Abstract
Objective
The plasmin/plasminogen system is involved in atherosclerosis. However, the mechanisms by which it stimulates disease are not fully defined. A key event in atherogenesis is the deposition of LDL on arterial walls where it is modified, aggregated and retained. Macrophages are recruited to clear the lipoproteins, and they become foam cells. The goal of this study was to assess the role of plasmin in macrophage uptake of aggregated LDL and foam cell formation.
Approach and Results
Plasminogen treatment of macrophages catabolizing aggregated LDL significantly accelerated foam cell formation. Macrophage interaction with aggregated LDL increased the surface expression of urokinase-type plasminogen activator receptor and plasminogen activator activity, resulting in increased ability to generate plasmin at the cell surface. The high local level of plasmin cleaves cell-associated aggregated LDL, allowing a portion of the aggregate to become sequestered in a nearly sealed, yet extracellular, acidic compartment. The low pH in the plasmin-induced compartment allows lysosomal enzymes, delivered via lysosome exocytosis, greater activity, resulting in more efficient cholesteryl ester hydrolysis and delivery of a large cholesterol load to the macrophage, thereby promoting foam cell formation.
Conclusion
These findings highlight a critical role for plasmin in the catabolism of aggregated LDL by macrophages and provide a new context for considering the atherogenic role of plasmin.
Keywords: Atherosclerosis, foam cell, plasmin, macrophage, aggregated low density lipoprotein
Multiple lines of evidence suggest that the plasmin/plasminogen system is involved in the initiation and progression of atherosclerosis1-9. Plasmin is a fibrinolytic serine protease capable of directly degrading components of the extracellular matrix as well as activating matrix metalloproteinases10-12. Local increases in fibrinolytic activity associated with human atherosclerotic vessels were originally reported more than 40 years ago13 and have since been confirmed1-4, 10. Plasmin is generated via the cleavage of plasminogen by either urokinase-type plasminogen activator (uPA) or tissue plasminogen activator. Monocytes/macrophages are a major source of uPA in atherosclerotic lesions10 where it is synthesized in an immature form, known as pro-uPA14. Pro-uPA becomes activated when it binds to the cell surface receptor urokinase-type plasminogen activator receptor (uPAR), thereby accelerating the conversion of plasminogen to plasmin15. In spite of extensive research, the role of the plasmin/plasminogen system in atherosclerotic lesion formation and progression remains incompletely characterized.
Studies in both humans and mouse models indicate an atherogenic role for plasmin1. In several large clinical studies elevated plasma concentrations of both plasmin and its precursor plasminogen were shown to be risk factors for both atherosclerosis and myocardial infarcts2-4. Further, the expression of uPA10, 16, 17 and uPAR18, 19 is upregulated in atherosclerotic lesions and correlates with disease severity16,18. Immunolocalization analyses have demonstrated uPAR staining localized to macrophages in the neointima of atherosclerotic lesions, but little uPAR is found on macrophages in non-atherosclerotic arteries19. Further, macrophage-targeted uPA overexpression is atherogenic in both ApoE−/− and LDLR−/− mice6,7, 8, while macrophage specific uPA knockout is atheroprotective in ApoE −/− mice9. These data strongly support the hypothesis that elevated uPA expression by artery wall macrophages accelerates atherosclerosis. Despite the fact that both animal and human studies have indicated a key role for macrophages in the atherogenic mechanisms of the plasmin/plasminogen system, most research has focused on plasmin mediated modification of the extracellular matrix20, 21. As such, a coherent picture of the role of the plasmin/plasminogen system in atherogenesis, in particular with respect to macrophage biology, has not yet emerged.
Our laboratory is interested in the conversion of macrophages to foam cells via their interaction with aggregated low density lipoprotein (agLDL). Although most studies examine foam cell formation via the incubation of macrophages with modified monomeric LDL (e.g., oxidized LDL), this does not accurately reflect the in vivo environment as the vast majority of the LDL in atherosclerotic plaques is aggregated and avidly bound to the subendothelial matrix22-24. For example, over 90% of lesional lipoproteins in human aortic fatty streaks were not released by extraction or by electrophoresis24, and monocyte/macrophage interaction with agLDL in atherosclerotic lesions has been visualized with electron microscopy25. Thus, mechanisms of foam cell formation based on ingestion of aggregated, rather than monomeric LDL, may be more physiologically relevant.
Previous studies in our laboratory and others have elucidated a novel pathway for macrophage foam cell formation via catabolism of agLDL26-28. We have shown that when macrophages come into contact with LDL aggregates, an extracellular, acidic, hydrolytic compartment (a lysosomal synapse) is formed. Lysosomes are delivered to the lysosomal synapse via targeted exocytosis, which results in the hydrolysis of LDL cholesteryl esters (CEs) and transfer of free cholesterol (FC) to the macrophage with subsequent foam cell formation27. The Kruth laboratory has examined the effects of plasmin on macrophages interacting with agLDL29. They found that plasmin treatment can disaggregate and release much, but not all, of the agLDL contained in the lysosomal synapse (also called a surface-connected compartment), generating lipoprotein structures similar to those observed extracellularly in atherosclerotic lesions. It is likely that the plasmin-mediated release of agLDL is caused by degradation of apolipoprotein B30.
In this study we examine the effects of plasmin on the interaction between macrophages and aggregated lipoproteins. Rather than examining the aggregate released by plasmin treatment, we focus on the portion of the aggregate that is not released from the lysosomal synapse and remains cell associated. Surprisingly, we found that plasminogen treatment of macrophages interacting with agLDL caused a significant increase in foam cell formation. Incubation of macrophages with agLDL increased the surface expression of uPAR and plasminogen activator (PA) activity, which would produce a high level of plasmin near the cell surface. To understand the mechanism by which plasmin promotes foam cell formation we visualized the effects of plasmin treatment on macrophage agLDL interactions using several microscopy and biochemical techniques. These experiments indicate that plasmin cleaves cell-associated agLDL, resulting in changes in the morphology of the lysosomal synapse and allowing a portion of the aggregate to be sequestered in a nearly sealed, yet extracellular, actin-dependent, acidic compartment. The morphologic changes in the compartment induced by plasmin facilitate generation of a more acidic environment, which in turn allows lysosomal enzymes greater activity. This results in more efficient CE hydrolysis and the delivery of a large cholesterol load to the macrophage. These findings indicate that physiological plasminogen concentrations are sufficient for plasmin-mediated agLDL processing and provide a mechanism for the ability of plasmin to accelerate foam cell formation and atherosclerosis. A detailed understanding of the mechanisms of foam cell formation is imperative for successful therapeutic targeting of atherosclerosis.
MATERIALS AND METHODS
The Materials and Methods is supplied in the Supplementary Material.
RESULTS
Macrophage incubation with plasminogen accelerates foam cell formation
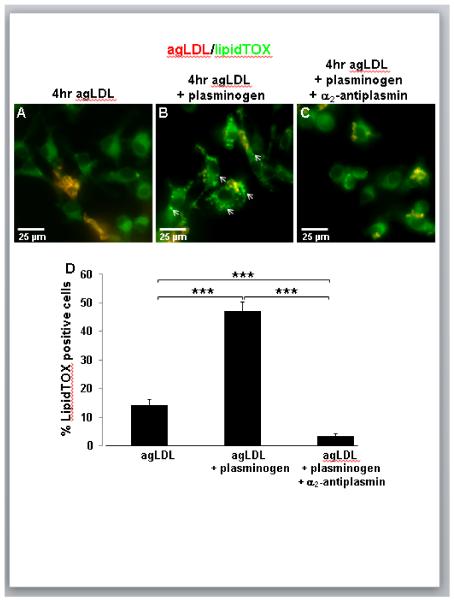
In order to examine the effects of plasmin on macrophage uptake of agLDL, bone marrow derived macrophages (BMMs) interacting with AlexaFluor-546 (Alexa546)-agLDL were incubated for 4 hr in the absence (Fig. 1A) or presence of a physiological concentration of plasminogen without (Fig. 1B) or with (Fig. 1C) α2-antiplasmin treatment. Following agLDL incubation, cells were fixed and labeled with LipidTOX Green to detect lipid droplets. LipidTOX Green will also stain the CE portion of the agLDL, so aggregates appear yellow/orange. In the absence of plasminogen most agLDL remained extracellular after 4 hr of incubation, and only a small percentage of cells containing LipidTOX-positive droplets could be seen (Fig. 1A). However, BMMs incubated with agLDL in the presence of plasminogen resulted in the formation of numerous cells containing neutral-lipid droplets (arrows, Fig. 1B), indicative of foam cell formation. The percentage of LipidTOX-positive cells was quantified for each condition. Treatment with a physiological concentration of plasminogen caused a three-fold increase in the number of lipid droplet containing BMMs (Fig. 1D). When the effects of plasminogen on foam cell formation in human monocyte derived macrophages (huMDMs) was examined, a two fold increase in the number of lipid droplet containing cells was observed (Supplemental Figure I).
Figure 1. Macrophage incubation with agLDL in the presence of a physiological concentration of plasminogen results in increased foam cell formation.
(A-C) BMMs were incubated for 4 hr with Alexa546-agLDL (red) in the absence (A) or presence of 2 μM plasminogen (B,C), fixed and labeled with LipidTOX Green (green). Cell incubation with agLDL in the presence of a physiological plasminogen concentration results in the formation of LipidTOX-positive lipid droplets (arrows, B). Application of α2-antiplasmin prevented the formation of LipidTOX-positive lipid droplets (C). (D) Quantification of the percentage of LipidTOX-positive cells demonstrates a three-fold increase in foam cell formation attributable to plasmin. *** P ≤ 0.001 two-way ANOVA. Data compiled from three independent experiments. Error bars represent the standard error of the mean (SEM).
Treatment with plasmin can lead to induction of pro-inflammatory cytokines that may cause cell death31. To investigate whether the increase in foam cell formation observed with the addition of plasminogen was partially due to macrophage phagocytosis of dead cells, we performed a control in which cells were incubated with plasminogen in the absence of agLDL. There was no difference in the amount of LipidTOX-positive BMMs in plasminogen treated cells and untreated cells (data not shown).
Cells incubated with agLDL and treated with both plasminogen and the plasmin inhibitor α2-antiplasmin did not show plasminogen-induced lipid droplet formation (Fig. 1C,D), indicating a specific role for plasmin in intracellular lipid accumulation and foam cell formation. The addition of α2-antiplasmin reduced foam cell formation to levels below that observed for agLDL alone (Fig. 1D). It has been reported that the amount of plasminogen contained in serum is sufficient to act on macrophage agLDL processing29. The experiments shown in Figure 1 were performed in serum-containing medium, so it is possible that both externally added and serum derived plasminogen contribute to the observed increase in foam cell formation. Consistent with this, when the same experiment was performed in serum-free medium, there was no statistically significant difference in foam cell formation between cells treated with agLDL alone and cells treated with plasminogen and α2-antiplasmin in addition to the agLDL (data not shown).
Macrophage incubation with agLDL results in increased surface uPAR expression and PA activity
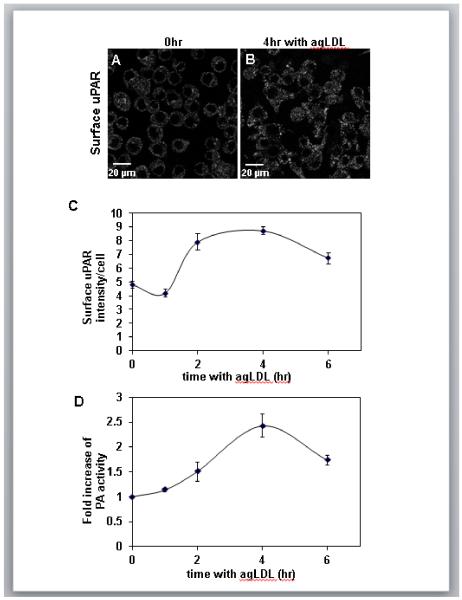
To understand the mechanism by which plasminogen promotes foam cell formation we first investigated the level of local plasmin activity at the macrophage plasma membrane. It is known that uPAR expression can be induced by fatty acid macrophage loading32, 33. Thus, we wondered if macrophage incubation with agLDL would cause an increase in surface uPAR expression. To test this we performed immunofluorescence surface labeling of uPAR in non-permeabilized J774 cells incubated with agLDL. In resting cells, a small amount of uPAR staining was observed at the cell surface (Fig. 2A). Upon treatment with agLDL for 4 hr, the amount of surface uPAR staining was significantly increased (Fig. 2B). Quantification of the surface levels of uPAR at different time points revealed a sharp increase in surface uPAR levels at 2 hr that reached a plateau at 4 hr (Fig. 2C). These results fit with the observation of increased uPAR staining in co-localization with macrophages in human atherosclerotic lesions18, 19.
Figure 2. Surface uPAR expression and PA activity increase during macrophage incubation with agLDL.
(A,B) J774 cells were incubated with agLDL for 0 (A) or 4 hr (B), fixed without permeabilization and labeled with an antibody against uPAR. (C) Quantification of surface uPAR staining of J774 cells incubated with agLDL for indicated periods of time. Values plotted are the integrated surface uPAR staining intensity per cell. Data shown is a representative experiment from three independent experiments. Error bars represent the SEM. (D) J774 cells were incubated in the absence or presence of agLDL for the indicated time periods followed by 30 min incubation with 2 μM plasminogen and the concentration of plasmin generated was measured using a plasmin-specific chromogenic substrate. Changes in PA activity resulting from agLDL incubation were quantified by dividing the plasmin concentration generated by cells incubated with agLDL by the plasmin concentration measured for cells incubated in media alone for the same amount of time. Data compiled from three independent experiments. Error bars represent the SEM.
Next we measured cell-surface associated PA activity as a function of agLDL incubation time. J774 macrophages were incubated with agLDL for various times followed by the addition of plasminogen for 30 min, and the concentration of plasmin generated was measured using a plasmin-specific chromogenic substrate. The increase in PA activity resulting from agLDL incubation was determined by the ratio of the amount of plasmin generated by macrophages incubated with agLDL divided by the plasmin concentration generated by macrophages incubated in media alone (Fig. 2D). Surface PA activity increased 2.5 fold after a 4 hr incubation with agLDL, indicating that macrophages are able to generate high local levels of plasmin at the plasma membrane in response to contact with agLDL.
Plasmin causes morphological changes in the lysosomal synapse that result in compartment tightening
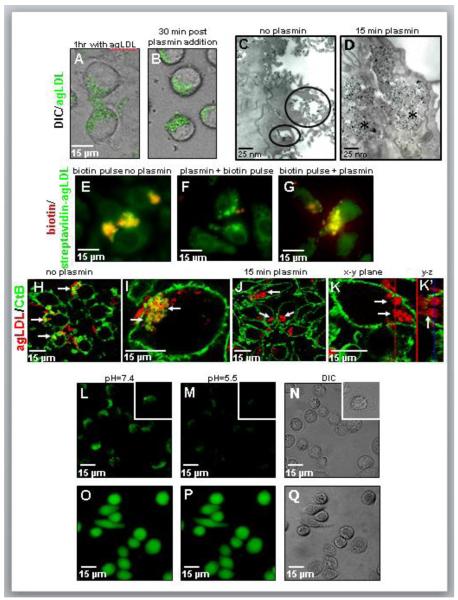
To visualize the consequences of these high local levels of plasmin, we performed time-lapse imaging of cells interacting with Alexa488-agLDL before and during plasmin treatment (Fig. 3A,B). Cells were incubated with Alexa488-agLDL for 1 hr to allow lysosomal synapse formation followed by the addition of 1 U/ml plasmin to the sample on the microscope stage. Consistent with previous studies29, time-lapse imaging shows cleavage and removal of much of the extracellular agLDL upon plasmin treatment, while a portion of the aggregate remains cell-associated (Fig. 3B, See also Supplemental Movie I). To further characterize the fraction of the aggregate that remains cell-associated following plasmin treatment, cells were incubated with colloidal gold labeled agLDL and imaged by transmission electron microscopy. Electron microscopy confirmed the presence of surface associated agLDL residing in compartments at or near the cell surface in the absence of plasmin treatment (circled in Fig. 3C). We have shown previously that these are surface-connected, acidified, hydrolase-containing compartments, which we have termed lysosomal synapses27. After removal of much of the extracellular aggregate by plasmin, the remaining agLDL was in a compartment near the cell surface that appeared to be intracellular in EM thin sections (asterisks in Fig. 3D).
Figure 3. Plasmin causes morphological changes in the lysosomal synapse that result in compartment tightening.
(A,B) Still frames from a time-lapse movie of cells before and after plasmin treatment. J774 cells were incubated with Alexa488-agLDL (green) for 60 min (A), 1 U/ml plasmin was added on the microscope stage and data acquisition was continued. (B) Cells 30 min after plasmin treatment. (C,D) J774 cells were incubated with colloidal gold-labeled agLDL for 45 min, left untreated (C) or treated with 1 U/ml plasmin during the last 15 min of incubation (D). Without plasmin treatment gold-agLDL resides in lysosomal synapses (circled in C). Plasmin treatment results in the formation of intra-cytoplasmic compartments containing condensed agLDL (asterisks in D). (E-G) J774 cells were incubated with streptavidin-Alexa488-agLDL (green) for 60 min and left untreated (E) or treated with 1 U/ml plasmin during the last 15 min of incubation (F,G). Cells were pulsed for 2 min with Alexa546-biotin (red) at the end of incubation (E), after (F) or before (G) plasmin treatment. In the absence of plasmin treatment agLDL in the lysosomal synapse is available for biotin labeling (yellow in E). However, agLDL in plasmin-induced compartments is unavailable for biotin binding after plasmin treatment (absence of yellow in F). AgLDL in plasmin-induced compartments was surface exposed and available for biotin binding before plasmin treatment (yellow in G). (H-K) J774 cells were incubated with Alexa546-agLDL (red) for 45 min (H,I) or for 30 min followed by a 15 min release with 1 U/ml plasmin (J,K) and labeled on ice with Alexa488-CtB (green) before fixation. In the absence of plasmin treatment agLDL residing in the lysosomal synapse is available for CtB labeling (yellow, indicated with arrows in H,I). Plasmin-induced compartments (arrows in J,K) are unavailable for CtB as indicated by the absence of green labeling around agLDL. (K,K’) High magnification confocal image of a plasmin treated cell. AgLDL in plasmin-induced CtB-negative compartments (arrows in K) preserves connection with extracellular agLDL (arrow in K’). The red line in image K indicates the position of the y-z reconstruction shown in K’. Similarly, the blue line in image K’ designates the position of the x-y plane shown in K. (L-N) J774 cells and huMDMs (inset) were incubated with FITC-agLDL (green in L,M) for 45 min, treated with 1 U/ml plasmin for 15 min and quenched with pH=5.5 MES buffer. FITC-agLDL (L) before quenching, (M) after quenching and (N) corresponding DIC. AgLDL in plasmin-induced compartment is quenched by cell impermeant low-pH buffer (M). (O-Q) Control cells, in which the cytoplasm was loaded with BCECF, did not show a decrease in fluorescence upon treatment with MES pH 5.5 (P), confirming that the treatment did not affect the pH of intracellular compartments. BCECF (O) before quenching, (P) after quenching and (Q) corresponding DIC.
To determine if the plasmin-induced compartments are intracellular or extracellular, we tested the accessibility of streptavidin-conjugated agLDL to biotin before and after plasmin treatment (Fig. 3E-G). J774 macrophages incubated with streptavidin-Alexa488-agLDL were exposed to a 2 min pulse of Alexa546-biotin to label extracellular biotin-accessible structures and then fixed. Consistent with previous studies, in the absence of plasmin treatment the biotin-Alexa546 staining clearly showed that the aggregate was largely extracellular following the 1 hr incubation (Fig. 3E)34. However, an Alexa546-biotin pulse performed after plasmin treatment results in the absence of biotin binding (Fig. 3F), indicating that the aggregate in these compartments was not labeled during a brief incubation with Alexa546-biotin. An Alexa546-biotin pulse followed by plasmin treatment results in the formation of biotin-positive compartments (Fig. 3G), confirming that the aggregate in these compartments was surface exposed prior to treatment. These data might indicate that the agLDL is in a fully sealed compartment, such as a phagosome, but we also considered the possibility that the surface-connected compartment had become nearly sealed but was still open to the extracellular space.
We have previously employed plasma membrane labeling with fluorescent cholera toxin subunit B (CtB) to characterize the topological organization of the lysosomal synapse27, 28. In this experiment the cells are not permeabilized, so the macromolecular CtB can only label glycolipids on the plasma membrane. We used this technique to compare the availability of agLDL-containing compartments to CtB with and without plasmin treatment (Fig. 3H-K). In contrast to CtB-positive compartments in non-treated cells (arrows Fig. 3H and I), agLDL-containing plasmin-induced compartments generally lack CtB labeling (arrows Fig. 3J and K). Plasmin-induced compartments in huMDMs also lack CtB labeling (data not shown). The ability of plasmin-induced compartments to exclude CtB and Alexa546-biotin suggests significant changes in their permeability to the extracellular space in comparison to compartments in non-treated cells. In light of these results, it appeared that plasmin treatment caused compartments to fully seal and become intracellular. Thus, we were surprised that in high magnification 3D confocal reconstruction, agLDL in plasmin-induced CtB-negative compartments (Fig. 3K arrows) appears continuous with extracellular portions of the aggregate (Fig. 3K’ arrow). The fact that agLDL in plasmin-induced CtB-negative compartments is still connected to extracellular remnants of the aggregate shows that plasmin-induced compartments preserve connection to the extracellular space.
To further investigate the surface connectivity of plasmin-induced compartments, we used a method based on pH-dependent quenching of extracellular fluorophores. This approach has been used previously to examine compartments that exclude large molecules yet preserve connection to the cell surface35, 36. For instance, endothelial cells form a subjunctional compartment during monocyte diapedesis that is not accessible to fluorescently labeled dextran but is sensitive to changes in extracellular pH36. To examine the surface connectivity of plasmin-induced compartments via modulation of extracellular pH, J774 macrophages and huMDMs were incubated with agLDL labeled with fluorescein isothiocyanate (FITC), a pH sensitive fluorophore, for 45 min, treated with 1U/ml plasmin for 15 min and examined in the absence (Fig. 3L) or presence (Fig. 3M) of a cell impermeant low pH buffer37. The fluorescence of FITC-agLDL in plasmin-induced compartments is efficiently quenched by 2-(N-mopholino)ethanesulfonic acid (MES) (pH 5.5) (Fig. 3L-N). Control cells, in which the cytoplasm was loaded with 2′7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF), a fluorescent pH sensor, did not show a decrease in fluorescence upon treatment with MES pH 5.5 (Fig. 3O-Q), confirming that the treatment did not affect the pH of intracellular compartments. Data from huMDMs also show that the fluorescence of FITC-agLDL in plasmin-induced compartments is efficiently quenched by MES (pH 5.5), indicating that in human macrophages the lysosomal synapse remains surface connected following plasmin treatment (inset Figure 3L-N). Taken together these data show that plasmin-induced compartments preserve connection to the cell surface, but this connection is more nearly sealed than in compartments in non-treated cells.
Formation of plasmin-induced compartments is an F-actin-dependent process
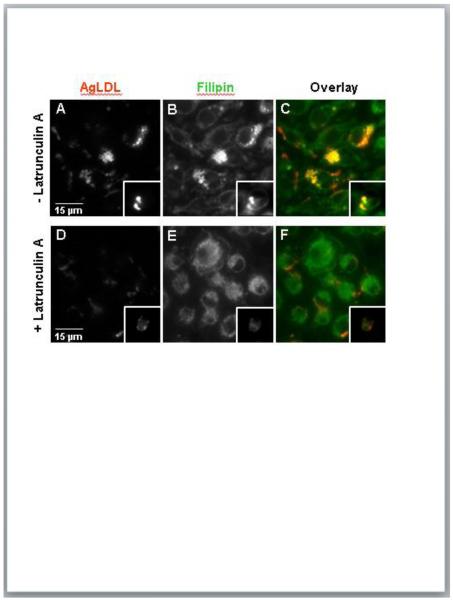
The lysosomal synapse is formed via F-actin-driven membrane protrusions surrounding the aggregate, and enhanced actin polymerization is detected during the first hour of the cell-aggregate interaction28. To test the role of actin polymerization in the formation and tightening of plasmin-induced compartments, we performed plasmin treatment on J774 macrophages and huMDMs in the presence of the F-actin disrupting drug latrunculin A (LatA) (Fig. 4). In the absence of LatA treatment, agLDL was observed primarily in plasmin-induced compartments (Fig. 4A). Consistent with the known function of the lysosomal synapse, the cholesterol-binding dye filipin indicates that these compartments contain high levels of FC (Fig. 4B). However, plasmin treatment in the presence of LatA removed almost all of the cell engaged agLDL and inhibited the formation of plasmin-induced compartments and the generation of FC (Fig. 4D-F). These data confirm the critical role of actin polymerization in the formation of plasmin-induced compartments.
Figure 4. Actin polymerization is required for the formation of plasmin-induced compartments.
J774 cells and huMDMs (inset) were incubated with Alexa546-agLDL (red) for 30 min and then treated with 1 U/ml plasmin during the last 15 min of aggregate incubation in the absence (A-C) or presence of 5 μM LatA (D-F). Cells were fixed and labeled with filipin (green) (B and E). The formation of plasmin-induced compartments is blocked in the presence of LatA.
Plasmin-induced compartment tightening results in increased compartment acidification
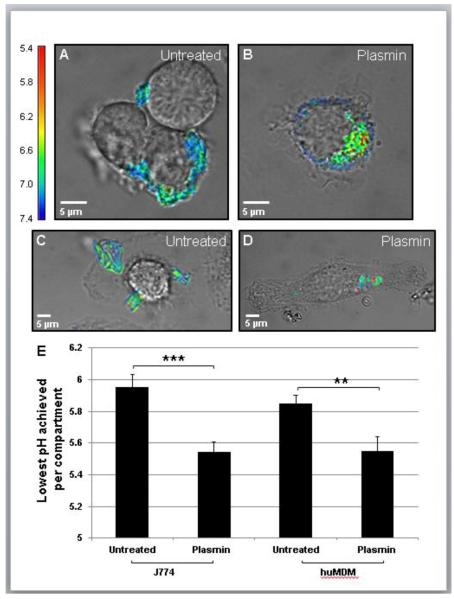
We have previously reported that the lysosomal synapse functions as an extracellular hydrolytic organelle due to targeted exocytosis of lysosomes and compartment acidification by vacuolar (H+)-ATPase on the plasma membrane27. The presence of lysosomal acid lipase (LAL) and a low pH lead to extracellular hydrolysis of CEs27, 28. However, pH values observed in the lysosomal synapse are typically higher than in lysosomes and fluctuate due to diffusion of H+ ions into the extracellular space27. In order to test how tightening of plasmin-induced compartments affects compartment acidification, we labeled LDL with CypHer 5E Mono N-hydroxysuccinimide ester (CypHer 5E) and Alexa488. The fluorescence of CypHer 5E increases as the pH decreases from 7 to 538, while Alexa488 is pH-independent in this range. Macrophages were incubated with dual labeled agLDL, and the pH of the aggregate containing compartment was determined from the ratio of CypHer 5E to Alexa488 fluorescence as compared to values obtained in pH calibration buffers. When J774 and huMDMs interacted with the dual labeled agLDL, regions of low pH could be seen at the contact sites (Fig. 5A-D). Measurements of the lowest pH achieved per compartment revealed that plasmin-induced compartments are significantly more acidic than their counterparts in non-treated cells (Fig. 5E). The optimal activity for LAL is at pH 5.539, which is the average value of the lowest pH recorded in plasmin-induced compartments. The pH in plasmin-induced compartments allows enhanced LAL activity and thereby more efficient CE hydrolysis than in untreated compartments.
Figure 5. Plasmin treatment results in a decrease in compartment pH.
(A-D) J774 cells (A,B) and huMDMs (C,D) were incubated with CypHer/Alexa488-labeled agLDL for 1 hr, left untreated (A,C) or treated with plasmin for the last 15 min of incubation (B,D), rinsed with media and imaged. (E) Quantification of lowest pH achieved in the lysosomal synapse and plasmin-induced compartments from 60 untreated and 60 plasmin-treated J774 cells from three independent experiments and 20 untreated and 20 plasmin-treated huMDMs from one experiment. *** P ≤ 0.001, ** P ≤ 0.005 student’s t test. Error bars represent the SEM.
CE hydrolysis in plasmin-induced compartments is more efficient, leading to the cellular delivery of large amounts of FC
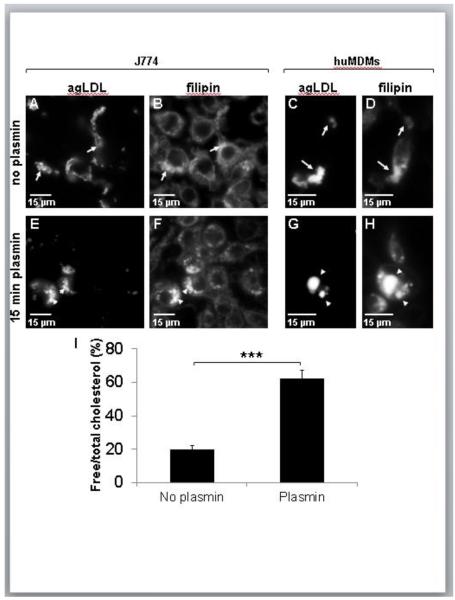
The lower pH of plasmin-induced compartments suggests increased activity of lysosomal enzymes, in particular LAL. To measure changes in agLDL-derived CE hydrolysis resulting from plasmin treatment, we fixed non-treated and plasmin treated J774 macrophages and huMDMs and labeled them with the cholesterol-binding dye filipin (Fig. 6A-H). In non-treated cells agLDL had the typical thread-like morphology, and the filipin signal was increased at sites of contact between aggregates and cells due to CE hydrolysis in the lysosomal synapse (arrows in Fig. 6A-D)27, 28. Plasmin treatment caused a change in agLDL morphology resulting in the formation of condensed, cell-associated structures significantly enriched in FC, as reflected by bright filipin labeling (arrowheads in Fig. 6E-H).
Figure 6. The concentration of agLDL-derived FC in macrophages increases after plasmin treatment.
(A-H) J774 cells (A,B,E,F) and huMDMs (C,D,G,H) were incubated with Alexa546-agLDL for 45 min and left untreated (A-D) or treated with 1 U/ml plasmin for the last 15 min of incubation (E-H). Cells were then fixed and labeled with filipin (B,D,F,H). In the absence of plasmin treatment, a major fraction of the agLDL stays in contact with the cell surface (arrows in A-D). Plasmin treatment results in formation of cell-associated compartments containing condensed agLDL and a significant amount of FC (arrowheads in E-H). (I) J774 cells were incubated with C14-cholesteryl oleate-labeled agLDL for 90 min, left untreated or treated with plasmin during the last 15 min of incubation. After rinsing out extracellular agLDL, cellular lipids were extracted and separated by thin layer chromatography. The amount of agLDL-derived FC and CE was measured by radioautography. *** P ≤ 0.001 student’s t test. Data compiled from two independent experiments. Error bars represent the SEM.
To measure changes in agLDL-derived CE hydrolysis upon plasmin treatment, J774 macrophages were incubated with agLDL reconstituted with 14C-labeled cholesteryl oleate, left untreated or treated with plasmin, and the hydrolysis of CE was measured as described previously27. We found that the fraction of cell-associated CE that was hydrolyzed to FC following plasmin treatment was 3 times higher than in non-treated cells (Fig. 6I). These data show that plasmin treatment of macrophages interacting with agLDL results in the delivery of large amounts of FC to the cell, which facilitates the increase in foam cell formation resulting from plasminogen treatment.
DISCUSSION
A key pathogenic event in the development of atherosclerosis is the retention of lipoprotein particles in the subintima40. These lipoprotein particles undergo oxidative modification, association with extracellular matrix proteoglycans, and aggregation23. Macrophages attempt to clear the lipoproteins and subsequently become foam cells. The physical features of the lipoproteins require distinctive mechanisms for their uptake. In particular, unlike monomeric LDL, the uptake of agLDL does not involve receptor-mediated endocytosis, but rather the aggregate is sequestered in deep invaginations at the cell surface, termed the lysosomal synapse26-28. Our studies elucidate the mechanism of a novel pathway by which the plasmin/plasminogen system may act on macrophage engaged agLDL contributing to accelerated foam cell formation and thereby atherogenesis. The results nicely reconcile the data of the Kruth group, showing that plasminogen releases agLDL from macrophages29, with the reported atherogenicity of the plasmin/plasminogen system.
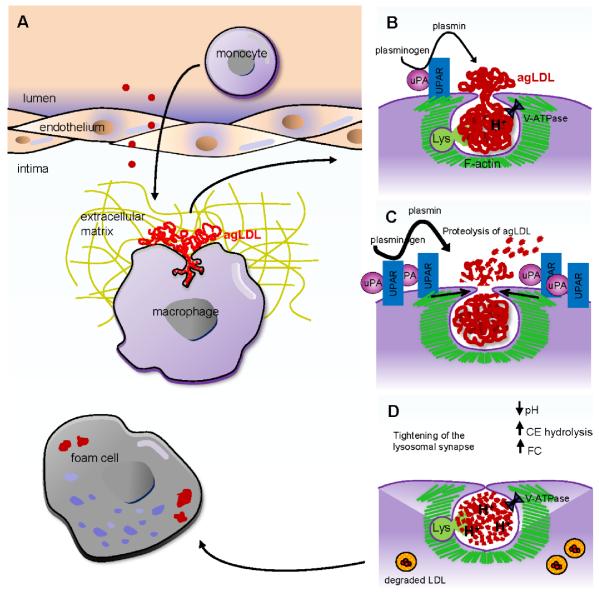
We have shown that plasmin can dramatically alter the early processing of cell-associated agLDL, its uptake by macrophages and foam cell formation. Figure 7 shows a schematic of the proposed mechanism of plasmin action. Monomeric LDL in the blood stream is deposited in the subintimal space, where it becomes oxidatively modified, retained and aggregated. When macrophages come into contact with agLDL, they form an extracellular acidic hydrolytic compartment, a lysosomal synapse (Fig. 7A,B). Interaction of macrophages with agLDL causes upregulation of uPAR and increased PA activity, resulting in the generation of local levels of plasmin sufficient to cleave the aggregate (Fig. 7C). Upregulation of macrophage uPAR is also observed in human atherosclerotic lesions18, 19, possibly due to macrophage interaction with agLDL. The aggregate located in acidic portions of the compartment probably is not cleaved further by plasmin due to the enzyme’s low activity at acidic pH39. Cleavage of macrophage-engaged agLDL results in tightening of the plasmin-induced compartment in an actin dependent manner. Compartment tightening allows more efficient generation of an acidic environment and enhanced activity of lysosomal enzymes that are exocytosed to the lysosomal synapse (Fig. 7D). This results in accelerated agLDL catabolism, leading to the delivery of large amounts of FC to the macrophage, thereby promoting foam cell formation. For clarity, we note that the lysosomal synapse forms independently of plasmin and is then modified by plasmin.
Figure 7. Proposed model explaining the mechanism of plasmin induced foam cell formation.
(A) Monomeric LDL (red) in the blood stream is deposited in the subintimal space, where it becomes oxidatively modified, retained and aggregated. When subintimal macrophages (purple) interact with agLDL (red), an extracellular, acidic, hydrolytic compartment, a lysosomal synapse, is created. (B) The lysosomal synapse is formed by F-actin (green) driven plasma membrane protrusions. The low pH of the compartment is maintained by V-ATPase in the macrophage plasma membrane. FC may be transferred to the macrophage plasma membrane after hydrolysis of LDL CEs by LAL that has been delivered to the compartment via lysosome exocytosis. (C) Macrophage interaction with agLDL results in upregulation of surface uPAR expression and increased PA activity resulting in the generation of local levels of plasmin sufficient to cleave the aggregate. The aggregate located in acidic portions of the compartment is not cleaved by plasmin due to its low activity at acidic pH. (D) Proteolysis of macrophage engaged agLDL results in tightening of the plasmin-induced compartment in an actin dependent process. Compartment tightening allow efficient generation of an acidic environment and enhanced activity of lysosomal enzymes which are exocytosed to the lysosomal synapse. This results in accelerated agLDL catabolism leading to the delivery of large amounts of FC to the macrophage and thereby promoting foam cell formation.
One aspect of the plasmin-induced changes in the physical organization of the lysosomal synapse that we find intriguing is the fact that the compartments do not fully seal. A proposed rationale for the need for extracellular hydrolysis in a lysosomal synapse was that macrophages could not internalize large species or moieties tightly linked to the extracellular matrix, even by phagocytosis. However, following plasmin treatment, there is no apparent reason that the compartments do not fully seal. Further, in the absence of plasmin we have repeatedly observed formation of the lysosomal synapse even with agLDL particles that are small enough to be internalized via phagocytosis. There is some precedence for macrophage surface-connected compartments containing lipoproteins that do not immediately seal. Macrophages sequester large beta-very low density lipoproteins in peripheral tubular compartments that remain surface connected for several minutes35. One possible reason the compartments don’t fully close is that moieties generated during the catabolism of agLDL inhibit compartment sealing. Phagosome sealing occurs through contractile activities regulated by Rho-family GTPases41. In particular, Rac and Cdc42 are transiently activated during phagocytosis42. Rac and/or Cdc42 are also involved in macrophage agLDL interactions inducing local actin polymerization to form the lysosomal synapse28. We have previously reported that loading of macrophages with FC causes increased Rac activity43. Interestingly, it was shown that constant activation of Rac causes a delay in phagosome closure44. Thus, it is possible that agLDL-derived FC and potentially other moieties generated during the catabolism of agLDL affect Rho-family GTPase activities in a way that interferes with their activation-inactivation cycle thus preventing compartment sealing. The detailed signaling mechanisms responsible for lysosomal synapse formation and function are currently under investigation.
The fact that the plasmin-induced compartments retain connection to the extracellular space is relevant to both atherogenesis and macrophage biology. Pharmacological approaches to inhibit lipid accumulation by macrophage foam cells have been pursued intensely as they are thought to be of value in preventing coronary artery disease45. However, successes have been limited, possibly due to an incomplete understanding of the mechanisms of foam cell formation in vivo. The cellular mechanisms for agLDL catabolism are likely to be significantly different for extracellular hydrolysis by lysosomal enzymes as opposed to degradation within a phagolysosome. After pinching off, phagosomes undergo a maturation process that alters their membrane composition46. As a consequence, different sets of soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) and other proteins are used for lysosome fusion with phagosomes and lysosome fusion with the plasma membrane47-49. In order to develop efficacious therapeutic approaches, the exact mechanism by which macrophages become foam cells must be understood.
Further, the extracellular catabolism of agLDL may play a role in the development of the atherosclerotic core itself. We have shown in our previous work that lysosomal synapses are dynamic27. They can be tightly sealed at times, but they also transiently open up, a process that would not happen with a fully sealed phagolysosome. This opening can release catabolic products, such as unesterified cholesterol, into the extracellular space. Significant increases in the ratio of free to total cholesterol have been observed during progression of plaques from fatty streaks to fibrolipid lesions50, 51. Extracellular FC is present in excess in advanced plaques, particularly those prone to rupture and therefore may represent an underlying sign of lesion instability. However, the source of this unesterified cholesterol is currently unknown. It is plausible that the extracellular hydrolysis of CEs contained in agLDL may contribute to the increased extracellular FC contained in the atherosclerotic core of fibrolipid lesions. The release of FC into the extracellular space may also play a role in the formation of extracellular cholesterol crystals, implicated as a mechanical factor that contributes directly to plaque vulnerability52. A number of additional enzymes are likely released into the extracellular space during the process of macrophage agLDL catabolism, including lysosomal hydrolases, which have been implicated in some studies to increase fusion of lipoproteins 53 and hydrolyze the extracellular matrix54.
In conclusion, the data presented herein indicate that in addition to remodeling the extracellular matrix, plasmin may be involved in lipoprotein modification and foam cell formation during genesis and progression of atherosclerotic lesions. Plasmin-induced macrophage cholesterol accumulation is a novel pathway by which the plasmin/plasminogen system may contribute to atherogenesis. These findings provide new insight into the atherogenicity of plasmin and implicate a specific mechanism by which plasmin can accelerate atherosclerosis. Elucidation of this pathway may enable the development of novel, targeted therapies for the prevention of atherosclerosis. This newly identified pathway for the plasmin/plasminogen system to regulate foam cell formation provides a new context in which to understand the mechanism by which plasmin plays a role in pro-atherogenic processes.
Supplementary Material
SIGNIFICANCE.
The plasmin/plasminogen system is involved in atherosclerosis. However, the mechanisms by which it stimulates disease are not fully defined. In atherogenesis, LDL is deposited on arterial walls where it is modified, aggregated and retained. Macrophages are recruited to clear the LDL, and they become foam cells. This study assesses the role of plasmin in macrophage uptake of aggregated LDL. We found that plasminogen treatment of macrophages catabolizing aggregated LDL accelerated foam cell formation. This occurs because plasmin proteolyses cell-associated aggregated LDL, allowing a portion of the aggregate to become sequestered in a nearly sealed, yet extracellular, acidic compartment. The low pH in the plasmin-induced compartment allows lysosomal enzymes, delivered via lysosome exocytosis, greater activity, resulting in more efficient cholesteryl ester hydrolysis and delivery of a large cholesterol load to the macrophage, thereby promoting foam cell formation. These findings provide a new context for considering the atherogenic role of plasmin.
ACKNOWLEDGEMENTS
We thank Leona Cohen-Gould for electron microscopy analysis and Haley Fraser for figure editing.
Sources of Funding
This work was supported by NIH grants R37-DK27083 and R01-HL093324. A.S.H. was supported by an NRSA Fellowship from the NIH.
Abbreviations
- ACAT
acyl-coenzyme A cholesterol acyltransferase
- Alexa
AlexaFluor
- agLDL
aggregated LDL
- BCECF-AM
2′7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester
- BMM
bone marrow derived macrophage
- CtB
cholera toxin subunit B
- CE
cholesteryl ester
- CypHer 5E
CypHer 5E Mono N-hydroxysuccinimide ester
- DMEM
Dulbecco’s Modified Eagle’s Medium
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- FC
free cholesterol
- huMDM
human monocyte derived macrophage
- LAL
lysosomal acid lipase
- LatA
latrunculin A
- MES
2-(N-mopholino)ethanesulfonic acid
- NA
numerical aperture
- PFA
paraformaldehyde
- PA
plasminogen activator
- SEM
standard error of the mean
- uPA
urokinase-type plasminogen activator
- uPAR
urokinase-type plasminogen activator receptor
Footnotes
Disclosures
None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Syrovets T, Simmet T. Novel aspects and new roles for the serine protease plasmin. Cell Mol Life Sci. 2004;61:873–885. doi: 10.1007/s00018-003-3348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cushman M, Lemaitre RN, Kuller LH, Psaty BM, Macy EM, Sharrett AR, Tracy RP. Fibrinolytic activation markers predict myocardial infarction in the elderly. The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19:493–498. doi: 10.1161/01.atv.19.3.493. [DOI] [PubMed] [Google Scholar]
- 3.Sakkinen PA, Cushman M, Psaty BM, Rodriguez B, Boineau R, Kuller LH, Tracy RP. Relationship of plasmin generation to cardiovascular disease risk factors in elderly men and women. Arterioscler Thromb Vasc Biol. 1999;19:499–504. doi: 10.1161/01.atv.19.3.499. [DOI] [PubMed] [Google Scholar]
- 4.Folsom AR, Aleksic N, Park E, Salomaa V, Juneja H, Wu KK. Prospective study of fibrinolytic factors and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb Vasc Biol. 2001;21:611–617. doi: 10.1161/01.atv.21.4.611. [DOI] [PubMed] [Google Scholar]
- 5.Moons L, Shi C, Ploplis V, Plow E, Haber E, Collen D, Carmeliet P. Reduced transplant arteriosclerosis in plasminogen-deficient mice. J Clin Invest. 1998;102:1788–1797. doi: 10.1172/JCI3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cozen AE, Moriwaki H, Kremen M, DeYoung MB, Dichek HL, Slezicki KI, Young SG, Veniant M, Dichek DA. Macrophage-targeted overexpression of urokinase causes accelerated atherosclerosis, coronary artery occlusions, and premature death. Circulation. 2004;109:2129–2135. doi: 10.1161/01.CIR.0000127369.24127.03. [DOI] [PubMed] [Google Scholar]
- 7.Kremen M, Krishnan R, Emery I, Hu JH, Slezicki KI, Wu A, Qian K, Du L, Plawman A, Stempien-Otero A, Dichek DA. Plasminogen mediates the atherogenic effects of macrophage-expressed urokinase and accelerates atherosclerosis in apoE-knockout mice. Proc Natl Acad Sci U S A. 2008;105:17109–17114. doi: 10.1073/pnas.0808650105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farris SD, Hu JH, Krishnan R, Emery I, Chu T, Du L, Kremen M, Dichek HL, Gold E, Ramsey SA, Dichek DA. Mechanisms of urokinase plasminogen activator (uPA)-mediated atherosclerosis: role of the uPA receptor and S100A8/A9 proteins. Journal of Biological Chemistry. 2011;286:22665–22677. doi: 10.1074/jbc.M110.202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnan R, Kremen M, Hu JH, Emery I, Farris SD, Slezicki KI, Chu T, Du L, Dichek HL, Dichek DA. Level of macrophage uPA expression is an important determinant of atherosclerotic lesion growth in Apoe−/−mice. Arterioscler Thromb Vasc Biol. 2009;29:1737–1744. doi: 10.1161/ATVBAHA.109.195529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneiderman J, Bordin GM, Engelberg I, Adar R, Seiffert D, Thinnes T, Bernstein EF, Dilley RB, Loskutoff DJ. Expression of fibrinolytic genes in atherosclerotic abdominal aortic aneurysm wall. A possible mechanism for aneurysm expansion. J Clin Invest. 1995;96:639–645. doi: 10.1172/JCI118079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmeliet P, Moons L, Lijnen R, Baes M, Lemaitre V, Tipping P, Drew A, Eeckhout Y, Shapiro S, Lupu F, Collen D. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nature Genetics. 1997;17:439–444. doi: 10.1038/ng1297-439. [DOI] [PubMed] [Google Scholar]
- 12.Shah PK. Inflammation, metalloproteinases, and increased proteolysis: an emerging pathophysiological paradigm in aortic aneurysm. Circulation. 1997;96:2115–2117. doi: 10.1161/01.cir.96.7.2115. [DOI] [PubMed] [Google Scholar]
- 13.Astrup T, Coccheri S. Thromboplastic and fibrinolytic activity of the arteriosclerotic human aorta. Nature. 1962;193:182–183. doi: 10.1038/193182a0. [DOI] [PubMed] [Google Scholar]
- 14.Binder BR, Mihaly J, Prager GW. uPAR-uPA-PAI-1 interactions and signaling: a vascular biologist’s view. Thromb Haemost. 2007;97:336–342. [PubMed] [Google Scholar]
- 15.Ellis V, Scully MF, Kakkar VV. Plasminogen activation initiated by single-chain urokinase-type plasminogen activator. Potentiation by U937 monocytes. Journal of Biological Chemistry. 1989;264:2185–2188. [PubMed] [Google Scholar]
- 16.Kienast J, Padro T, Steins M, Li CX, Schmid KW, Hammel D, Scheld HH, van de Loo JC. Relation of urokinase-type plasminogen activator expression to presence and severity of atherosclerotic lesions in human coronary arteries. Thromb Haemost. 1998;79:579–586. [PubMed] [Google Scholar]
- 17.Falkenberg M, Tjarnstrom J, Ortenwall P, Olausson M, Risberg B. Localization of fibrinolytic activators and inhibitors in normal and atherosclerotic vessels. Thromb Haemost. 1996;75:933–938. [PubMed] [Google Scholar]
- 18.Steins MB, Padro T, Schwaenen C, Ruiz S, Mesters RM, Berdel WE, Kienast J. Overexpression of urokinase receptor and cell surface urokinase-type plasminogen activator in the human vessel wall with different types of atherosclerotic lesions. Blood Coagul Fibrinolysis. 2004;15:383–391. doi: 10.1097/01.mbc.0000114441.59147.56. [DOI] [PubMed] [Google Scholar]
- 19.Raghunath PN, Tomaszewski JE, Brady ST, Caron RJ, Okada SS, Barnathan ES. Plasminogen activator system in human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:1432–1443. doi: 10.1161/01.atv.15.9.1432. [DOI] [PubMed] [Google Scholar]
- 20.Plow EF, Hoover-Plow J. The functions of plasminogen in cardiovascular disease. Trends in Cardiovascular Medicine. 2004;14:180–186. doi: 10.1016/j.tcm.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Fay WP, Garg N, Sunkar M. Vascular functions of the plasminogen activation system. Arteriosclerosis, Thrombosis & Vascular Biology. 2007;27:1231–1237. doi: 10.1161/ATVBAHA.107.140046. [DOI] [PubMed] [Google Scholar]
- 22.Boren J, Gustafsson M, Skalen K, Flood C, Innerarity TL. Role of extracellular retention of low density lipoproteins in atherosclerosis. Curr Opin Lipidol. 2000;11:451–456. doi: 10.1097/00041433-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Tabas I. Nonoxidative modifications of lipoproteins in atherogenesis. Annu Rev Nutr. 1999;19:123–139. doi: 10.1146/annurev.nutr.19.1.123. [DOI] [PubMed] [Google Scholar]
- 24.Smith EB, Massie IB, Alexander KM. The release of an immobilized lipoprotein fraction from atherosclerotic lesions by incubation with plasmin. Atherosclerosis. 1976;25:71–84. doi: 10.1016/0021-9150(76)90049-6. [DOI] [PubMed] [Google Scholar]
- 25.Tamminen M, Mottino G, Qiao JH, Breslow JL, Frank JS. Ultrastructure of early lipid accumulation in ApoE-deficient mice. Arteriosclerosis, Thrombosis & Vascular Biology. 1999;19:847–853. doi: 10.1161/01.atv.19.4.847. [DOI] [PubMed] [Google Scholar]
- 26.Zhang WY, Gaynor PM, Kruth HS. Aggregated low density lipoprotein induces and enters surface-connected compartments of human monocyte-macrophages. Uptake occurs independently of the low density lipoprotein receptor. J Biol Chem. 1997;272:31700–31706. doi: 10.1074/jbc.272.50.31700. [DOI] [PubMed] [Google Scholar]
- 27.Haka AS, Grosheva I, Chiang E, Buxbaum AR, Baird BA, Pierini LM, Maxfield FR. Macrophages create an acidic extracellular hydrolytic compartment to digest aggregated lipoproteins. Mol Biol Cell. 2009;20:4932–4940. doi: 10.1091/mbc.E09-07-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grosheva I, Haka AS, Qin C, Pierini LM, Maxfield FR. Aggregated LDL in contact with macrophages induces local increases in free cholesterol levels that regulate local actin polymerization. Arterioscler Thromb Vasc Biol. 2009;29:1615–1621. doi: 10.1161/ATVBAHA.109.191882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang WY, Ishii I, Kruth HS. Plasmin-mediated macrophage reversal of low density lipoprotein aggregation. J Biol Chem. 2000;275:33176–33183. doi: 10.1074/jbc.M908714199. [DOI] [PubMed] [Google Scholar]
- 30.Piha M, Lindstedt L, Kovanen PT. Fusion of proteolyzed low-density lipoprotein in the fluid phase: a novel mechanism generating atherogenic lipoprotein particles. Biochemistry. 1995;34:10120–10129. doi: 10.1021/bi00032a004. [DOI] [PubMed] [Google Scholar]
- 31.Li Q, Laumonnier Y, Syrovets T, Simmet T. Plasmin triggers cytokine induction in human monocyte-derived macrophages. Arterioscler Thromb Vasc Biol. 2007;27:1383–1389. doi: 10.1161/ATVBAHA.107.142901. [DOI] [PubMed] [Google Scholar]
- 32.Assmann A, Mohlig M, Osterhoff M, Pfeiffer AF, Spranger J. Fatty acids differentially modify the expression of urokinase type plasminogen activator receptor in monocytes. Biochemical & Biophysical Research Communications. 2008;376:196–199. doi: 10.1016/j.bbrc.2008.08.115. [DOI] [PubMed] [Google Scholar]
- 33.Oka H, Kugiyama K, Doi H, Matsumura T, Shibata H, Miles LA, Sugiyama S, Yasue H. Lysophosphatidylcholine induces urokinase-type plasminogen activator and its receptor in human macrophages partly through redox-sensitive pathway. Arteriosclerosis, Thrombosis & Vascular Biology. 2000;20:244–250. doi: 10.1161/01.atv.20.1.244. [DOI] [PubMed] [Google Scholar]
- 34.Buton X, Mamdouh Z, Ghosh R, Du H, Kuriakose G, Beatini N, Grabowski GA, Maxfield FR, Tabas I. Unique cellular events occurring during the initial interaction of macrophages with matrix-retained or methylated aggregated low density lipoprotein (LDL). Prolonged cell-surface contact during which ldl-cholesteryl ester hydrolysis exceeds ldl protein degradation. J Biol Chem. 1999;274:32112–32121. doi: 10.1074/jbc.274.45.32112. [DOI] [PubMed] [Google Scholar]
- 35.Myers JN, Tabas I, Jones NL, Maxfield FR. Beta-very low density lipoprotein is sequestered in surface-connected tubules in mouse peritoneal macrophages. Journal of Cell Biology. 1993;123:1389–1402. doi: 10.1083/jcb.123.6.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature. 2003;421:748–753. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- 37.Yamashiro DJ, Maxfield FR. Kinetics of endosome acidification in mutant and wild-type Chinese hamster ovary cells. J Cell Biol. 1987;105:2713–2721. doi: 10.1083/jcb.105.6.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beletskii A, Cooper M, Sriraman P, Chiriac C, Zhao L, Abbot S, Yu L. High-throughput phagocytosis assay utilizing a pH-sensitive fluorescent dye. Biotechniques. 2005;39:894–897. doi: 10.2144/000112001. [DOI] [PubMed] [Google Scholar]
- 39.Sheriff S, Du H, Grabowski GA. Characterization of lysosomal acid lipase by site-directed mutagenesis and heterologous expression. Journal of Biological Chemistry. 1995;270:27766–27772. doi: 10.1074/jbc.270.46.27766. [DOI] [PubMed] [Google Scholar]
- 40.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 41.Swanson JA. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol. 2008;9:639–649. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swanson JA, Hoppe AD. The coordination of signaling during Fc receptor-mediated phagocytosis. J Leukoc Biol. 2004;76:1093–1103. doi: 10.1189/jlb.0804439. [DOI] [PubMed] [Google Scholar]
- 43.Qin C, Nagao T, Grosheva I, Maxfield FR, Pierini LM. Elevated plasma membrane cholesterol content alters macrophage signaling and function. Arteriosclerosis, Thrombosis & Vascular Biology. 2006;26:372–378. doi: 10.1161/01.ATV.0000197848.67999.e1. [DOI] [PubMed] [Google Scholar]
- 44.Nakaya M, Kitano M, Matsuda M, Nagata S. Spatiotemporal activation of Rac1 for engulfment of apoptotic cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9198–9203. doi: 10.1073/pnas.0803677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McLaren JE, Michael DR, Ashlin TG, Ramji DP. Cytokines, macrophage lipid metabolism and foam cells: implications for cardiovascular disease therapy. Prog Lipid Res. 2011;50:331–347. doi: 10.1016/j.plipres.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Scott CC, Botelho RJ, Grinstein S. Phagosome maturation: a few bugs in the system. J Membr Biol. 2003;193:137–152. doi: 10.1007/s00232-002-2008-2. [DOI] [PubMed] [Google Scholar]
- 47.Stow JL, Manderson AP, Murray RZ. SNAREing immunity: the role of SNAREs in the immune system. Nature Reviews. Immunology. 2006;6:919–929. doi: 10.1038/nri1980. [DOI] [PubMed] [Google Scholar]
- 48.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nature Reviews Molecular Cell Biology. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 49.Rao SK, Huynh C, Proux-Gillardeaux V, Galli T, Andrews NW. Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J Biol Chem. 2004;279:20471–20479. doi: 10.1074/jbc.M400798200. [DOI] [PubMed] [Google Scholar]
- 50.Guyton JR, Klemp KF. Development of the atherosclerotic core region. Chemical and ultrastructural analysis of microdissected atherosclerotic lesions from human aorta. Arterioscler Thromb. 1994;14:1305–1314. doi: 10.1161/01.atv.14.8.1305. [DOI] [PubMed] [Google Scholar]
- 51.Guyton JR, Klemp KF. Development of the lipid-rich core in human atherosclerosis. Arterioscler Thromb Vasc Biol. 1996;16:4–11. doi: 10.1161/01.atv.16.1.4. [DOI] [PubMed] [Google Scholar]
- 52.Abela GS, Aziz K, Vedre A, Pathak DR, Talbott JD, Dejong J. Effect of cholesterol crystals on plaques and intima in arteries of patients with acute coronary and cerebrovascular syndromes. Am J Cardiol. 2009;103:959–968. doi: 10.1016/j.amjcard.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 53.Devlin CM, Leventhal AR, Kuriakose G, Schuchman EH, Williams KJ, Tabas I. Acid sphingomyelinase promotes lipoprotein retention within early atheromata and accelerates lesion progression. Arterioscler Thromb Vasc Biol. 2008;28:1723–1730. doi: 10.1161/ATVBAHA.108.173344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reddy VY, Zhang QY, Weiss SJ. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1995;92:3849–3853. doi: 10.1073/pnas.92.9.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.