Abstract
Oxidative stress has been linked to the pathogenesis of diabetic nephropathy, the complication of diabetes in the kidney. NADPH oxidases of the Nox family are a major source of reactive oxygen species in the diabetic kidney and are critical mediators of redox signaling in glomerular and tubulointerstitial cells exposed to the diabetic milieu. Here, we present an overview of the current knowledge related the understanding of the role of Nox catalytic and regulatory subunits in the processes that control mesangial cell, podocyte and tubulointerstitial cell injury induced by hyperglycemia and other predominant factors enhanced in the diabetic milieu, including the renin-angiotensin system and transforming growth factor-ß. The role of the Nox isoform, Nox4, in the redox processes that alter renal biology in diabetes will be highlighted.
Keywords: Diabetic complications, Diabetic nephropathy, Hyperglycemia, Oxidative stress, NADPH oxidases of the Nox family, Nox4, Reactive oxygen species, Glomerular cell injury, Tubulointerstitial cell injury
Introduction
Diabetic nephropathy (DN) is a major microvascular complication of type 1 or type 2 diabetes and the most common cause of end-stage renal disease affecting approximately 20 to 40 % of diabetic patients [1]. Oxidative stress has emerged as a critical pathogenic factor in the initiation and development of diabetic complications, including DN [2-10]. Diabetes is accompanied by increased generation of reactive oxygen species (ROS) in the kidney [3, 6, 8, 10-14]. A deleterious role of ROS in the diabetic kidney is suggested by the findings that antioxidants are relatively effective to prevent glomerular and tubular alterations in experimental animal models of diabetes [10, 13, 15-24]. A number of redox sensitive mechanisms orchestrate key events of DN such as glomerular and tubular hypertrophy, mesangial cell injury, extracellular matrix accumulation, thickening of glomerular or tubular basement membranes as well as podocyte dysfunction leading ultimately to proteinuria, glomerulosclerosis and tubulointerstitial fibrosis [6, 8, 10-13, 15-17, 19, 20, 24-31]. While chronic hyperglycemia alone may be sufficient to trigger renal pathological response, data from animal models as well as cultured cells indicate that a combination of growth factors, hormones and cytokines, in addition to glucose, act on renal cells to generate ROS that induce and maintain tissue or cell injury in the diabetic kidney [6, 8, 11, 12, 32-34]. The ROS that can mediate renal injury during diabetes include superoxide anion (O2-•), hydrogen peroxide (H2O2), hydroxyl radical (OH.) and peroxynitrite (ONOO-) [8, 12, 35, 36]. Under physiological conditions, ROS mediate normal cellular functions. Tissue and cell damage occur once pathological circumstances such as prolonged exposure to glucose cause an elevation in ROS levels and a reduction of antioxidants production [8, 10]. Although multiple sources of ROS exist in cells and tissues such as xanthine oxidase or uncoupled nitric oxide synthase, the primary sources of ROS in renal cells and the diabetic kidney appear to be the mitochondrial electron transport chain [27, 37-41] and the NADPH oxidase of the Nox family [26, 42-46]. Blockade of mitochondrial- or Nox oxidase-derived ROS generation both ameliorate diabetes-induced renal cell injury [25-28, 31, 41, 47-49]. There is evidence of crosstalk between these sources of ROS during the pathogenesis of various human diseases. The present review will focus on the role of the NADPH oxidases in the pathogenesis of DN with emphasis on a member of the Nox family, Nox4.
NADPH oxidases of the Nox family as a source of ROS in the renal system
Nox oxidases predominantly expressed in the renal system
Early studies of NADPH oxidases were performed in neutrophils and phagocytic cells to investigate the respiratory burst NADPH oxidase system [50]. This enzyme catalyzes the NADPH-dependent reduction of molecular oxygen to generate superoxide anion which then dismutates to hydrogen peroxide [50, 51]. The phagocyte oxidase consists of two plasma membrane-associated proteins, Nox2 (a.k.a. gp91phox) (the catalytic subunit) and p22phox, as well as regulatory factors, p47phox, p67phox, p40phox, and the small GTPase Rac. Homologs of Nox2 have been found in all vascular and renal cells [43, 44, 52-57]. To date, the Nox family comprises seven members: Nox1-5, and the dual oxidases, Duox1 and -2 [43, 46, 58, 59]. Nox1, Nox2, and Nox4 are the NADPH oxidase isoforms predominantly expressed in the renal system (Figure 1) [42-44, 52-57, 60, 61]. Importantly, the calcium-dependent isoform Nox5 is also found in the human vasculature and kidney; however, the fact that the enzyme is not present in rodents has rendered the investigation of its role in renal pathologies more difficult [53, 56, 62]. Additionally, no data related to Nox3 or Duox1/2 expression in the kidney are yet available.
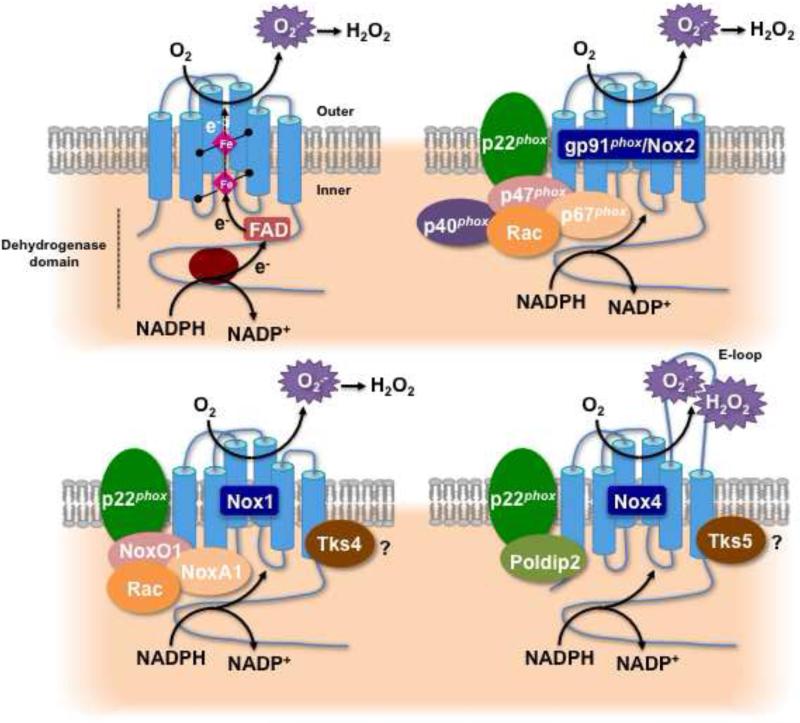
Figure 1.
Structure and molecular organization of the renal NADPH oxidases of the Nox family. The top right left panel illustrates the topology of and the enzymatic reaction catalyzed by the Nox enzymes. The other panels represent the molecular structure of the Nox oxidase isoforms predominantly expressed in the kidney, Nox2 (a.k.a. gp91phox), Nox1, and Nox4. The regulatory subunits differ from a Nox isoform to another.
Although the mechanisms by which the activity of the Nox enzymes is regulated in cardiovascular or renal cells is not fully understood, a number of regulatory subunits have been identified. The isoforms Nox2 and Nox1 require p22phox as an activating, stabilizing and/or regulatory subunit for binding to p47phox or NoxO1 [43, 58, 59]. Activation mechanisms for Nox1 are similar to those of Nox2, and involve complex formation with regulatory subunits upon agonist stimulation. Although Nox1 seems to primarily interact with the p47phox homolog NoxO1 (Nox organizer 1), the p67phox homolog NoxA1 (Nox activator 1), and Rac upon activation, it was reported that p47phox and p67phox can partially replace NoxO1 and NoxA1, respectively [43, 46, 52, 53, 56, 58, 59] (Figure 1). In contrast, the activity of Nox5 is not controlled by any other subunits and is strictly dependent on calcium binding to the enzyme through its EF hand domains [43].
Idiosyncrasy of the isoform Nox4
The most abundant Nox isoform in the renal system is Nox4 [43, 44, 57, 58]. The isoform Nox4 was cloned from the kidney and is highly expressed in renal tubules, renal fibroblasts, glomerular mesangial cells, and podocytes [26, 61, 63-68]. Before examining what is known about the role of Nox4 in renal cell injury, it is important to consider the unique properties of the oxidase.
Nox4 is a 578-amino-acid protein that exhibits 39% identity to Nox2 with special conservation in the six membrane-spanning regions and binding sites for NADPH, flavin adenine dinucleotide (FAD), and heme, [43, 54, 56, 58-60, 65, 68]. Evidence suggests that Nox4 heterodimerization with p22phox enhances the enzyme activity and/or stability without the requisite regulatory subunits essential to other Nox isoforms [43, 58, 59, 69, 70]. Moreover, it has been shown that the Nox4 dehydrogenase domain exists in a conformation which allows the spontaneous transfer of electrons from NADPH to FAD [71]. Hence, Nox4 has been referred to as a “constitutively active” enzyme regulated primarily at the level of its expression in response to various stimuli [43, 54, 59]. As a corollary, the overall ROS output of Nox4 may be directly governed by its expression level. Interestingly, transcriptional events are involved in the chronic control of Nox4 protein expression [64, 72-81]. On the other hand, Nox4 is acutely regulated through translational mechanisms without change in its mRNA levels [63, 82-86]. It has been suggested that stimulation with certain agonists causes early Nox4 protein accumulation independent of mRNA transcription by promoting translation of existing mRNA copies [82, 84, 86]. Indeed, Nox4 expression is rapidly upregulated by the binding of agonists implicated in renal diseases such as transforming growth factor-ß (TGF-ß) [63, 73], angiotensin II (Ang II) [82], insulin, and insulin-like growth factor (IGF-I) [84, 85, 87] to their membrane receptors. These types of translational regulations were also proposed for other proteins in the kidney and implicated in the pathogenesis of renal diseases such as DN [82, 88-90]. Regulatory proteins that enhance Nox4 activity, Poldip2 (initially known as NoxR1), and the p47phox-related adaptor protein Tks5, have recently been identified, however their role in Nox4-mediated renal cell injury remains to be studied [91-93]. A potential implication of Rac in the control of Nox4 function was suggested in endothelial and mesangial cells [66, 72, 94]; however, it remains a controversial topic because, unlike Nox2 or Nox1, Rac1 does not activate Nox4 in transfected cells [43].
In contrast to Nox2 and Nox1, hydrogen peroxide rather than superoxide has been suggested as the major ROS product detected for Nox4 [43, 70, 78, 95-98]. However, several independent studies demonstrate Nox4 generates superoxide. Studies in vascular smooth cells as well as in cardiac, vascular or renal cells and tissue detected Nox4-dependent superoxide production [26, 64, 68, 73, 83, 99-106]. Importantly, some of these studies measured superoxide production by high performance liquid chromatography (HPLC) analysis of 2-hydroxyethidium, the dihydroethidium-derived oxidation product specific for superoxide [73, 100], or electron paramagnetic/spin resonance, two “gold standard” for superoxide measurement [100, 107, 108]. Silencing of Nox4 significantly reduced the signal detected. Additionally, the studies with lucigenin-enhanced chemiluminescence assays are also reasonably good indicators of Nox4 capacity to generate superoxide [26, 83, 99, 102, 103] as long as the probe is used at low concentrations in order to avoid artifactual events. Beside the vasculature or the kidney, Nox4-mediated superoxide production was also reported in other systems such as the brain [109, 110]. On the other hand, other studies suggest that hydrogen peroxide formation occurs through Nox4 third extracytosolic loop (E-loop) [111]. Here, it was demonstrated that the structure of the E-loop may obstruct superoxide release as well as provide a source for protons, thus permitting rapid dismutation of superoxide to generate hydrogen peroxide [111]. Alteration of E-loop makes Nox4 produce superoxide rather than hydrogen peroxide [111].
Nox4 has been shown to be associated with intracellular membranes of compartments or organelles such as focal adhesions, endoplasmic reticulum, plasma membrane, mitochondria, and nucleus [43, 54, 56, 60, 70, 77, 83, 99, 112]. However, the localization of Nox4 remains controversial and there is no general agreement on Nox4 subcellular localizations. This is likely to be due to the use of different antibodies, some of them poorly characterized, and with the possible cross-reactivities against other cellular proteins. In addition, tagged Nox proteins used in heterologous overexpression systems may mis-localize, further confounding the issue. Also, subcellular fractionations are not always accompanied by marker enzymes, making contamination with other subcellular fractions a possibility. More studies are needed to characterize binding proteins that may direct Nox4 to specific subcellular localization or amino acids within the molecule necessary and sufficient to direct its localization. Importantly, a mitochondrial targeting sequence was identified in Nox4 [43, 54, 56, 60, 70, 77, 83, 99, 112].
Roles of Nox4 and other Nox oxidase subunits in renal cell injury during the pathogenesis of diabetic kidney disease
Evidence suggests that Nox enzymes contribute to the pathogenesis of DN. This is because multiple stimuli and agonists implicated in this pathology, such as hyperglycemia, Ang II, TGF-ß, advanced glycation end products (AGEs), advanced oxidation protein products (AOPPs), platelet-derived growth factor (PDGF), oxidized low-density lipoprotein, IGF-I, vascular endothelial growth factor (VEGF), endothelin, and aldosterone, have been shown to alter the activity or expression of the Nox proteins and their regulatory subunits, and ultimately the amount of ROS produced [26, 31, 43, 44, 46, 63, 64, 72, 73, 79, 80, 82-85, 101, 105, 113-119]. Renal cells from the two major compartments of the kidney, glomeruli and tubulointerstitium, are particularly sensitive to chronic hyperglycemia that is an effective inducer and/or activator of Nox oxidases in these cells. Upregulation of Nox4, Nox2, Nox1, and p22phox (mRNA and protein) together with increased superoxide or hydrogen peroxide generation has been reported in response to high glucose concentrations in renal cells as well as in experimental models of diabetes [26, 31, 48, 64, 83]. Chronic hyperglycemia further amplifies Nox-derived ROS generation in renal cells and tissue via an elevation in the concentration of the agonists and mediators mentioned above, especially Ang II and TGF-ß. For instance, elevation of Nox4, Nox2, p22phox, p47phox, or p67phox expression as well as increased oxidative stress in glomeruli and tubules of animals with diabetes are inhibited by treatment with angiotensin-converting enzyme inhibitor or angiotensin II type 1 (AT1) receptor blocker [48, 49, 120-124]. Similarly, TGF-ß redox signaling pathways are triggered by the mediators upregulated by hyperglycemia, including Ang II and AGEs [11, 49, 125, 126]. Nox oxidase subunits (e.g. p22phox, Nox4) can be simultaneous targets and regulators of agonists like TGF-ß in renal cells [126, 127]. The essential role of Nox4 or other Nox subunits in diabetes-induced renal damage is further supported by the observation that most of the agents improving renal pathologies in DN prevent the increased expression of these Nox components in diabetic glomeruli and tubules [26, 47, 101, 124, 128-130].
In this section, we will consider the role of Nox4 or other relevant Nox oxidase subunits in renal cell injury induced by hyperglycemia and the various stimuli known to be involved in the pathogenesis of DN.
Roles of Nox4 and other relevant Nox oxidase subunits in diabetes-induced glomerular cell injury
The initial morphological alteration that takes place during the early phase of DN are characterized by an increase in mesangial matrix accumulation accompanied by mesangial cell hypertrophy that contribute to glomerular basement membrane thickening [8, 11, 131]. As the disease advances, the deposition of matrix protein (e.g. fibronectin, type IV collagen or laminin) is further exacerbated and damage of glomerular epithelial cells or podocytes occurs with widening of their foot processes following the loss of key proteins implicated in the structural organization of the slit diaphragm [8, 11]. Podocyte injury and reduction of podocyte number due to apoptotic cell death also occurs during early DN and plays a key role in early proteinuria [8, 11]. Nox-derived ROS and particularly that produced by Nox4 appear to participate to each of these pathological events. Figures 2 and 3 are an overview of the major redox signaling pathways engaged by diabetic stimuli that modulate the function of Nox4 and other critical Nox oxidases subunits with the resultant type of glomerular mesangial cell and podocyte injury.
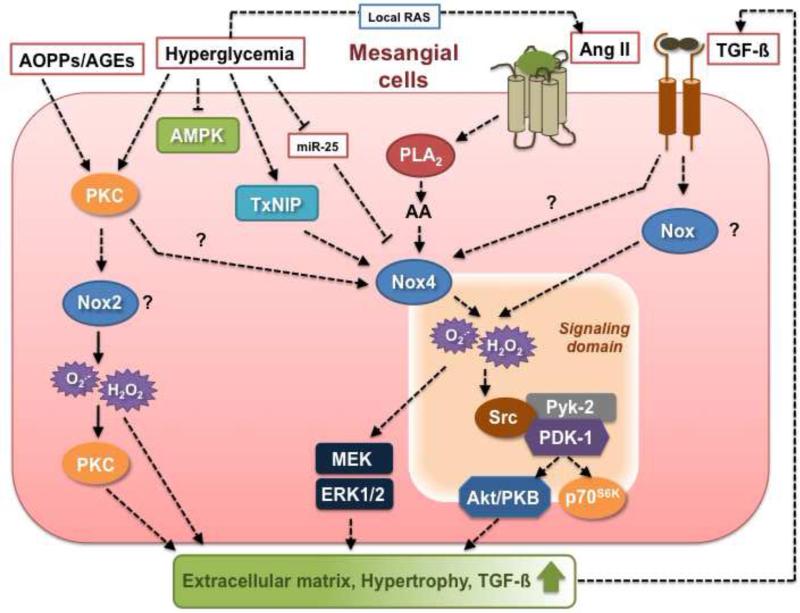
Figure 2.
Nox-dependent signaling pathways implicated in glomerular mesangial cell injury triggered by diabetic stimuli. See text for detail.
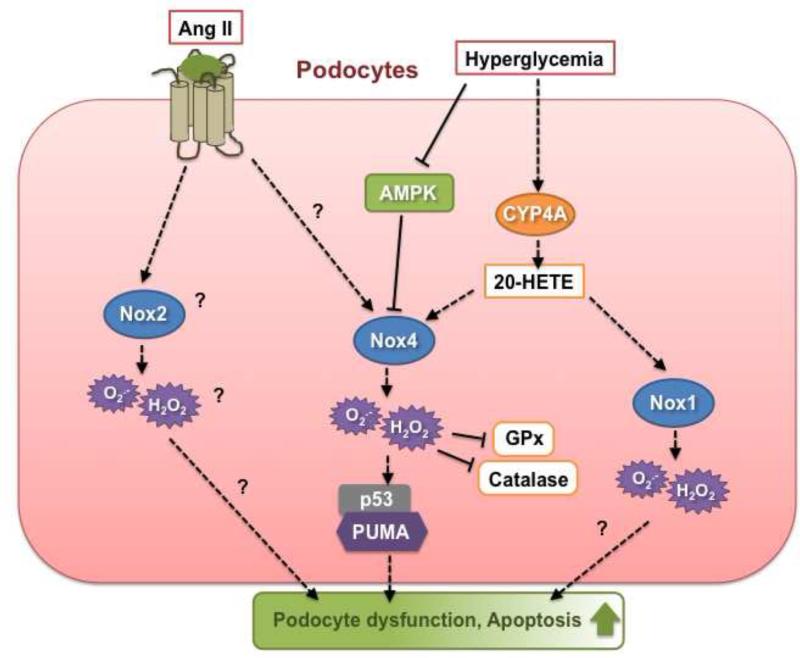
Figure 3.
Nox-dependent signaling pathways implicated in podocyte injury triggered by diabetic stimuli. See text for detail.
Role of Nox4 and other Nox oxidase subunits in glomerular mesangium/mesangial cell injury
Nox4 protein expression increases in the glomeruli, including the mesangium, and Nox4-derived ROS contribute to oxidative stress during the initial and chronic stages of diabetes [26, 47, 64, 101, 124, 128-130]. The molecular mechanisms by which diabetes and high glucose concentrations induce Nox4 protein expression and subsequent Nox4-mediated ROS production in the glomeruli remain speculative. However, the elevation in Nox4 protein and ROS generation are reversed by insulin treatment, confirming that hyperglycemia and hyperglycemia-induced mediators most likely accounted for these effects [26, 128]. Studies by our group provided evidence that Nox4-dependent ROS generation mediates glomerular hypertrophy and mesangial matrix accumulation [26]. Inhibition of Nox4 oxidase by administration of antisense oligonucleotides for Nox4 reduced glomerular enlargement as well as fibronectin accumulation in glomeruli from type 1 diabetic rats [26]. In cultured mesangial cells, glucose elicits a rapid upregulation in Nox4 protein levels, including in the mitochondrial fraction, which is associated with an increase in cellular and mitochondrial ROS production [83, 132]. Moreover, prolonged exposure of mesangial cells to high glucose has also been described to augment Nox4 mRNA and protein expression [128, 133, 134]. Nox4 is required for high glucose-induced (acute or chronic) increase in ROS production and accumulation of fibronectin in these cells [26]. Furthermore, Nox4 participates to high glucose-mediated mitochondrial ROS generation in mesangial cells [83], suggesting that Nox4-derived ROS may affect mitochondrial function. This contention is supported by the recent observation that ROS generated by overexpression of Nox4 are able to oxidize and affect the activity of mitochondrial proteins in cardiac myocytes [99]. Interestingly, Nox4 and p22phox both contribute to high glucose-dependent oxidative stress and fibronectin or collagen IV accumulation in mesangial cells [127, 135, 136]. The mRNA and protein expression of p22phox are upregulated by glucose in these cells as well as in glomeruli from diabetic animals [130, 135-137].
Other studies have demonstrated that statins and Rho kinase inhibitors prevent increased Nox4 expression and oxidative stress in diabetic kidney, including glomeruli, concomitantly to the amelioration of mesangial matrix expansion and renal function, indicate that small GTPases Rho and Rac pathways (known targets of statins) may contribute to the modulation of Nox4 expression and Nox4-dependent ROS release [47, 138]. microRNAs also play a role in Nox4 expression and serves as an endogenous silencer for Nox4 gene in mesangial cells [133]. The downregulation of microRNA-25 (miR-25) by high glucose in mesangial cells or by hyperglycemia in diabetic kidney results in the relief of Nox4 gene silencing that lead to increased Nox4 expression and ROS production [133]. Thioredoxin-interacting protein (TxNIP) has been recently identified as an upstream regulator of Nox4 protein expression in mesangial cells exposed to high glucose. TxNIP mediates high glucose-induced ROS generation by Nox4 in mesangial cells [132].
Nox4 also confers Ang II-mediated harmful effects in mesangial cells. Ang II elicits an acute increase in Nox4 protein expression as well as a chronic prolonged upregulation of Nox4 mRNA and protein levels associated with enhanced ROS production in these cells [129]. Ang II-induced ROS generation in mesangial cells acts principally through Nox4 as an upstream activator of ERK1/ERK2, Pyk-2/Src/PDK-1, Akt/PKB, and/or p70S6K pathways that lead to cell hypertrophy and increased protein synthesis and/or fibronectin expression [42, 66, 67, 82]. More specifically, Pyk-2 appears to act as a molecular scaffold binding to both PDK-1 and Src, thereby allowing Src to tyrosine phosphorylate and activate PDK-1, which in turn activate its downstream effectors, Akt/PKB and p70S6K [82]. Interestingly, PDK-1/Src/Pyk-2 complex may favor the formation of signaling platforms bringing key intermediates into proximity, thereby facilitating the contact between Nox4-derived ROS and downstream effectors [82, 118]. It was reported that activation of Rac1 by phospholipase A2-mediated arachidonic acid (AA) release appears to be implicated in Nox4-dependent ROS production and subsequent Akt/PKB-mediated cellular hypertrophy in mesangial cell treated with Ang II [66, 67]. The p22phox subunit is also required for the hypertrophic and fibrotic actions of Ang II in mesangial cells [139]
Although TGF-ß specifically increases the expression of Nox4 and ROS production in a myriad of cell types [42, 63, 73], the role of Nox4 in TGF-ß-mediated ROS generation and matrix protein accumulation has not been directly established in mesangial cells. However, p22phox is required for TGF-ß-induced ROS production in mesangial cells [127].
Protein kinase C (PKC) isozymes are positioned upstream and downstream of p22phox-containing NADPH oxidases and ROS production in mesangial cells exposed to high glucose or TGF-ß [135, 140]. Furthermore, in mesangial cells, high glucose-induced oxidative stress involves autocrine TGF-ß stimulation of PKC and resultant generation of ROS by p22phox-based Nox oxidase via p22phox protein upregulation [127]. Other studies with mesangial cells exposed to AOPPs place an NADPH oxidase, distal to PKC activation, resulting in extracellular matrix protein overproduction and upregulation of TGF-ß [126].
The role of other Nox catalytic isoforms or regulatory subunits in mesangial cell injury in the diabetic kidney has been less studied. A role for Nox2 or Nox1 is indirectly suggested by the observation that administration of apocynin significantly reduced glomerular fibronectin and collagen accumulation in type 1 diabetic rats [25]. However, the involvement of Nox2 in the mesangium is less clear as some studies indicate that Nox2 is not detected in cultured human and rat mesangial cells whereas Nox2 is detected in isolated mouse mesangial cells and in diabetic glomeruli [114, 141, 142]. Nox2 regulatory subunits p47phox and p67phox are systematically found in mesangial cells [141, 143] and the expression and translocation of p47phox and p67phox to the membrane is increased in the diabetic glomeruli [144]. High glucose causes an increase in p47phox protein expression in mesangial cells and downregulation of p47phox blunts high glucose-induced ROS and extracellular matrix accumulation [135, 140, 145]. Importantly, diabetes-induced oxidative stress and glomerular injury are attenuated in p47phox-deficient type 1 diabetic Akita mice [142]. Moreover, deletion of p47phox attenuates diabetes-induced and high glucose-induced Nox2 expression in glomeruli and mesangial cells, respectively [142]. This indicates that p47phox-dependent activation of Nox oxidase, likely Nox2, is determinant for the promotion of DN. However, a recent study showed that glomerular mesangial matrix expansion and albuminuria are not attenuated in type 1 diabetic Nox2 knockout mice [146]. Interestingly, an upregulation of Nox4 is observed in these mice [146]. Together, these findings warrant a reassessment of the role of Nox2 in DN. While Nox1 is thought to promote the deleterious effects of glucose or Ang II in the vasculature [147, 148], its function in mesangial cell injury in the diabetic milieu has not been reported. However, p47phox and p67phox recruitment to the membrane contributes to Ang II-mediated oxidative stress and mesangial cell growth [149].
AOPPs also increase p22phox, p47phox and Nox4 protein expression [126]. AOPPs promote an increase in p47phox expression and translocation as well as its interaction with p22phox expression [126]. These events are associated with enhanced extracellular matrix protein synthesis.
Role of Nox4 and other Nox oxidases in glomerular epithelial cell/podocyte injury
Upregulation of Nox4 and Nox1 protein expression in diabetic glomeruli including podocytes is accompanied by increased oxidative stress, loss of podocyte or foot process effacement as well as albuminuria in OVE26 type 1 diabetic mice [64, 101, 150]. In cultured podocytes exposed to high glucose for prolonged time period, Nox4 protein is augmented and Nox4-derived ROS play a key role in apoptotic cell death [64, 101, 150, 151]. Unlike what is observed in mesangial cells, glucose does not acutely regulate Nox4 protein expression in podocytes [64]. A sequential regulation of Nox oxidases by cytochrome P450 of the 4A family (CYP4A) was recently identified in podocytes in which 20-hydroxyeicosatetraenoic acid (20-HETE) generation by CYP4A mediates the stimulatory effect of high glucose on Nox4 and Nox1 expression and the resultant ROS production at later time points [64]. In the presence of high glucose concentrations, Nox4 promotes podocyte cell death via activation of p53- and PUMA-dependent apoptotic pathway [101]. The oxidative stress triggered by high glucose appears to be exacerbated by the fact that the ROS generated by Nox4 affects the balance between oxidants and antioxidants by decreasing the activity of key antioxidant enzymes such as glutathione peroxidase (GPx) and catalase [151]. While Nox4 was detected within the mitochondria in podocytes, its contribution to ROS production has not been characterized [83]. Importantly, inactivation of AMPK-activated protein kinase (AMPK) by high glucose accounts for the increase in Nox4 mRNA and protein expression as well as subsequent ROS production and podocyte apoptosis [101]. AMPK activators, e.g. AICAR (5-aminoimidazole-4-carboxamide-1-riboside) or adiponectin, significantly reduced Nox4 expression, oxidative stress and podocyte injury in vitro or in vivo [101, 150, 152]. AMPK activation is also able to counteract the induction of ROS production by high glucose via blockade of a p22phox-based NADPH oxidase in mesangial cells [137]. These observations are consistent with the recent concept proposing that AMPK can act as a suppressor of oxidative stress via inhibition of NADPH oxidase subunits expression in various biological systems including vascular tissues [153-155].
Although podocytes also express Nox2, p22phox, p47phox and p67phox [57, 156], there is no evidence of regulation of these subunits by high glucose. While Ang II- or TGF-ß-induced oxidative stress mediates podocyte injury [33, 57, 157], very little is known regarding the role of the Nox oxidases in the podocyte dysfunction promoted by these agonists or the other major mediators of DN. Similar to what is observed in MCs, Ang II-dependent increase in NADPH oxidase activity is associated with the upregulation of Nox4, Nox2, Rac and p22phox expression in podocytes [57, 158]. To date, the modulation of NADPH oxidase subunits by TGF-ß, including Nox4, has been not reported in podocytes.
An important consideration is the fact that most of the factors induced by the diabetic milieu that account for Nox-mediated glomerular injury can be generated by one of the glomerular cell type and act in a paracrine manner on a second type of glomerular cell to mobilize Nox-dependent signaling in these neighboring cells. For instance, since both mesangial cells and podocytes possess a local renin-angiotensin system activated by glucose [11], Ang II generated by one of these cell type may promote autocrine or pacracrine activation of Nox enzymes leading to damage in both cell types. The same observation can be applied to TGF-ß that can target Nox oxidases in both mesangial cells and podocytes in paracrine or autocrine manner. Therefore, complex interplay exists between mesangial cells and podocytes in diabetes and it is reasonable to think that activation of Nox oxidases plays a central role in these reciprocal interactions contributing to glomerular damage.
Role of Nox4 and other relevant Nox oxidase subunits in diabetes-induced tubular and interstitial cell injury
Excessive generation of ROS induced by hyperglycemia in association with Ang II-mediated activation of TGF-ß actively participates in tubular and interstitial cell dysfunction [8, 11]. These events results in fibrotic processes in the tubulointerstitium due to exacerbated extracellular matrix protein synthesis in tubular cells and activated resident interstitial fibroblasts as well as tubular epithelial-to-mesenchymal transition (EMT) that contribute to the thickening of the tubular basement membrane and tubulointerstitial fibrosis [8, 11, 42]. In addition, tubular cells undergo apoptosis and hypertrophy in response to diabetes-induced oxidative stress [8, 11]. Figure 4 is an overview of the major redox signaling pathways engaged by diabetic stimuli that modulate the function of Nox4 and other critical Nox oxidases subunits with the resultant type of tubulointerstitial cell injury.
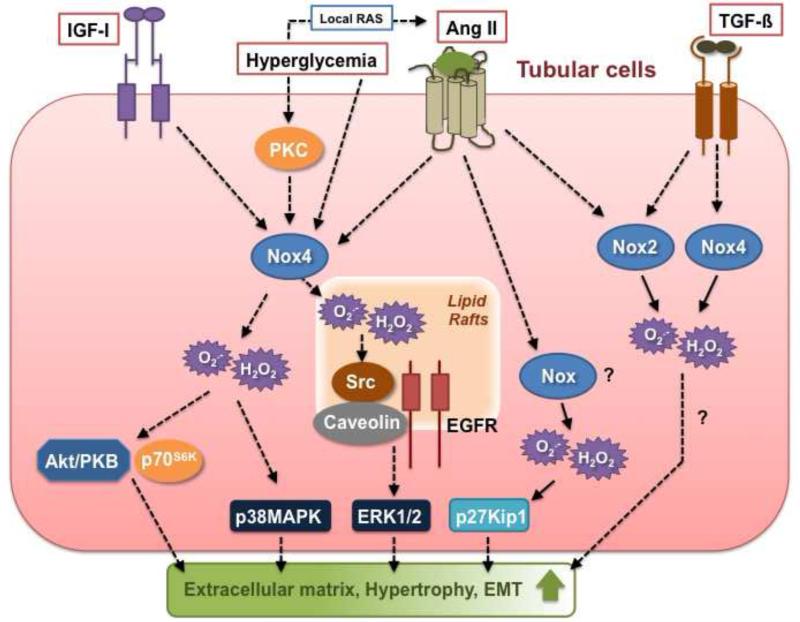
Figure 4.
Nox-dependent signaling pathways implicated in tubular cell injury triggered by diabetic stimuli. See text for detail.
Role of Nox4 and other Nox oxidases in tubular cell injury
Similar to glomeruli, tubules from type 1 diabetic rats show an increase in Nox4 mRNA and protein expression and downregulation of tubular Nox4 levels with in vivo administration of antisense oligonucleotides reduces diabetes-mediated ROS production and extracellular matrix protein synthesis in the renal cortex that is mainly composed of tubular epithelial cells [26, 128, 129]. Interestingly, Nox4 protein expression is increased in renal cortex but is unchanged in medulla from type 2 diabetic mice [159]. Increased Nox4 expression in diabetic tubules correlates with an augmentation in p22phox levels [128, 159]. Whilst the levels of Nox2 and p47phox are not affected in renal cortex from type 2 diabetic mice [159], Nox2 is increased in the cortex from type 1 diabetic rats [26]. A role for Nox2 is challenged by a recent report showing that tubulointerstitial injury is not ameliorated in type 1 diabetic Nox2 knockout mice [146]. Exposure of cultured renal proximal tubular epithelial cells to high glucose leads to the upregulation of Nox4 protein expression but seems to have no effect on Nox2, Nox1, p22phox or p47phox expression [159]. Furthermore, Nox4-dependent ROS production is required for glucose-induced increase in fibronectin accumulation and TGF-ß expression in these cells [159]. The profibrotic action of the oxidase is corroborated by the finding that overexpression of Nox4 in tubular cells causes a robust increase in fibronectin synthesis [85]. A potential upstream regulator of Nox4 expression in the tubules is protein kinase C-ß since diabetes-induced Nox4 expression and oxidative stress are attenuated in renal cortex from PKC-ß-null mice kidney [160]. Similar to podocytes, AMPK activation attenuates high glucose-mediated Nox4 protein upregulation in tubular epithelial cells, supporting the concept that the kinase acts as a suppressor of oxidative stress [161]. p38MAPK has been also identified as a critical downstream effector of Nox4 in the signaling pathway linking high glucose and tubular cell injury [159].
Ang II has been shown to utilize Nox4 as a mediator of its injurious effects in tubular epithelial cells. Chronic angiotensin II treatment upregulates Nox4 expression and induces epithelial-to-mesenchymal transition in renal epithelial cells through Nox4-dependent ROS production and resultant Src/caveolin-mediated activation of epidermal growth factor receptor/ERK signaling pathway [162-164]. Furthermore, Ang II upregulates Nox4 expression in the mitochondrial fraction of renal tubular cells and Nox4 is required for Ang II-mediated ROS mitochondrial production [165]. ROS derived from Nox4 contribute to Ang II-dependent apoptosis in these cells [165]. Unlike glucose, Ang II seems to be able to enhance the expression of Nox2, p22phox and p47phox subunits along with Nox4 in tubular cells [164, 166]. The effects on p22phox, p47phox and Nox4 appear to be mediated by lectin-like oxidized low-density lipoprotein receptor-1 [164, 166]. Another report shows that high glucose induces p22phox expression and oxidative stress via AT1 receptor activation, further underlining the important role of Ang II as a regulator of Nox signaling in tubular cells [167]. Furthermore, a p22phox-containing NADPH oxidase is critical for Ang II-mediated tubular cell hypertrophy through induction of p27Kip1, a factor promoting cell cycle arrest [166]. As in mesangial cells, Src and ERK pathways appears to be a primary target of Nox4-derived ROS and plays a key role in the resultant fibrotic or hypertrophic response in tubular cells. AMPK also inhibits Ang II-induced increase in Nox4 expression and epithelial-to-mesenchymal transition [161].
Although it is clear that oxidative stress is implicated in TGF-ß-mediated tubular cell injury [168], there is a deficit of causal evidence supporting the role of the oxidase or other Nox enzymes in TGF-ß signaling in tubular cells. Stimulation of tubular cells by TGF-ß results in an increase in Nox4 protein expression, an effect prevented by AMPK activation [161]. Conflicting results exist concerning the regulation of other Nox subunits by TGF-ß in these cells. On one hand, TGF-ß has no effect on Nox2, p22phox and p47phox expression and that only p67phox seems to be required for the deleterious actions of the cytokine in renal tubular cells [169]. On the other hand, TGF-ß was shown to upregulate p22phox [170].
Among the other factors known to mediate tubular damage in DN, one of the best agonist identified as a regulator of Nox oxidase activity in tubular cells is IGF-I. Hence, Nox4-derived ROS serve as signal transducer for the fibrotic effects of IGF-I in renal tubular epithelial cells via Akt/PKB and mTOR/p70S6K signaling pathways activation [85].
Role of Nox4 and other Nox oxidases in interstitial cell injury
The information regarding the roles of hyperglycemia-mediated oxidative stress or Nox-derived ROS in interstitial cell injury, particularly in the activation of renal fibroblast into myofibroblasts, remains very sparse. Although glucose has been shown to elicit extracellular matrix protein synthesis in kidney fibroblasts, there is no evidence that the ROS produced by Nox4 or any other Nox oxidases mediate these effects. Most of the data available concern the effect of TGF-ß. Studies with fibroblasts derived from heart, lung, and kidney indicate that Nox4 is central to TGF-ß-induced ROS generation and myofibroblast differentiation to a profibrotic phenotype [42]. For example, TGF-ß-induced transition of cardiac or lung fibroblasts to myofibroblasts is dependent on Nox4 regulation of Smad2/3, transcription factors that binds and activate the promoter region of extracellular matrix protein genes such as collagens [42, 73, 74]. Importantly, Nox4 is also the predominant Nox oxidase isoform implicated in kidney myofibroblast differentiation and expression of fibronectin in response to TGF-ß [42, 63]. However, unlike cardiac and lung myofibroblasts, Nox4 is positioned downstream of Smad3 and proximal to ERK in renal fibroblasts. These results are in agreement with what is reported in pulmonary vascular smooth muscle cells, where TGF-β-induced proliferation occurs through a Nox4-dependent pathway downstream of Smad3 [171].
Similar to the glomeruli, it is important to consider the existence of reciprocal paracrine activation between tubulointerstitial cells [172]. Tubular cells may modulate Nox-dependent pathways and the biological behavior of neighboring fibroblasts through paracrine mechanisms, which include the production and release of TGF-ß or Ang II as tubular cells also contain a locally active renin-angiotensin system. On the other hand, it is likely that secretion of IGF-I by fibroblasts regulates Nox oxidase signaling in adjacent tubules.
Nox4, with a friend like this who needs enemies?
Although significant progress has been made in the investigation of the role of Nox4 and other relevant oxidases in diabetic kidney disease, there is still a need for more direct and less circumstantial evidence establishing which Nox homologs or subunits are implicated in redox-dependent pathologies in different tissue and cells. This necessity is underscored by the data obtained using transgenic and Nox4 knockout mice. Experiments inducing a variety of kidney diseases, including streptozotocin-induced type 1 diabetes, in global and inducible Nox4 knockout mice indicate that Nox4 does not promote renal disease but may have a small protective effect against proteinuria, fibrosis and inflammation [173, 174].
Although the aforementioned Nox4 knockout animal studies are in contrast to the plethora of in vitro and in vivo examples outlined above, the results are neither surprising nor controversial. Indeed, when comparing in vitro and in vivo results as well as in vivo findings, to one another, several factors should be considered.
First, we must reemphasize that diabetes induces systemic hyperglycemia, which effect organs differently. Exposure of the kidney to chronic hyperglycemia induces expression of growth factors, vasoactive peptides, and cytokines from the various cell types of this heterogeneous organ resulting in paracrine activation and cross talks between these cell types. This cannot be compared to in vitro experimental conditions where an agonist is added to one renal cell type (mesangial, tubular epithelial, or podocyte cell) with subsequent measurement of a biological output. However, these variations do not detract from studies documenting agonist-induced, Nox4-mediated cellular injury in vitro.
Second, it is important to stress that genetic knockdown models represents very artificial situations in that: (i) over the course of development and growth, compensatory pathways may emerge which can alter signaling pathways more globally, and (ii) the continuous absence of a gene can perturb the developmental sequence and therefore can affect the response of the adult organism to stress or disease in ways that may not relate directly to the role of that gene in the adult animal. On the other hand, a phenotype can be more reliably associated with inactivation of the target gene when considering a tissue specific knockout. This is stressed by the finding that Nox4 global knockout and Nox4 tissue-specific knockout animals give rise to two opposite phenotypes when induced with the same disease. Here, cardiac-specific knockout of Nox4 protects against pressure overload-induced cardiac injury whereas the global Nox4 knockout showed no protection when subjected to pressure overload [175]. This suggests that the group of proteins or signaling intermediates affected by Nox4 may be cell specific. Some of these pathways may protect cell function basally or in pathological conditions. Thus, global deletion of Nox4 may be deleterious independently of the disease induced. Note that cell-specific knockout may be challenging for some renal cells, such as mesangial cells, since there are no specific promoters available for these cells.
Finally, and importantly, not all rodent models of DN are the same. For example, the severity of the renal damages seen in DN differs considerably in the different mice model of type 1 (OVE26, Akita mice and streptozotocin-induced diabetes models) or type 2 diabetes (db/db and BTBR ob/ob mice) [176, 177]. Importantly, similar variations exist between the mice backgrounds with some animals including C57BL6 mice, the background of most of the Nox4-deficient mice described in the literature, being more resistant to DN than others [176, 177]. It is also conceivable that the role of Nox4 varies during the stages and the duration of the disease, i.e., early or advanced diabetes as well as the severity of the disease at these different phases of diabetic complications.
Collectively, we suggest that interpretation of data by direct comparisons from independent studies using different rodent models, different genetic backgrounds, and induced with different agents to promote diabetic kidney disease should be approached and interpreted with caution.
Nox4 and other Nox oxidases as therapeutic targets for DN
It is apparent from this review that the ample in vivo and in vitro experimental evidence support a role for the Nox family of NADPH oxidases, particularly Nox4, in the pathogenesis and pathophysiology of DN. The corollary of these observations is the consideration of Nox4 and the other relevant Nox homologs as therapeutic targets for the treatment of diabetic complications in the kidney. There has been a considerable effort for the generation and development of agents able to inhibit the Nox enzymes in a homolog-specific manner [178-184]. The data obtained in the kidney presented in this review suggest that the catalytic subunits Nox4 and Nox2 may be privileged targets for these inhibitors. Among these inhibitors, the orally administrable small-molecules inhibitors from the Pyrazolo pyridine chemical series, particularly the compounds referred as GKT136901 and GKT137831, have drawn considerable attention. In contrast with most of the other compounds that have been described as Nox inhibitors but have not undergone or completed preclinical studies, intensive research is currently conducted to test the bioefficacy of the GKT inhibitors in animals models of disease and they have recently been used in a phase 1 clinical trial [183, 185-189]. They are presented as dual Nox4 and Nox1 inhibitors with to a lesser extent some inhibitory action on Nox5 and Nox2 [159, 180, 183, 185]. While the mechanisms by which these compounds inhibit Nox4 and Nox1 remain unclear, they may act as competitive inhibitors since their structure resemble to NADPH [179]. Numerous preclinical studies performed with GKT136901 or GKT137831 in experimental animal models indicate that the inhibitors effectively attenuate the pathological changes observed in renal complication of diabetes, atherosclerosis, liver fibrosis and idiopathic pulmonary fibrosis [183, 185-189]. Interestingly, the efficacy of the dual Nox4/1 inhibitors was primarily demonstrated in disease models for which the role of Nox4 as mediator of the pathologies is established (i.e. lung or liver fibrosis) [74, 186, 190], suggesting that the protective actions of the compound may be predominantly due to the targeting of Nox4 in these conditions. Importantly, the observation that treatment of normal mice with the inhibitors is not interfering with physiological processes together with the fact that Nox4 knockout studies produce a healthy animal, for which it is difficult to elicit a positive or negative phenotype except under specific stressor conditions [178], speak to the lack of adverse effects of these inhibitors.
Beside stressing the importance of direct inhibition of relevant Nox oxidases, the present review also suggests that adjunct therapies targeting the agonists or signaling intermediates that regulate the expression or function of Nox subunits and subsequent ROS production should be considered for the treatment of diabetic complications. In regards to the observation reported above, such strategies could involve the use of PKC inhibitors, agents that disrupt the AGE signaling, inhibitors of the RAS (angiotensin-converting enzyme inhibitors and Ang II receptor blockers), statins (that inhibit Rac1 and Rho), Rho/Rho kinase inhibitors as well as AMPK activators (i.e. metformin).
Conclusion
The wide range of Nox4 and other Nox oxidase actions in diabetic-induced pathological processes imply that the therapeutic potential of Nox inhibitors for the treatment of diabetic complications could be considerable. However, the fact that the homologs expressed in rat or mice kidney may not be the only Nox homologs contributing to diabetic kidney disease in human should be considered. This concern is justified in regards to recent observations showing that the homolog Nox5, which is not expressed in rodents, seems to be critical for redox-mediated damages in human renal cells, including podocytes and tubular cells in diabetic environment [62]. Therefore, it is becoming clear that defining the contribution of Nox5 homolog to diabetes-induced injury in human tissue and cells is a prerequisite for the design of successful therapy based on the targeting of Nox oxidases. The understanding of the individual role of Nox homologs, especially Nox5, in the different molecular mechanisms and signaling cascades altered by diabetes should be pursued concomitantly to the translation of the information gathered into therapeutic intervention.
Highlights.
Oxidative stress is a major player in diabetic nephropathy.
Nox4 is a member of the NADPH oxidases of the Nox family with unique properties.
Here, we review the role of Nox4 and other relevant NADPH oxidase subunits in the pathogenesis of diabetic kidney disease.
Acknowledgements
Supported by Juvenile Diabetes Research Foundation Multiproject Grants (Y.G and K.B.), a NIH RO1 DK 079996 (Y.G.), NIH RO1 CA 131272 (K.B), and the Veterans Administration (K.B.).
Abbreviations
- 20-HETE
20-hydroxyeicosatetraenoic acid
- AA
arachidonic acid
- AGEs
advanced glycation end-products
- AICAR
5-aminoimidazole-4-carboxamide-1-riboside
- Akt/PKB
Akt/protein kinase B
- AMPK
AMP-activated protein kinase
- Ang II
angiotensin II
- AOPPs
advanced oxidation protein products
- AT1
receptor angiotensin II type 1 receptor
- CTGF
connective tissue growth factor
- CYP4A
cytochrome P450 of the 4A family
- DHE
dihydroethidium
- DN
diabetic nephropathy
- Duox
dual oxidase
- EGF
epidermal growth factor
- eNOS
endothelial nitric oxide synthase
- ERK
extracellular signal-regulated kinase
- FAD
flavin adenine dinucleotide
- GPx
glutathione peroxidase
- H2O2
hydrogen peroxide
- HIF-1
hypoxia inducible factor
- IGF-I
insulin-like growth factor
- MEK
mitogen-activated protein kinase/ERK kinase
- miR-25
microRNA-25
- NADPH
nicotinamide adenine dinucleotide phosphate
- Nox
NADPH oxidase
- NoxA1
Nox activator 1
- NoxO1
Nox organizer 1
- O2-•
superoxide anion
- p38MAPK
p38 mitogen-activated protein kinase
- p70S6K
p70 S6 kinase
- PDGF
platelet-derived growth factor
- PDK-1
3-phosphoinositide-dependent protein kinase-1
- Phox
phagocyte oxidase
- PI3-K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- PLA2
phospholipase A2
- Poldip2
polymerase delta-interacting protein 2
- Pyk-2
proline-rich tyrosine kinase-2
- ROS
reactive oxygen species
- Smad
Sma and Mad homolog
- SOD
superoxide dismutase
- TGF-ß
transforming growth factor-ß
- Tks
tyrosine kinase substrate with five SH3 domains
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hakim FA, Pflueger A. Role of oxidative stress in diabetic kidney disease. Medical science monitor : international medical journal of experimental and clinical research. 2010;16:RA37–48. [PubMed] [Google Scholar]
- 2.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 3.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 4.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circulation research. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinokio Y, Suzuki S, Hirai M, Chiba M, Hirai A, Toyota T. Oxidative DNA damage in diabetes mellitus: its association with diabetic complications. Diabetologia. 1999;42:995–998. doi: 10.1007/s001250051258. [DOI] [PubMed] [Google Scholar]
- 6.Kashihara N, Haruna Y, Kondeti VK, Kanwar YS. Oxidative stress in diabetic nephropathy. Current medicinal chemistry. 2010;17:4256–4269. doi: 10.2174/092986710793348581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnackenberg CG. Oxygen radicals in cardiovascular-renal disease. Current opinion in pharmacology. 2002;2:121–125. doi: 10.1016/s1471-4892(02)00133-9. [DOI] [PubMed] [Google Scholar]
- 8.Singh DK, Winocour P, Farrington K. Oxidative stress in early diabetic nephropathy: fueling the fire. Nature reviews. Endocrinology. 2011;7:176–184. doi: 10.1038/nrendo.2010.212. [DOI] [PubMed] [Google Scholar]
- 9.Son SM, Whalin MK, Harrison DG, Taylor WR, Griendling KK. Oxidative stress and diabetic vascular complications. Current diabetes reports. 2004;4:247–252. doi: 10.1007/s11892-004-0075-8. [DOI] [PubMed] [Google Scholar]
- 10.Stanton RC. Oxidative stress and diabetic kidney disease. Current diabetes reports. 2011;11:330–336. doi: 10.1007/s11892-011-0196-9. [DOI] [PubMed] [Google Scholar]
- 11.Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annual review of pathology. 2011;6:395–423. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood) 2008;233:4–11. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 13.Koya D, Hayashi K, Kitada M, Kashiwagi A, Kikkawa R, Haneda M. Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. Journal of the American Society of Nephrology : JASN. 2003;14:S250–253. doi: 10.1097/01.asn.0000077412.07578.44. [DOI] [PubMed] [Google Scholar]
- 14.Vasavada N, Agarwal R. Role of oxidative stress in diabetic nephropathy. Advances in chronic kidney disease. 2005;12:146–154. doi: 10.1053/j.ackd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Brezniceanu ML, Liu F, Wei CC, Chenier I, Godin N, Zhang SL, Filep JG, Ingelfinger JR, Chan JS. Attenuation of interstitial fibrosis and tubular apoptosis in db/db transgenic mice overexpressing catalase in renal proximal tubular cells. Diabetes. 2008;57:451–459. doi: 10.2337/db07-0013. [DOI] [PubMed] [Google Scholar]
- 16.Craven PA, DeRubertis FR, Kagan VE, Melhem M, Studer RK. Effects of supplementation with vitamin C or E on albuminuria, glomerular TGF-beta, and glomerular size in diabetes. Journal of the American Society of Nephrology : JASN. 1997;8:1405–1414. doi: 10.1681/ASN.V891405. [DOI] [PubMed] [Google Scholar]
- 17.Craven PA, Melhem MF, Phillips SL, DeRubertis FR. Overexpression of Cu2+/Zn2+ superoxide dismutase protects against early diabetic glomerular injury in transgenic mice. Diabetes. 2001;50:2114–2125. doi: 10.2337/diabetes.50.9.2114. [DOI] [PubMed] [Google Scholar]
- 18.DeRubertis FR, Craven PA, Melhem MF. Acceleration of diabetic renal injury in the superoxide dismutase knockout mouse: effects of tempol. Metabolism: clinical and experimental. 2007;56:1256–1264. doi: 10.1016/j.metabol.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 19.DeRubertis FR, Craven PA, Melhem MF, Salah EM. Attenuation of renal injury in db/db mice overexpressing superoxide dismutase: evidence for reduced superoxide-nitric oxide interaction. Diabetes. 2004;53:762–768. doi: 10.2337/diabetes.53.3.762. [DOI] [PubMed] [Google Scholar]
- 20.Melhem MF, Craven PA, Liachenko J, DeRubertis FR. Alpha-lipoic acid attenuates hyperglycemia and prevents glomerular mesangial matrix expansion in diabetes. Journal of the American Society of Nephrology : JASN. 2002;13:108–116. doi: 10.1681/ASN.V131108. [DOI] [PubMed] [Google Scholar]
- 21.Siu B, Saha J, Smoyer WE, Sullivan KA, Brosius FC., 3rd Reduction in podocyte density as a pathologic feature in early diabetic nephropathy in rodents: prevention by lipoic acid treatment. BMC nephrology. 2006;7:6. doi: 10.1186/1471-2369-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winiarska K, Malinska D, Szymanski K, Dudziak M, Bryla J. Lipoic acid ameliorates oxidative stress and renal injury in alloxan diabetic rabbits. Biochimie. 2008;90:450–459. doi: 10.1016/j.biochi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Yi X, Nickeleit V, James LR, Maeda N. alpha-Lipoic acid protects diabetic apolipoprotein E-deficient mice from nephropathy. Journal of diabetes and its complications. 2011;25:193–201. doi: 10.1016/j.jdiacomp.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng S, Carlson EC, Yang L, Kralik PM, Huang Y, Epstein PN. Podocyte-specific overexpression of the antioxidant metallothionein reduces diabetic nephropathy. Journal of the American Society of Nephrology : JASN. 2008;19:2077–2085. doi: 10.1681/ASN.2007080967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, Fujita T, Wilcox CS. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney international. 2005;67:1890–1898. doi: 10.1111/j.1523-1755.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 26.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. The Journal of biological chemistry. 2005;280:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 27.Hwang I, Lee J, Huh JY, Park J, Lee HB, Ho YS, Ha H. Catalase deficiency accelerates diabetic renal injury through peroxisomal dysfunction. Diabetes. 2012;61:728–738. doi: 10.2337/db11-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nam SM, Lee MY, Koh JH, Park JH, Shin JY, Shin YG, Koh SB, Lee EY, Chung CH. Effects of NADPH oxidase inhibitor on diabetic nephropathy in OLETF rats: the role of reducing oxidative stress in its protective property. Diabetes research and clinical practice. 2009;83:176–182. doi: 10.1016/j.diabres.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Satriano J. Kidney growth, hypertrophy and the unifying mechanism of diabetic complications. Amino acids. 2007;33:331–339. doi: 10.1007/s00726-007-0529-9. [DOI] [PubMed] [Google Scholar]
- 30.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 31.Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, Sourris KC, Penfold SA, Bach LA, Cooper ME, Forbes JM. Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes. 2008;57:460–469. doi: 10.2337/db07-1119. [DOI] [PubMed] [Google Scholar]
- 32.Abboud HE. Growth factors and diabetic nephrology: an overview. Kidney international. Supplement. 1997;60:S3–6. [PubMed] [Google Scholar]
- 33.Campbell KN, Raij L, Mundel P. Role of angiotensin II in the development of nephropathy and podocytopathy of diabetes. Current diabetes reviews. 2011;7:3–7. doi: 10.2174/157339911794273973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rincon-Choles H, Kasinath BS, Gorin Y, Abboud HE. Angiotensin II and growth factors in the pathogenesis of diabetic nephropathy. Kidney international. Supplement. 2002:S8–11. doi: 10.1046/j.1523-1755.62.s82.3.x. [DOI] [PubMed] [Google Scholar]
- 35.Djordjevic VB. Free radicals in cell biology. International review of cytology. 2004;237:57–89. doi: 10.1016/S0074-7696(04)37002-6. [DOI] [PubMed] [Google Scholar]
- 36.Droge W. Free radicals in the physiological control of cell function. Physiological reviews. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 37.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 38.Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, Tan AL, Fukami K, Thallas-Bonke V, Nawroth PP, Brownlee M, Bierhaus A, Cooper ME, Forbes JM. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. Journal of the American Society of Nephrology : JASN. 2009;20:742–752. doi: 10.1681/ASN.2008050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Craven PA, Phillips SL, Melhem MF, Liachenko J, DeRubertis FR. Overexpression of manganese superoxide dismutase suppresses increases in collagen accumulation induced by culture of mesangial cells in high-media glucose. Metabolism: clinical and experimental. 2001;50:1043–1048. doi: 10.1053/meta.2001.25802. [DOI] [PubMed] [Google Scholar]
- 40.Kiritoshi S, Nishikawa T, Sonoda K, Kukidome D, Senokuchi T, Matsuo T, Matsumura T, Tokunaga H, Brownlee M, Araki E. Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: potential role in diabetic nephropathy. Diabetes. 2003;52:2570–2577. doi: 10.2337/diabetes.52.10.2570. [DOI] [PubMed] [Google Scholar]
- 41.Kitada M, Kume S, Imaizumi N, Koya D. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes. 2011;60:634–643. doi: 10.2337/db10-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnes JL, Gorin Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney international. 2011;79:944–956. doi: 10.1038/ki.2010.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological reviews. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 44.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxidants & redox signaling. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 45.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free radical biology & medicine. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free radical biology & medicine. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujii M, Inoguchi T, Maeda Y, Sasaki S, Sawada F, Saito R, Kobayashi K, Sumimoto H, Takayanagi R. Pitavastatin ameliorates albuminuria and renal mesangial expansion by downregulating NOX4 in db/db mice. Kidney international. 2007;72:473–480. doi: 10.1038/sj.ki.5002366. [DOI] [PubMed] [Google Scholar]
- 48.Onozato ML, Tojo A, Goto A, Fujita T, Wilcox CS. Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEI and ARB. Kidney international. 2002;61:186–194. doi: 10.1046/j.1523-1755.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 49.Tojo A, Asaba K, Onozato ML. Suppressing renal NADPH oxidase to treat diabetic nephropathy. Expert opinion on therapeutic targets. 2007;11:1011–1018. doi: 10.1517/14728222.11.8.1011. [DOI] [PubMed] [Google Scholar]
- 50.Clark RA. Activation of the neutrophil respiratory burst oxidase. The Journal of infectious diseases. 1999;179(Suppl 2):S309–317. doi: 10.1086/513849. [DOI] [PubMed] [Google Scholar]
- 51.Leusen JH, Verhoeven AJ, Roos D. Interactions between the components of the human NADPH oxidase: a review about the intrigues in the phox family. Frontiers in bioscience : a journal and virtual library. 1996;1:d72–90. doi: 10.2741/a117. [DOI] [PubMed] [Google Scholar]
- 52.Brandes RP, Schroder K. Composition and functions of vascular nicotinamide adenine dinucleotide phosphate oxidases. Trends in cardiovascular medicine. 2008;18:15–19. doi: 10.1016/j.tcm.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Brandes RP, Weissmann N, Schroder K. NADPH oxidases in cardiovascular disease. Free radical biology & medicine. 2010;49:687–706. doi: 10.1016/j.freeradbiomed.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 54.Brown DI, Griendling KK. Nox proteins in signal transduction. Free radical biology & medicine. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. American journal of physiology. Regulatory, integrative and comparative physiology. 2003;285:R277–297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 56.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nistala R, Whaley-Connell A, Sowers JR. Redox control of renal function and hypertension. Antioxidants & redox signaling. 2008;10:2047–2089. doi: 10.1089/ars.2008.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geiszt M. NADPH oxidases: new kids on the block. Cardiovascular research. 2006;71:289–299. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Selemidis S, Sobey CG, Wingler K, Schmidt HH, Drummond GR. NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacology & therapeutics. 2008;120:254–291. doi: 10.1016/j.pharmthera.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 60.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circulation research. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schreck C, O'Connor PM. NAD(P)H oxidase and renal epithelial ion transport. American journal of physiology. Regulatory, integrative and comparative physiology. 2011;300:R1023–1029. doi: 10.1152/ajpregu.00618.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holterman C, Saleem M, Towaij C, Touyz R, Cooper M, Kennedy C. Nox5-dependent reactive oxygen species production in human podocytes exposed to diabetic stimuli [Abstract]. Journal of the American Society of Nephrology : JASN. 2011;22 [Google Scholar]
- 63.Bondi CD, Manickam N, Lee DY, Block K, Gorin Y, Abboud HE, Barnes JL. NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. Journal of the American Society of Nephrology : JASN. 2010;21:93–102. doi: 10.1681/ASN.2009020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K, Abboud HE. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes. 2009;58:1201–1211. doi: 10.2337/db08-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. American journal of physiology. Renal physiology. 2003;285:F219–229. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- 67.Gorin Y, Ricono JM, Wagner B, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Angiotensin II-induced ERK1/ERK2 activation and protein synthesis are redox-dependent in glomerular mesangial cells. The Biochemical journal. 2004;381:231–239. doi: 10.1042/BJ20031614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. The Journal of biological chemistry. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 69.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. The Journal of biological chemistry. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 70.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cellular signalling. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 71.Nisimoto Y, Jackson HM, Ogawa H, Kawahara T, Lambeth JD. Constitutive NADPH-dependent electron transferase activity of the Nox4 dehydrogenase domain. Biochemistry. 2010;49:2433–2442. doi: 10.1021/bi9022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chai D, Wang B, Shen L, Pu J, Zhang XK, He B. RXR agonists inhibit high-glucose-induced oxidative stress by repressing PKC activity in human endothelial cells. Free radical biology & medicine. 2008;44:1334–1347. doi: 10.1016/j.freeradbiomed.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 73.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circulation research. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 74.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nature medicine. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circulation research. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 76.Moe KT, Aulia S, Jiang F, Chua YL, Koh TH, Wong MC, Dusting GJ. Differential upregulation of Nox homologues of NADPH oxidase by tumor necrosis factor-alpha in human aortic smooth muscle and embryonic kidney cells. Journal of cellular and molecular medicine. 2006;10:231–239. doi: 10.1111/j.1582-4934.2006.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O'Dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, Ogier-Denis E. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Molecular and cellular biology. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. The Biochemical journal. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tong X, Schroder K. NADPH oxidases are responsible for the failure of nitric oxide to inhibit migration of smooth muscle cells exposed to high glucose. Free radical biology & medicine. 2009;47:1578–1583. doi: 10.1016/j.freeradbiomed.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wingler K, Wunsch S, Kreutz R, Rothermund L, Paul M, Schmidt HH. Upregulation of the vascular NAD(P)H-oxidase isoforms Nox1 and Nox4 by the renin-angiotensin system in vitro and in vivo. Free radical biology & medicine. 2001;31:1456–1464. doi: 10.1016/s0891-5849(01)00727-4. [DOI] [PubMed] [Google Scholar]
- 81.Yamagishi S, Nakamura K, Ueda S, Kato S, Imaizumi T. Pigment epithelium-derived factor (PEDF) blocks angiotensin II signaling in endothelial cells via suppression of NADPH oxidase: a novel anti-oxidative mechanism of PEDF. Cell and tissue research. 2005;320:437–445. doi: 10.1007/s00441-005-1094-8. [DOI] [PubMed] [Google Scholar]
- 82.Block K, Eid A, Griendling KK, Lee DY, Wittrant Y, Gorin Y. Nox4 NAD(P)H oxidase mediates Src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II: role in mesangial cell hypertrophy and fibronectin expression. The Journal of biological chemistry. 2008;283:24061–24076. doi: 10.1074/jbc.M803964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meng D, Lv DD, Fang J. Insulin-like growth factor-I induces reactive oxygen species production and cell migration through Nox4 and Rac1 in vascular smooth muscle cells. Cardiovascular research. 2008;80:299–308. doi: 10.1093/cvr/cvn173. [DOI] [PubMed] [Google Scholar]
- 85.New DD, Block K, Bhandhari B, Gorin Y, Abboud HE. IGF-I increases the expression of fibronectin by Nox4-dependent Akt phosphorylation in renal tubular epithelial cells. American journal of physiology. Cell physiology. 2012;302:C122–130. doi: 10.1152/ajpcell.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peshavariya H, Jiang F, Taylor CJ, Selemidis S, Chang CW, Dusting GJ. Translation-linked mRNA destabilization accompanying serum-induced Nox4 expression in human endothelial cells. Antioxidants & redox signaling. 2009;11:2399–2408. doi: 10.1089/ars.2009.2579. [DOI] [PubMed] [Google Scholar]
- 87.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Molecular and cellular biology. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kasinath BS, Feliers D, Sataranatarajan K, Ghosh Choudhury G, Lee MJ, Mariappan MM. Regulation of mRNA translation in renal physiology and disease. American journal of physiology. Renal physiology. 2009;297:F1153–1165. doi: 10.1152/ajprenal.90748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kasinath BS, Mariappan MM, Sataranatarajan K, Lee MJ, Feliers D. mRNA translation: unexplored territory in renal science. Journal of the American Society of Nephrology : JASN. 2006;17:3281–3292. doi: 10.1681/ASN.2006050488. [DOI] [PubMed] [Google Scholar]
- 90.Mariappan MM, Feliers D, Mummidi S, Choudhury GG, Kasinath BS. High glucose, high insulin, and their combination rapidly induce laminin-beta1 synthesis by regulation of mRNA translation in renal epithelial cells. Diabetes. 2007;56:476–485. doi: 10.2337/db05-1334. [DOI] [PubMed] [Google Scholar]
- 91.Diaz B, Shani G, Pass I, Anderson D, Quintavalle M, Courtneidge SA. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Science signaling. 2009;2:ra53. doi: 10.1126/scisignal.2000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circulation research. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sedeek M, Hebert RL, Kennedy CR, Burns KD, Touyz RM. Molecular mechanisms of hypertension: role of Nox family NADPH oxidases. Current opinion in nephrology and hypertension. 2009;18:122–127. doi: 10.1097/MNH.0b013e32832923c3. [DOI] [PubMed] [Google Scholar]
- 94.Wu RF, Ma Z, Myers DP, Terada LS. HIV-1 Tat activates dual Nox pathways leading to independent activation of ERK and JNK MAP kinases. The Journal of biological chemistry. 2007;282:37412–37419. doi: 10.1074/jbc.M704481200. [DOI] [PubMed] [Google Scholar]
- 95.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free radical biology & medicine. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:1368–1376. doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- 97.von Lohneysen K, Noack D, Jesaitis AJ, Dinauer MC, Knaus UG. Mutational analysis reveals distinct features of the Nox4-p22 phox complex. The Journal of biological chemistry. 2008;283:35273–35282. doi: 10.1074/jbc.M804200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circulation research. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G, Barnes JL, Abboud HE. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. The Journal of biological chemistry. 2010;285:37503–37512. doi: 10.1074/jbc.M110.136796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes to cells : devoted to molecular & cellular mechanisms. 2005;10:1139–1151. doi: 10.1111/j.1365-2443.2005.00907.x. [DOI] [PubMed] [Google Scholar]
- 104.Liu RM, Choi J, Wu JH, Gaston Pravia KA, Lewis KM, Brand JD, Mochel NS, Krzywanski DM, Lambeth JD, Hagood JS, Forman HJ, Thannickal VJ, Postlethwait EM. Oxidative modification of nuclear mitogen-activated protein kinase phosphatase 1 is involved in transforming growth factor beta1-induced expression of plasminogen activator inhibitor 1 in fibroblasts. The Journal of biological chemistry. 2010;285:16239–16247. doi: 10.1074/jbc.M110.111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maalouf RM, Eid AA, Gorin YC, Block K, Escobar GP, Bailey S, Abboud HE. Nox4-derived reactive oxygen species mediate cardiomyocyte injury in early type 1 diabetes. American journal of physiology. Cell physiology. 2012;302:C597–604. doi: 10.1152/ajpcell.00331.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peshavariya HM, Dusting GJ, Selemidis S. Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free radical research. 2007;41:699–712. doi: 10.1080/10715760701297354. [DOI] [PubMed] [Google Scholar]
- 107.Spencer NY, Yan Z, Boudreau RL, Zhang Y, Luo M, Li Q, Tian X, Shah AM, Davisson RL, Davidson B, Banfi B, Engelhardt JF. Control of hepatic nuclear superoxide production by glucose 6-phosphate dehydrogenase and NADPH oxidase-4. The Journal of biological chemistry. 2011;286:8977–8987. doi: 10.1074/jbc.M110.193821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49:717–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Infanger DW, Cao X, Butler SD, Burmeister MA, Zhou Y, Stupinski JA, Sharma RV, Davisson RL. Silencing nox4 in the paraventricular nucleus improves myocardial infarction-induced cardiac dysfunction by attenuating sympathoexcitation and periinfarct apoptosis. Circulation research. 2010;106:1763–1774. doi: 10.1161/CIRCRESAHA.109.213025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, Barthel K, Schuhmann MK, Herrmann AM, Meuth SG, Stoll G, Meurer S, Schrewe A, Becker L, Gailus-Durner V, Fuchs H, Klopstock T, de Angelis MH, Jandeleit-Dahm K, Shah AM, Weissmann N, Schmidt HH. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS biology. 2010;8 doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. The Journal of biological chemistry. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 113.Lee HS, Song CY. Oxidized low-density lipoprotein and oxidative stress in the development of glomerulosclerosis. American journal of nephrology. 2009;29:62–70. doi: 10.1159/000151277. [DOI] [PubMed] [Google Scholar]
- 114.Miyata K, Rahman M, Shokoji T, Nagai Y, Zhang GX, Sun GP, Kimura S, Yukimura T, Kiyomoto H, Kohno M, Abe Y, Nishiyama A. Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. Journal of the American Society of Nephrology : JASN. 2005;16:2906–2912. doi: 10.1681/ASN.2005040390. [DOI] [PubMed] [Google Scholar]
- 115.Shi XY, Hou FF, Niu HX, Wang GB, Xie D, Guo ZJ, Zhou ZM, Yang F, Tian JW, Zhang X. Advanced oxidation protein products promote inflammation in diabetic kidney through activation of renal nicotinamide adenine dinucleotide phosphate oxidase. Endocrinology. 2008;149:1829–1839. doi: 10.1210/en.2007-1544. [DOI] [PubMed] [Google Scholar]
- 116.Suzuki H, Usui I, Kato I, Oya T, Kanatani Y, Yamazaki Y, Fujisaka S, Senda S, Ishii Y, Urakaze M, Mahmood A, Takasawa S, Okamoto H, Kobayashi M, Tobe K, Sasahara M. Deletion of platelet-derived growth factor receptor-beta improves diabetic nephropathy in Ca(2)(+)/calmodulin-dependent protein kinase IIalpha (Thr286Asp) transgenic mice. Diabetologia. 2011;54:2953–2962. doi: 10.1007/s00125-011-2270-x. [DOI] [PubMed] [Google Scholar]
- 117.Taira M, Toba H, Murakami M, Iga I, Serizawa R, Murata S, Kobara M, Nakata T. Spironolactone exhibits direct renoprotective effects and inhibits renal renin-angiotensin-aldosterone system in diabetic rats. European journal of pharmacology. 2008;589:264–271. doi: 10.1016/j.ejphar.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 118.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Science's STKE : signal transduction knowledge environment. 2006;2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 119.Wagner B, Ricono J, Gorin Y, Block K, Arar M, Riley D, Choudhury G, HE. A. Mitogenic signaling via platelet-derived growth factor beta in metanephric mesenchymal cells. J Am Soc Nephrol. 2007;18:2903–2911. doi: 10.1681/ASN.2006111229. [DOI] [PubMed] [Google Scholar]
- 120.Arozal W, Watanabe K, Veeraveedu PT, Ma M, Thandavarayan RA, Suzuki K, Tachikawa H, Kodama M, Aizawa Y. Effects of angiotensin receptor blocker on oxidative stress and cardio-renal function in streptozotocin-induced diabetic rats. Biological & pharmaceutical bulletin. 2009;32:1411–1416. doi: 10.1248/bpb.32.1411. [DOI] [PubMed] [Google Scholar]
- 121.Fan Q, Liao J, Kobayashi M, Yamashita M, Gu L, Gohda T, Suzuki Y, Wang LN, Horikoshi S, Tomino Y. Candesartan reduced advanced glycation end-products accumulation and diminished nitro-oxidative stress in type 2 diabetic KK/Ta mice. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association -European Renal Association. 2004;19:3012–3020. doi: 10.1093/ndt/gfh499. [DOI] [PubMed] [Google Scholar]
- 122.Lakshmanan AP, Watanabe K, Thandavarayan RA, Sari FR, Harima M, Giridharan VV, Soetikno V, Kodama M, Aizawa Y. Telmisartan attenuates oxidative stress and renal fibrosis in streptozotocin induced diabetic mice with the alteration of angiotensin-(1-7) mas receptor expression associated with its PPAR-gamma agonist action. Free radical research. 2011;45:575–584. doi: 10.3109/10715762.2011.560149. [DOI] [PubMed] [Google Scholar]
- 123.Nagai Y, Yao L, Kobori H, Miyata K, Ozawa Y, Miyatake A, Yukimura T, Shokoji T, Kimura S, Kiyomoto H, Kohno M, Abe Y, Nishiyama A. Temporary angiotensin II blockade at the prediabetic stage attenuates the development of renal injury in type 2 diabetic rats. Journal of the American Society of Nephrology : JASN. 2005;16:703–711. doi: 10.1681/ASN.2004080649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sonta T, Inoguchi T, Matsumoto S, Yasukawa K, Inuo M, Tsubouchi H, Sonoda N, Kobayashi K, Utsumi H, Nawata H. In vivo imaging of oxidative stress in the kidney of diabetic mice and its normalization by angiotensin II type 1 receptor blocker. Biochemical and biophysical research communications. 2005;330:415–422. doi: 10.1016/j.bbrc.2005.02.174. [DOI] [PubMed] [Google Scholar]
- 125.Onozato ML, Tojo A, Kobayashi N, Goto A, Matsuoka H, Fujita T. Dual blockade of aldosterone and angiotensin II additively suppresses TGF-beta and NADPH oxidase in the hypertensive kidney. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007;22:1314–1322. doi: 10.1093/ndt/gfl780. [DOI] [PubMed] [Google Scholar]
- 126.Wei XF, Zhou QG, Hou FF, Liu BY, Liang M. Advanced oxidation protein products induce mesangial cell perturbation through PKC-dependent activation of NADPH oxidase. American journal of physiology. Renal physiology. 2009;296:F427–437. doi: 10.1152/ajprenal.90536.2008. [DOI] [PubMed] [Google Scholar]
- 127.Xia L, Wang H, Munk S, Kwan J, Goldberg HJ, Fantus IG, Whiteside CI. High glucose activates PKC-zeta and NADPH oxidase through autocrine TGF-beta1 signaling in mesangial cells. American journal of physiology. Renal physiology. 2008;295:F1705–1714. doi: 10.1152/ajprenal.00043.2008. [DOI] [PubMed] [Google Scholar]
- 128.Etoh T, Inoguchi T, Kakimoto M, Sonoda N, Kobayashi K, Kuroda J, Sumimoto H, Nawata H. Increased expression of NAD(P)H oxidase subunits, NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibity by interventive insulin treatment. Diabetologia. 2003;46:1428–1437. doi: 10.1007/s00125-003-1205-6. [DOI] [PubMed] [Google Scholar]
- 129.Fujii M, Inoguchi T, Sasaki S, Maeda Y, Zheng J, Kobayashi K, Takayanagi R. Bilirubin and biliverdin protect rodents against diabetic nephropathy by downregulating NAD(P)H oxidase. Kidney international. 2010;78:905–919. doi: 10.1038/ki.2010.265. [DOI] [PubMed] [Google Scholar]
- 130.Maeda Y, Inoguchi T, Takei R, Sawada F, Sasaki S, Fujii M, Kobayashi K, Urata H, Nishiyama A, Takayanagi R. Inhibition of chymase protects against diabetes-induced oxidative stress and renal dysfunction in hamsters. American journal of physiology. Renal physiology. 2010;299:F1328–1338. doi: 10.1152/ajprenal.00337.2010. [DOI] [PubMed] [Google Scholar]
- 131.Abboud HE. Mesangial cell biology. Experimental cell research. 2012;318:979–985. doi: 10.1016/j.yexcr.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 132.Shah A, Xia L, Goldberg H, Lee KW, Quaggin SE, Fantus IG. Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species (ROS) generation by mitochondria and the NADPH oxidase, Nox4, in mesangial cells. The Journal of biological chemistry. 2013 doi: 10.1074/jbc.M112.419101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fu Y, Zhang Y, Wang Z, Wang L, Wei X, Zhang B, Wen Z, Fang H, Pang Q, Yi F. Regulation of NADPH oxidase activity is associated with miRNA-25-mediated NOX4 expression in experimental diabetic nephropathy. American journal of nephrology. 2010;32:581–589. doi: 10.1159/000322105. [DOI] [PubMed] [Google Scholar]
- 134.Jeong SI, Kim SJ, Kwon TH, Yu KY, Kim SY. Schizandrin prevents damage of murine mesangial cells via blocking NADPH oxidase-induced ROS signaling in high glucose. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2012;50:1045–1053. doi: 10.1016/j.fct.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 135.Xia L, Wang H, Goldberg HJ, Munk S, Fantus IG, Whiteside CI. Mesangial cell NADPH oxidase upregulation in high glucose is protein kinase C dependent and required for collagen IV expression. American journal of physiology. Renal physiology. 2006;290:F345–356. doi: 10.1152/ajprenal.00119.2005. [DOI] [PubMed] [Google Scholar]
- 136.Zhang L, Pang S, Deng B, Qian L, Chen J, Zou J, Zheng J, Yang L, Zhang C, Chen X, Liu Z, Le Y. High glucose induces renal mesangial cell proliferation and fibronectin expression through JNK/NF-kappaB/NADPH oxidase/ROS pathway, which is inhibited by resveratrol. The international journal of biochemistry & cell biology. 2012;44:629–638. doi: 10.1016/j.biocel.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 137.Whiteside C, Wang H, Xia L, Munk S, Goldberg HJ, Fantus IG. Rosiglitazone prevents high glucose-induced vascular endothelial growth factor and collagen IV expression in cultured mesangial cells. Experimental diabetes research. 2009;2009:910783. doi: 10.1155/2009/910783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gojo A, Utsunomiya K, Taniguchi K, Yokota T, Ishizawa S, Kanazawa Y, Kurata H, Tajima N. The Rho-kinase inhibitor, fasudil, attenuates diabetic nephropathy in streptozotocin-induced diabetic rats. European journal of pharmacology. 2007;568:242–247. doi: 10.1016/j.ejphar.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 139.Block K, Ricono JM, Lee DY, Bhandari B, Choudhury GG, Abboud HE, Gorin Y. Arachidonic acid-dependent activation of a p22(phox)-based NAD(P)H oxidase mediates angiotensin II-induced mesangial cell protein synthesis and fibronectin expression via Akt/PKB. Antioxidants & redox signaling. 2006;8:1497–1508. doi: 10.1089/ars.2006.8.1497. [DOI] [PubMed] [Google Scholar]
- 140.Kwan J, Wang H, Munk S, Xia L, Goldberg HJ, Whiteside CI. In high glucose protein kinase C-zeta activation is required for mesangial cell generation of reactive oxygen species. Kidney international. 2005;68:2526–2541. doi: 10.1111/j.1523-1755.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 141.Jones SA, Hancock JT, Jones OT, Neubauer A, Topley N. The expression of NADPH oxidase components in human glomerular mesangial cells: detection of protein and mRNA for p47phox, p67phox, and p22phox. Journal of the American Society of Nephrology : JASN. 1995;5:1483–1491. doi: 10.1681/ASN.V571483. [DOI] [PubMed] [Google Scholar]
- 142.Liu GC, Fang F, Zhou J, Koulajian K, Yang S, Lam L, Reich HN, John R, Herzenberg AM, Giacca A, Oudit GY, Scholey JW. Deletion of p47 ( phox ) attenuates the progression of diabetic nephropathy and reduces the severity of diabetes in the Akita mouse. Diabetologia. 2012 doi: 10.1007/s00125-012-2586-1. [DOI] [PubMed] [Google Scholar]
- 143.Pleskova M, Beck KF, Behrens MH, Huwiler A, Fichtlscherer B, Wingerter O, Brandes RP, Mulsch A, Pfeilschifter J. Nitric oxide down-regulates the expression of the catalytic NADPH oxidase subunit Nox1 in rat renal mesangial cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:139–141. doi: 10.1096/fj.05-3791fje. [DOI] [PubMed] [Google Scholar]
- 144.Kitada M, Koya D, Sugimoto T, Isono M, Araki S, Kashiwagi A, Haneda M. Translocation of glomerular p47phox and p67phox by protein kinase C-beta activation is required for oxidative stress in diabetic nephropathy. Diabetes. 2003;52:2603–2614. doi: 10.2337/diabetes.52.10.2603. [DOI] [PubMed] [Google Scholar]
- 145.Hua H, Munk S, Goldberg H, Fantus IG, Whiteside CI. High glucose-suppressed endothelin-1 Ca2+ signaling via NADPH oxidase and diacylglycerol-sensitive protein kinase C isozymes in mesangial cells. The Journal of biological chemistry. 2003;278:33951–33962. doi: 10.1074/jbc.M302823200. [DOI] [PubMed] [Google Scholar]
- 146.You YH, Okada S, Ly S, Jandeleit-Dahm KA, Barit D, Namikoshi T, Sharma K. Role of Nox2 in Diabetic Kidney Disease. American journal of physiology. Renal physiology. 2013 doi: 10.1152/ajprenal.00511.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells : nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circulation research. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 148.Lavrentyev EN, Malik KU. High glucose-induced Nox1-derived superoxides downregulate PKC-betaII, which subsequently decreases ACE2 expression and ANG(1-7) formation in rat VSMCs. American journal of physiology. Heart and circulatory physiology. 2009;296:H106–118. doi: 10.1152/ajpheart.00239.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ding G, Zhang A, Huang S, Pan X, Zhen G, Chen R, Yang T. ANG II induces c-Jun NH2-terminal kinase activation and proliferation of human mesangial cells via redox-sensitive transactivation of the EGFR. American journal of physiology. Renal physiology. 2007;293:F1889–1897. doi: 10.1152/ajprenal.00112.2007. [DOI] [PubMed] [Google Scholar]
- 150.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ. Adiponectin regulates albuminuria and podocyte function in mice. The Journal of clinical investigation. 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Piwkowska A, Rogacka D, Audzeyenka I, Jankowski M, Angielski S. High glucose concentration affects the oxidant-antioxidant balance in cultured mouse podocytes. Journal of cellular biochemistry. 2011;112:1661–1672. doi: 10.1002/jcb.23088. [DOI] [PubMed] [Google Scholar]
- 152.Piwkowska A, Rogacka D, Jankowski M, Dominiczak MH, Stepinski JK, Angielski S. Metformin induces suppression of NAD(P)H oxidase activity in podocytes. Biochemical and biophysical research communications. 2010;393:268–273. doi: 10.1016/j.bbrc.2010.01.119. [DOI] [PubMed] [Google Scholar]
- 153.McCarty MF, Barroso-Aranda J, Contreras F. AMP-activated kinase may suppress NADPH oxidase activation in vascular tissues. Medical hypotheses. 2009;72:468–470. doi: 10.1016/j.mehy.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 154.Schuhmacher S, Foretz M, Knorr M, Jansen T, Hortmann M, Wenzel P, Oelze M, Kleschyov AL, Daiber A, Keaney JF, Jr., Wegener G, Lackner K, Munzel T, Viollet B, Schulz E. alpha1AMP-activated protein kinase preserves endothelial function during chronic angiotensin II treatment by limiting Nox2 upregulation. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:560–566. doi: 10.1161/ATVBAHA.110.219543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu C, Viollet B, Yan D, Zou MH. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circulation research. 2010;106:1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Greiber S, Munzel T, Kastner S, Muller B, Schollmeyer P, Pavenstadt H. NAD(P)H oxidase activity in cultured human podocytes: effects of adenosine triphosphate. Kidney international. 1998;53:654–663. doi: 10.1046/j.1523-1755.1998.00796.x. [DOI] [PubMed] [Google Scholar]
- 157.Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Current diabetes reviews. 2008;4:39–45. doi: 10.2174/157339908783502370. [DOI] [PubMed] [Google Scholar]
- 158.Whaley-Connell A, Habibi J, Nistala R, Cooper SA, Karuparthi PR, Hayden MR, Rehmer N, DeMarco VG, Andresen BT, Wei Y, Ferrario C, Sowers JR. Attenuation of NADPH oxidase activation and glomerular filtration barrier remodeling with statin treatment. Hypertension. 2008;51:474–480. doi: 10.1161/HYPERTENSIONAHA.107.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, Hebert RL. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. American journal of physiology. Renal physiology. 2010;299:F1348–1358. doi: 10.1152/ajprenal.00028.2010. [DOI] [PubMed] [Google Scholar]
- 160.Ohshiro Y, Ma RC, Yasuda Y, Hiraoka-Yamamoto J, Clermont AC, Isshiki K, Yagi K, Arikawa E, Kern TS, King GL. Reduction of diabetes-induced oxidative stress, fibrotic cytokine expression, and renal dysfunction in protein kinase Cbeta-null mice. Diabetes. 2006;55:3112–3120. doi: 10.2337/db06-0895. [DOI] [PubMed] [Google Scholar]
- 161.Lee JH, Kim JH, Kim JS, Chang JW, Kim SB, Park JS, Lee SK. AMP-activated protein kinase inhibits TGF-beta-, angiotensin II-, aldosterone-, high glucose- and albumin-induced epithelial-mesenchymal transition. American journal of physiology. Renal physiology. 2013 doi: 10.1152/ajprenal.00148.2012. [DOI] [PubMed] [Google Scholar]
- 162.Chen J, Chen JK, Harris RC. Angiotensin II induces epithelial-to-mesenchymal transition in renal epithelial cells through reactive oxygen species/Src/caveolin-mediated activation of an epidermal growth factor receptor-extracellular signal-regulated kinase signaling pathway. Molecular and cellular biology. 2012;32:981–991. doi: 10.1128/MCB.06410-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Peng ZZ, Hu GY, Shen H, Wang L, Ning WB, Xie YY, Wang NS, Li BX, Tang YT, Tao LJ. Fluorofenidone attenuates collagen I and transforming growth factor-beta1 expression through a nicotinamide adenine dinucleotide phosphate oxidase-dependent way in NRK-52E cells. Nephrology (Carlton) 2009;14:565–572. doi: 10.1111/j.1440-1797.2009.01129.x. [DOI] [PubMed] [Google Scholar]
- 164.Xu Y, Ruan S, Xie H, Lin J. Role of LOX-1 in Ang II-induced oxidative functional damage in renal tubular epithelial cells. International journal of molecular medicine. 2010;26:679–690. doi: 10.3892/ijmm_00000514. [DOI] [PubMed] [Google Scholar]
- 165.Kim SM, Kim YG, Jeong KH, Lee SH, Lee TW, Ihm CG, Moon JY. Angiotensin II-induced mitochondrial Nox4 is a major endogenous source of oxidative stress in kidney tubular cells. PloS one. 2012;7:e39739. doi: 10.1371/journal.pone.0039739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hannken T, Schroeder R, Stahl RA, Wolf G. Angiotensin II-mediated expression of p27Kip1 and induction of cellular hypertrophy in renal tubular cells depend on the generation of oxygen radicals. Kidney international. 1998;54:1923–1933. doi: 10.1046/j.1523-1755.1998.00212.x. [DOI] [PubMed] [Google Scholar]
- 167.Takao T, Horino T, Kagawa T, Matsumoto R, Shimamura Y, Ogata K, Inoue K, Taniguchi Y, Taguchi T, Morita T, Terada Y. Possible involvement of intracellular angiotensin II receptor in high-glucose-induced damage in renal proximal tubular cells. Journal of nephrology. 2011;24:218–224. doi: 10.5301/jn.2010.5785. [DOI] [PubMed] [Google Scholar]
- 168.Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh ST, Lee HB. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. Journal of the American Society of Nephrology : JASN. 2005;16:667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- 169.Zhang H, Jiang Z, Chang J, Li X, Zhu H, Lan HY, Zhou SF, Yu X. Role of NAD(P)H oxidase in transforming growth factor-beta1-induced monocyte chemoattractant protein-1 and interleukin-6 expression in rat renal tubular epithelial cells. Nephrology (Carlton) 2009;14:302–310. doi: 10.1111/j.1440-1797.2008.01072.x. [DOI] [PubMed] [Google Scholar]
- 170.Lee HB, Yu MR, Yang Y, Jiang Z, Ha H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. Journal of the American Society of Nephrology : JASN. 2003;14:S241–245. doi: 10.1097/01.asn.0000077410.66390.0f. [DOI] [PubMed] [Google Scholar]
- 171.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. American journal of physiology. Lung cellular and molecular physiology. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 172.Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. American journal of physiology. Regulatory, integrative and comparative physiology. 2011;300:R1009–1022. doi: 10.1152/ajpregu.00809.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nlandu Khodo S, Dizin E, Sossauer G, Szanto I, Martin PY, Feraille E, Krause KH, de Seigneux S. NADPH-Oxidase 4 Protects against Kidney Fibrosis during Chronic Renal Injury. Journal of the American Society of Nephrology : JASN. 2012;23:1967–1976. doi: 10.1681/ASN.2012040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Babelova A, Avaniadi D, Jung O, Fork C, Beckmann J, Kosowski J, Weissmann N, Anilkumar N, Shah AM, Schaefer L, Schroder K, Brandes RP. Role of Nox4 in murine models of kidney disease. Free radical biology & medicine. 2012;53:842–853. doi: 10.1016/j.freeradbiomed.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 175.Matsushima S, Kuroda J, Ago T, Zhai P, Park JY, Xie LH, Tian B, Sadoshima J. Increased Oxidative Stress in the Nucleus Caused by Nox4 Mediates Oxidation of HDAC4 and Cardiac Hypertrophy. Circulation research. 2012 doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Alpers CE, Hudkins KL. Mouse models of diabetic nephropathy. Current opinion in nephrology and hypertension. 2011;20:278–284. doi: 10.1097/MNH.0b013e3283451901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Brosius FC, 3rd, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T. Mouse models of diabetic nephropathy. Journal of the American Society of Nephrology : JASN. 2009;20:2503–2512. doi: 10.1681/ASN.2009070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, Schmidt HH. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cellular and molecular life sciences : CMLS. 2012;69:2327–2343. doi: 10.1007/s00018-012-1010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nature reviews. Drug discovery. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gaggini F, Laleu B, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, Page P. Design, synthesis and biological activity of original pyrazolo-pyrido-diazepine, -pyrazine and -oxazine dione derivatives as novel dual Nox4/Nox1 inhibitors. Bioorganic & medicinal chemistry. 2011;19:6989–6999. doi: 10.1016/j.bmc.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 181.Jaquet V, Scapozza L, Clark RA, Krause KH, Lambeth JD. Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxidants & redox signaling. 2009;11:2535–2552. doi: 10.1089/ars.2009.2585. [DOI] [PubMed] [Google Scholar]
- 182.Kim JA, Neupane GP, Lee ES, Jeong BS, Park BC, Thapa P. NADPH oxidase inhibitors: a patent review. Expert opinion on therapeutic patents. 2011;21:1147–1158. doi: 10.1517/13543776.2011.584870. [DOI] [PubMed] [Google Scholar]
- 183.Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, Page P. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. Journal of medicinal chemistry. 2010;53:7715–7730. doi: 10.1021/jm100773e. [DOI] [PubMed] [Google Scholar]
- 184.Lambeth JD, Krause KH, Clark RA. NOX enzymes as novel targets for drug development. Seminars in immunopathology. 2008;30:339–363. doi: 10.1007/s00281-008-0123-6. [DOI] [PubMed] [Google Scholar]
- 185.Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, Brenner DA. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. 2012;56:2316–2327. doi: 10.1002/hep.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jiang JX, Chen X, Serizawa N, Szyndralewicz C, Page P, Schroder K, Brandes RP, Devaraj S, Torok NJ. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free radical biology & medicine. 2012 doi: 10.1016/j.freeradbiomed.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sedeek M, Gutsol A, Montezano AC, Burger D, Nguyen Dinh Cat A, Kennedy CR, Burns KD, Cooper ME, Jandeleit-Dahm K, Page P, Szyndralewiez C, Heitz F, Hebert RL, Touyz RM. Renoprotective effects of a novel Nox1/4 inhibitor in a mouse model of Type 2 diabetes. Clin Sci (Lond) 2013;124:191–202. doi: 10.1042/CS20120330. [DOI] [PubMed] [Google Scholar]
- 188.Sedeek M, Montezano AC, Hebert RL, Gray SP, Di Marco E, Jha JC, Cooper ME, Jandeleit-Dahm K, Schiffrin EL, Wilkinson-Berka JL, Touyz RM. Oxidative stress, Nox isoforms and complications of diabetes--potential targets for novel therapies. Journal of cardiovascular translational research. 2012;5:509–518. doi: 10.1007/s12265-012-9387-2. [DOI] [PubMed] [Google Scholar]
- 189.Vendrov AE, Madamanchi NR, Niu XL, Molnar KC, Runge M, Szyndralewiez C, Page P, Runge MS. NADPH oxidases regulate CD44 and hyaluronic acid expression in thrombin-treated vascular smooth muscle cells and in atherosclerosis. The Journal of biological chemistry. 2010;285:26545–26557. doi: 10.1074/jbc.M110.143917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Carnesecchi S, Deffert C, Donati Y, Basset O, Hinz B, Preynat-Seauve O, Guichard C, Arbiser JL, Banfi B, Pache JC, Barazzone-Argiroffo C, Krause KH. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxidants & redox signaling. 2011;15:607–619. doi: 10.1089/ars.2010.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]