral regulatory proteins. Dysregulation of E6 expression, as well as of another major papillomavirus oncoprotein, E7, often accompanies viral integration into the host chromosome and is required for the development of CIN lesions and neoplasia (Ostrow et al. 1993). Thus, other detection methods for subclinical/early cases, in particular, should be considered. Serology is a relatively insensitive method for detecting infection as seroconversion may not occur until months after exposure, if at all (Edelstein et al. 2011). Other methods of detection include polymerase chain reaction (PCR) amplification of papillomavirus DNA and immunohistochemistry for the cellular p16 protein that increases with papillomaviral interference with the retinoblastoma pathway (Reuschenbach et al. 2010). PCR using conserved primers amplifying part of the capsid protein L1 was attempted on DNA extracts from the formalin-fixed tissues within the case series, but was unsuccessful at amplifying a product, likely due to the poor quality of DNA often extracted from these samples, or possibly due to sequence differences from the type species used to design the primer set.
Significance
The results of this retrospective study are of relevance to the increasing number of studies using nonhuman primates, particularly rhesus macaques, as experimental models for reproductive diseases. Knowledge of common background lesions and the spectrum of individual variation in anatomic and immunologic features is essential for accurate interpretation of experimental outcomes. In studies where the local immune response is of key importance or where concurrent spontaneous infections may preclude complete data analysis, thorough prescreening of animals should be undertaken. This screening could include one or more of the following: recording and/or synchronizing the stage of reproductive cycle, serology testing for various infectious agents, gynecologic examination, cytologic evaluation of cervicovaginal cells and/or PCR amplification of papillomavirus DNA from these samples, and evaluation of vaginal secretions for the presence of inflammatory cells, flora overgrowth, or variations in pH.
In addition, variable leukocyte infiltrates were present within the inflammatory and proliferative lesions identified in this study. However, in many cases, these infiltrates were also seen in normal control tissues. The diversity of mucosal immune response of the lower female reproductive tract is incompletely understood, and characterizing the quantity and quality of the immune cell responses to different pathogens is an important area of ongoing study.
Acknowledgments
The authors would like to thank Nilsa Silva and Karen Boisvert for histology support, Ronald Desrosiers for reagents, and Elizabeth Curran, Andrew Miller, and previous NEPRC pathologists for tissue collection and contributing to the NEPRC tissue archives.
The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the NEPRC Base grant (8P51OD011103-51) and the T32 Training Grant (RR0070000-36).
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- CIN
cervical intraepithelial neoplasia
- DAB
diaminobenzidine
- ELISA
enzyme-linked immunosorbent assay
- MPO
myeloperoxidase
- NBF
neutral buffered formalin
- NEPRC
New England Primate Research Center
- SIV
simian immunodeficiency virus
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Ambrose Z, KewalRamani VN, Bieniasz PD, Hatziioannou T. HIV/AIDS: In search of an animal model. Trends Biotechnol. 2007;25:333–37. doi: 10.1016/j.tibtech.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Baroncelli S, Negri DR, Michelini Z, Cara A. Macaca mulatta, fascicularis and nemestrina in AIDS vaccine development. Expert Rev Vaccines. 2008;7:1419–34. doi: 10.1586/14760584.7.9.1419. [DOI] [PubMed] [Google Scholar]
- Bennett MW, Dick EJ, Jr., Schlabritz-Loutsevitch NE, Lopez-Alvarenga JC, Williams PC, Mark Sharp R, Hubbard GB. Endometrial and cervical polyps in 22 baboons (Papio sp.), 5 cynomolgus macaques (Macaca fascicularis) and one marmoset (Callithrix jacchus) J Med Primatol. 2009;38:257–62. doi: 10.1111/j.1600-0684.2009.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergin IL, Bell JD, Chen Z, Zochowski MK, Chai D, Schmidt K, Culmer DL, Aronoff DM, Patton DL, Mwenda JM, Wood CE, Burk RD. Novel genital alphapapillomaviruses in baboons (Papio hamadryas Anubis) with cervical dysplasia. Vet Pathol. 2013;50:200–8. doi: 10.1177/0300985812439725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjercke S, Scott H, Braathen LR, Thorsby E. HLA-DR-expressing Langerhans’-like cells in vaginal and cervical epithelium. Acta Obstet Gynecol Scand. 1983;62:585–89. doi: 10.3109/00016348309156253. [DOI] [PubMed] [Google Scholar]
- Campaner AB, Nadais RF, Galvao MA, Santos RE, Aoki T. Evaluation of density of Langerhans cells in human cervical intraepithelial neoplasia. Acta Obstet Gynecol Scand. 2007;86:361–66. doi: 10.1080/00016340601133871. [DOI] [PubMed] [Google Scholar]
- Chen Z, van Doorslaer K, DeSalle R, Wood CE, Kaplan JR, Wagner JD, Burk RD. Genomic diversity and interspecies host infection of alpha12 Macaca fascicularis papillomaviruses (MfPVs) Virology. 2009;393:304–10. doi: 10.1016/j.virol.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline JM, Wood CE, Vidal JD, Tarara RP, Buse E, Weinbauer GF, de Rijk EP, van Esch E. Selected background findings and interpretation of common lesions in the female reproductive system in macaques. Toxicol Pathol. 2008;36:142s–63s. doi: 10.1177/0192623308327117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fabio S, Corrias F, Monardo F, Titti F. Flow cytometry analysis of immune cell populations isolated from cervicovaginal secretions of cynomolgus monkeys. J Immunol Methods. 2004;284:7–14. doi: 10.1016/j.jim.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Drijkoningen M, De Wolf-Peeters C, Degreef H, Desmet V. Epidermal Langerhans cells, dermal dendritic cells, and keratinocytes in viral lesions of skin and mucous membranes: An immunohistochemical study. Arch Dermatol Res. 1988;280:220–27. doi: 10.1007/BF00513961. [DOI] [PubMed] [Google Scholar]
- Edelstein ZR, Carter JJ, Garg R, Winer RL, Feng Q, Galloway DA, Koutsky LA. Serum antibody response following genital {alpha}9 human papillomavirus infection in young men. J Infect Dis. 2011;204:209–16. doi: 10.1093/infdis/jir242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JN, Morris HB. Langerhans’ cells and lymphocyte subsets in the female genital tract. Br J Obstet Gynaecol. 1985;92:974–82. doi: 10.1111/j.1471-0528.1985.tb03080.x. [DOI] [PubMed] [Google Scholar]
- Fais S, Delle Fratte F, Mancini F, Cioni V, Guadagno M, Vetrano G, Pallone F. Human cervical epithelial cells that express HLA-DR associated with viral infection and activated mononuclear cell infiltrate. J Clin Pathol. 1991;44:290–92. doi: 10.1136/jcp.44.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini SL, Hubert P, Doyen J, Boniver J, Delvenne P. Influence of the mucosal epithelium microenvironment on Langerhans cells: Implications for the development of squamous intraepithelial lesions of the cervix. Int J Cancer. 2002;97:654–59. doi: 10.1002/ijc.10084. [DOI] [PubMed] [Google Scholar]
- Hickey DK, Patel MV, Fahey JV, Wira CR. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: Stratification and integration of immune protection against the transmission of sexually transmitted infections. J Reprod Immunol. 2011;88:185–94. doi: 10.1016/j.jri.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes KK, Sparling P, Mardh P, Lemon SM, Stamm WE, Piot P, Wasserheit JN. Sexually Transmitted Diseases. McGraw-Hill; New York, NY: 1999. [Google Scholar]
- Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087–95. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Greenblatt RM, Anastos K, Minkoff H, Massad LS, Young M, Levine AM, Darragh TM, Weinberg V, Smith-McCune KK. Functional attributes of mucosal immunity in cervical intraepithelial neoplasia and effects of HIV infection. Cancer Res. 2004;64:6766–74. doi: 10.1158/0008-5472.CAN-04-1091. [DOI] [PubMed] [Google Scholar]
- Levy J. HIV and the Pathogenesis of AIDS. ASM Press; Herndon, VA: 2007. [Google Scholar]
- Lu FX, Abel K, Ma Z, Rourke T, Lu D, Torten J, McChesney M, Miller CJ. The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin Exp Immunol. 2002;128:10–20. doi: 10.1046/j.1365-2249.2002.01780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Lu FX, Torten M, Miller CJ. The number and distribution of immune cells in the cervicovaginal mucosa remain constant throughout the menstrual cycle of rhesus macaques. Clin Immunol. 2001;100:240–49. doi: 10.1006/clim.2001.5058. [DOI] [PubMed] [Google Scholar]
- Martin DH. The microbiota of the vagina and its influence on women’s health and disease. Am J Med Sci. 2012;343:2–9. doi: 10.1097/MAJ.0b013e31823ea228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain KL, Leach CT, Jenson HB, Joshi VV, Pollock BH, Parmley RT, DiCarlo FJ, Chadwick EG, Murphy SB. Association of Epstein-Barr virus with leiomyosarcomas in children with AIDS. N Engl J Med. 1995;332:12–18. doi: 10.1056/NEJM199501053320103. [DOI] [PubMed] [Google Scholar]
- Mselle TF, Meadows SK, Eriksson M, Smith JM, Shen L, Wira CR, Sentman CL. Unique characteristics of NK cells throughout the human female reproductive tract. Clin Immunol. 2007;124:69–76. doi: 10.1016/j.clim.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Oakley BB, Fiedler TL, Marrazzo JM, Fredricks DN. Diversity of human vaginal bacterial communities and associations with clinically defined bacterial vaginosis. Appl Environ Microbiol. 2008;74:4898–909. doi: 10.1128/AEM.02884-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrow RS, Liu Z, Schneider JF, McGlennen RC, Forslund K, Faras AJ. The products of the E5, E6, or E7 open reading frames of RhPV 1 can individually transform NIH 3T3 cells or in cotransfections with activated ras can transform primary rodent epithelial cells. Virology. 1993;196:861–67. doi: 10.1006/viro.1993.1547. [DOI] [PubMed] [Google Scholar]
- Ostrow RS, McGlennen RC, Shaver MK, Kloster BE, Houser D, Faras AJ. A rhesus monkey model for sexual transmission of a papillomavirus isolated from a squamous cell carcinoma. Proc Natl Acad Sci USA. 1990;87:8170–74. doi: 10.1073/pnas.87.20.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr EL, Parr MB, Zheng LM, Young JD. Mouse granulated metrial gland cells originate by local activation of uterine natural killer lymphocytes. Biol Reprod. 1991;44:834–41. doi: 10.1095/biolreprod44.5.834. [DOI] [PubMed] [Google Scholar]
- Patel S, Chiplunkar S. Host immune responses to cervical cancer. Curr Opin Obstet Gynecol. 2009;21:54–59. doi: 10.1097/GCO.0b013e32831a9890. [DOI] [PubMed] [Google Scholar]
- Poonia B, Walter L, Dufour J, Harrison R, Marx PA, Veazey RS. Cyclic changes in the vaginal epithelium of normal rhesus macaques. J Endocrinol. 2006;190:829–35. doi: 10.1677/joe.1.06873. [DOI] [PubMed] [Google Scholar]
- Poppe WA, Drijkoningen M, Ide PS, Lauweryns JM, Van Assche FA. Lymphocytes and dendritic cells in the normal uterine cervix. An immunohistochemical study. Eur J Obstet Gynecol Reprod Biol. 1998;81:277–82. doi: 10.1016/s0301-2115(98)00202-4. [DOI] [PubMed] [Google Scholar]
- Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: Mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253–63. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- Raju GC, Teh M, Wee A. The expression of HLA-DR antigen in cervical neoplasia. Cancer Detect Prev. 1994;18:367–73. [PubMed] [Google Scholar]
- Reuschenbach M, Clad A, von Knebel Doeberitz C, Wentzensen N, Rahmsdorf J, Schaffrath F, Griesser H, Freudenberg N, von Knebel Doeberitz M. Performance of p16INK4a-cytology, HPV mRNA, and HPV DNA testing to identify high grade cervical dysplasia in women with abnormal screening results. Gynecol Oncol. 2010;119:98–105. doi: 10.1016/j.ygyno.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Roberts JN, Kines RC, Katki HA, Lowy DR, Schiller JT. Effect of Pap smear collection and carrageenan on cervicovaginal human papillomavirus-16 infection in a rhesus macaque model. J Natl Cancer Inst. 2011;103:737–43. doi: 10.1093/jnci/djr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188:2445–54. doi: 10.4049/jimmunol.1102736. [DOI] [PubMed] [Google Scholar]
- Sonoda Y, Mukaida N, Wang JB, Shimada-Hiratsuka M, Naito M, Kasahara T, Harada A, Inoue M, Matsushima K. Physiologic regulation of postovulatory neutrophil migration into vagina in mice by a C-X-C chemokine(s) J Immunol. 1998;160:6159–65. [PubMed] [Google Scholar]
- Stevceva L, Kelsall B, Nacsa J, Moniuszko M, Hel Z, Tryniszewska E, Franchini G. Cervicovaginal lamina propria lymphocytes: Phenotypic characterization and their importance in cytotoxic T-lymphocyte responses to simian immunodeficiency virus SIVmac251. J Virol. 2002;76:9–18. doi: 10.1128/JVI.76.1.9-18.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwin LH, McGonagle D, Martin IG, Blair GE. Dendritic cells: Immunological sentinels with a central role in health and disease. Immunol Cell Biol. 2000;78:91–102. doi: 10.1046/j.1440-1711.2000.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrjanen K, Vayrynen M, Mantyjarvi R, Castren O, Saarikoski S. Natural killer (NK) cells with HNK-1 phenotype in the cervical biopsies of women followed-up for human papillomavirus (HPV) lesions. Acta Obstet Gynecol Scand. 1986;65:139–45. doi: 10.3109/00016348609158369. [DOI] [PubMed] [Google Scholar]
- Van Esch E, Cline JM, Buse E, Wood CE, de Rijk EPCT, Weinbauer GF. Summary comparison of female reproductive system in human and the cynomolgus monkey (Macaca fascicularis) Toxicol Pathol. 2008;36:171S–72S. [Google Scholar]
- Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: Cellular responses and interactions. Immunol Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- Wood CE. Morphologic and immunohistochemical features of the cynomolgus macaque cervix. Toxicol Pathol. 2008;36:119S–29S. [Google Scholar]
- Wood CE, Chen Z, Cline JM, Miller BE, Burk RD. Characterization and experimental transmission of an oncogenic papillomavirus in female macaques. J Virol. 2007;81:6339–345. doi: 10.1128/JVI.00233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamai L, Ponti C, Mirandola P, Gobbi G, Papa S, Galeotti L, Cocco L, Vitale M. NK cells and cancer. J Immunol. 2007;178:4011–16. doi: 10.4049/jimmunol.178.7.4011. [DOI] [PubMed] [Google Scholar]