Abstract
There are several murine models described with features similar to human primary biliary cirrhosis (PBC). Amongst these models, the one which has the closest serologic features to PBC is a mouse with a T cell-restricted expression of the dominant negative transforming growth factor β receptor type II (dnTGFβRII). Our work has demonstrated that CD8+ T cells from dnTGFβRII mice transfer autoimmune cholangitis to Rag1-/- recipients. However, it remained unclear whether the autoimmune cholangitis was secondary to an intrinsic function within CD8+ T cells or due to the abnormal TGFβR environment within which CD8+ T cells were generated. To address this mechanistic issue, we utilized our dnTGFβRII, OT-I/Rag1-/-, OT-II/Rag1-/- mice and in addition generated OT-I/dnTGFβRII/Rag1-/-, and OT-II/dnTGFβRII/Rag1-/- mice in which the entire T cell repertoire was replaced with ovalbumin (OVA) specific CD8+ or CD4+ T cells, respectively. Importantly, neither the parental OT-I/dnTGFβRII/Rag1-/- mice and/or OT-II/dnTGFβRII/Rag1-/- mice developed cholangitis. However, adoptive transfer demonstrated that only transfer of CD8+ T cells from dnTGFβRII mice but not CD8+ T cells from OT-I/Rag -/- mice or from OT-I/dnTGFβRII/Rag1-/- mice transferred disease. These data were not secondary to absence of CD4+ T cell help since a combination of CD8+ T cells from OT-I/dnTGFβRII/Rag1-/- and CD4+ T cells from OT II/dnTGFβRII/Rag1-/- or CD8+ T cells from OT-I/dnTGFβRII/Rag1-/- with CD4+ T cells from OT-II/Rag1-/- mice failed to transfer disease. In conclusion, defective TGFβRII signaling, in addition to clonal CD8+ T cells that target biliary cells, are required for induction of autoimmune cholangitis.
Keywords: primary biliary cirrhosis, murine models, autoimmunity, cholangitis
Primary biliary cirrhosis (PBC) is a progressive autoimmune liver disease characterized by portal tract lymphocytic infiltration, selective destruction of biliary epithelial cells (1, 2) and the presence of anti-mitochondrial antibodies (AMAs) to autoantigens of the family of 2-oxo-acid dehydrogenase complexes located in the inner mitochondrial membrane (3-5), including and, in particular, the dominant autoepitope of the pyruvate dehydrogenase E2 complex (PDC-E2) (6-8). A major obstacle in dissecting the molecular basis of PBC has been the absence of suitable animal models. We have previously reported that mice transgenic for directed expression of a dominant negative form of transforming growth factor beta receptor type II (dnTGFβRII), under the control of the CD4 promoter lacking the CD8 silencer, spontaneously developed an autoimmune biliary ductular disease similar to human PBC, including development of AMA (9-13). This observation is critical because our previous work on PDC-E2 specific CD4+ and CD8+ T cells in human PBC suggests that autoimmune cholangitis in patients is mediated by autoantigen specific T cells (14-17).
Earlier work has demonstrated that adoptive transfer of CD8+ T cells from dnTGFβRII mice induces autoimmune cholangitis in recipients (11, 18). However, both in humans and in the murine models, there has always been the question as to whether the multi-lineage response to mitochondrial autoantigens and, in particular, PDC-E2, is specifically involved in tissue damage or whether it is part of a non-specific loss of tolerance and therefore an epiphenomenon. To address this issue, we took advantage of our dnTGFβRII model and developed unique murine constructs in which we introduced the dnTGFβRII gene, along with either OT-I TCR or OT-II TCR transgenes into Rag1-/- mice. In other words, we developed two dnTGFβRII strains in which the T cell repertoire was replaced with either ovalbumin (OVA)-specific CD8+ T cells (OT-I) or OVA-specific CD4+ T cells (OT-II). We report herein that autoimmune cholangitis requires T cell antigen specificity for the development of autoimmune cholangitis. These data have importance not only for this mouse model, but highlight the significance of breach of tolerance to PDC-E2 in humans with PBC.
Materials and Methods
Animals
Our colony of dnTGFβRII mice on a C57BL/B6 (B6) background was maintained at the University of California at Davis animal facility (Davis, CA) (9). C57BL/6-Tg (TcrαTcrβ) 1100Mjb/J (OT-I), C57BL/6-Tg (TcrαTcrβ) 425Cbn/J (OT-II) and B6.129S7-Rag1tm1Mom/J (Rag1-/-) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). To generate OT-I/dnTGFβRII/Rag1-/- mice, male dnTGFβRII mice and OT-I mice were mated with female Rag1-/- mice to obtain dnTGFβRII/Rag1+/- mice and OT-I/Rag1+/- mice, which were subsequently back-crossed with female Rag1-/- mice to obtain dnTGFβRII/Rag1-/- mice and OT-I/Rag1-/- mice, respectively. Male dnTGFβRII/Rag1-/- mice were then mated with female OT-I/Rag1-/- mice to obtain OT-I/Rag1-/- and OT-I/dnTGFβRII/Rag1-/- mice. OT-II/dnTGFβRII/Rag1-/- mice were similarly generated. In all cases, genotypes were confirmed via the polymerase chain reaction (9). Mice were fed sterile rodent Helicobacter Medicated Dosing System (three-drug combination) diets (Bio-Serv, Frenchtown, NJ) and maintained in individually ventilated cages under specific pathogen-free conditions. Sulfatrim (Hi-tech Pharmacal, Amityville, NY) was delivered through drinking water. The experimental protocols were approved by the University of California Animal Care and Use Committee.
Experimental Protocol
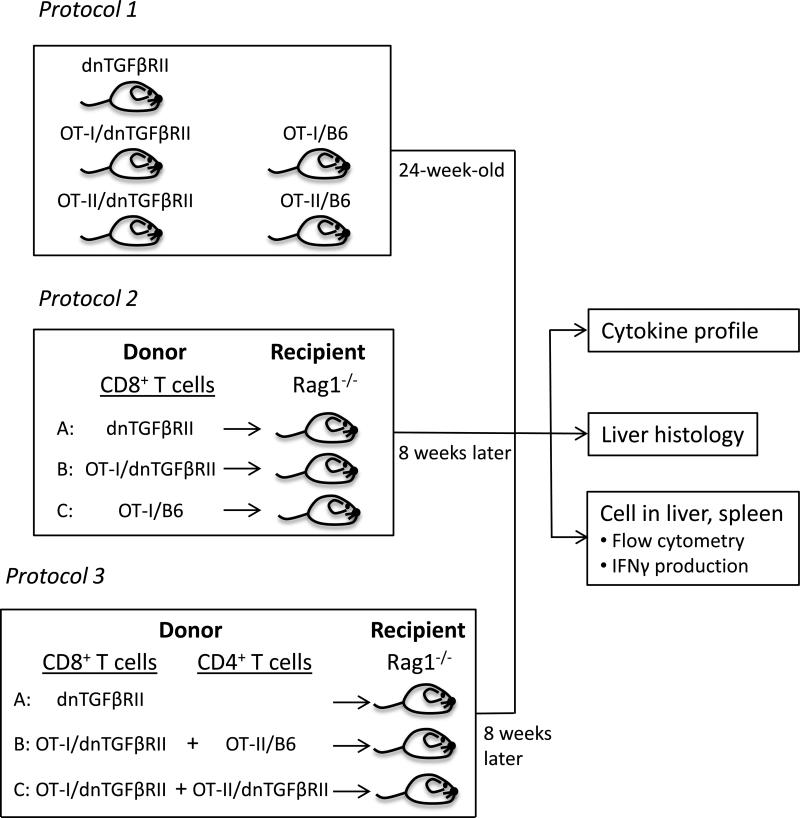
1. Natural history in unmanipulated dnTGFβRII genetically modified mice
In the first phase of this work we monitored the natural history of immunopathology in groups of 7-12 mice, including dnTGFβRII, OT-I/dnTGFβRII/Rag1-/-, OT-I/Rag1-/-, OT-II/dnTGFβRII/Rag1-/-, and OT-II/Rag1-/-; only female mice were studied. The mice were left unmanipulated to minimize infection and loss of animals until 24 weeks of age, when liver and spleen were collected on all mice. The liver specimens were examined for histopathology. Splenic and hepatic mononuclear cells (MNCs) were isolated for phenotypic analysis by flow cytometry as described below (Fig.1). To confirm that CD8+ T cells are immunologically functional and, as further controls for this work, we performed ex vivo stimulation with anti-CD3 and anti-CD28 or the OVA peptide 257-264, followed by measurement of IFNγ production.
Figure 1.
Schematic illustration of the experimental protocol.
2. Expression of autoimmune cholangitis following adoptive CD8+ T cells transfer
In the second phase of the protocol female Rag1-/- mice at 8 weeks of age underwent adoptive transfer with purified splenic CD8+ T cells from donor dnTGFβRII, OT-I/dnTGFβRII/Rag1-/- or OT-I/Rag1-/- mice. The adoptive transfer was performed by collection of splenic cells from 8-week-old dnTGFβRII, OT-I/dnTGFβRII /Rag1-/- or OT-I /Rag1-/- mice. Purified CD8+ T cells were prepared using CD8 microbeads (Miltenyi Biotec, Auburn, CA) and aliquots of 1 × 106 CD8+ T cells were thence transferred by intravenous injection. Eight weeks following this adoptive transfer, all recipients were sacrificed and sera, liver and spleen were collected. The liver specimens were examined for histopathology. Splenic and hepatic MNCs were analyzed by flow cytometry. The concentration of serum TNFα, IFNγ, MCP-1 (monocyte chemoattractant protein-1), and IL-6 was determined using the mouse Cytometry Bead Array kit (CBA; BD Biosciences, San Jose, CA) (19) (Fig.1).
3. Expression of autoimmune cholangitis following adoptive CD8+ and CD4+ T cells transfer
In the third phase of this experiment we determined the role of CD4+ helper T cells in CD8+ T cell mediated autoimmune cholangitis. Purified splenic CD4+ T cells from donor OT-II/dnTGFβRII/Rag1-/- or OT-II/Rag1-/- mice underwent transfer into Rag1-/- recipient mice as noted in Figure 1. Specifically, splenic T cells were collected from 8-week-old dnTGFβRII, OTI/dnTGFβRII /Rag1-/-, OT-II/dnTGFβRII/Rag1-/- or OT-II/Rag1-/- mice. Purified CD8+ or CD4+ T cells were prepared using CD8 or CD4 microbeads (Miltenyi Biotec, Auburn, CA), respectively. Eight-week-old female Rag1-/- mice were used as recipients. Aliquots of 1 × 106 of CD8+ or CD4+ T cells were then transferred by intravenous injection. Eight weeks following the adoptive transfer, all recipient animals were sacrificed and analyzed by histopathology, flow cytometry and the mouse Cytometry Bead Array kit (Fig.1).
Flow Cytometry
Splenocytes and liver infiltrating MNCs were isolated as described (20) and resuspended in staining buffer consisting of 0.2 % BSA, 0.04% EDTA, and 0.05 % sodium azide in PBS. The cells were dispensed into 25 μL aliquots and incubated with anti-mouse Fc receptor blocking reagent (eBioscience, San Diego, CA) for 15 minutes at 4°C. Cells were washed and stained for 30 minutes at 4°C with cocktails containing combinations of fluorochrome conjugated monoclonal antibodies for the cell surface markers CD4, CD8a, CD44, CD62L, NK1.1, TCR Vα2, TCR Vβ5.1, 5.2 (Biolegend, San Diego, CA), and TCR-β (eBioscience). After staining the cells were washed once with PBS containing 0.2 % BSA. For intracellular cytokine staining, splenic MNCs from dnTGFβRII, OT-I/dnTGFβRII/Rag1-/- and OT-I/Rag1-/- mice were resuspended in RPMI 1640 medium with 10 % heat-inactivated fetal bovine serum (GIBCO-Invitrogen Corp., Grand Island, NY), 100 μg/mL streptomycin, 100 U/mL penicillin, and 0.5 μg/mL each of anti-CD3 (Biolegend) and anti-CD28 (Biolegend) or 10 μg/ml the OVA amino acid 257-264 peptide (GenScript Inc., Piscataway, NJ). The cells were incubated at 37 °C in a humidified 5 % CO2 incubator. Brefeldin A (1 μg/ml) (Sigma-Aldrich Co., St. Louis, MO) was added after 1 hour incubation. The cells were then incubated for 4 hours. The cells were stained for surface CD8a, NK1.1 and TCRβ, fixed and permeabilized with BD Cytofix/Cytoperm Solution (BD Biosciences), then stained for intracellular IFNγ (BioLegend). Normal IgG isotype controls were used in parallel. A FACScan flow cytometer (BD Immunocytometry Systems, San Jose, CA) upgraded for the detection of five colors by Cytek Development (Fremont, CA) was used to acquire data, which were analyzed with Cellquest PRO software (BD Immunocytometry Systems).
Histopathology
The liver from sacrificed mice were fixed in 4 % paraformaldehyde, embedded in paraffin, cut into 4 μm sections, deparaffinized, stained with hematoxylin and eosin (H&E), and evaluated using light microscopy (12). Portal inflammation were evaluated by a “blinded” pathologist using the following scoring system we have previously defined: 0, no inflammation; 1, minimal inflammation; 2, mild inflammation; 3, moderate inflammation; and 4, severe inflammation. Bile duct damage was graded as: 0, no significant changes; 1, mild change; 2, moderate to severe bile duct damage or bile duct loss.
Statistical Analysis
These data were expressed as the mean ± standard deviation (SD) and were evaluated with a two-tailed unpaired Mann-Whitney test, one-way ANOVA followed by a Bonferroni multiple comparison test, or a Kruskal-Wallis test followed by Dunn's multiple comparisons test, as appropriate.
Results
Natural history in unmanipulated dnTGFβRII genetically modified mice
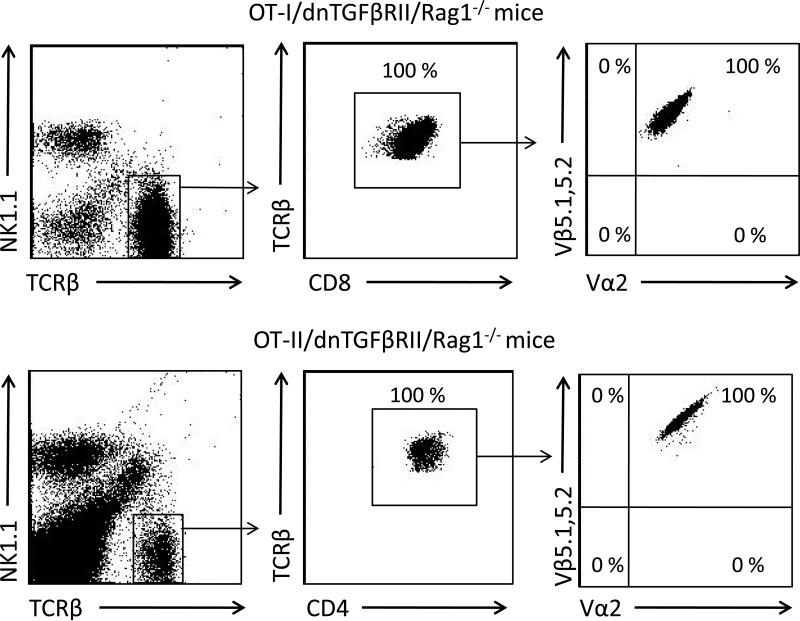
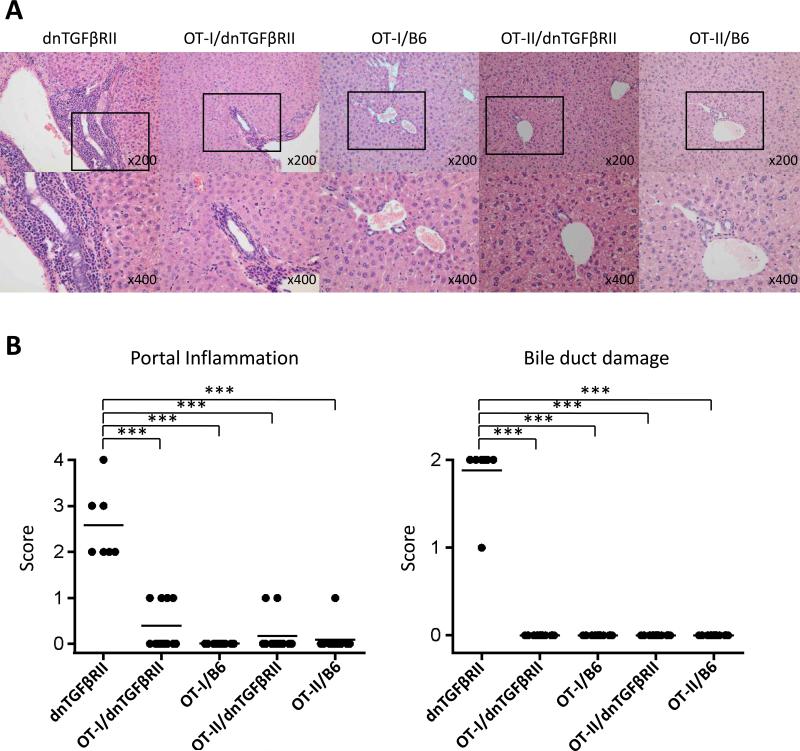
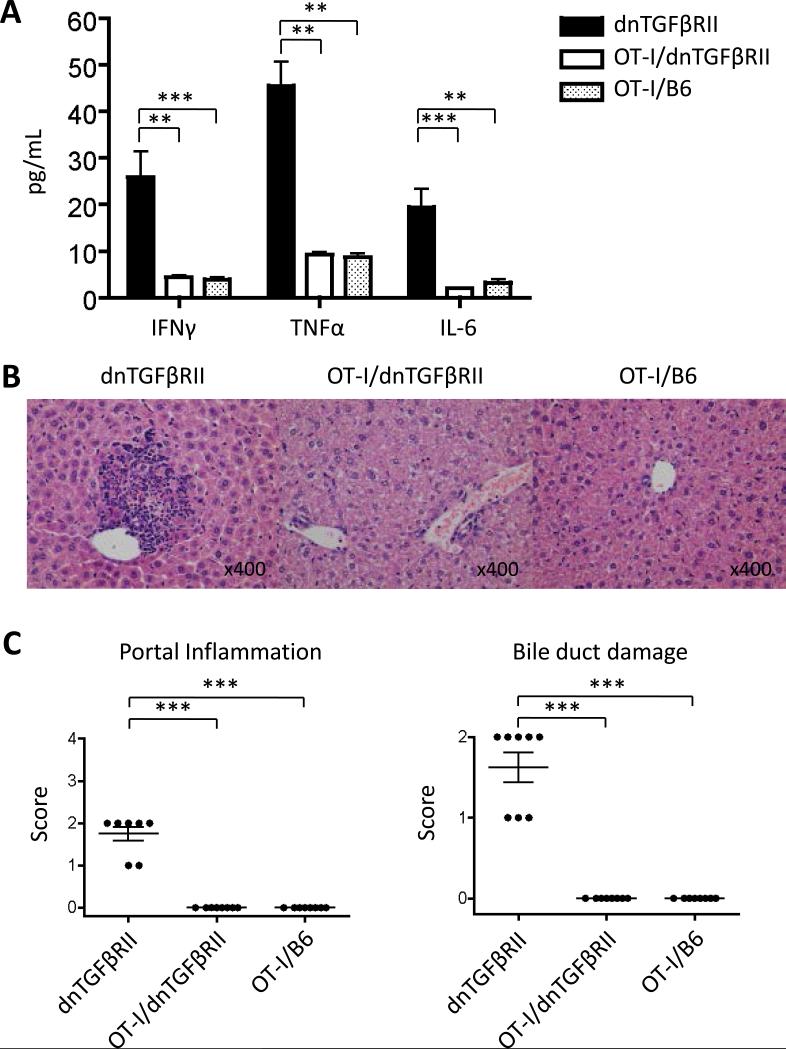
We confirmed the composition of the splenic T cell compartment in OT-I/dnTGFβRII/Rag1-/- and OT-II/dnTGFβRII/Rag1-/- mice. All T cells from OT-I/dnTGFβRII/Rag1-/- mice are CD8 positive and exclusively express the TCR Vα2 and Vβ5.1, 5.2, while T cells in OT-II/dnTGFβRII/Rag1-/- mice are CD4 positive and express Vα2 and Vβ5.1, 5.2 (Fig. 2). Histological examination of liver sections in dnTGFβRII mice demonstrated significant autoimmune cholangitis with MNCs infiltration in hepatic portal tracts and bile duct damage. In contrast, there was no significant hepatic pathology in either OT-II/dnTGFβRII/Rag1-/- or OT-II/Rag1-/- mice (Fig. 3A and 3B). These results indicate that OVA-specific CD4+ T cells with the TGFβ signaling deficiency were not associated with autoimmune biliary disease. Some (4 out of 12 mice) of the OT-I/dnTGFβRII/Rag1-/- mice had detectable lymphocytic infiltration in the portal tracts, but it was significantly less than in dnTGFβRII mice. However, there was no bile duct damage in any of the OT-I/dnTGFβRII/Rag1-/- mice (Fig. 3A and 3B).
Figure 2.
Representive flow cytometry analysis of T cells in spleen from OT-I/dnTGFβRII/Rag1-/- mice and OT-II/dnTGFβRII/Rag1-/- mice.
Figure 3.
(A) Representative H&E stained of liver sections of dnTGFβRII mice, OT-I/dnTGFβRII/Rag1-/- mice (OT-I/dnTGFβRII), OT-I/Rag1-/- mice (OT-I/B6), OT-II/dnTGFβRII/Rag1-/- mice (OT-II/dnTGFβRII) and OT-II/Rag1-/- mice (OT-II/B6). (B) Scoring of portal inflammation and bile duct damage in dnTGFβRII (n=7), OT-I/dnTGFβRII (n=12), OT-I/B6 (n=12), OT-II/dnTGFβRII (n=12), and OT-II/B6 mice (n=12). ***p<0.001 determined using Kruskal-Wallis test followed by Dunn's multiple comparisons test.
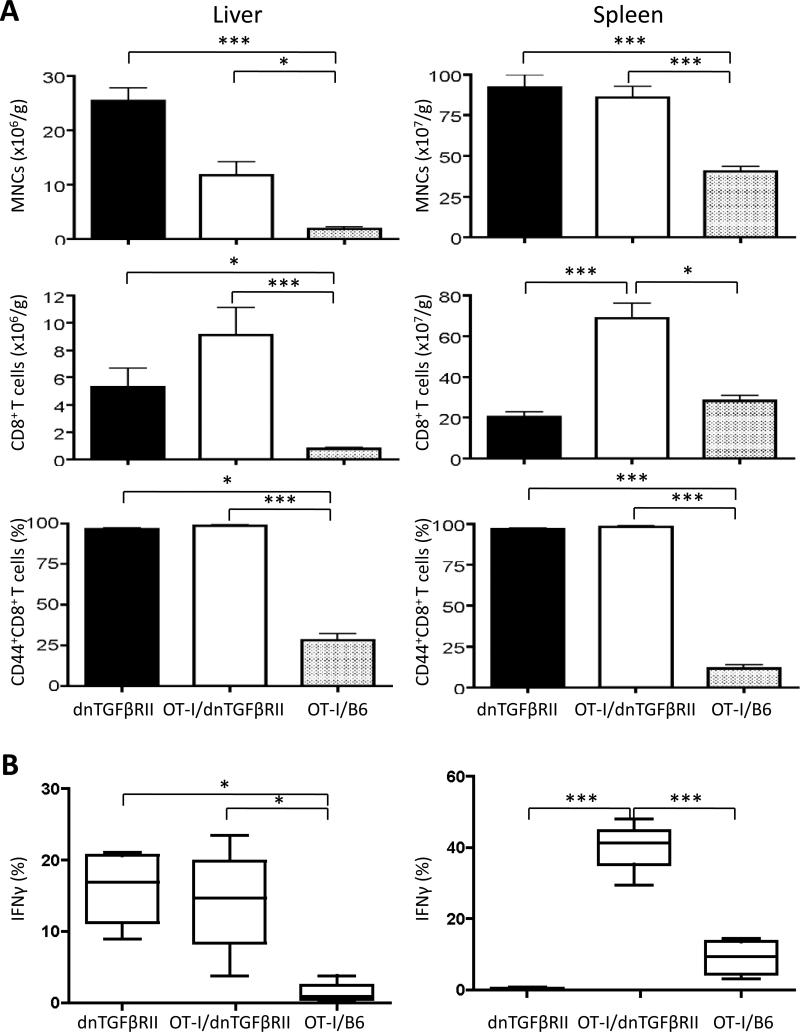
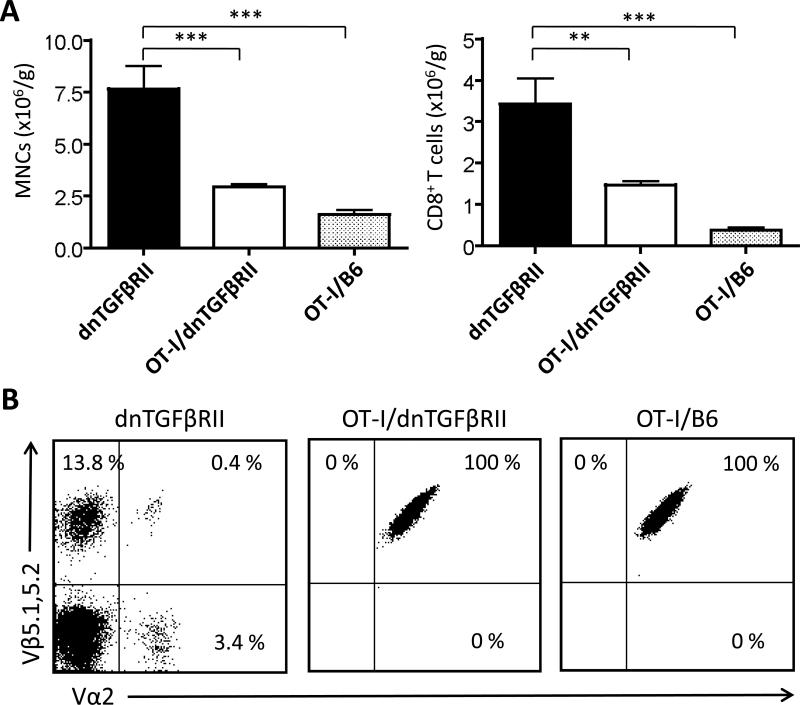
The absolute numbers of MNCs were significantly increased in the liver and spleen of OT-I/dnTGFβRII/Rag1-/- mice and dnTGFβRII mice compared to OT-I/Rag1-/- mice (Fig. 4A). The absolute numbers of CD8+ T cells were significantly increased in the liver of OT-I/dnTGFβRII/Rag1-/- mice and dnTGFβRII mice compared to OT-I/Rag1-/- mice, suggesting that the TGFβRII transgene caused autonomous cell proliferation in both strains. In both liver and spleen, almost 100% of CD8+ T cells from OT-I/dnTGFβRII/Rag1-/- mice and dnTGFβRII mice were CD44+ memory T cells, while most of CD8+ T cells from OT-I/Rag1-/- mice were CD44- naive T cells (Fig. 4A). To prove that the OT-I/dnTGFβRII/Rag1-/- CD8 cells were immunologically functional, we performed ex vivo stimulation with anti-CD3 and anti-CD28 or the OVA peptide 257-264 that is recognized by the OT-I TCR. dnTGFβRII and OT-I/dnTGFβRII/Rag1-/- IFNγ producing CD8+ T cells were significantly increased compared to OT-I/Rag1-/- mice after anti-CD3 and anti-CD28 stimulation. In addition, OT-I/dnTGFβRII/Rag1-/- IFNγ producing CD8+ T cells were significantly increased compared to OT-I/Rag1-/- mice and dnTGFβRII mice after OVA stimulation, proving that these cells were not only functionally intact, but were producing massive amounts of Th1 cytokines compared to OT-1 cells in a non-transgenic B6 background (Fig. 4B). These results indicate that although OVA-specific CD8+ T cells with TGFβ signaling deficiency accumulated massively, and were immunologically activated and capable of a substantial Th1 response, they were associated with only a mild lymphoid cell infiltration in portal area and were not associated with autoimmune cholangitis.
Figure 4.
Flow cytometric analysis of the MNCs in the liver and spleen of dnTGFβRII, OT-I/dnTGFβRII/Rag1-/- (OT-I/ dnTGFβRII), and OT-I/Rag1-/- (OT-I/B6) mice. (A) Number and phenotype of total MNCs and CD8+ T cells in the spleen and liver of dnTGFβRII mice (n=7), OT-I/ dnTGFβRII (n=8), and OT-I/B6 (n=8) mice. (B) The frequency of IFNγ producing CD8+ T cells in the spleen of dnTGFβRII (n=4), OT-I/dnTGFβRII (n=4), and OT-I/B6 (n=4) mice. The cells were stimulated ex vivo with anti-CD3 and anti-CD28 (left panel) or the OVA peptide 257-264 (right panel). *p<0.05, **p<0.01, ***p<0.001 determined using one-way ANOVA followed by a Bonferroni multiple comparisons test or Kruskal-Wallis test followed by Dunn's multiple comparisons test.
Expression of autoimmune cholangitis following adoptive CD8+ T cells transfer
To further determine the role of antigen specific CD8+ T cells in autoimmune cholangitis in dnTGFβRII mice, 1 × 106 CD8+ T cells from the spleens of OT-I/dnTGFβRII/Rag1-/-, OT-I/Rag1-/- or dnTGFβRII mice underwent transfer into Rag1-/- mice. We measured the production of inflammatory cytokines in the serum of these recipients at eight weeks following the adoptive transfer. IFNγ, TNFα and IL-6 production were significantly higher in the recipients of CD8+ T cells from dnTGFβRII mice than those received OT-I/dnTGFβRII/Rag1-/- and OT-I/Rag1-/- CD8+ T cells (Fig. 5A). Thus although OT-I/dnTGFβRII/Rag1-/- were capable of a substantial Th1 response, they did not develop it in vivo.
Figure 5.
Adoptive transfer of OVA-specific CD8+ T cells into Rag1-/- recipient mice. (A) Serum cytokine profiles of recipients transferred with CD8+ T cells from dnTGFβRII, OT-I/dnTGFβRII/Rag1-/- (OT-I/dnTGFβRII), and OT-I/Rag1-/- (OT-I/B6) donors at 8 weeks after transfer. (B) Representative H&E stained liver sections of recipient mice transferred with CD8+ T cell from dnTGFβRII mice, OT-I/dnTGFβRII, or OT-I/B6. (C) Scoring of portal inflammation and bile duct damage in the recipients. Each group included 8 mice. **p<0.01, ***p<0.001 determined using Kruskal-Wallis test followed by Dunn's multiple comparisons test.
Inflammatory MNCs infiltration and bile duct damage were detected in the liver from recipients of dnTGFβRII CD8+ T cells but not in the recipients of OT-I/dnTGFβRII/Rag1-/- and OT-I/Rag1-/- CD8+ T cells (Fig. 5B and 5C). The number of liver infiltrating MNCs and CD8+ T cells was significantly higher in the recipients of dnTGFβRII CD8+ T cells than the recipients of OT-I/dnTGFβRII/Rag1-/- and OT-I/Rag1-/- CD8+ T cells (Fig. 6A). Flow cytometric analysis confirmed that the CD8+ T cells recovered from the recipients of OT-I/dnTGFβRII/Rag1-/- and OT-I/Rag1-/- CD8+ T cells exclusively expressed the TCR Vα2 and Vβ5.1, 5.2, while such specific TCR only comprised a small fraction in the CD8+ T cell repertoire derived from the dnTGFβRII mice (Fig. 6B). These results indicate that adoptive transfer of dnTGFβRII CD8+ T cells into Rag1-/- mice induced cholangitis in the liver of recipients; in contrast, the same number of CD8+ T cells from OT-I/dnTGFβRII/Rag1-/- donors did not cause cholangitis in the recipient mice.
Figure 6.
Flow cytometric analysis of the total MNCs and CD8+ T cells in liver of recipients transferred with CD8+ T cells from dnTGFβRII, OT-I/dnTGFβRII/Rag1-/- (OT-I/dnTGFβRII), and OT-I/Rag1-/- (OT-I/B6) donors at 8 weeks after transfer. (A) Number of total MNCs and CD8+ T cells in the liver. Each group included 8 mice. (B) Analysis of TCR Vα2 and Vβ5.1, 5.2 for hepatic CD8+ T cells. Data are expressed as the mean ± standard deviation. **p<0.01,***p<0.001 determined using one-way ANOVA followed by a Bonferroni multiple comparisons test or Kruskal-Wallis test followed by Dunn's multiple comparisons test.
Expression of autoimmune cholangitis following adoptive CD8+ and CD4+ T cells transfer
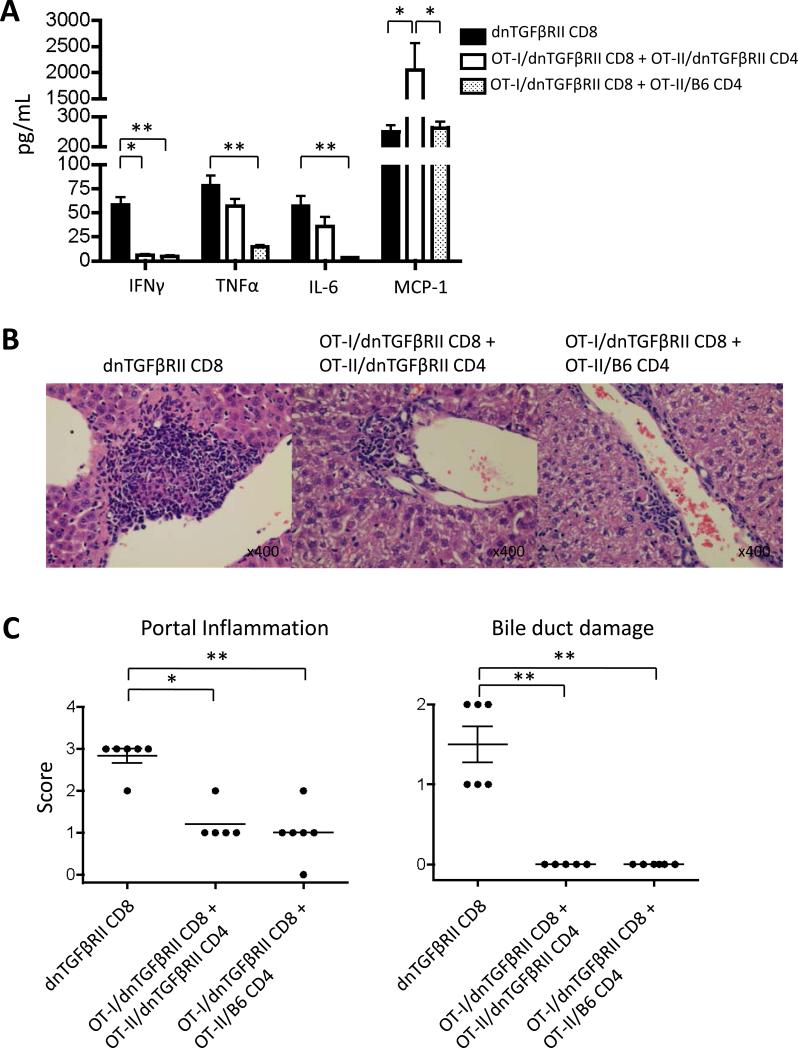
CD8+ T cells from OT-I/dnTGFβRII/Rag1-/- and OT-I/Rag1-/- do not receive CD4+ T cell help throughout development, while CD8+ T cells from dnTGFβRII do receive CD4+ T cell help. To determine the role of CD4+ helper cells in CD8+ T cell mediated autoimmune cholangitis, 1 × 106 CD8+ T cells from the spleen of dnTGFβRII, 1 × 106 CD8+ T cells from OT-I/dnTGFβRII/Rag1-/- mice with 1 × 106 CD4+ T cells from OT-II/dnTGFβRII/Rag1-/- or 1 × 106 CD8+ T cells from OT-I/dnTGFβRII/Rag1-/- mice with 1 × 106 CD4+ T cells from OT-II/Rag1-/- mice underwent transfer into Rag1-/- mice. IFNγ, TNFα and IL-6 production were significantly higher in the recipients of CD8+ T cells from dnTGFβRII mice than those receiving OT-I/dnTGFβRII/Rag1-/- CD8+ T cells with OT-II/Rag1-/- CD4+ T cells at eight weeks following the adoptive transfer. MCP-1 production was significantly higher in the recipients of OT-I/dnTGFβRII/Rag1-/- CD8+ T cells with OT-II/dnTGFβRII/Rag1-/- CD4+ T cells compared to mice receiving dnTGFβRII CD8+ T cells and OT-I/dnTGFβRII/Rag1-/- CD8+ T cells with OT-II/Rag1-/- CD4+ T cells (Fig. 7A). Some recipient mice in each of the transfer groups had minimal detectable lymphocytic infiltration in the portal tracts, however, portal inflammation in the liver from recipients of dnTGFβRII CD8+ T cells was significantly more severe than in the other recipients. Bile duct damage, however, was only detected in the liver transferred with dnTGFβRII CD8+ T cells (Fig. 7B and 7C). These results suggest that the autoimmune biliary disease is induced by antigen-specific CD8+ T cells within the natural CD8+ T cell repertoire of dnTGFβRII mice.
Figure 7.
Adoptive transfer of OVA-specific CD8+ T cells and CD4+ T cells into recipient mice. (A) Serum cytokine profiles of recipients transferred CD8+ T cells from dnTGFβRII (dnTGFβRII CD8), CD8+ T cells from OT-I/dnTGFβRII/Rag1-/- with CD4+ T cells from OT-II/dnTGFβRII/Rag1-/- (OT-I/dnTGFβRII CD8 + OT-II/dnTGFβRII CD4) and CD8+ T cells from OT-I/dnTGFβRII/Rag1-/- with CD4+ T cells from OT-II/Rag1-/- (OT-I/dnTGFβRII CD8 + OT-II/B6 CD4) at 8 weeks after transfer. (B) Representative H&E stained liver sections of dnTGFβRII CD8, OT-I/dnTGFβRII CD8 + OT-II/dnTGFβRII CD4 and OT-I/dnTGFβRII CD8 + OT-II/B6 CD4. (C) Scoring of portal inflammation and bile duct damage in the recipients. Each group included 5-6 mice. *p<0.05, **p<0.01 determined using Kruskal-Wallis test followed by Dunn's multiple comparisons test.
Discussion
PBC is an organ-specific autoimmune disease characterized by destruction of intrahepatic small bile duct biliary epithelial cells (1, 2). We have demonstrated that PDC-E2, along with other mitochondrial autoantigens, are present within the apoptotic blebs from human intrahepatic biliary epithelial cells (HiBECs), but not detected in apoptotic blebs from other human tissues (21, 22). We have also demonstrated that PDC-E2-specific autoreactive CD4+ and CD8+ T cells exist in peripheral blood and are highly enriched in the liver of PBC patients (14-17, 23). Taken together, these data suggest that autoreactive T cells play a critical role in the tissue-specific immunopathogenesis of PBC. In addition to these studies based on human clinical specimens, we have used the dnTGFβRII mice with TGFβ signaling deficiency in the T cells, a mouse model of autoimmune cholangitis that resembles human PBC (9), to demonstrate that the CD8+ cytotoxic T cell population with the impaired TGFβ signaling is essential for the development of autoimmune biliary epithelial damage in this model (18). However, it is unclear whether the pathogenic CD8+ T cells in the liver of dnTGFβRII mice require antigen specificity.
To examine the role of antigen specificity in the T cell-mediated autoimmune cholangitis in the dnTGFβRII mice, we generated two mouse strains OT-I/dnTGFβRII/Rag1-/- and OT-II/dnTGFβRII/Rag1-/-, in which the entire T cell repertoire was replaced with either CD8+ or CD4+ T cells specific for a single irrelevant antigen OVA. We demonstrated that OT-II/dnTGFβRII/Rag1-/- mice had no inflammation in liver at 24 weeks of age, while the OT-I/dnTGFβRII/Rag1-/- mice had minimal inflammation in portal tract but no autoimmune cholangitis. We further demonstrated that adoptive transfer of CD8+ T cells from OT-I/dnTGFβRII/Rag1-/- mice did not induce cholangitis in the recipient mice.
A previous study demonstrated that Rag1-/- recipient mice transferred with CD8+ T cell from Tgfbr2f/f dLcK-Cre mice plus CD4+ T cell from control mice developed more severe autoimmunity compared to the recipients of Tgfbr2f/f dLcK-Cre CD8+ T cells alone (24). Indeed, isolated CD8+ T cells from OT-I/dnTGFβRII/Rag1-/- had not received CD4+ T cell help during development, while isolated CD8+ T cells from dnTGFβRII had received CD4+ T cell help during development. In addition, consequently, we confirmed that adoptive transfer of CD8+ T cells from OT-I/dnTGFβRII/Rag1-/- mice with CD4+ T cells from OT-II/dnTGFβRII/Rag1-/- mice did not induce cholangitis in recipient mice. We also showed that the TGFβ signaling defect had the same effect on the OT-I/dnTGFβRII/Rag1-/- peripheral CD8 cells as on dnTGFβRII cells—i.e. excess accumulation (higher cell numbers), spontaneous activation (increased CD44) and excessive cytokine production (increased Th1 cytokines). Despite these abnormalities, these cells did not mediate disease upon transfer, nor did they produce excess cytokines without CD4 help. In the presence of CD4 help, they produced more cytokines, but still did not mediate disease. These results strongly suggest that antigen specificity for autoantigens is a critical aspect of dnTGFβRII mediated liver disease. The irrelevant antigen OVA-specific CD4+ and CD8+ T cells with TGFβ signaling deficiency do not cause autoimmune cholangitis. Therefore, the organ-specific autoimmune cholangitis spontaneously developed in the dnTGFβRII mice is not the consequence of a non-antigen specific, cell intrinsic loss of tolerance.
It has been reported that the T cell limited deficiency of TGFβ signaling resulted in spontaneous T cell differentiation, as demonstrated by the overwhelming CD44+ memory phenotype and the capacity of IFN-γ production of T cells in the dnTGFβRII mouse model (25). Similarly, we found that while the OVA-specific CD8+ T cells in the OT-I/Rag1-/- mice were mostly naïve T cells with poor IFNγ production capability, those in the OT-I/dnTGFβRII/Rag1-/- mice were almost exclusively CD44+ memory T cells with the capacity for excess IFN-γ production, although the mice had never been exposed to OVA. Of note, although the OT-I/dnTGFβRII/Rag1-/- mice were free of bile duct damage, they did develop mild inflammation in portal tract. This is in agreement with the notion that liver serves as a “graveyard” for activated CD8+ T cells (26), and that hepatitis could be induced by influenza-specific CD8+ T cells even though influenza antigens were not detected in the liver (27, 28). It is possible that even under the specific pathogen-free condition, some OVA-specific CD8+ T cells in the OT-I/dnTGFβRII/Rag1-/- mice could be activated by non-specific environmental factors, resulting in the mild liver inflammation.
Recently, several studies using transgenic mouse models that expressed various model autoantigens demonstrated that autoantigen specific T cells induced autoimmune diseases. For example, OVA-specific CD4+ T cells induced bladder autoimmune inflammation in transgenic URO-OVA mice that express the model self antigen OVA on the bladder urothelium (29). A study using skin-directed expression of OVA demonstrated that GVHD-like inflammatory skin disease was induced by transferring OVA-specific OT-I CD8+ T cells (30). Furthermore, transfer of OT-I T cells led to cholangitis in the liver of transgenic mouse in which the model antigen OVA was expressed in cholangiocytes (31). These experimental models of autoimmune diseases demonstrated the critical role of autoantigen-specific T cells in the pathogenesis of the tissues or organs that express the specific antigens. Our previous and current studies clearly demonstrate that CD8+ T cells are critical for the autoimmune cholangitis in the dnTGFβRII mice; however this organ-specific pathogenesis in the bile duct tissue that does not express OVA cannot be induced by the OVA-specific CD8+ T cells. Taken together, these results strongly suggest that the autoimmune cholangitis in the dnTGFβRII mouse model is induced by effector CD8+ T cells specific for autoantigens expressed in the bile duct tissue. Identifying these autoantigens as well as CD8+ T cells specific for these autoantigens in future studies will be important for understanding the mechanism of autoimmune cholangitis in the mouse model, as well as that of PBC in humans. Indeed, future studies should focus on establishment of antigen specific CD8+ T cells and appropriate vector for delivery and subsequent in vivo expression; such a model will provide a novel venue for therapeutic intervention and dissection of pathogenic mechanisms.
Acknowledgments
The authors thank Masanobu Tsuda and Yoko Miyamoto Ambrosini for technical support in this experiment. The authors thank Ms. Nikki Phipps for support in preparing this article.
Financial support provided by National Institutes of Health grant, DK090019.
Abbreviations
- dnTGFβRII
transforming growth factor β receptor type II
- PBC
primary biliary cirrhosis
- OVA
ovalbumin
- AMAs
antimitochondrial antibodies
- PDC-E2
the pyruvate dehydrogenase E2 complex
- MNCs
mononuclear cells
- H&E
hematoxylin and eosin
References
- 1.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 2.Gershwin ME, Mackay IR. The causes of primary biliary cirrhosis: Convenient and inconvenient truths. Hepatology. 2008;47:737–745. doi: 10.1002/hep.22042. [DOI] [PubMed] [Google Scholar]
- 3.Van de Water J, Fregeau D, Davis P, Ansari A, Danner D, Leung P, Coppel R, et al. Autoantibodies of primary biliary cirrhosis recognize dihydrolipoamide acetyltransferase and inhibit enzyme function. J Immunol. 1988;141:2321–2324. [PubMed] [Google Scholar]
- 4.Rieger R, Leung PS, Jeddeloh MR, Kurth MJ, Nantz MH, Lam KS, Barsky D, et al. Identification of 2-nonynoic acid, a cosmetic component, as a potential trigger of primary biliary cirrhosis. J Autoimmun. 2006;27:7–16. doi: 10.1016/j.jaut.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. Journal of immunology. 1987;138:3525–3531. [PubMed] [Google Scholar]
- 6.Gershwin ME, Ansari AA, Mackay IR, Nakanuma Y, Nishio A, Rowley MJ, Coppel RL. Primary biliary cirrhosis: an orchestrated immune response against epithelial cells. Immunol Rev. 2000;174:210–225. doi: 10.1034/j.1600-0528.2002.017402.x. [DOI] [PubMed] [Google Scholar]
- 7.Moteki S, Leung PS, Dickson ER, Van Thiel DH, Galperin C, Buch T, Alarcon-Segovia D, et al. Epitope mapping and reactivity of autoantibodies to the E2 component of 2-oxoglutarate dehydrogenase complex in primary biliary cirrhosis using recombinant 2-oxoglutarate dehydrogenase complex. Hepatology. 1996;23:436–444. doi: 10.1002/hep.510230307. [DOI] [PubMed] [Google Scholar]
- 8.Kawata K, Kobayashi Y, Gershwin ME, Bowlus CL. The Immunophysiology and Apoptosis of Biliary Epithelial Cells: Primary Biliary Cirrhosis and Primary Sclerosing Cholangitis. Clin Rev Allergy Immunol. 2012 doi: 10.1007/s12016-012-8324-0. [DOI] [PubMed] [Google Scholar]
- 9.Oertelt S, Lian ZX, Cheng CM, Chuang YH, Padgett KA, He XS, Ridgway WM, et al. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177:1655–1660. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- 10.Tsuda M, Zhang W, Yang GX, Tsuneyama K, Ando Y, Kawata K, Park O, et al. Deletion of IL-12p35 induces liver fibrosis in dominant negative transforming growth factor beta receptor type II mice. Hepatology. 2012 doi: 10.1002/hep.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moritoki Y, Zhang W, Tsuneyama K, Yoshida K, Wakabayashi K, Yang GX, Bowlus C, et al. B cells suppress the inflammatory response in a mouse model of primary biliary cirrhosis. Gastroenterology. 2009;136:1037–1047. doi: 10.1053/j.gastro.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida K, Yang GX, Zhang W, Tsuda M, Tsuneyama K, Moritoki Y, Ansari AA, et al. Deletion of interleukin-12p40 suppresses autoimmune cholangitis in dominant negative transforming growth factor beta receptor type II mice. Hepatology. 2009;50:1494–1500. doi: 10.1002/hep.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ando Y, Yang GX, Tsuda M, Kawata K, Zhang W, Nakajima T, Tsuneyama K, et al. The immunobiology of colitis and cholangitis in interleukin-23p19 and interleukin-17a deleted dominant negative form of transforming growth factor beta receptor type ii mice. Hepatology. 2012;56:1418–1426. doi: 10.1002/hep.25803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kita H, Matsumura S, He XS, Ansari AA, Lian ZX, Van de Water J, Coppel RL, et al. Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. J Clin Invest. 2002;109:1231–1240. doi: 10.1172/JCI14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimoda S, Van de Water J, Ansari A, Nakamura M, Ishibashi H, Coppel RL, Lake J, et al. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest. 1998;102:1831–1840. doi: 10.1172/JCI4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kita H, Lian ZX, Van de Water J, He XS, Matsumura S, Kaplan M, Luketic V, et al. Identification of HLA-A2-restricted CD8(+) cytotoxic T cell responses in primary biliary cirrhosis: T cell activation is augmented by immune complexes cross-presented by dendritic cells. J Exp Med. 2002;195:113–123. doi: 10.1084/jem.20010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van de Water J, Ansari A, Prindiville T, Coppel RL, Ricalton N, Kotzin BL, Liu S, et al. Heterogeneity of autoreactive T cell clones specific for the E2 component of the pyruvate dehydrogenase complex in primary biliary cirrhosis. J Exp Med. 1995;181:723–733. doi: 10.1084/jem.181.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang GX, Lian ZX, Chuang YH, Moritoki Y, Lan RY, Wakabayashi K, Ansari AA, et al. Adoptive transfer of CD8(+) T cells from transforming growth factor beta receptor type II (dominant negative form) induces autoimmune cholangitis in mice. Hepatology. 2008;47:1974–1982. doi: 10.1002/hep.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuang YH, Lian ZX, Yang GX, Shu SA, Moritoki Y, Ridgway WM, Ansari AA, et al. Natural killer T cells exacerbate liver injury in a transforming growth factor beta receptor II dominant-negative mouse model of primary biliary cirrhosis. Hepatology. 2008;47:571–580. doi: 10.1002/hep.22052. [DOI] [PubMed] [Google Scholar]
- 20.Lian ZX, Okada T, He XS, Kita H, Liu YJ, Ansari AA, Kikuchi K, et al. Heterogeneity of dendritic cells in the mouse liver: identification and characterization of four distinct populations. Journal of immunology. 2003;170:2323–2330. doi: 10.4049/jimmunol.170.5.2323. [DOI] [PubMed] [Google Scholar]
- 21.Lleo A, Selmi C, Invernizzi P, Podda M, Coppel RL, Mackay IR, Gores GJ, et al. Apotopes and the biliary specificity of primary biliary cirrhosis. Hepatology. 2009;49:871–879. doi: 10.1002/hep.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rong G, Zhong R, Lleo A, Leung PS, Bowlus CL, Yang GX, Yang CY, et al. Epithelial cell specificity and apotope recognition by serum autoantibodies in primary biliary cirrhosis. Hepatology. 2011;54:196–203. doi: 10.1002/hep.24355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimoda S, Nakamura M, Ishibashi H, Hayashida K, Niho Y. HLA DRB4 0101-restricted immunodominant T cell autoepitope of pyruvate dehydrogenase complex in primary biliary cirrhosis: evidence of molecular mimicry in human autoimmune diseases. J Exp Med. 1995;181:1835–1845. doi: 10.1084/jem.181.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang N, Bevan MJ. TGF-beta signaling to T cells inhibits autoimmunity during lymphopenia-driven proliferation. Nat Immunol. 2012;13:667–673. doi: 10.1038/ni.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 26.Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunological reviews. 2000;174:47–62. doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- 27.Duchini A, Hendry RM, Redfield DC, Pockros PJ. Influenza infection in patients before and after liver transplantation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2000;6:531–542. doi: 10.1053/jlts.2000.9738. [DOI] [PubMed] [Google Scholar]
- 28.Polakos NK, Cornejo JC, Murray DA, Wright KO, Treanor JJ, Crispe IN, Topham DJ, et al. Kupffer cell-dependent hepatitis occurs during influenza infection. The American journal of pathology. 2006;168:1169–1178. doi: 10.2353/ajpath.2006.050875. quiz 1404-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu W, Chen X, Evanoff DP, Luo Y. Urothelial antigen-specific CD4+ T cells function as direct effector cells and induce bladder autoimmune inflammation independent of CD8+ T cells. Mucosal Immunol. 2011;4:428–437. doi: 10.1038/mi.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shibaki A, Sato A, Vogel JC, Miyagawa F, Katz SI. Induction of GVHD-like skin disease by passively transferred CD8(+) T-cell receptor transgenic T cells into keratin 14-ovalbumin transgenic mice. J Invest Dermatol. 2004;123:109–115. doi: 10.1111/j.0022-202X.2004.22701.x. [DOI] [PubMed] [Google Scholar]
- 31.Derkow K, Loddenkemper C, Mintern J, Kruse N, Klugewitz K, Berg T, Wiedenmann B, et al. Differential priming of CD8 and CD4 T-cells in animal models of autoimmune hepatitis and cholangitis. Hepatology. 2007;46:1155–1165. doi: 10.1002/hep.21796. [DOI] [PubMed] [Google Scholar]