Abstract
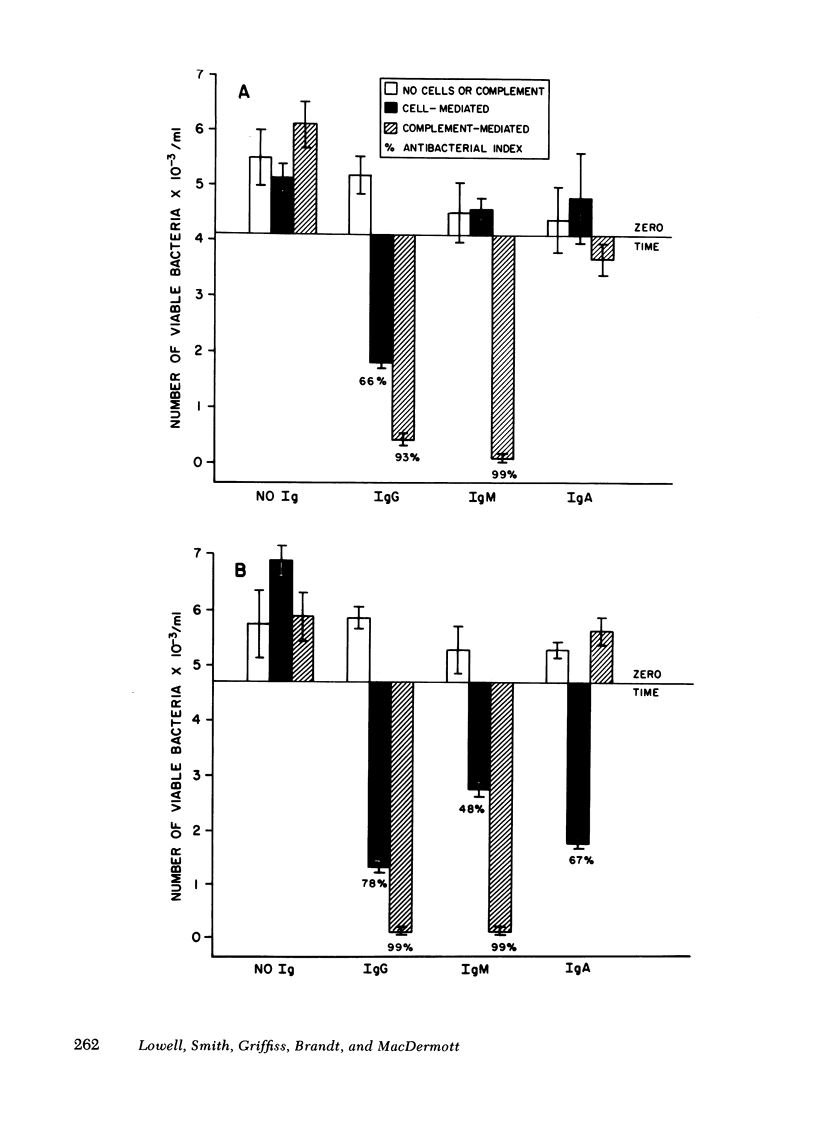
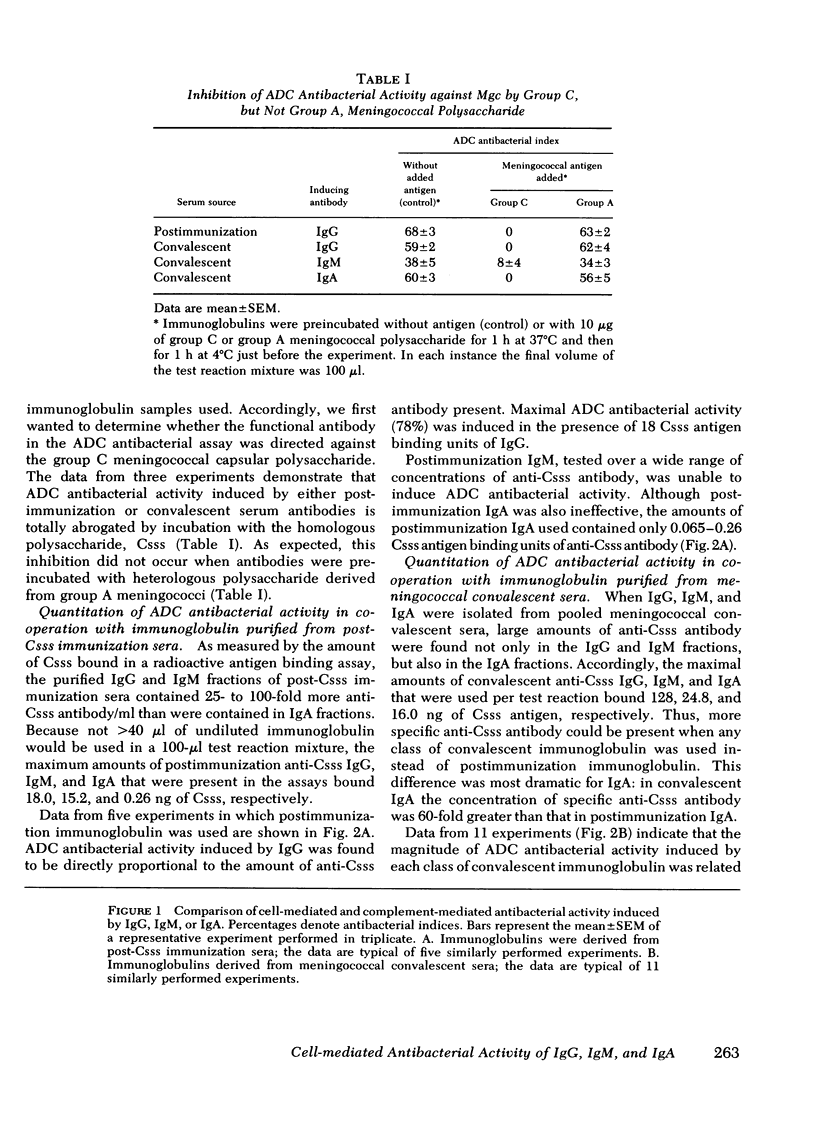
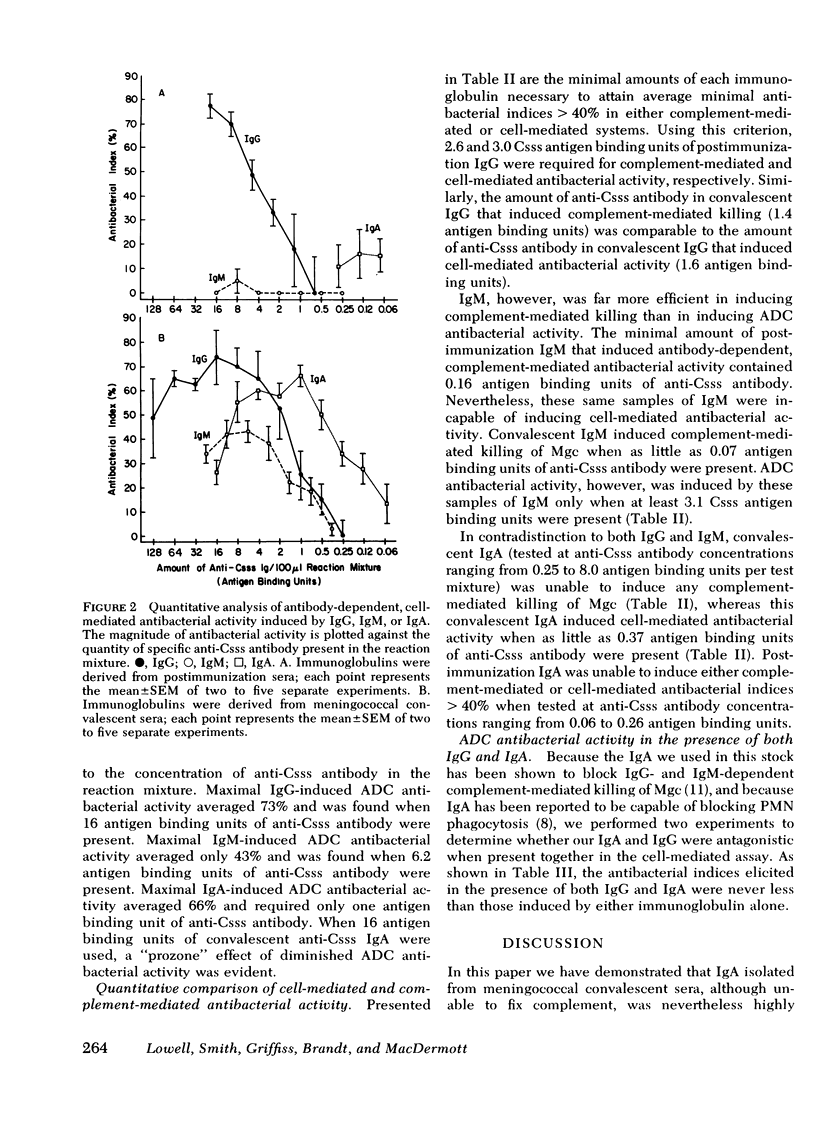
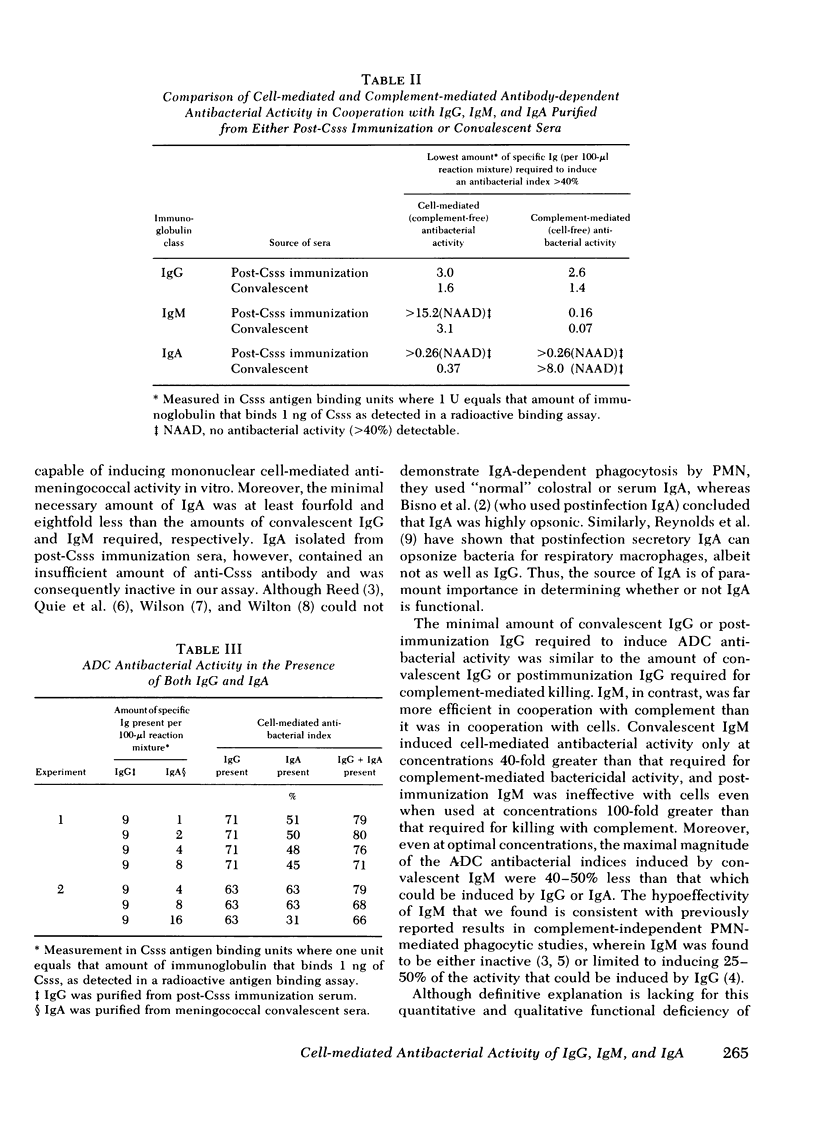
We have compared the abilities of immunoglobulin (Ig)G, IgM, and IgA to induce either mononuclear cell-mediated (complement-independent) or complement-mediated (cell-free) antibacterial activity against group C meningococci. In each of these assays, immunoglobulins purified from the sera of individuals immunized with meningococcal group C polysaccharide were compared with those purified from sera of patients convalescing from disseminated meningococcal disease. Our data support three conclusions. First, although nonbactericidal in cooperation with complement, IgA can induce cell-mediated antibacterial activity as well as IgG. Second, the amount of IgG required to induce cell-mediated antibacterial activity is similar to the amount required for complement-mediated killing. Third, although the amount of either postimmunization or convalescent IgM required to induce complement-mediated killing is 16- to 20-fold less than the amount of respective IgG required, IgM is inferior to IgG in its ability to induce cell-mediated antibacterial activity because in the cell-mediated system (a) postimmunization IgM is ineffective; (b) the amount of convalescent IgM required for minimal activity is eightfold more than the amount of convalescent IgG required; and (c) the maximal antibacterial index induced by convalescent IgM is 50% less than that which can be induced by IgG. These data suggest that IgG and IgA may play a greater role than IgM in mononuclear cell-mediated antibacterial host immune defense.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaud-Battandier F., Bundy B. M., O'Neill M., Bienenstock J., Nelson D. L. Cytotoxic activities of gut mucosal lymphoid cells in guinea pigs. J Immunol. 1978 Sep;121(3):1059–1065. [PubMed] [Google Scholar]
- Bisno A. L., Ofek I., Beachey E. H., Chandler R. W., Curran J. W. Human immunity to Neisseria gonorrhoeae: acquired serum opsonic antibodies. J Lab Clin Med. 1975 Aug;86(2):221–229. [PubMed] [Google Scholar]
- Brandt B. L., Wyle F. A., Artenstein M. S. A radioactive antigen-binding assay for Neisseria meningitidis polysaccharide antibody. J Immunol. 1972 Apr;108(4):913–920. [PubMed] [Google Scholar]
- Frasch C. E., Parkes L., McNelis R. M., Gotschlich E. C. Protection against group B meningococcal disease. I. Comparison of group-specific and type-specific protection in the chick embryo model. J Exp Med. 1976 Aug 1;144(2):319–329. doi: 10.1084/jem.144.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fubara E. S., Freter R. Protection against enteric bacterial infection by secretory IgA antibodies. J Immunol. 1973 Aug;111(2):395–403. [PubMed] [Google Scholar]
- Gold R., Wyle F. A. New Classification of Neisseria meningitidis by Means of Bactericidal Reactions. Infect Immun. 1970 May;1(5):479–484. doi: 10.1128/iai.1.5.479-484.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M. Bactericidal activity of meningococcal antisera. Blocking by IgA of lytic antibody in human convalescent sera. J Immunol. 1975 Jun;114(6):1779–1784. [PubMed] [Google Scholar]
- Griffiss J. M., Bertram M. A., Broud D. D. Separation and purification of immunoglobulins M,A and G from small volumes of human sera by a continuous, inline chromatographic process. J Chromatogr. 1978 Aug 21;156(1):121–130. doi: 10.1016/s0021-9673(00)83132-5. [DOI] [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Ca++-dependent binding of antigen-19 S antibody complexes to macrophages. J Immunol. 1969 May;102(5):1172–1178. [PubMed] [Google Scholar]
- Lowell G. H., Smith L. F., Artenstein M. S., Nash G. S., MacDermott R. P., Jr Antibody-dependent cell-mediated antibacterial activity of human mononuclear cells. I. K lymphocytes and monocytes are effective against meningococi in cooperation with human imune sera. J Exp Med. 1979 Jul 1;150(1):127–137. doi: 10.1084/jem.150.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melewicz F. M., Shore S. L., Ades E. W., Phillips D. J. The mononuclear cell in human blood which mediates antibody-dependent cellular cytotoxicity to virus-infected target cells. II. Identification as a K cell. J Immunol. 1977 Feb;118(2):567–573. [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann H., Perlmann P., Pape G. R., Halldén G. Purification, fractionation and assay of antibody-dependent lymphocytic effector cells (K cells) in human blood. Scand J Immunol. 1976 Jun;Suppl 5:57–68. doi: 10.1111/j.1365-3083.1976.tb03856.x. [DOI] [PubMed] [Google Scholar]
- Quie P. G., Messner R. P., Williams R. C., Jr Phagocytosis in subacute bacterial endocarditis. Localization of the primary opsonic site to Fc fragment. J Exp Med. 1968 Oct 1;128(4):553–570. doi: 10.1084/jem.128.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed W. P. Serum factors capable of opsonizing Shigella for phagocytosis by polymorphonuclear neutrophils. Immunology. 1975 Jun;28(6):1051–1059. [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y., Kazmierowski J. A., Newball H. H. Specificity of opsonic antibodies to enhance phagocytosis of Pseudomonas aeruginosa by human alveolar macrophages. J Clin Invest. 1975 Aug;56(2):376–385. doi: 10.1172/JCI108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y., Thompson R. E. Pulmonary host defenses. I. Analysis of protein and lipids in bronchial secretions and antibody responses after vaccination with pseudomonas aeruginosa. J Immunol. 1973 Aug;111(2):358–368. [PubMed] [Google Scholar]
- Roberts R. B. The relationship between group A and group C meningococcal polysaccharides and serum opsonins in man. J Exp Med. 1970 Mar 1;131(3):499–513. doi: 10.1084/jem.131.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Barnett J. A., May F. P., Sanford J. P. Comparison of the opsonic activity of gamma-G- and gamma-M-anti-Proteus globulins. J Immunol. 1967 Feb;98(2):336–343. [PubMed] [Google Scholar]
- Steigbigel R. T., Lambert L. H., Jr, Remington J. S. Phagocytic and bacterial properties of normal human monocytes. J Clin Invest. 1974 Jan;53(1):131–142. doi: 10.1172/JCI107531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. D. Studies on the opsonic activity of human secretory IgA using an in vitro phagocytosis system. J Immunol. 1972 Mar;108(3):726–730. [PubMed] [Google Scholar]
- Wilton J. M. Suppression by IgA of IgG-mediated phagocytosis by human polymorphonuclear leucocytes. Clin Exp Immunol. 1978 Dec;34(3):423–428. [PMC free article] [PubMed] [Google Scholar]
- Wåhlin B., Perlmann H., Perlmann P. Analysis by a plaque assay of IgG- or IgM- dependent cytolytic lymphocytes in human blood. J Exp Med. 1976 Nov 2;144(5):1375–1380. doi: 10.1084/jem.144.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Pennington C. L., Artenstein M. S. Human antibody response to three meningococcal outer membrane antigens: comparison by specific hemagglutination assays. Infect Immun. 1974 Nov;10(5):975–984. doi: 10.1128/iai.10.5.975-984.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



