Abstract
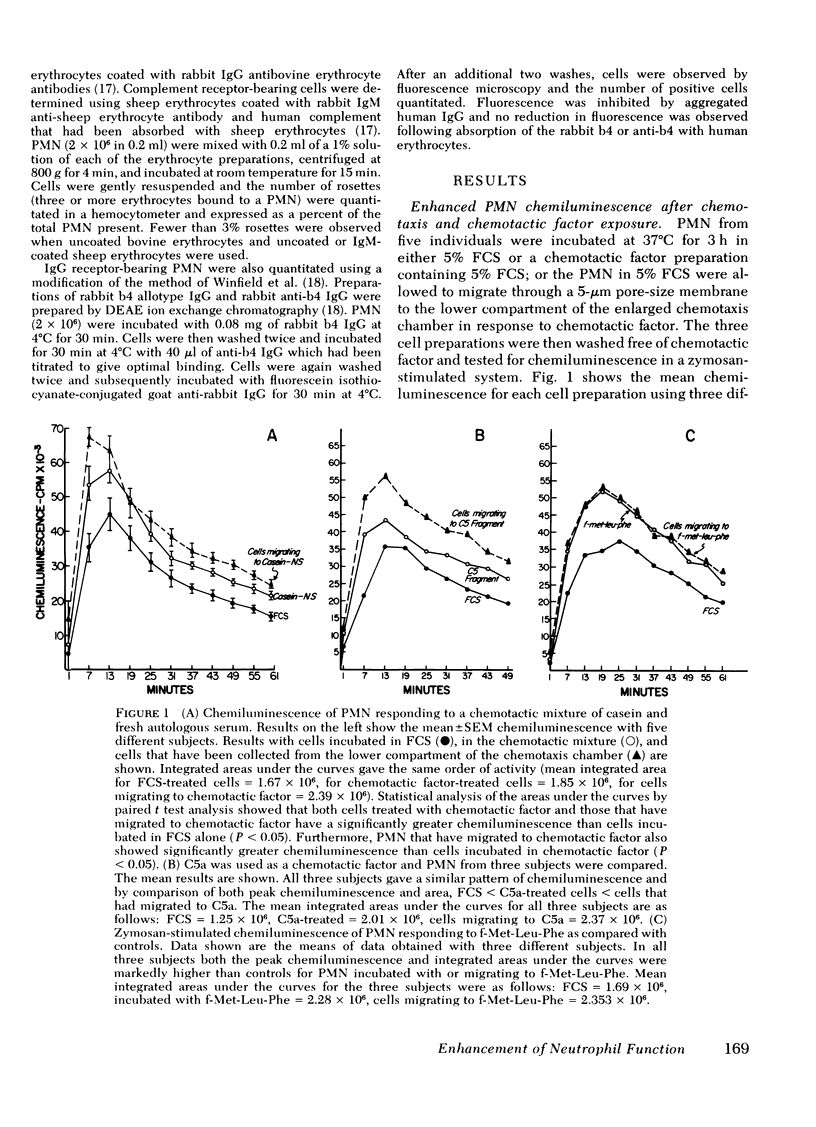
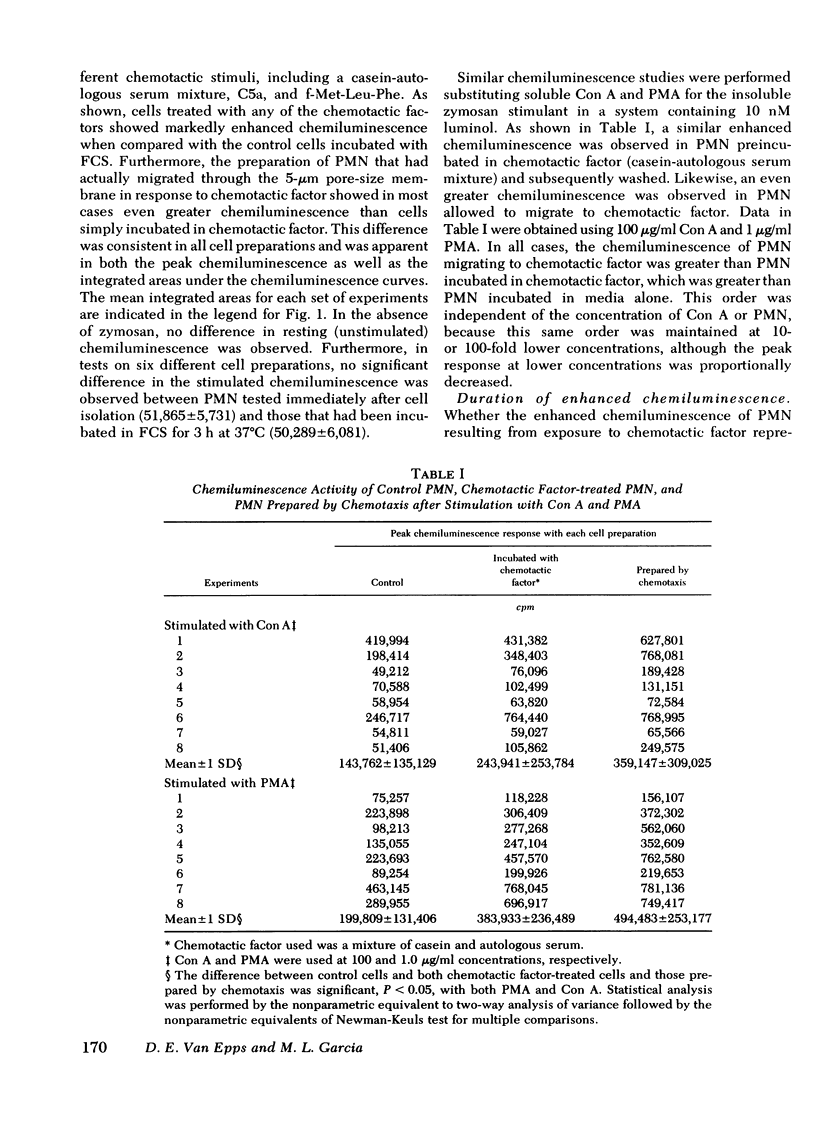
Exposure of human polymorphonuclear leukocytes (PMN) to chemotactic factor, as well as the migration of PMN through a 5-μm pore-size membrane, results in a PMN population with enhanced chemiluminescence, enhanced capacity for superoxide anion production, and increased Escherichia coli bactericidal activity. The enhanced PMN response resulting from exposure to chemotactic factor was observed with several chemotactic stimuli, including a mixture of casein and autologous serum, chemotactic C5 fragment, and formyl-l-methionyl-l-leucine-l-phenylalanine (f-Met-Leu-Phe). Enhanced levels of chemiluminescence were observed with both soluble stimuli (concanavalin A and phorbol myristate acetate) as well as particulate stimuli (opsonized zymosan).
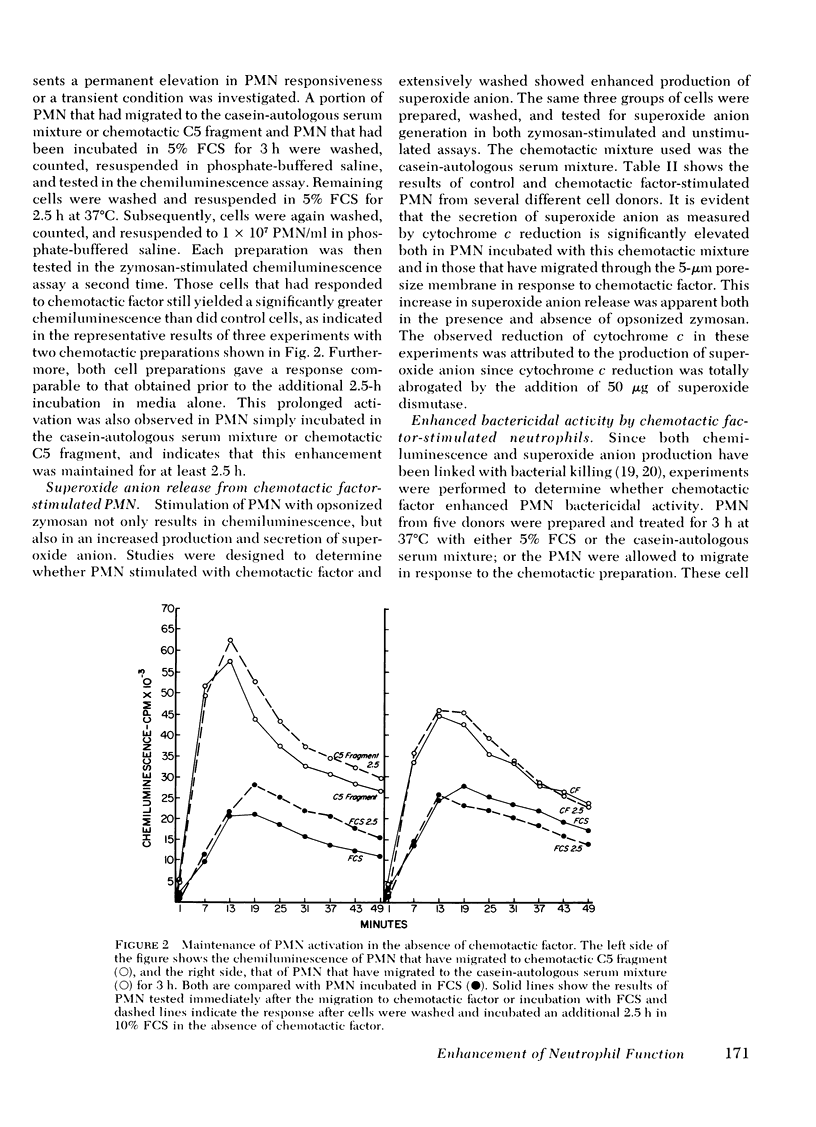
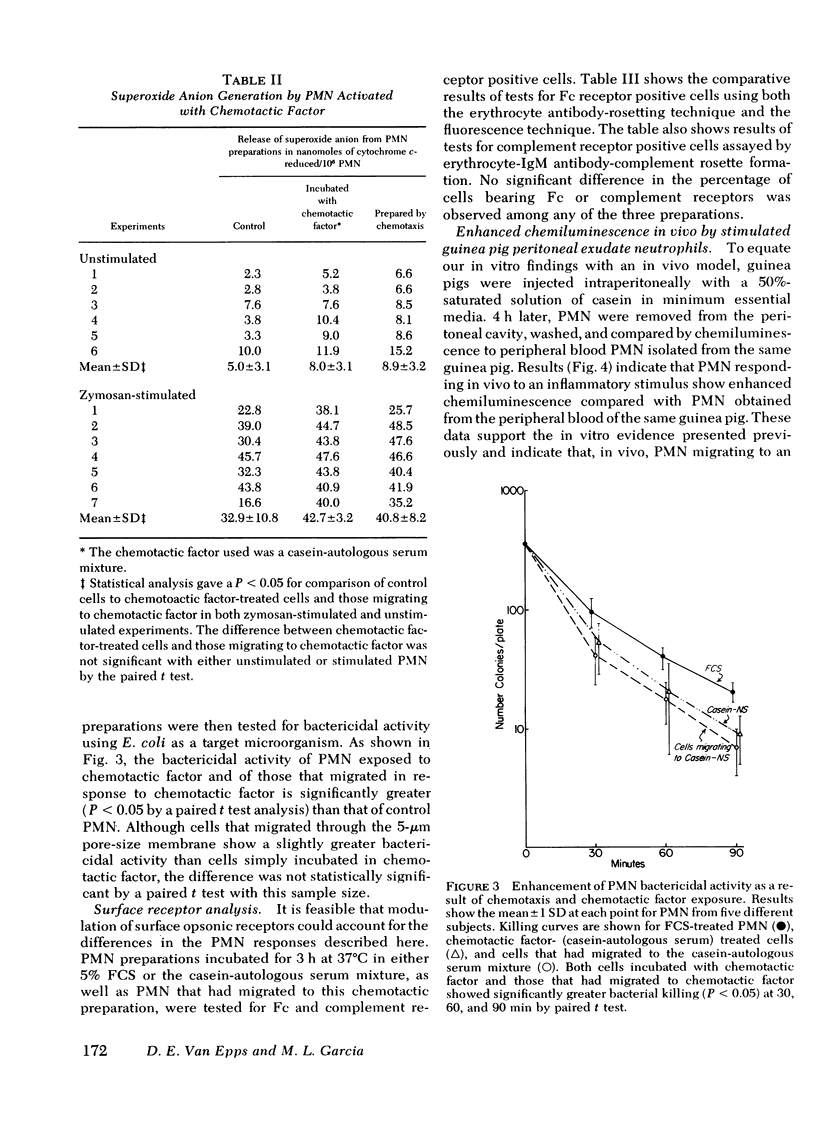
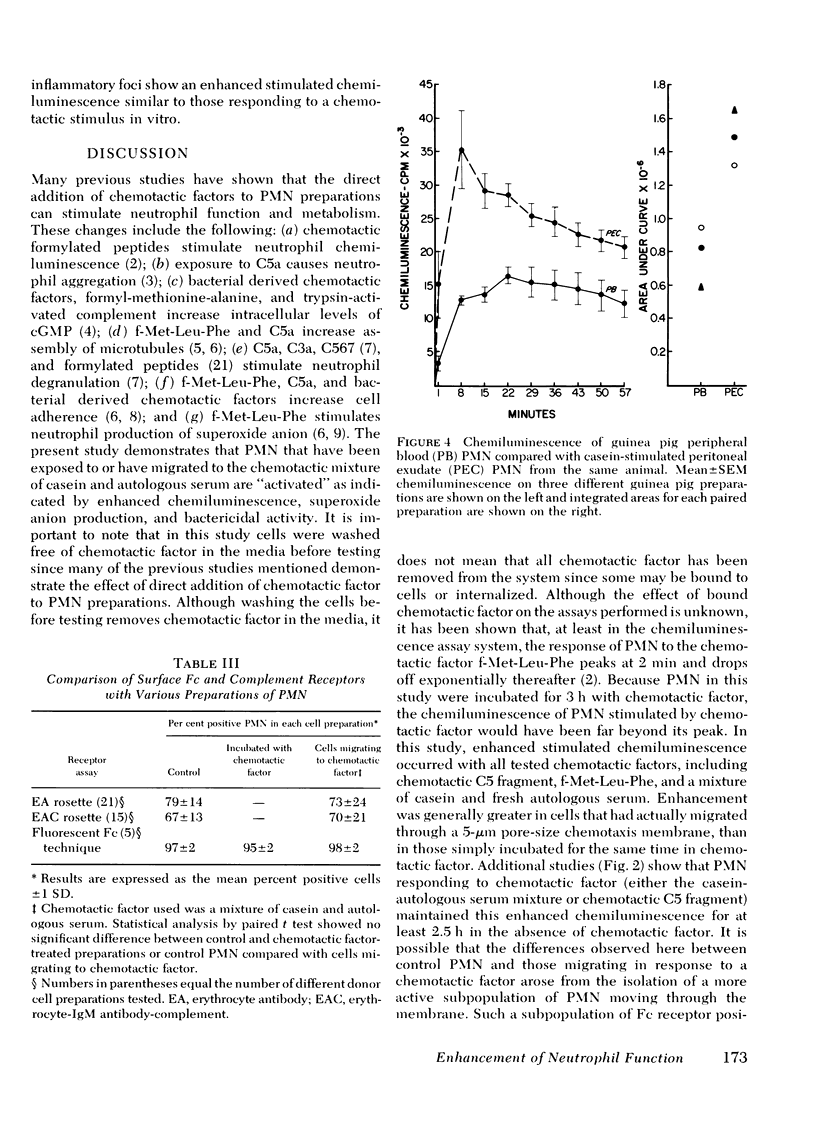
Once activated by chemotactic factor, PMN retained their enhanced stimulated chemiluminescence in the absence of chemotactic factor for at least 2.5 h. Enhanced activity could not be correlated with a shift in the number of immunoglobulin (Ig)G Fc receptor positive or complement receptor positive PMN. In vivo studies with guinea pigs indicated that PMN attracted to an intraperitoneal injection of casein, like those attracted through a chemotaxis membrane in vitro in response to casein, showed markedly enhanced stimulated chemiluminescence when compared with peripheral blood PMN from the same animal. Such a mechanism to stimulated PMN function may enhance the effectiveness of PMN in host defense at inflammatory foci.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Yevich S. J., Orth R. W., Steele R. H. The superoxide anion and singlet molecular oxygen: their role in the microbicidal activity of the polymorphonuclear leukocyte. Biochem Biophys Res Commun. 1974 Oct 8;60(3):909–917. doi: 10.1016/0006-291x(74)90401-x. [DOI] [PubMed] [Google Scholar]
- Becker E. L., Showell H. J., Henson P. M., Hsu L. S. The ability of chemotactic factors to induce lysosomal enzyme release. I. The characteristics of the release, the importance of surfaces and the relation of enzyme release to chemotactic responsiveness. J Immunol. 1974 Jun;112(6):2047–2054. [PubMed] [Google Scholar]
- Boxer L. A., Yoder M., Bonsib S., Schmidt M., Ho P., Jersild R., Baehner R. L. Effects of a chemotactic factor, N-formylmethionyl peptide, on adherence, superoxide anion generation, phagocytosis, and microtubule assembly of human polymorphonuclear leukocytes. J Lab Clin Med. 1979 Mar;93(3):506–514. [PubMed] [Google Scholar]
- Cross A. S., Lowell G. H. Stimulation of polymorphonuclear leukocyte bactericidal activity by supernatants of activated human mononuclear cells. Infect Immun. 1978 Nov;22(2):502–507. doi: 10.1128/iai.22.2.502-507.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Wright D. G., Schiffmann E. Role of secretory events in modulating human neutrophil chemotaxis. J Clin Invest. 1978 Dec;62(6):1364–1374. doi: 10.1172/JCI109257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvath L., Andersen B. R. Defective initiation of oxidative metabolism in polymorphonuclear leukocytes. N Engl J Med. 1979 May 17;300(20):1130–1135. doi: 10.1056/NEJM197905173002003. [DOI] [PubMed] [Google Scholar]
- Hatch G. E., Gardner D. E., Menzel D. B. Chemiluminescence of phagocytic cells caused by N-formylmethionyl peptides. J Exp Med. 1978 Jan 1;147(1):182–195. doi: 10.1084/jem.147.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch G. E., Nichols W. K., Hill H. R. Cyclic nucleotide changes in human neutrophils induced by chemoattractants and chemotactic modulators. J Immunol. 1977 Aug;119(2):450–456. [PubMed] [Google Scholar]
- Issekutz A. C., Lee K. Y., Biggar W. D. Enhancement of human neutrophil bactericidal activity by chemotactic factors. Infect Immun. 1979 May;24(2):295–301. doi: 10.1128/iai.24.2.295-301.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson D., DeChatelet L. R., Spitznagel J. K., Wang P. Comparison of NADH and NADPH oxidase activities in granules isolated from human polymorphonuclear leukocytes with a fluorometric assay. J Clin Invest. 1977 Feb;59(2):282–290. doi: 10.1172/JCI108639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempner M. S., Gallin J. I. Separation and functional characterization of human neutrophil subpopulations. Blood. 1978 Apr;51(4):659–669. [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. Neutrophil aggregation and swelling induced by chemotactic agents. J Immunol. 1977 Jul;119(1):232–239. [PubMed] [Google Scholar]
- Schleupner C. J., Glasgow L. A. Peritoneal macrophage activation indicated by enhanced chemiluminescence. Infect Immun. 1978 Sep;21(3):886–895. doi: 10.1128/iai.21.3.886-895.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Generation of superoxide radicals by human peripheral neutrophils activated by chemotactic factor. Evidence for the role of calcium. J Lab Clin Med. 1979 Apr;93(4):583–593. [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C., Patrick R. A., Hassett C. Motility and adhesiveness in human neutrophils. Effects of chemotactic factors. J Clin Invest. 1979 Feb;63(2):221–229. doi: 10.1172/JCI109293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps D. E., Goodwin J. S., Murphy S. Age-dependent variations in polymorphonuclear leukocyte chemiluminescence. Infect Immun. 1978 Oct;22(1):57–61. doi: 10.1128/iai.22.1.57-61.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps D. E., Reed K., Williams R. C., Jr Suppression of human PMN bactericidal activity by human IgA paraproteins. Cell Immunol. 1978 Mar 15;36(2):363–376. doi: 10.1016/0008-8749(78)90280-0. [DOI] [PubMed] [Google Scholar]
- Van Epps D. E., Williams R. C., Jr Suppression of leukocyte chemotaxis by human IgA myeloma components. J Exp Med. 1976 Nov 2;144(5):1227–1242. doi: 10.1084/jem.144.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van D. E., Paxton L. L. Chemotaxis as a preparative technique for human polymorphonuclear leukocytes. J Lab Clin Med. 1975 Aug;86(2):309–314. [PubMed] [Google Scholar]
- Winfield J. B., Lobo P. I., Hamilton M. E. Fc receptor heterogeneity: immunofluorescent studies of B, T, and "third population" lymphocytes in human blood with rabbit IgG b4/anti-b4 complexes. J Immunol. 1977 Nov;119(5):1778–1784. [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]



