Abstract
FAS/FASL altered expression may cause tumor protecting immunomodulation, with a direct impact on patient prognosis. FAS expression was studied in 60 squamous cell carcinomas of the oral cavity. FAS expression did not show a significant association with tumor histopathological characteristics, but was significantly associated with lymph node positivity. FAS expression was significantly associated with disease specific death and negative FAS expression was an independent risk factor, increasing risk 4 times when compared to positive expression. When FAS and FASL expression results were combined, we were able to define high, intermediate and low risk profiles. Disease-free and disease-specific survival were significantly correlated with FAS/FASL expression profiles. The high risk category was an independent marker for earlier disease relapse and disease-specific death, with approximately 4- and 6-fold increased risk, respectively, when compared to the low risk profile. Risk profiles based on FAS/FASL expression showed that high risk was significantly associated with increased disease relapse and death, as well as shorter disease-free or disease-specific survival. This categorization, added to patient clinical data, may facilitate the choice of therapy, minimizing treatment failure and increasing disease control.
Introduction
Head and neck cancer (HNC) is a significant cause of mortality and morbidity worldwide, presenting approximately 600,000 new cases yearly [1], whereas tumors of the oral cavity contribute with 389,000 new cases per year, with a mortality rate of 50% [2].
Currently, the most important HNC prognostic factor is the presence of regional lymph node metastases, which correlates with a 50% reduction in life expectancy [2]–[4], however, micrometastases may not be detected by routine histology [5].
Several factors are responsible for the modulation of tumoral growth and patient prognosis. Throughout the years, factors that alter proliferation and apoptosis have received a lot of attention. It is believed that disequilibrium between proliferation and apoptosis may be the key factor in tumor development and prognosis [6].
Programmed cell death plays a critical role in the development and homeostasis of multicelullar organisms [6]. This complex process involves several genes, as well as mutations and polymorphisms that may lead to deficient death signaling and potentiation of tumor aggressiveness. Some tumor cells have acquired the ability to overcome apoptosis or to induce apoptosis of tumor-specific lymphocytes, favoring tumor progression [7]. Apoptosis resistance is a capacity shared by most malignancies. Subversion of apoptotic pathways is a major mechanism in cancer devopment, being also related with tumor aggressiveness, histological differentiation and prognosis [8], [9].
FAS (CD95), a member of the TNF family, is a transmembrane protein with cystein rich extracellular domains and a death cytoplasmatic domain, common to all family members and essential in the translation of the death stimulus [10], [11]. Immediately after the receptor stimulation by the FASL ligand (CD95L), the apoptotic signal is transmitted through the adapter FADD (FAS Associated Death Domain), which converts caspase 8 zymogen into its active form, triggering the apoptosis start. Activation of this cascade will culminate into DNA fragmentation, causing radical morphological and biochemical intracellular changes [11]–[12].
FAS/FASL altered expression may cause tumor protecting immunomodulation, with a direct impact on patient prognosis [13]. In a previous study, microarray experiments compared gene expression between more aggressive oral tumors (tumors with premature metastasis; T1/T2, N+) and more benign tumors (advanced tumors without metastasis; T3/T4, N0). These results generated a list of genes with differential expression, where FAS and FASL were among the least expressed in more benign tumors, suggesting a role in tumor apoptosis resistance [14]. Owing to these results, the present study aimed to correlate FAS/FASL tumor expression with clinical variables, tumor histology and prognosis of squamous cell carcinoma of the oral cavity.
Materials and Methods
Ethics
This study was approved by the Research Ethics Committee of the Heliopolis Hospital on 08/12/2008 (CEP no 637) and an informed consent was obtained from all patients enrolled.
Samples
Samples were collected by the Head and Neck Genome Project (GENCAPO), a collaborative consortium created in 2002 with more than 50 researchers from 9 institutions in São Paulo State, Brazil, whose aim is to develop clinical, genetic and epidemiological analysis of HNSCC. In this study, 60 tumoral tissue samples were obtained and used for immunohistochemical analysis of the FAS and FASL gene, within a total of 60 patients with oral squamous cell carcinomas, surgically treated at the Head and Neck Surgery Department of Heliópolis Hospital, São Paulo, Brazil, during the period of January/2002 to December/2008. The clinical follow-up was at least 48 months after surgery. Previous surgical or chemotherapic treatment, distant metastasis, no removal of cervical lymph nodes and positive surgical margins were exclusion criteria. Histopathological slides were reviewed by a senior pathologist to confirm the diagnosis and select appropriate areas for immunohistochemical analysis. Tumors were classified according to the TNM system (3rd edition) [15]. Clinical, epidemiological and pathological tumor characteristics are described in Table 1 and 2.
Table 1. Epidemiological features.
| Epidemiological features | Total | |
| No. | (%) | |
| Gender | ||
| Female | 8 | (13.3) |
| Male | 52 | (86.7) |
| Age, yr | ||
| Median 55, df ±10.7 | ||
| Tobacco and Alcohol habits | ||
| Smoker and alcoholic | 50 | (83.3) |
| Only smoker | 7 | (11.7) |
| Only alcoholic | 1 | (1.7) |
| Tumor sub-sities | ||
| Tongue | 22 | (36.7) |
| Gum | 12 | (20.0) |
| Floor mouth | 21 | (35.0) |
| Retromolar area | 5 | (8.3) |
| Treatment | ||
| Only operated | 60 | (100.0) |
| Operated+irradiated | 31 | (51.7) |
| Total | 60 | (100.0) |
Table 2. Epidemiological, clinical and pathological tumor features and their association with FAS and FASL expression.
| Clinical and pathological features | Total | FAS expression | FASL expression | |||||
| Negative | Positive | p | Negative | Positive | p | |||
| No. | (%) | No. | No. | No. | No. | |||
| Stage | ||||||||
| 2 | 17 | (28.3) | 6 | 11 | 0.025 | 7 | 10 | 0.177 |
| 3 | 17 | (28.3) | 7 | 10 | 5 | 12 | ||
| 4 | 26 | (43.4) | 19 | 7 | 15 | 11 | ||
| Tumor size (T) ¥ | ||||||||
| T1+T2 | 24 | (40.0) | 11 | 13 | 0.233 | 8 | 16 | 0.297 |
| T3 | 12 | (20.0) | 5 | 7 | 7 | 5 | ||
| T4 | 24 | (40.0) | 16 | 8 | 12 | 12 | ||
| Lymph nodes (N) ¥ | ||||||||
| Absent | 27 | (45.0) | 9 | 18 | 0.004 | 11 | 16 | 0.548 |
| Present | 33 | (55.0) | 23 | 10 | 16 | 17 | ||
| Diferentiation | ||||||||
| Well | 26 | (43.4) | 16 | 10 | 0.441 | 18 | 8 | 0.003 |
| Moderately | 29 | (48.3) | 13 | 16 | 7 | 22 | ||
| Poorly | 5 | (8.3) | 3 | 2 | 2 | 3 | ||
| Disease specific death | ||||||||
| No | 32 | (53.3) | 11 | 21 | <0.001 | 9 | 23 | 0.006 |
| Yes | 25 | (41.7) | 20 | 5 | 16 | 9 | ||
| Not available* | 3 | (5.0) | 1 | |||||
| Disease relapse | ||||||||
| No | 26 | (43.4) | 10 | 16 | 0.080 | 6 | 20 | 0.007 |
| Yes | 29 | (48.3) | 18 | 11 | 17 | 12 | ||
| Not available* | 5 | (8.3) | ||||||
| Total | 60 | (100.0) | 32 | 28 | 27 | 33 | ||
TNM classification 3rd edition.
Not available (not considered in the statistical calculations).
Tissue Microarray
Formalin-fixed, paraffin-embedded tissue sections of 60 primary oral squamous cell carcinomas treated at the Head and Neck Surgery Department of Heliópolis Hospital, São Paulo, Brazil, were used for immunohistochemistry (IHC) analysis. Histological characterization of all samples was done by hematoxylin and eosin staining, followed by immunohistochemistry analysis of tissue microarrays (TMA). Two 1 mm cylinders were used to represent each sample in the TMA slide (Beecher Instruments®, Silver Spring, MD, USA).
Immunohistochemistry
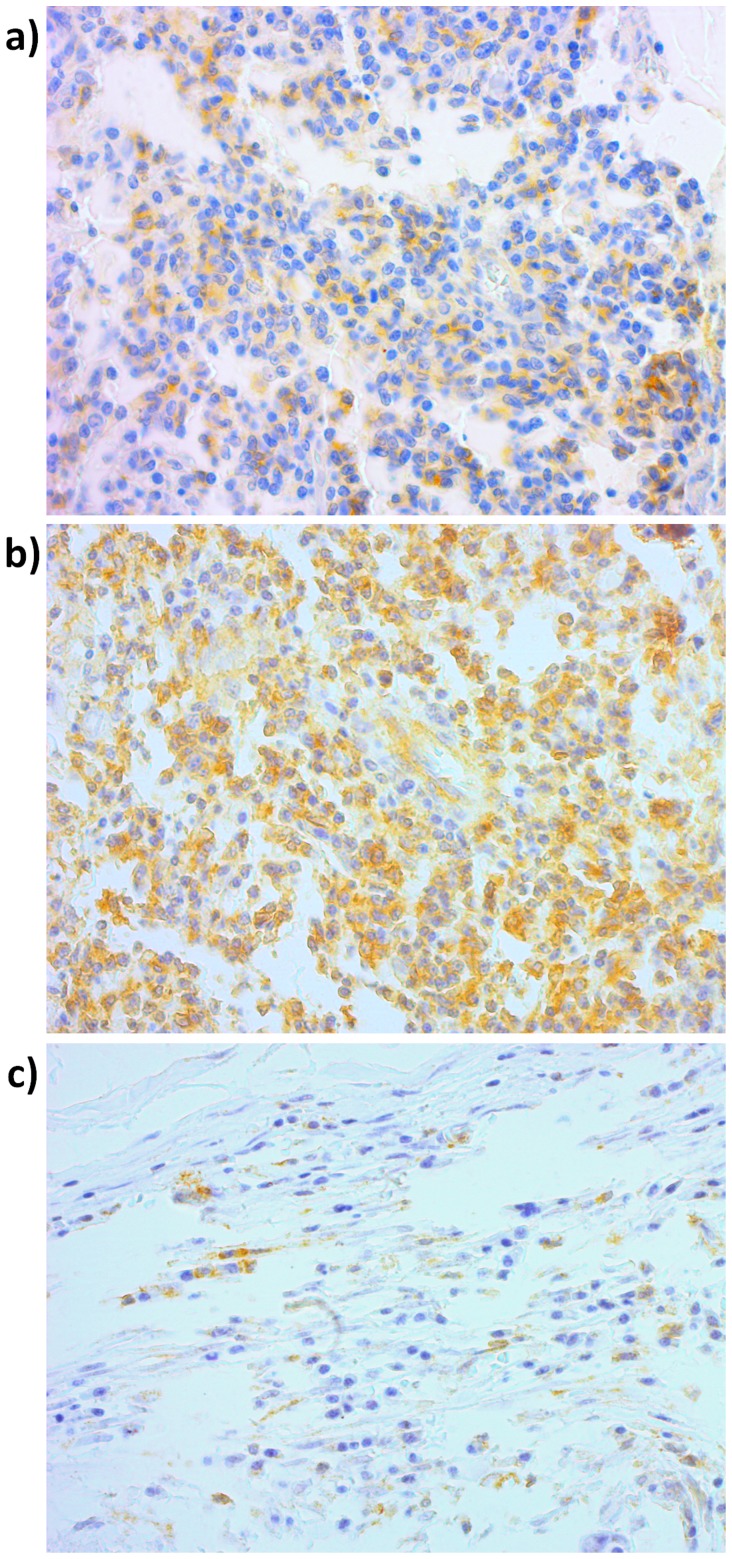
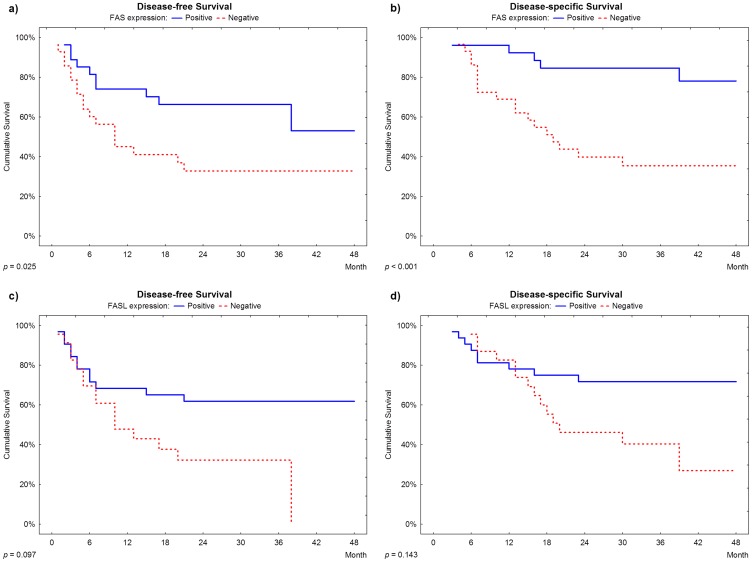
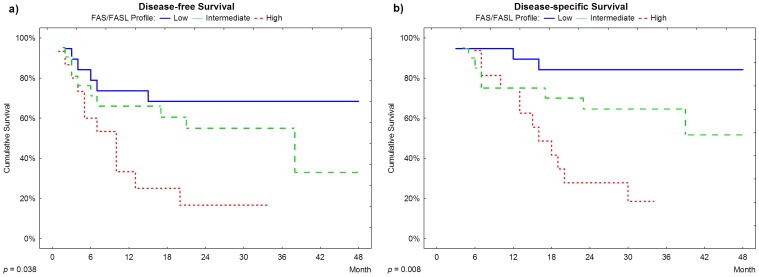
Anti-FAS monoclonal antibody and anti-FASL monoclonal antibody (Santa Cruz Biotechnology®, USA) were used in the IHC reaction, at a 1∶400 dilution [16]–[18]. Positive and negative controls were used. Sample scoring was performed by semi-quantitative microscopic analysis, considering the number of stained cells and signal intensity. Two spots were evaluated for each sample and a mean score was calculated. Considering the percentage of immune-positive tumor cells, a score of 1 was given when ≤10% of cells were positive; 2 when 10–50% of cells were positive and 3 when ≥50% of cells were positive. Signal intensity was scored as negative (0), weak (1), moderate (2) and strong (3). Both scores were multiplied [19], [20] and the resulting score was used to categorize FAS and FASL expression as positive (>3, Figure 1a and 1b, respectively) and negative (≤3, Figure 1c).
Figure 1. Immunohistochemical.
a. Positive FAS expression; b. Positive FASL expression; c. Negative immunostaining. Magnification was 400×.
Statistical Analysis
The chi square and Fisher exact tests were used for association analysis and confirmation was obtained by the Lilliefors test (significance considered when p<0.05). Multivariate logistic regression was used to obtain odds ratio (OR) and confidence intervals (CI 95%). Survival was calculated by the number of months between surgery and death for each patient or the last appointment in case the patient was alive. In order to calculate disease-free survival, the time endpoint was the date of disease relapse. The Kaplan-Meier model was used for survival analysis, using the Wilcoxon p-value and the Cox Proportional Hazards to adjust p-values and obtain hazard ratio (HR). Statistical calculations were performed using the Epi Info® v3.4.3, 2007 and Statsoft Statistica® v7.0.61.0 softwares.
Results
FAS Expression
FAS expression was studied in 60 tumors, of which 28 were positive (46.7%) and 32 were negative (53.3%). FAS expression did not show a significant association with tumor characteristics such as size (p = 0.233) and differentiation grade (p = 0.441), but was significantly associated with positive lymph nodes (p = 0.004, Table 2). Multivariate analysis showed that negative FAS expression was an independent marker for lymph node positivity (OR = 5.02, CI = 1.34–18.75, Table 3).
Table 3. Multivariate analysis of the relationship between clinical, pathological tumor features and survival with FAS and FASL expression.
| Variables | Logistic regression | Cox proportional hazard | ||||||||
| Lymph-nodes | Disease relapse | Disease specific death | Disease-free survival | Disease-specific survival | ||||||
| OR (95% CI)¥ | p ¥ | OR (95% CI)¥ | p ¥ | OR (95% CI)¥ | p ¥ | HR (95% CI)§ | p § | HR (95% CI)§ | p § | |
| FAS expression | ||||||||||
| Positive | 1 | 1 | 1 | 1 | 1 | |||||
| Negative | 5.02 (1.34–18.75) | 0.017 | 1.49 (0.39–5.78) | 0.561 | 4.59 (1.01–21.51) | 0.050 | 1.66 (0.69–3.97) | 0.257 | 3.73 (1.16–11.95) | 0.027 |
| FASL expression | ||||||||||
| Positive | 1 | 1 | 1 | 1 | 1 | |||||
| Negative | 1.22 (0.30–5.00) | 0.780 | 5.51 (1.32–23.04) | 0.019 | 6.06 (1.05–35.06) | 0.044 | 2.58 (1.03–6.46) | 0.044 | 2.14 (0.73–6.30) | 0.166 |
| Tumor size (T) | ||||||||||
| T1+T2 | 1 | 1 | 1 | 1 | 1 | |||||
| T3 | 1.62 (0.30–8.67) | 0.576 | 1.63 (0.29–9.25) | 0.581 | 2.32 (0.33–16.20) | 0.395 | 2.31 (0.73–7.35) | 0.156 | 3.00 (0.76–11.91) | 0.118 |
| T4 | 4.44 (1.08–18.20) | 0.038 | 2.68 (0.62–11.55) | 0.186 | 2.76 (0.51–14.84) | 0.236 | 2.05 (0.77–5.50) | 0.152 | 1.97 (0.63–6.22) | 0.245 |
| Differentiation | ||||||||||
| Well | 1 | 1 | 1 | 1 | 1 | |||||
| Moderately | 3.56 (0.81–15.63) | 0.092 | 1.09 (0.24–4.96) | 0.909 | 1.66 (0.26–10.44) | 0.589 | 1.57 (0.57–4.35) | 0.385 | 1.84 (0.56–6.05) | 0.318 |
| Poorly | 6.07 (0.45–81.73) | 0.174 | 0.28 (0.03–2.97) | 0.291 | 7.19 (0.37–139.86) | 0.193 | 0.54 (0.11–2.79) | 0.465 | 1.94 (0.41–9.17) | 0.405 |
| Lymph-nodes | ||||||||||
| Absent | – | – | 1 | 1 | 1 | 1 | ||||
| Present | – | – | 4.07 (0.48–34.40) | 0.197 | 13.55 (0.94–195.73) | 0.056 | 2.28 (0.62–8.33) | 0.214 | 3.49 (0.78–15.65) | 0.102 |
| Irradiated | ||||||||||
| No | – | – | 1 | 1 | 1 | 1 | ||||
| Yes | – | – | 0.17 (0.02–1.27) | 0.085 | 0.30 (0.02–3.68) | 0.344 | 0.30 (0.09–0.97) | 0.044 | 0.52 (0.17–1.56) | 0.241 |
OR – Odds ratio; HR – Hazard ratio; CI – Confidence interval.
Values adjusted by multivariate logistic regression.
Values adjusted by Cox proportional hazards.
FAS expression was significantly associated with disease specific death (p<0.001, Table 2) and multivariate analysis showed that negative expression was an independent death risk factor, increasing risk 4 times when compared to positive expression (OR = 4.59, CI = 1.01–21.51, Table 3). Nonetheless, FAS expression was not correlated with disease relapse (p = 0.080, Table 2).
Disease-free and disease-specific survival were significantly correlated with FAS expression (p = 0.025 and p<0.001, respectively). According to a 24 month after surgery follow up, approximately 70% of cases with negative expression presented disease relapse, as compared to approximately 25% of recurrence in patients with positive expression of FAS (Figure 2a). Additionally, according to a 36 month after surgery follow up, approximately 65% of cases with negative expression died of disease specific causes, as compared to 15% of deaths in patients with positive expression of FAS (Figure 2b). Multivariate analysis revealed that a negative expression of FAS was an independent marker for earlier disease specific death, showing a 3-fold increased risk when compared to positive expression (HR = 3.73, CI = 1.16–11.95, Table 3), but the same association was not found for disease relapse (HR = 1.66, CI = 0.69–3.97, Table 3).
Figure 2. Survival plots.
a. and b.: Disease-free survival and disease-specific survival according to FAS expression; c. and d.: Disease-free survival and disease-specific survival according to FASL expression.
FASL Expression
Regarding FASL, 33 (55.0%) tumors showed positive expression, whereas 27 (45.0%) were negative. FASL expression was significantly associated with differentiation grade (p = 0.003), but was not associated with tumor size (p = 0.297) or positive lymph nodes (p = 0.548, Table 2).
FASL expression did significantly correlate with disease relapse (p = 0.007) and disease specific death (p = 0.006, Table 2). Multivariate analysis showed that negative FASL was an independent marker of disease relapse and disease specific death, representing an increased risk of over 6 times for each, when compared to a positive expression (respectively, OR = 5.51, CI = 1.32–23.04 and OR = 6.06, CI = 1.05–35.06; Table 3).
In contrast, disease-free and disease-specific survival were not associated with FASL expression (p = 0.143 and p = 0.097, respectively, Figure 2c and 2d).
FAS/FASL Profiles
In an attempt to combine FAS and FASL expression results, we categorized the FAS/FASL profile in three classes: low risk (positive FAS and FASL expression); intermediate risk (negative expression of one marker) and high risk (negative expression of both markers). Frequencies of each FAS/FASL profiles were 20 (33.3%), 21 (35.0%) and 19 (31.7%), respectively for low, intermediate and high risk.
Disease-free and disease-specific survival were significantly correlated with FAS/FASL profiles (p = 0.038 and p = 0.008, respectively). On a 24 month after surgery follow up, 80% of cases classified as high risk had relapsed and approximately 70% died of disease-specific causes, compared to approximately 30% of relapse and 15% of death in patients classified as low risk (Figure 3a and 3b). Multivariate analysis revealed that the high risk category is an independent marker for earlier disease relapse and disease-specific death, with approximately 4- and 6-fold increased risk, respectively, when compared to the low risk profile (HR = 3.80, CI = 1.19–12.52 and HR = 6.43, CI = 1.45–28.55).
Figure 3. Survival plots.
a. and b.: Disease-free survival and disease-specific survival according to FAS/FASL profile.
Discussion and Conclusions
Apoptosis is a physiological process of cell number control, which plays an important role in cellular homeostasis and embryonic development [21]–[24]. Cell population is defined by a balance between proliferation and survival and disruption of this balance can lead to cancer growth [25]–[32].
The extrinsic apoptosis pathway can be triggered by enzymes of the TNF family, including FAS and FASL. FASL positive T-cells can eliminate FAS positive tumoral cells by inducing apoptosis [10], [12]. Therefore, reduction or loss of FAS expression may result in decreased sensitivity of tumoral cells to cytotoxic activity, impairing apoptosis.
FAS expression has been previously associated with tumor apoptosis in the stomach [13], esophagus [33] and liver [34]. In addition, FAS/FASL diminished expression correlates with worse prognosis in lung [35] esophageal [36], larynx [37], colorectal [38] and gastric [39] tumors.
In agreement with the literature, our results show that negative FAS expression correlates with lymph node metastasis (5 times increased risk). When compared with positive expression, negative expression was significantly associated with cancer related deaths and shorter disease-free and disease-specific survival. Multivariate analysis confirmed that negative FAS expression was an independent risk factor for death and disease-specific survival reduction, increasing risk approximately 5 times for each. Our results also showed that negative FASL expression was associated with increased disease relapse and disease-related deaths. Multivariate analysis confirmed that FASL negative expression was an independent risk factor for disease relapse and death, increasing risk up to 6 times when compared to positive expression. However, FASL expression was not related to worse disease-free survival or disease-specific survival.
In contrast with our results, other studies have revealed higher FASL expression as a marker of worse prognosis in esophageal [36] and lung [40]–[42] tumors. Their hypothesis relies on tumor FASL expression as a T-cell apoptosis inducer, resulting in lower tumor attack by the immune system [43]–[45]. However, our results support the hypothesis that the immune system response is already compromised in oral cancer, most likely because it is a tobacco/alcohol associated disease [46]. As previously reported, chronic alcohol consumption impairs Natural Killer cell (NK) activity and decreases NK cell number, therefore affecting their ability to destroy tumor cells. [47]. In addition, several studies have reported a similar decrease in number and activity of NK cells in smokers [48]; [49], in which cases a lower production of interferon-c and TNF-a cytokines is observed [50]. Based on these facts, our hypothesis predicts that the oral immune response is attenuated in patients with chronic tobacco and alcohol consumption, therefore in these individuals, a lack of FASL may represent a loss of the extrinsic apoptosis signal in tumor cells, conferring a worse prognosis.
In summary, our results correlate a negative FAS/FASL expression with worse prognosis in oral squamous cell carcinoma patients, suggesting that these proteins play important roles in oral cancer cell apoptosis.
Acknowledgments
We thank the GENCAPO (Head and Neck Genome Project - http://www.gencapo.famerp.br/) team for the invaluable discussions that motivated the present study.
Funding Statement
Authors acknowledge the financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Grants 04/12054-9) and researcher fellowships from Conselho Nacional de Pesquisas (CNPq) and Fundação de Amparo à Pesquisa do Estado do Espírito Santo (FAPES). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bauman JE, Michel LS, Chung CH (2012) New promising molecular targets in head and neck squamous cell carcinoma. Curr Opin Oncol 24: 235–242. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. (2010) GLOBOCAN 2008, cancer incidence and mortality worldwide: IARC CancerBase No. 10. International Agency for Research on Cancer: Available: http://globocan.iarc.fr. Accessed 20 July 2012.
- 3. Myers EM, Fagan JJ (1998) Treatment of the N+ neck in squamous cell carcinoma of the upper aerodigestive tract. Otolaryngol Clin North Am 31: 671–686. [DOI] [PubMed] [Google Scholar]
- 4. Zhen W, Karnell LH, Hoffman HT, Funk GF, Buatti JM, et al. (2004) The National Cancer Data Base report on squamous cell carcinoma of the base of tongue. Head Neck 26: 660–674. [DOI] [PubMed] [Google Scholar]
- 5. Pentenero M, Gandolfo S, Carrozzo M (2005) Importance of tumor thickness and depth of invasion in nodal involvement and prognosis of oral squamous cell carcinoma: a review of the literature. Head Neck 27: 1080–1091. [DOI] [PubMed] [Google Scholar]
- 6. Shibakita M, Tachibana M, Dhar DK, Ohno S, Kubota H, et al. (2000) Spontaneous apoptosis in advanced esophageal carcinoma: its relation to Fas expression. Clin Cancer Res 6: 4755–4759. [PubMed] [Google Scholar]
- 7. Zhang X, Miao X, Sun T, Tan W, Qu S, et al. (2005) Functional polymorphisms in cell death pathway genes FAS and FASL contribute to risk of lung cancer. J Med Genet 42: 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Volm M, Koomagi R (2000) Relevance of proliferative and pro-apoptotic factors in non-small-cell lung cancer for patient survival. Br J Cancer 82: 1747–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun T, Miao X, Zhang X, Tan W, Xiong P, et al. (2004) Polymorphisms of death pathway genes FAS and FASL in esophageal squamous-cell carcinoma. J Natl Cancer Inst 96: 1030–1036. [DOI] [PubMed] [Google Scholar]
- 10. Ashkenazi A, Dixit VM (1999) Apoptosis control by death and decoy receptors. Curr Opin Cell Bio 11: 255–260. [DOI] [PubMed] [Google Scholar]
- 11. French LE, Tschopp J (2003) Protein-based therapeutic approaches targeting death receptors. Cell Death Differ 10: 117–123. [DOI] [PubMed] [Google Scholar]
- 12. Ashe PC, Berry MD (2003) Apoptotic signaling cascades. Prog Neuropsychopharmacol Biol Psychiatry 27: 199–214. [DOI] [PubMed] [Google Scholar]
- 13. Ohno S, Tachibana M, Shibakita M, Dhar DK, Yoshimura H, et al. (2000) Prognostic significance of Fas and Fas ligand system-associated apoptosis in gastric cancer. Ann Surg Oncol 7: 750–757. [DOI] [PubMed] [Google Scholar]
- 14. Severino P, Alvares AM, Michaluart P Jr, Okamoto OK, Nunes FD, et al. (2008) Global gene expression profiling of oral cavity cancers suggests molecular heterogeneity within anatomic subsites. BMC Res Notes 1: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deschler DG, Day T (2008) Pocket Guide to Neck Dissection and Classification and TNM Staging of Head and Neck Cancer. American Academy of Otolaryngology-Head and Neck Surgery Foundation. 28 p.
- 16. Rimm DL, Camp RL, Charette LA, Olsen DA, Provost E (2001) Amplification of tissue by construction of tissue microarrays. Exp Mol Pathol 70: 255–264. [DOI] [PubMed] [Google Scholar]
- 17. Hedvat CV, Hegde A, Chaganti RS, Chen B, Qin J, et al. (2002) Application of tissue microarray technology to the study of non-Hodgkin's and Hodgkin's lymphoma. Hum Pathol 33: 968–974. [DOI] [PubMed] [Google Scholar]
- 18. Hsu FD, Nielsen TO, Alkushi A, Dupuis B, Huntsman D, et al. (2002) Tissue microarrays are an effective quality assurance tool for diagnostic immunohistochemistry. Mod Pathol 15: 1374–1380. [DOI] [PubMed] [Google Scholar]
- 19. Soini Y, Kahlos K, Puhakka A, Lakari E, Saily M, et al. (2000) Expression of inducible nitric oxide synthase in healthy pleura and in malignant mesothelioma. Br J Cancer 3: 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Campos AH, Aldred VL, Ribeiro KC, Vassallo J, Soares FA (2009) Role of immunoexpression of nitric oxide synthases by Hodgkin and Reed-Sternberg cells on apoptosis deregulation and on clinical outcome of classical Hodgkin lymphoma. Mol Cell Biochem 321: 95–102. [DOI] [PubMed] [Google Scholar]
- 21. DeLong MJ (1998) Apoptosis: a modulator of cellular homeostasis and disease states. Ann NY Acad Sci 842: 82–90. [DOI] [PubMed] [Google Scholar]
- 22. Brill A, Torchinsky A, Carp H, Toder V (1999) The role of apoptosis in normal and abnormal embryonic development. J Assist Reprod Genet 16: 512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morita Y, Tsutsumi O, Taketani Y (2001) Regulatory mechanisms of female germ cell apoptosis during embryonic development. Endocr J 48: 289–301. [DOI] [PubMed] [Google Scholar]
- 24. Doseff AI (2004) Apoptosis: the sculptor of development. Stem Cells Dev 13: 473–483. [DOI] [PubMed] [Google Scholar]
- 25. McGill G, Fisher DE (1997) Apoptosis in tumorigenesis and cancer therapy. Front Biosci 2: 353–379. [DOI] [PubMed] [Google Scholar]
- 26. Sjostrom J, Bergh J (2001) How apoptosis is regulated, and what goes wrong in cancer. BMJ 322: 1538–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koornstra JJ, de Jong S, Hollema H, de Vries EG, Kleibeuker JH (2003) Changes in apoptosis during the development of colorectal cancer: a systematic review of the literature. Crit Rev Oncol Hematol 45: 37–53. [DOI] [PubMed] [Google Scholar]
- 28. Gerl R, Vaux DL (2005) Apoptosis in the development and treatment of cancer. Carcinogenesis 26: 263–270. [DOI] [PubMed] [Google Scholar]
- 29. Vermeulen K, Van Bockstaele DR, Berneman ZN (2005) Apoptosis: mechanisms and relevance in cancer. Ann Hematol 84: 627–639. [DOI] [PubMed] [Google Scholar]
- 30. King KL, Cidlowski JA (1995) Cell cycle and apoptosis: common pathways to life and death. J Cell Biochem 58: 175–180. [DOI] [PubMed] [Google Scholar]
- 31. Yuo A (2001) Differentiation, apoptosis, and function of human immature and mature myeloid cells: intracellular signaling mechanism. Int J Hematol 73: 438–452. [DOI] [PubMed] [Google Scholar]
- 32. Blagosklonny MV (2003) Apoptosis, proliferation, differentiation: in search of the order. Semin Cancer Biol 13: 97–105. [DOI] [PubMed] [Google Scholar]
- 33. Gratas C, Tohma Y, Barnas C, Taniere P, Hainaut P, et al. (1998) Up-regulation of Fas (APO-1/CD95) ligand and down-regulation of Fas expression in human esophageal cancer. Cancer Res 58: 2057–2062. [PubMed] [Google Scholar]
- 34. Nagao M, Nakajima Y, Hisanaga M, Kayagaki N, Kanehiro H, et al. (1999) The alteration of Fas receptor and ligand system in hepatocellular carcinomas: how do hepatoma cells escape from the host immune surveillance in vivo? Hepatology 30: 413–421. [DOI] [PubMed] [Google Scholar]
- 35. Fan CF, Xu HT, Lin XY, Yu JH, Wang EH (2011) A multiple marker analysis of apoptosis-associated protein expression in non-small cell lung cancer in a Chinese population. Folia Histochemica et Cytobiologica 49: 231–239. [DOI] [PubMed] [Google Scholar]
- 36. Watson GA, Naran S, Zhang X, Stang MT, Oliveira PEQ, et al. (2011) Cytoplasmic Overexpression of CD95L in Esophageal Adenocarcinoma Cells Overcomes Resistance to CD95-Mediated Apoptosis. Neoplasia 13: 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Asensio C, Zapata A, García-Ahijado J, Gil B, Salvadores P (2007) Fas expression is associated with a better prognosis in laryngeal squamous cell carcinoma. Anticancer Res 27: 4083–4086. [PubMed] [Google Scholar]
- 38. Pryczynicz A, Guziñska-Ustymowicz K, Kemona A (2010) Fas/FasL expression in colorectal cancer. An immunohistochemical study. Folia Histochem Cytobiol 48: 425–429. [DOI] [PubMed] [Google Scholar]
- 39. Li Q, Peng J, Li XH, Liu T, Liang QC, et al. (2010) Clinical significance of Fas and FasL protein expression in gastric carcinoma and local lymph node tissues. World J Gastroenterol 16: 1274–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee SH, Shin MS, Park WS, Kim SY, Kim HS, et al. (1999) Alterations of Fas (Apo-1/CD95) gene in non-small cell lung cancer. Oncogene 18: 3754–3760. [DOI] [PubMed] [Google Scholar]
- 41. Nambu Y, Hughes SJ, Rehemtulla A, Hamstra D, Orringer MB, et al. (1998) Lack of cell surface Fas/APO-1 expression in pulmonary adenocarcinoma. J Clin Invest 101: 1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Niehans GA, Brunner T, Frizelle SP, Liston JC, Salerno CT, et al. (1997) Human lung carcinomas express Fas ligand. Cancer Res 57: 1007–1012. [PubMed] [Google Scholar]
- 43. Owen-Schaub LB, van Golen KL, Hill LL, Price JE (1998) Fas and Fas ligand interactions suppress melanoma lung metastasis. J Exp Med 188: 1717–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Möller P, Koretz K, Leithäuser F, Brüderlein S, Henne C, et al. (1994) Expression of APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in normal and neoplastic colon epithelium. Int J Cancer 57: 371–377. [DOI] [PubMed] [Google Scholar]
- 45. Gastman BR, Atarshi Y, Reichert TE, Saito T, Balkir L, et al. (1999) Fas ligand is expressed on human squamous cell carcinomas of the head and neck, and it promotes apoptosis of T lymphocytes. Cancer Res 59: 5356–5364. [PubMed] [Google Scholar]
- 46. Farshadpour F, Kranenborg H, Calkoen EVB, Hordijk GJ, Koole R, et al. (2011) Survival analysis of head and neck squamous cell Carcinoma: influence of smoking and drinking. Head Neck 33: 817–823. [DOI] [PubMed] [Google Scholar]
- 47. Szabo G (1999) Consequences of alcohol consumption on host defence. Alcohol and Alcoholism 34: 830–841. [DOI] [PubMed] [Google Scholar]
- 48. Mian MF, Lauzon NM, Stampfli MR, Mossman KL, Ashkar AA (2008) Impairment of human NK cell cytotoxic activity and cytokine release by cigarette smoke. J Leukoc Biol 83: 774–874. [DOI] [PubMed] [Google Scholar]
- 49. Accomando WP, Wiencke JK, Houseman EA, Butler RA, Zheng S, et al. (2012) Decreased NK Cells in Patients with Head and Neck Cancer Determined in Archival DNA. Clin Cancer Res 18: 6147–6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gonçalves RB, Coletta RD, Silvério KG, Benevides L, Casati MZ, et al. (2011) Impact of smoking on inflammation: overview of molecular. Mechanisms Inflamm. Res 60: 409–424. [DOI] [PubMed] [Google Scholar]