Abstract
Natural killer (NK) cells belong to the innate immune system and are potent cytolytic and cytokine-producing effector cells in response to tumor targets. NK cell based anti-tumor immunotherapy was so far mainly successful in patients with different types of leukemia. For instance, acute myeloid leukemia (AML) patients displayed a prolonged survival if transplanted with haploidentical stem cells giving rise to NK cells with a mismatch in inhibitory killer immunoglobulin receptors (KIRs) and recipients’ HLA class I. Although promising results have been achieved with hematological tumors, solid tumors are in most cases poorly controlled by NK cells. Therapeutic protocols that aimed at improving NK cell responses in patients with solid malignancies succeeded in increasing NK cell numbers and functional responses of NK cells isolated from the patients’ peripheral blood. However, in the majority of cases tumor progression and overall survival of patients were not significantly improved. There is increasing evidence that tumor-associated NK cells become gradually impaired during tumor progression compared to NK cells from peripheral blood and healthy tissues. Future protocols of NK cell based immunotherapy should integrate three important aspects to improve NK cell anti-tumor activity: facilitating NK cell migration to the tumor site, enhancing their infiltration into the tumor tissue and ensuring subsequent efficient activation in the tumor. This review summarizes the current knowledge of tumor-infiltrating NK cells and the influence of the tumor microenvironment on their phenotype and function.
Keywords: NK cells, Tumor immunology, Tumor microenvironment
Introduction
Tumor initiation and progression is a complex process characterized by the interaction of malignant cells and infiltrating immune cells that shape each others’ phenotype and responses. In many cases, infiltration of high numbers of certain immune cells, especially cytotoxic effectors such as NK or CD8+ T cells, was shown to correlate with an improved prognosis for cancer patients [1, 2]. However, recent studies described phenotypic and functional features of immune cells in the tumor microenvironment that rather supported tumor growth [3]. Examples are tumor-associated macrophages (TAMs) that are often polarized towards an immunoregulatory phenotype, immature dendritic cells (iDCs) that promote tolerogenic responses and CD8+ T cells that acquire an exhausted phenotype. In addition, tumors can promote the generation and recruitment of suppressive immune cells, such as regulatory T cells (Tregs) [4] and myeloid-derived suppressor cells (MDSCs) [5] that can suppress responses of anti-tumor effector cells. Thus, strategies of cancer immunotherapy should aim not only at increasing the numbers of effector cells in the tumors, but also at circumventing the immunosuppressive mechanisms that hamper effective anti-tumor immune responses at the tumor site.
NK cell based therapy relies on the ability of NK cells to efficiently distinguish tumor cells from healthy cells [6]. Transformed cells often express increased levels of ligands that engage activating NK cell receptors, while at the same time ligands of inhibitory receptors are downregulated. MHC class I molecules, that are expressed at high levels on normal cells and protect them from NK cell lysis, represent the main class of inhibitory ligands. It was shown that the majority of acute myeloid leukemia (AML) cells expressed ligands for the activating NK cell receptors DNAM-1, NKp30 and NKp46 and thus, were susceptible to NK cell lysis [7]. Patients suffering from AML, who received grafts with a mismatch in NK cell expressed inhibitory killer immunoglobulin receptors (KIRs) and recipient expressed HLA class I ligands, survived significantly longer than recipients of grafts with an absence of mismatch [8]. Lessons from KIR-mismatched haploidentical transplantation indicated the importance to circumvent KIR-mediated inhibition in order to achieve full NK cell activation. Fully humanized antibodies against inhibitory KIRs have been developed and their safety was evaluated in phase I and II clinical trials with AML and multiple myeloma patients [9, 10]. Moreover, Venstrom et al. [11] recently demonstrated that the outcome of allogeneic hematopoetic stem cell transplantation in AML patients correlated with the expression of activating KIR genes of the donors. Donor KIR2DS1 positivity was associated with protection against relapse and donor KIR3DS1 with reduced mortality. Further examples of anti-cancer therapies that involve NK cells are the treatment of bladder cancer with Mycobacterium bovis bacillus Calmette-Guerin (BCG), the tyrosine kinase inhibitor Imatinib Mesylate (Gleevec) treatment of gastrointestinal tumors [12], DC-based immunotherapies [13] and antibody-based therapies [14]. Since in many cases NK cell based therapies of solid tumors remained unsuccessful, a better knowledge of the impact of the tumor microenvironment on NK cell activation is critical for the design of improved therapeutic protocols.
Tumor Cell Recognition by NK Cells
NK cells are often described as potent cytotoxic effectors that can eliminate tumor cells without prior sensitization [15, 16]. However, increasing evidence exists that effector functions of NK cells are more complex and regulated at multiple levels. During development, NK cells pass through a process of education, which results in the generation of mature effectors that attack malignant or stressed cells, but not healthy cells. It was shown that resting human NK cells can respond to certain stimuli [17], but their full activation is only achieved when multiple signals are properly integrated. Target cells initiate NK cell activation if they express sufficient amounts of ligands for activating NK cell receptors and low levels of ligands that engage inhibitory receptors [6]. In addition, NK cell priming with DCs [18], their interaction with CD4+ T cells ([19] and our unpublished observations) or neutrophils [20, 21] or the presence of certain cytokines, such as IL-2, IL-12, IL-15, IL-18 or IL-21 [22], can further enhance their effector function.
The expression of numerous activating receptors (summarized in Fig. 1) enables recognition of an array of ligands widely expressed on transformed cells, while mainly absent in healthy tissues [6]. Activating receptors include NCRs (NKp30 and NKp44 in human, NKp46 in human and mouse), NKR (NK1.1 in mouse), NKG2D and DNAM-1 (in human and mouse). Other receptors, such as 2B4, CD48 or NTBA can also trigger and/or support NK cell activation. NKG2D, the best-characterized NK cell receptor in the context of tumor immunity, recognizes stress-induced ligands of the Rae1 protein family, H60 and MULT1 in mice, and MICA, MICB and members of ULBP family in humans. NKG2D ligands (NKG2D-Ls) are rarely expressed on healthy cells, but upregulated upon cellular transformation or viral infection [23, 24]. Furthermore, chemotherapeutic drugs or ionizing radiation that cause activation of the DNA damage pathway can further upregulate NKG2D-L expression on tumor cells [25]. In addition, activation of the DNA damage pathway also increases expression of ligands for the activating receptor DNAM-1 facilitating tumor cell recognition by NK cells [26]. DNAM-1- and NKG2D-mediated anti-tumor responses can be further enhanced by treatment with IL-2 or/and IL-12, respectively [27, 28]. Importantly, it was reported recently that the NKG2D receptor was essential for effective immunosurveillance of lymphoma and prostate carcinoma in mouse models of spontaneously arising malignancies [29].
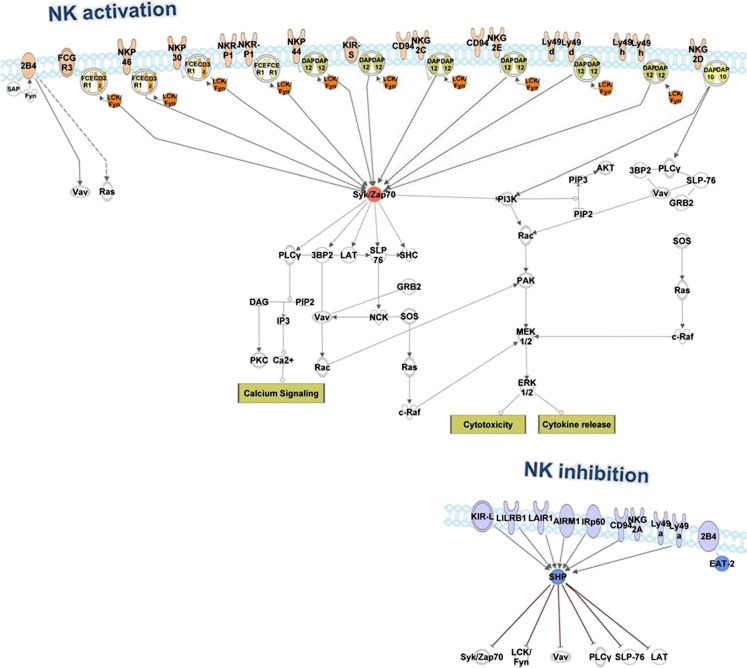
Fig. 1.
NK cell activating and inhibitory receptors and their downstream signaling molecules. Signaling pathways downstream of activating NK cell receptors typically lead to NK cell cytotoxic responses and/or cytokine production. Central molecules involved in NK cell activation are often downregulated in the tumor microenvironment. These molecules include: CD3ζ, an adaptor molecule coupled to several activating receptors; contains an immunoreceptor tyrosine-based activation motif (ITAM) that becomes phosphorylated by Src family kinases upon engagement of activating receptor(s) by its ligands; Lck kinase that phosphorylates ITAMs of CD3ζ and FcεRIγ adaptor molecules; Syk kinase that is recruited to phosphorylated ITAMs and mediates downstream signaling; additional downstream molecules such as Vav, PI3K or PLCγ. Inhibitory NK cell receptors contain immunoreceptor tyrosine-based inhibition motifs (ITIMs) and recruit phosphatases such as SHP-1 and SHIP upon ligand recognition. These events lead to the dephosphorylation of active components downstream of activating receptors counteracting NK cell activation. Tumor-associated NK cells often upregulate expression of inhibitory receptors that result in an enhanced threshold for NK cell activation. The network was generated using the Ingenuity Pathway Analysis (IPA) software (Ingenuity Systems, www.ingenuity.com)
The NCRs NKp46 and NKp30 are expressed on most NK cells, whereas NKp44 is induced after activation. Tumor-associated ligands for most NCRs remain unknown. Recently, BAT3 (the nuclear factor HLA-B-associated transcript 3, also called Bag6, BCL2-associated athanogene 6) and B7-H6, a B7-family member, were defined as ligands for NKp30. BAT3 is an intracellular protein that is released via exosomes from DCs and activates NK cells [30, 31]. BAT3 neutralization was shown to decrease the lysis of Raji cells by primary NK cells and to prevent tumor cell rejection by PBMCs in a multiple myeloma mouse model [30]. So far, the importance of BAT3 during anti-tumor immune responses in cancer patients remains unclear. B7-H6 is detected on the cell surface of several tumor cell lines and at low percentages of primary tumor cells of hematological origin derived from a small subset of patients [32]. B7-H6 was shown to activate cytotoxic NK cell responses by its interaction with NKp30. Three different isoforms of NKp30 were described. The final outcome of NKp30 activation depends on the NKp30 isoform expressed on the surface and can result in a quantitatively and qualitatively different response of NK cells [33].
Recently, Rosental et al. reported that proliferating cell nuclear antigen (PCNA) binds to NKp44 and inhibits NK cell function [34]. Expression of PCNA was associated with shorter overall survival of breast cancer patients [35] and might be involved in tumor immune evasion. The full effector potential of NK cells is mainly achieved upon simultaneous engagement of several activating receptors [36]. As an example, the simultaneous blockade of DNAM-1 and NCRs leads to the complete abrogation of lysis of certain tumor cells by NK cells. Thus, the identification of additional ligands for activating receptors such as for the NCRs will open new possibilities for therapeutic targeting.
Tumor cells employ multiple mechanisms to escape from the direct recognition by NK cells. Tumor-infiltrating NK cells in cancer patients often display reduced surface expression of activating NK cell receptors compared to NK cells from peripheral blood [37]. The NKG2D-Ls can be shed from the surface of tumor cells, leading to increased levels of soluble ligands in serum [38–40]. Patients with high levels of NKG2D-Ls in serum show decreased expression of the NKG2D receptor on both NK and CD8+ T cells. Reduction of NKG2D levels on tumor-infiltrating and peripheral blood CD8+ T cells from individuals with cancer was associated with circulating tumor-derived soluble MICA [39]. Paschen et al. showed that high serum levels of soluble ULBP2 in melanoma patients were associated with disease progression and poor prognosis [41]. The downregulation of NKG2D expression in response to the continuous exposure to its ligands can also lead to the impaired function of several other activating NK cell receptors including NKp46 and CD16 [42]. Moreover, NKG2D expression can be reduced by soluble factors such as TGF-β [43, 44] and L-kynurenine, a product of tryptophan degradation. L-kynurenine was also shown to reduce the expression of NKp46 on human NK cells [45]. Pietra et al. reported that the co-culture of NK cells with cell lines derived from melanoma skin lesions in the presence of IL-2 led to the inhibition of the IL-2-induced upregulation of NKp30, NKp44 and NKG2D, all of which are involved in melanoma cell killing [46]. The observed effect was mediated by tumor-derived indoleamine 2,3-dioxygenase (IDO) and prostaglandin E2 (PGE2) secreted by tumor cells. Expression of inhibitory KIRs and CD94/NKG2A receptors remained unchanged indicating that activating receptors were main targets of tumor-mediated suppression. We have evidence that expression of activating NK cell receptors in the tumor microenvironment is also regulated at the transcriptional level (our unpublished observation). In addition to the reduced expression of activating receptors, we also observed downregulation of the downstream signaling molecules indicating that the function of NK cells receptors in the tumor tissue can be modulated at multiple levels.
Efficient recognition of tumor cells by NK cells is instrumental for NK cell activation. Thus, up-regulation of activating ligands and/or down-regulation of inhibitory ligands on tumor cells would increase NK cell activation against tumors. For instance, we showed that activation of p53 with the small molecular compound, RITA, led to the upregulation of ULBP1 and ULBP2 in tumor cells resulting in enhanced NK cell-mediated target recognition [47]. Similarly, certain MIC and ULBP proteins can be upregulated by the oncogene Ras [48] or by application of histone-deacetylase inhibitors [49, 50]. The expression of NKG2D-Ls was shown to be controlled by certain miRNAs [51–53], some of which have broad tumor-suppressive functions. Several other compounds such as dacarbazine, a cytotoxic drug used for treatment of melanoma patients [54], hydralazine and valproate, tested in cervical cancer studies [55], 5-fluorouracil used for pancreatic cancer patients or IFN-α applied in melanoma patients [56] were shown to increase NKG2D-L expression on tumor cells. A detailed analysis of the molecular network that regulates expression of ligands for activating NK cell receptors might lead to the identification of novel targets for the modulation of NK cell/tumor cell recognition. Furthermore, a combination of agents known to upregulate activating ligands with other approaches of NK cell based therapies might improve the therapeutic benefit.
Anti-Tumor Effector Function of NK cells
NK cells lyse tumor cells upon engagement of activating receptors by the release of cytotoxic mediators, perforin and granzymes. Expression of death receptor ligands, FasL and TRAIL, enable NK cells to induce apoptosis in tumor cells expressing the corresponding receptors. Engagement of FcγRIII (CD16) by Ab-coated targets induces NK cell degranulation and is an important mode of action of Ab-based therapies. In addition, other mechanisms such as the release of exosomes that contain perforin and death receptor ligands [57] and the formation of nanotubes that enables contact with target cells over long distances [58] contributes to NK cell effector responses. Depending on the tumor origin and expression of cognate ligands, NK cells might use different mechanisms for tumor cell lysis. Besides tumor cells, immune cells infiltrating the tumor tissue can be direct targets for NK cells. Our previous study revealed that the mononuclear subset of myeloid derived suppressor cells (MDSCs) from mouse subcutaneous lymphoma expressed the NKG2D-L, Rae1, rendering those cells susceptible to NK cell lysis [59]. Similarly, NK cells were shown to kill immature DCs [60] that accumulate in the tumor tissue and thereby might prevent DC-mediated tolerance induction. In addition, NK cell cytolytic activity can also be directed towards activated T cells [61]. Although killing of activated T cells by NK cells can be beneficial for the control of immune responses and prevention of immunopathology, it might be detrimental for anti-tumor immune responses.
In addition to their cytolytic function, NK cells secrete IFN-γ, a cytokine with pleiotropic anti-tumor activity [62], which include direct suppression of tumor cell growth and metastases, induction of tumor cell apoptosis, anti-angiogenic activity, induction of T cell and macrophage differentiation and effector functions and upregulation of MHC class I and components of the antigen presenting machinery. NK cells are considered as early IFN-γ producers promoting the development of subsequent adaptive immune responses [63, 64]. Accordingly, protective immune responses in several transplantable tumor models in mice were abolished in the absence of IFN-γ [63, 65]. Recently, O’Sullivan et al. demonstrated that in the absence of adaptive immunity, NK cell derived IFN-γ contributed to tumor immunoediting mediated by innate immune system, through the induction of macrophage polarization [66]. Besides IFN-γ, NK cells secrete other pro-inflammatory cytokines including TNF-α and GM-CSF and chemokines such as CCL3 and CCL4 that recruit and activate other immune cells at the site of inflammation [67]. In the tumor tissue, NK cells can produce soluble factors that support tumor angiogenesis, such as VEGF and PDGF (our unpublished observation). In addition, human CD56bright NK cells can secrete IL-10 and IL-13 when stimulated with IL-2 and IL-15 [68, 69].
Many cytokines can have both immunoactivating and inhibitory function in the context of malignant disease. For example, IFN-γ was shown to induce the expression of the tryptophan-degrading enzyme, IDO, prostaglandin E2 (PGE2) and to enhance expression of HLA-G on tumor cells. High expression of IDO, cyclooxigenase-2, an enzyme that catalyzes key steps in PGE2 production, and HLA-G has been correlated with poor prognosis for cancer patients [70–72]. Activation of IDO leads to depletion of tryptophan from the microenvironment that is essential for immune cell functions. In addition, products of tryptophan degradation such as L-kynerunine and kynerunic acid can directly inhibit various immune cells, including T cells and NK cells. Both membrane-bound and soluble form of HLA-G that are increased in cancer patients bind to the inhibitory receptor ILT-2 expressed by NK cells [72–74]. Besides IFN-γ, other cytokines, such as GM-CSF, IL-10 and LIF contribute to the induction of HLA-G expression on malignant cells [75]. NK cell engagement of HLA-G can raise the threshold for NK cell activation by shifting the balance of inhibitory versus activating signals or can induce apoptosis of NK cells. In addition, HLA-G can be transferred by intercellular membrane exchange (trogocytosis) from APCs and tumor cells to NK cells creating HLA-G+ NK cells with regulatory functions [76].
In conclusion, NK cell effector functions such as cytolysis and cytokine production can have both immunostimulatory and immunosuppressive effects in the tumor. The dynamics and nature of NK cell responses in the tumor tissue need to be further explored and factors shifting the balance towards immunosuppression need to be defined.
Shaping of NK Cell Function by the Tumor Microenvironment
There is increasing evidence that the phenotype and function of different immune cells is shaped by the tumor microenvironment. For instance, it was shown that tumor-specific T cells from the peripheral blood of cancer patients exert potent responses in vitro, but T cells isolated from the tumor tissue were rather unresponsive [77]. T cells exposed to chronic antigen stimulation, such as during viral infection and cancer progression, became functionally impaired and displayed an exhausted phenotype [78]. Importantly, exhausted T cells were detected in the tumor tissue, but not in the blood of melanoma patients [79, 80]. Transcriptional profiling revealed expression of certain markers including the inhibitory receptors PD-1, CTLA-4, LAG-3, CD160 and Tim-3 on exhausted T cells. Blockade of PD-1, CTLA-4, LAG-3 or Tim-3 as single treatments or in combinations restored T cell function both in vitro and in vivo. We observed that several T cell exhaustion markers such as PD-1, CTLA-4, LAG-3 and CD160 were up-regulated by tumor-infiltrating NK cells compared to peripheral blood NK cells in a mouse model of subcutaneously transplanted lymphoma (our unpublished observation). Previously, Terme et al. [81] reported that tumor-derived IL-18 induced PD-1 expression on mouse NK cells that was associated with increased dissemination of metastases in mice. In multiple myeloma patients, tumor-associated NK cells were shown to express PD-1. Upon PD-1 blockade, increased killing of B7-H1-expressing tumor cells by NK cells was observed [82]. CTLA-4 inhibits the function of both T cells [83] and NK cells (our unpublished observation) underlining its importance as a therapeutic target. Of note, while LAG-3 inhibits T cell responses, evidence exists that it might have an activating role in NK cells [84]. Similarly, it was shown that in NK cells CD160 could trigger cytotoxicity and secretion of IFN-γ, TNF-α, IL-6, IL-8 and MIP1-β [85], while having mainly inhibitory function in T cells [86]. Different roles of Tim-3 in the regulation of NK cell responses has been reported. Ndhlovu et al. showed that cross-linking of Tim-3 diminished NK cell cytolytic responses [87], whereas engagement of Tim-3 by its natural ligand, galectin-9, induced IFN-γ release by human NK cells [88]. In conclusion, several molecules such as CTLA-4 and PD-1, were identified as inhibitory receptors in both NK cells and T cells, whereas others, such as LAG-3 and CD160, might exert different functions on NK cells or T cells. The blockade of inhibitory receptors that can simultaneously enhance functions of both tumor-infiltrating NK cells and T cells represents a promising approach to restore the activity of tumor-infiltrating lymphocytes against tumors.
In addition to the increased expression of inhibitory receptors, the impairment of T cell responses in the tumor tissue is characterized by changes in the signaling machinery of the TCR complex [77]. These include decreased expression of the adaptor molecule CD3ζ, tyrosine kinases Lck and Fyn and reduced expression of NF-κB family members that were correlated with impaired cytokine production by cytotoxic T lymphocytes. Similar signatures were observed in tumor-infiltrating NK cells. The signaling network downstream of activating and inhibitory NK cell receptors and central molecules targeted by tumor immunosuppressive mechanisms are depicted in Fig. 1. NK cells isolated from the ascites of ovarian carcinoma patients expressed decreased levels of the adaptor proteins CD3ζ and FcεRIγ and Lck kinase compared to NK cells from peripheral blood [89]. These features correlated with their reduced ability to produce IFN-γ upon IL-2 stimulation. Similarly, in ovarian cancer patients, tumor-infiltrating NK cells displayed reduced levels of CD3ζ, produced less IFN-γ, IL-2 and IL-4, but more IL-10, compared to peripheral blood NK cells [90]. We also observed that in RMA-S lymphoma-infiltrating NK cells, mRNA levels of several signaling molecules downstream of activating NK cell receptors including Lck, PI3K, PLCγ and Vav, were decreased when compared to peripheral blood NK cells (our unpublished observations). Strategies that target signaling components are very challenging due to their promiscuous expression and function. Thus, it is important to identify the tumor-derived factors that cause impairments in the signaling machinery. Targeting these factors can potentially improve the function of multiple immune effector cells including NK and T cells.
In addition to the immunosuppressive mechanisms exerted by tumor cells, multiple immune cells with suppressive functions are detected within the tumor tissue (Fig. 2). These include immature dendritic cells (iDCs), tumor-associated macrophages (TAMs), myeloid derived suppressor cells (MDSCs) and regulatory T cells (Treg). In the tumor tissue, NK cells can interact with different immune cells. The cross-talk of NK cells with iDCs leads to DC maturation and NK cell activation but, under certain conditions, influenced by the cell-to-cell ratio, previous activation status and environmental stimuli, results in the elimination of iDCs [91]. DCs in solid tumors often display an immature phenotype characterized by low expression of co-stimulatory molecules [92]. Rather than stimulating immune cells, iDCs in tumors often induce tolerance [93]. It is possible that activated NK cells in the tumor promote T cell activation by eliminating immature tolerogenic DCs and secreting IFN-γ that supports DC maturation. However, the outcome of the interaction between tumor-infiltrating DCs and tumor-infiltrating NK cells in vivo is currently unknown. Several factors produced within the tumor tissue such as VEGF, GM-CSF, M-CSF and IL-6 affect normal myelopoiesis and support the accumulation of immature myeloid cells in tumor-bearing mice and cancer patients [94]. A common feature of these cells is their ability to suppress T cell responses and these cells were called “myeloid-derived suppressor cells” (MDSCs). Depending on the tumor model and experimental set-up studied, MDSCs were reported to suppress [95] or promote NK cell responses in mice [59]. Similar to MDSCs, Tregs are enriched in the tumor tissue of cancer patients and tumor-bearing mice. Tregs were shown to control the homeostatic proliferation of NK cells and their anti-tumor immune responses [96]. Active recruitment and in situ proliferation contribute to the accumulation of Tregs in the tumor tissue that is further supported by other suppressive cells in tumors, such as iDCs and MDSCs. It was demonstrated that Tregs downregulated NKG2D and suppressed IL-12-induced IFN-γ production by NK cells in a TGF-β dependent manner [97]. TGF-β is an important immunosuppressive factor produced by tumor and immune cells with broad effects on tumor-infiltrating immune cells [98]. It can also indirectly influence NK cell responses by affecting DC function, by inducing Tregs or by modulating the expression of adhesion molecules on endothelial cells in tumor vessels. Within the tumor microenvironment, TGF-β exerts its function mainly by its membrane-bound form presented by Tregs and MDSCs [95, 97].
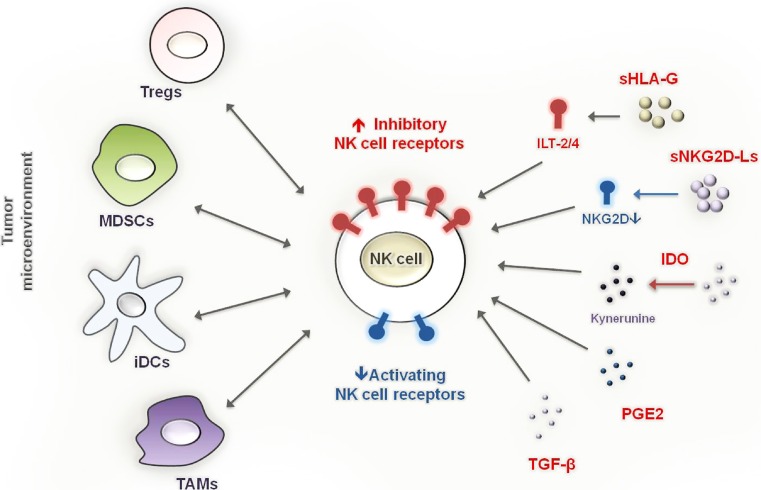
Fig. 2.
Components of the tumor microenvironment that inhibit NK cell effector functions. Cells with suppressive function that are recruited and/or expanded in the tumor tissue such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), immature dendritic cells (iDCs) and tumor-associated macrophages (TAMs) interact with NK cells within the tumor tissue and can suppress NK cell effector function. The activation of NK cell cytotoxic responses and cytokine production can prevent the accumulation of immunosuppressive cells, e.g. by elimination of iDCs and monocytic MDSCs or by IFN-γ-mediated M1-polarization of TAMs. Soluble factors released by tumor cells as well as by infiltrating immune cells, such as soluble HLA-G (sHLA-G), soluble NKG2D ligands (sNKG2D-Ls), TGF-β, PGE2, the tryptophan-degrading enzyme, IDO, and its product kynurenine can inhibit NK cells by direct engagement of inhibitory receptors or by downregulation of activating NK cell receptors
In conclusion, inhibitory networks that impede NK cell activation in the tumor tissue include tumor cells, tumor cell derived factors and tumor-induced immune cells with suppressive functions (Fig. 2). The outcome is an impairment of NK cell function at multiple levels including expression of activating receptors, signaling molecules and effector molecules such as cytokines. Dissecting and counteracting this inhibitory network is critical for the design of novel strategies of anti-cancer immunotherapy.
NK Cell Recruitment to the Tumor Site
In peripheral blood of healthy individuals, two subsets of NK cells are defined: CD56dim, comprising ~90 % and CD56bright, comprising ~10 % of total NK cells. CD56bright NK cells are enriched in several tissues, such as in the lymph nodes and placenta. When stimulated, the CD56bright subset produces cytokines, whereas CD56dim NK cells exert potent cytolytic activity. However, depending on the stimuli, both cell subsets can display various effector functions. Several studies revealed that CD56bright NK cells were the dominant subset in the tumor tissue [99, 100]. It is currently unknown whether the enrichment of CD56bright NK cells is a consequence of differential recruitment to the tumor site or increased proliferation and survival. Moreover, it is possible that CD56dim NK cells acquire CD56bright features in response to factors produced within the tumor microenvironment. Accordingly, it was shown that the MUC16 glycoprotein that is present in ovarian tumors down-regulated CD16 expression, which represents a hallmark of the CD56dim NK cell subset [101]. Furthermore, CD56bright NK cells display improved survival under conditions of oxidative stress, often present within tumor tissue [102]. We demonstrated that, similar to human malignancies, in mouse solid tumors, CD27-expressing NK cells were preferentially detected [103]. Mouse CD27high NK cells are considered to be the equivalent of the human CD56bright population.
In early studies addressing the NK cell infiltration of solid tumors and its association with prognosis, CD57 was used to identify NK cells. Of note, CD57 is expressed only by a subset of CD56dim NK cells and by a small subpopulation of T cells. On NK cells, CD57 expression correlates with high expression of inhibitory KIRs, low expression of the activating receptors NKp30, NKp46 and NKG2D and of the cytokine receptor chains IL-2Rβ and IL-12Rβ [104, 105]. Functionally, CD57+ NK cells display reduced proliferative capacity, but are potent IFN-γ producers and show high lytic capacity when stimulated via CD16. Thus, high numbers of tumor-infiltrating CD57+ cells were correlated with improved prognosis of cancer patients with several malignancies including lung, gastric and colorectal cancer [2, 106, 107]. These early observations suggested that CD56dimCD57+ NK cells might be effective during anti-tumor responses. However, the contribution of other NK cell subsets remained unclear. Several recent studies used NKp46 as marker to stain NK cells within the tumor. These data suggest that certain solid malignancies are infiltrated by a significant number of NK cells. Those include renal cell carcinoma [108] and gastrointestinal sarcoma (GIST) [33], where frequencies of NK cells among tumor-infiltrating leukocytes are found to be higher than in peripheral blood. Renal cell carcinoma-infiltrating NK cells displayed reduced cytotoxicity and increased surface levels of NKG2A/CD94 inhibitory receptor complex [108]. In GIST patients, NK cells were found to express predominantly the immunosuppressive isoform of the NKp30 receptor named NKp30c [33]. Compared to NKp30 isoforms a and b, which dominate in healthy individuals, triggering of NK30c with tumor cells or iDCs that express NKp30 ligands led to increased IL-10 production and diminished release of IFN-γ. Furthermore, the differential expression of NKp30 isoforms served as a predictive marker for the clinical outcome of GIST patients.
An important prerequisite for successful targeting of tumors is the efficient NK cell homing to the tumor site and infiltration of tumor tissue. In this context it was shown that certain cytokines such as IL-2, IL-12, IL-21, IFN-α as well as agents such as CpG or therapeutic mAbs increased numbers and/or activity of NK cells from peripheral blood [12]. However, low overall responses seen in treated patients with solid tumors might be the consequence of inefficient NK cell trafficking to the tumor site. In addition, NK cells are often not located in direct contact with tumor cells, but rather in the proximity of the blood vessels [109, 110]. Processes of cell migration and tissue infiltration are controlled by chemokines and chemokine receptors and factors that regulate cell–cell, cell–extracellular matrix (ECM) interactions and the modulation of ECM components [111]. Several of these molecules have been evaluated as therapeutic targets. CXCR3 and CX3CR1 are among the most important chemokine receptors implicated in NK cell recruitment to the tumor tissue. CD27high NK cells in mice and CD56bright NK cells in human express CXCR3 that might be responsible for their preferential accumulation in tumors [112]. We have shown that CD27high NK cells accumulation in mouse subcutaneous lymphoma is CXCR3-dependent [103]. Lavergne and colleagues demonstrated increased infiltration of NK cells in CX3CL1-producing tumors correlating with reduced tumor growth [113]. A recent study by Pachynski et al. identified the protein chemerin as an important chemoattractant for NK cells [114]. Expression of chemerin correlated with good prognosis for melanoma patients and was downregulated during tumor progression in several types of solid tumors in humans. In mice, over-expression or exogenous application of chemerin led to higher NK cell infiltration of B16 melanoma tumors and to inhibition of tumor growth in a NK cell dependent manner.
Matrix metalloproteinase proteins (MMPs) have been implicated in processes such as destruction of extracellular matrix, basement membrane invasion and angiogenesis. Members of a disintegrin and metalloproteinase (ADAM) family proteins are membrane-bound proteases that cut and release ectodomains of transmembrane proteins including cytokines, growth factors and cell adhesion molecules [115–117]. Many ADAMs are overexpressed in tumors and affect different steps of tumor progression [117]. NK cell recognition of target cells is hampered by ADAM10 and ADAM17 activation, which were shown to be involved in the proteolytic release of soluble MICA and MICB from tumor cells [118, 119]. Grzywacz et al. suggested that NK cell encounter of target cells led to activation of MMPs that subsequently shed CD16 from the surface of NK cells [120]. Therefore, increased expression of MMP and ADAM enzymes in the tumor microenvironment might affect NK cell function and migration indirectly, by modifying the expression of cytokines, growth factors and cell adhesion molecules, or directly, by targeting NK cell receptors and their ligands.
Conclusions
Several studies support the view that NK cells are important effector cells against tumors. Individuals with a high NK cell activity have a reduced risk to develop cancer [121]. Moreover, increased numbers of NK cells in different types of tumors correlate with improved prognosis for cancer patients [2, 106, 107]. However, emerging evidence exists that NK cell function in the tumor microenvironment becomes impaired during tumor progression. Phenotypic and functional changes were observed at multiple levels including surface receptors, signaling and effector molecules. The suppressive network that affects NK cell function is complex and comprises tumor cells, tumor-derived factors and other immunosuppressive cells.
Successful strategies of NK cell based cancer therapy should integrate measures of efficient NK cell trafficking to the tumor site, infiltration of the tumor tissue, efficient recognition of target cells and activation of anti-tumor effector functions. To achieve these goals, a comprehensive knowledge about the suppressive networks impeding NK cell responses in different tumor entities during tumor progression is needed. In addition, markers for immunomonitoring that predict NK cell function within the tumor microenvironment need to be defined. The identification of checkpoints of both NK cell activation and loss of function in the tumors could identify novel targets expressed by NK cells. Their therapeutic manipulation could potentially unleash high effector function of NK cells against tumors within the tumor microenvironment.
References
- 1.Deschoolmeester V, Baay M, Van Marck E, Weyler J, Vermeulen P, Lardon F, Vermorken JB. Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC Immunol. 2010;11:19. doi: 10.1186/1471-2172-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, Martos JA, Moreno M. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79(12):2320–2328. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 3.Kerkar SP, Restifo NP. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. 2012;72(13):3125–3130. doi: 10.1158/0008-5472.CAN-11-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72(9):2162–2171. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68(8):2561–2563. doi: 10.1158/0008-5472.CAN-07-6229. [DOI] [PubMed] [Google Scholar]
- 6.Bryceson YT, Long EO. Line of attack: NK cell specificity and integration of signals. Curr Opin Immunol. 2008;20(3):344–352. doi: 10.1016/j.coi.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moretta L, Locatelli F, Pende D, Mingari MC, Moretta A. Natural killer alloeffector responses in haploidentical hemopoietic stem cell transplantation to treat high-risk leukemias. Tissue Antigens. 2010;75(2):103–109. doi: 10.1111/j.1399-0039.2009.01404.x. [DOI] [PubMed] [Google Scholar]
- 8.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 9.Benson DM Jr, Hofmeister CC, Padmanabhan S, Suvannasankha A, Jagganath S, Abonour R, Bakan C, Andre P, Efebera Y, Tiollier J, Caligiuri MA, Farag SS (2012) A phase I trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood. doi:10.1182/blood-2012-06-438028, Published online Oct 1 [DOI] [PMC free article] [PubMed]
- 10.Vey N, Bourhis JH, Boissel N, Bordessoule D, Prebet T, Charbonnier A, Etienne A, Andre P, Romagne F, Benson D, Dombret H, Olive D (2012) A phase I trial of the anti-inhibitory KIR monoclonal antibody IPH2101 for acute myeloid leukemia (AML) in complete remission. Blood. doi:10.1182/blood-2012-06-437558, Published online Sep 21 [DOI] [PubMed]
- 11.Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M, Gallagher MM, Malkki M, Petersdorf E, Dupont B, Hsu KC. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367(9):805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol. 2008;9(5):486–494. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- 13.Andrews DM, Maraskovsky E, Smyth MJ. Cancer vaccines for established cancer: how to make them better? Immunol Rev. 2008;222:242–255. doi: 10.1111/j.1600-065X.2008.00612.x. [DOI] [PubMed] [Google Scholar]
- 14.Alderson KL, Sondel PM. Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. J Biomed Biotechnol. 2011;2011:379123. doi: 10.1155/2011/379123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- 16.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 17.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26(4):503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bihl F, Pecheur J, Breart B, Poupon G, Cazareth J, Julia V, Glaichenhaus N, Braud VM. Primed antigen-specific CD4+ T cells are required for NK cell activation in vivo upon Leishmania major infection. J Immunol. 2010;185(4):2174–2181. doi: 10.4049/jimmunol.1001486. [DOI] [PubMed] [Google Scholar]
- 20.Sporri R, Joller N, Hilbi H, Oxenius A. A novel role for neutrophils as critical activators of NK cells. J Immunol. 2008;181(10):7121–7130. doi: 10.4049/jimmunol.181.10.7121. [DOI] [PubMed] [Google Scholar]
- 21.Jaeger BN, Donadieu J, Cognet C, Bernat C, Ordonez-Rueda D, Barlogis V, Mahlaoui N, Fenis A, Narni-Mancinelli E, Beaupain B, Bellanne-Chantelot C, Bajenoff M, Malissen B, Malissen M, Vivier E, Ugolini S. Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis. J Exp Med. 2012;209(3):565–580. doi: 10.1084/jem.20111908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zwirner NW, Domaica CI. Cytokine regulation of natural killer cell effector functions. Biofactors. 2010;36(4):274–288. doi: 10.1002/biof.107. [DOI] [PubMed] [Google Scholar]
- 23.Nausch N, Cerwenka A. NKG2D ligands in tumor immunity. Oncogene. 2008;27(45):5944–5958. doi: 10.1038/onc.2008.272. [DOI] [PubMed] [Google Scholar]
- 24.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3(10):781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 25.Raulet DH, Guerra N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat Rev Immunol. 2009;9(8):568–580. doi: 10.1038/nri2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini A, Foa R, Santoni A. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood. 2009;113(15):3503–3511. doi: 10.1182/blood-2008-08-173914. [DOI] [PubMed] [Google Scholar]
- 27.Chan CJ, Andrews DM, McLaughlin NM, Yagita H, Gilfillan S, Colonna M, Smyth MJ. DNAM-1/CD155 interactions promote cytokine and NK cell-mediated suppression of poorly immunogenic melanoma metastases. J Immunol. 2010;184(2):902–911. doi: 10.4049/jimmunol.0903225. [DOI] [PubMed] [Google Scholar]
- 28.Smyth MJ, Swann J, Kelly JM, Cretney E, Yokoyama WM, Diefenbach A, Sayers TJ, Hayakawa Y. NKG2D recognition and perforin effector function mediate effective cytokine immunotherapy of cancer. J Exp Med. 2004;200(10):1325–1335. doi: 10.1084/jem.20041522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28(4):571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pogge von Strandmann E, Simhadri VR, von Tresckow B, Sasse S, Reiners KS, Hansen HP, Rothe A, Boll B, Simhadri VL, Borchmann P, McKinnon PJ, Hallek M, Engert A. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity. 2007;27(6):965–974. doi: 10.1016/j.immuni.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Simhadri VR, Reiners KS, Hansen HP, Topolar D, Simhadri VL, Nohroudi K, Kufer TA, Engert A, Pogge von Strandmann E. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PLoS One. 2008;3(10):e3377. doi: 10.1371/journal.pone.0003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C, Moretta A, West R, Xu W, Vivier E, Levin SD. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206(7):1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delahaye NF, Rusakiewicz S, Martins I, Menard C, Roux S, Lyonnet L, Paul P, Sarabi M, Chaput N, Semeraro M, Minard-Colin V, Poirier-Colame V, Chaba K, Flament C, Baud V, Authier H, Kerdine-Romer S, Pallardy M, Cremer I, Peaudecerf L, Rocha B, Valteau-Couanet D, Gutierrez JC, Nunes JA, Commo F, Bonvalot S, Ibrahim N, Terrier P, Opolon P, Bottino C, Moretta A, Tavernier J, Rihet P, Coindre JM, Blay JY, Isambert N, Emile JF, Vivier E, Lecesne A, Kroemer G, Zitvogel L. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med. 2011;17(6):700–707. doi: 10.1038/nm.2366. [DOI] [PubMed] [Google Scholar]
- 34.Rosental B, Brusilovsky M, Hadad U, Oz D, Appel MY, Afergan F, Yossef R, Rosenberg LA, Aharoni A, Cerwenka A, Campbell KS, Braiman A, Porgador A. Proliferating cell nuclear antigen is a novel inhibitory ligand for the natural cytotoxicity receptor NKp44. J Immunol. 2011;187(11):5693–5702. doi: 10.4049/jimmunol.1102267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stuart-Harris R, Caldas C, Pinder SE, Pharoah P. Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast. 2008;17(4):323–334. doi: 10.1016/j.breast.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107(1):159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Maux CB, Moretta A, Vergnon I, Opolon P, Lecluse Y, Grunenwald D, Kubin M, Soria JC, Chouaib S, Mami-Chouaib F. NK cells infiltrating a MHC class I-deficient lung adenocarcinoma display impaired cytotoxic activity toward autologous tumor cells associated with altered NK cell-triggering receptors. J Immunol. 2005;175(9):5790–5798. doi: 10.4049/jimmunol.175.9.5790. [DOI] [PubMed] [Google Scholar]
- 38.Hilpert J, Grosse-Hovest L, Grunebach F, Buechele C, Nuebling T, Raum T, Steinle A, Salih HR. Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. J Immunol. 2012;189(3):1360–1371. doi: 10.4049/jimmunol.1200796. [DOI] [PubMed] [Google Scholar]
- 39.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419(6908):734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 40.Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169(8):4098–4102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 41.Paschen A, Sucker A, Hill B, Moll I, Zapatka M, Nguyen XD, Sim GC, Gutmann I, Hassel J, Becker JC, Steinle A, Schadendorf D, Ugurel S. Differential clinical significance of individual NKG2D ligands in melanoma: soluble ULBP2 as an indicator of poor prognosis superior to S100B. Clin Cancer Res. 2009;15(16):5208–5215. doi: 10.1158/1078-0432.CCR-09-0886. [DOI] [PubMed] [Google Scholar]
- 42.Coudert JD, Scarpellino L, Gros F, Vivier E, Held W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood. 2008;111(7):3571–3578. doi: 10.1182/blood-2007-07-100057. [DOI] [PubMed] [Google Scholar]
- 43.Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004;172(12):7335–7340. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 44.Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, Biassoni R, Bottino C, Moretta L, Moretta A. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci U S A. 2003;100(7):4120–4125. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Della Chiesa M, Carlomagno S, Frumento G, Balsamo M, Cantoni C, Conte R, Moretta L, Moretta A, Vitale M. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood. 2006;108(13):4118–4125. doi: 10.1182/blood-2006-03-006700. [DOI] [PubMed] [Google Scholar]
- 46.Pietra G, Manzini C, Rivara S, Vitale M, Cantoni C, Petretto A, Balsamo M, Conte R, Benelli R, Minghelli S, Solari N, Gualco M, Queirolo P, Moretta L, Mingari MC. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res. 2012;72(6):1407–1415. doi: 10.1158/0008-5472.CAN-11-2544. [DOI] [PubMed] [Google Scholar]
- 47.Textor S, Fiegler N, Arnold A, Porgador A, Hofmann TG, Cerwenka A. Human NK cells are alerted to induction of p53 in cancer cells by upregulation of the NKG2D ligands ULBP1 and ULBP2. Cancer Res. 2011;71(18):5998–6009. doi: 10.1158/0008-5472.CAN-10-3211. [DOI] [PubMed] [Google Scholar]
- 48.Liu XV, Ho SS, Tan JJ, Kamran N, Gasser S. Ras activation induces expression of Raet1 family NK receptor ligands. J Immunol. 2012;189(4):1826–1834. doi: 10.4049/jimmunol.1200965. [DOI] [PubMed] [Google Scholar]
- 49.Armeanu S, Bitzer M, Lauer UM, Venturelli S, Pathil A, Krusch M, Kaiser S, Jobst J, Smirnow I, Wagner A, Steinle A, Salih HR. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005;65(14):6321–6329. doi: 10.1158/0008-5472.CAN-04-4252. [DOI] [PubMed] [Google Scholar]
- 50.Kato N, Tanaka J, Sugita J, Toubai T, Miura Y, Ibata M, Syono Y, Ota S, Kondo T, Asaka M, Imamura M. Regulation of the expression of MHC class I-related chain A, B (MICA, MICB) via chromatin remodeling and its impact on the susceptibility of leukemic cells to the cytotoxicity of NKG2D-expressing cells. Leukemia. 2007;21(10):2103–2108. doi: 10.1038/sj.leu.2404862. [DOI] [PubMed] [Google Scholar]
- 51.Heinemann A, Zhao F, Pechlivanis S, Eberle J, Steinle A, Diederichs S, Schadendorf D, Paschen A. Tumor suppressive microRNAs miR-34a/c control cancer cell expression of ULBP2, a stress-induced ligand of the natural killer cell receptor NKG2D. Cancer Res. 2012;72(2):460–471. doi: 10.1158/0008-5472.CAN-11-1977. [DOI] [PubMed] [Google Scholar]
- 52.Nachmani D, Lankry D, Wolf DG, Mandelboim O. The human cytomegalovirus microRNA miR-UL112 acts synergistically with a cellular microRNA to escape immune elimination. Nat Immunol. 2010;11(9):806–813. doi: 10.1038/ni.1916. [DOI] [PubMed] [Google Scholar]
- 53.Stern-Ginossar N, Gur C, Biton M, Horwitz E, Elboim M, Stanietsky N, Mandelboim M, Mandelboim O. Human microRNAs regulate stress-induced immune responses mediated by the receptor NKG2D. Nat Immunol. 2008;9(9):1065–1073. doi: 10.1038/ni.1642. [DOI] [PubMed] [Google Scholar]
- 54.Hervieu A, Rebe C, Vegran F, Chalmin F, Bruchard M, Vabres P, Apetoh L, Ghiringhelli F, Mignot G (2012) Dacarbazine-mediated upregulation of NKG2D ligands on tumor cells activates NK and CD8 T cells and restrains melanoma growth. J Invest Dermatol [DOI] [PubMed]
- 55.Chavez-Blanco A, De la Cruz-Hernandez E, Dominguez GI, Rodriguez-Cortez O, Alatorre B, Perez-Cardenas E, Chacon-Salinas R, Trejo-Becerril C, Taja-Chayeb L, Trujillo JE, Contreras-Paredes A, Duenas-Gonzalez A. Upregulation of NKG2D ligands and enhanced natural killer cell cytotoxicity by hydralazine and valproate. Int J Oncol. 2011;39(6):1491–1499. doi: 10.3892/ijo.2011.1144. [DOI] [PubMed] [Google Scholar]
- 56.Khallouf H, Marten A, Serba S, Teichgraber V, Buchler MW, Jager D, Schmidt J. 5-Fluorouracil and interferon-alpha immunochemotherapy enhances immunogenicity of murine pancreatic cancer through upregulation of NKG2D ligands and MHC class I. J Immunother. 2012;35(3):245–253. doi: 10.1097/CJI.0b013e31824b3a76. [DOI] [PubMed] [Google Scholar]
- 57.Lugini L, Cecchetti S, Huber V, Luciani F, Macchia G, Spadaro F, Paris L, Abalsamo L, Colone M, Molinari A, Podo F, Rivoltini L, Ramoni C, Fais S. Immune surveillance properties of human NK cell-derived exosomes. J Immunol. 2012;189(6):2833–2842. doi: 10.4049/jimmunol.1101988. [DOI] [PubMed] [Google Scholar]
- 58.Chauveau A, Aucher A, Eissmann P, Vivier E, Davis DM. Membrane nanotubes facilitate long-distance interactions between natural killer cells and target cells. Proc Natl Acad Sci U S A. 2010;107(12):5545–5550. doi: 10.1073/pnas.0910074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nausch N, Galani IE, Schlecker E, Cerwenka A. Mononuclear myeloid-derived “suppressor” cells express RAE-1 and activate natural killer cells. Blood. 2008;112(10):4080–4089. doi: 10.1182/blood-2008-03-143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wehner R, Dietze K, Bachmann M, Schmitz M. The bidirectional crosstalk between human dendritic cells and natural killer cells. J Innate Immun. 2011;3(3):258–263. doi: 10.1159/000323923. [DOI] [PubMed] [Google Scholar]
- 61.Rabinovich BA, Li J, Shannon J, Hurren R, Chalupny J, Cosman D, Miller RG. Activated, but not resting, T cells can be recognized and killed by syngeneic NK cells. J Immunol. 2003;170(7):3572–3576. doi: 10.4049/jimmunol.170.7.3572. [DOI] [PubMed] [Google Scholar]
- 62.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13(2):95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 63.Kelly JM, Darcy PK, Markby JL, Godfrey DI, Takeda K, Yagita H, Smyth MJ. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat Immunol. 2002;3(1):83–90. doi: 10.1038/ni746. [DOI] [PubMed] [Google Scholar]
- 64.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5(12):1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 65.Kelly JM, Takeda K, Darcy PK, Yagita H, Smyth MJ. A role for IFN-gamma in primary and secondary immunity generated by NK cell-sensitive tumor-expressing CD80 in vivo. J Immunol. 2002;168(9):4472–4479. doi: 10.4049/jimmunol.168.9.4472. [DOI] [PubMed] [Google Scholar]
- 66.O’Sullivan T, Saddawi-Konefka R, Vermi W, Koebel CM, Arthur C, White JM, Uppaluri R, Andrews DM, Ngiow SF, Teng MW, Smyth MJ, Schreiber RD, Bui JD. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J Exp Med. 2012;209(10):1869–1882. doi: 10.1084/jem.20112738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maghazachi AA. Role of chemokines in the biology of natural killer cells. Curr Top Microbiol Immunol. 2010;341:37–58. doi: 10.1007/82_2010_20. [DOI] [PubMed] [Google Scholar]
- 68.Terabe M, Park JM, Berzofsky JA. Role of IL-13 in regulation of anti-tumor immunity and tumor growth. Cancer Immunol Immunother. 2004;53(2):79–85. doi: 10.1007/s00262-003-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97(10):3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 70.Godin-Ethier J, Hanafi LA, Piccirillo CA, Lapointe R. Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res. 2011;17(22):6985–6991. doi: 10.1158/1078-0432.CCR-11-1331. [DOI] [PubMed] [Google Scholar]
- 71.Kim HS, Moon HG, Han W, Yom CK, Kim WH, Kim JH, Noh DY. COX2 overexpression is a prognostic marker for Stage III breast cancer. Breast Cancer Res Treat. 2012;132(1):51–59. doi: 10.1007/s10549-011-1521-3. [DOI] [PubMed] [Google Scholar]
- 72.Amiot L, Ferrone S, Grosse-Wilde H, Seliger B. Biology of HLA-G in cancer: a candidate molecule for therapeutic intervention? Cell Mol Life Sci. 2011;68(3):417–431. doi: 10.1007/s00018-010-0583-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borrego F, Kabat J, Kim DK, Lieto L, Maasho K, Pena J, Solana R, Coligan JE. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol. 2002;38(9):637–660. doi: 10.1016/s0161-5890(01)00107-9. [DOI] [PubMed] [Google Scholar]
- 74.Zilberman S, Schenowitz C, Agaugue S, Benoit F, Riteau B, Rouzier R, Carosella ED, Rouas-Freiss N, Menier C. HLA-G1 and HLA-G5 active dimers are present in malignant cells and effusions: the influence of the tumor microenvironment. Eur J Immunol. 2012;42(6):1599–1608. doi: 10.1002/eji.201141761. [DOI] [PubMed] [Google Scholar]
- 75.Campoli M, Ferrone S. Tumor escape mechanisms: potential role of soluble HLA antigens and NK cells activating ligands. Tissue Antigens. 2008;72(4):321–334. doi: 10.1111/j.1399-0039.2008.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rouas-Freiss N, Moreau P, Menier C, LeMaoult J, Carosella ED. Expression of tolerogenic HLA-G molecules in cancer prevents antitumor responses. Semin Cancer Biol. 2007;17(6):413–421. doi: 10.1016/j.semcancer.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 77.Frey AB, Monu N. Signaling defects in anti-tumor T cells. Immunol Rev. 2008;222:192–205. doi: 10.1111/j.1600-065X.2008.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 79.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zippelius A, Batard P, Rubio-Godoy V, Bioley G, Lienard D, Lejeune F, Rimoldi D, Guillaume P, Meidenbauer N, Mackensen A, Rufer N, Lubenow N, Speiser D, Cerottini JC, Romero P, Pittet MJ. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004;64(8):2865–2873. doi: 10.1158/0008-5472.can-03-3066. [DOI] [PubMed] [Google Scholar]
- 81.Terme M, Ullrich E, Aymeric L, Meinhardt K, Desbois M, Delahaye N, Viaud S, Ryffel B, Yagita H, Kaplanski G, Prevost-Blondel A, Kato M, Schultze JL, Tartour E, Kroemer G, Chaput N, Zitvogel L. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 2011;71(16):5393–5399. doi: 10.1158/0008-5472.CAN-11-0993. [DOI] [PubMed] [Google Scholar]
- 82.Benson DM, Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J, Smith MK, Greenfield CN, Porcu P, Devine SM, Rotem-Yehudar R, Lozanski G, Byrd JC, Caligiuri MA. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116(13):2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/B7 family. Immunol Rev. 2011;241(1):180–205. doi: 10.1111/j.1600-065X.2011.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miyazaki T, Dierich A, Benoist C, Mathis D. Independent modes of natural killing distinguished in mice lacking Lag3. Science. 1996;272(5260):405–408. doi: 10.1126/science.272.5260.405. [DOI] [PubMed] [Google Scholar]
- 85.Le Bouteiller P, Tabiasco J, Polgar B, Kozma N, Giustiniani J, Siewiera J, Berrebi A, Aguerre-Girr M, Bensussan A, Jabrane-Ferrat N. CD160: a unique activating NK cell receptor. Immunol Lett. 2011;138(2):93–96. doi: 10.1016/j.imlet.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 86.Cai G, Freeman GJ. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol Rev. 2009;229(1):244–258. doi: 10.1111/j.1600-065X.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 87.Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF, Lanier LL. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119(16):3734–3743. doi: 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gleason MK, Lenvik TR, McCullar V, Felices M, O’Brien MS, Cooley SA, Verneris MR, Cichocki F, Holman CJ, Panoskaltsis-Mortari A, Niki T, Hirashima M, Blazar BR, Miller JS. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012;119(13):3064–3072. doi: 10.1182/blood-2011-06-360321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lai P, Rabinowich H, Crowley-Nowick PA, Bell MC, Mantovani G, Whiteside TL. Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin Cancer Res. 1996;2(1):161–173. [PubMed] [Google Scholar]
- 90.Rabinowich H, Suminami Y, Reichert TE, Crowley-Nowick P, Bell M, Edwards R, Whiteside TL. Expression of cytokine genes or proteins and signaling molecules in lymphocytes associated with human ovarian carcinoma. Int J Cancer. 1996;68(3):276–284. doi: 10.1002/(SICI)1097-0215(19961104)68:3<276::AID-IJC2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 91.Moretta L, Ferlazzo G, Bottino C, Vitale M, Pende D, Mingari MC, Moretta A. Effector and regulatory events during natural killer-dendritic cell interactions. Immunol Rev. 2006;214:219–228. doi: 10.1111/j.1600-065X.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- 92.Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, Banchereau J. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190(10):1417–1426. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hurwitz AA, Watkins SK. Immune suppression in the tumor microenvironment: a role for dendritic cell-mediated tolerization of T cells. Cancer Immunol Immunother. 2012;61(2):289–293. doi: 10.1007/s00262-011-1181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182(1):240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 96.Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+ CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176(3):1582–1587. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 97.Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay JY, Bernard J, Caillat-Zucman S, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L. CD4+ CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202(8):1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10(8):554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Textor S, Durst M, Jansen L, Accardi R, Tommasino M, Trunk MJ, Porgador A, Watzl C, Gissmann L, Cerwenka A. Activating NK cell receptor ligands are differentially expressed during progression to cervical cancer. Int J Cancer. 2008;123(10):2343–2353. doi: 10.1002/ijc.23733. [DOI] [PubMed] [Google Scholar]
- 100.Schleypen JS, Baur N, Kammerer R, Nelson PJ, Rohrmann K, Grone EF, Hohenfellner M, Haferkamp A, Pohla H, Schendel DJ, Falk CS, Noessner E. Cytotoxic markers and frequency predict functional capacity of natural killer cells infiltrating renal cell carcinoma. Clin Cancer Res. 2006;12(3 Pt 1):718–725. doi: 10.1158/1078-0432.CCR-05-0857. [DOI] [PubMed] [Google Scholar]
- 101.Patankar MS, Jing Y, Morrison JC, Belisle JA, Lattanzio FA, Deng Y, Wong NK, Morris HR, Dell A, Clark GF. Potent suppression of natural killer cell response mediated by the ovarian tumor marker CA125. Gynecol Oncol. 2005;99(3):704–713. doi: 10.1016/j.ygyno.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 102.Harlin H, Hanson M, Johansson CC, Sakurai D, Poschke I, Norell H, Malmberg KJ, Kiessling R. The CD16- CD56(bright) NK cell subset is resistant to reactive oxygen species produced by activated granulocytes and has higher antioxidative capacity than the CD16+ CD56(dim) subset. J Immunol. 2007;179(7):4513–4519. doi: 10.4049/jimmunol.179.7.4513. [DOI] [PubMed] [Google Scholar]
- 103.Wendel M, Galani IE, Suri-Payer E, Cerwenka A. Natural killer cell accumulation in tumors is dependent on IFN-gamma and CXCR3 ligands. Cancer Res. 2008;68(20):8437–8445. doi: 10.1158/0008-5472.CAN-08-1440. [DOI] [PubMed] [Google Scholar]
- 104.Bjorkstrom NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, Bjorklund AT, Flodstrom-Tullberg M, Michaelsson J, Rottenberg ME, Guzman CA, Ljunggren HG, Malmberg KJ. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK cell differentiation uncoupled from NK cell education. Blood. 2010;116(19):3853–3864. doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- 105.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, Lanier LL. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK cell subset. Blood. 2010;116(19):3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, Aridome K, Hokita S, Aikou T. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88(3):577–583. [PubMed] [Google Scholar]
- 107.Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, Zuil M, Callol L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35(1):23–28. doi: 10.1016/s0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 108.Schleypen JS, Von Geldern M, Weiss EH, Kotzias N, Rohrmann K, Schendel DJ, Falk CS, Pohla H. Renal cell carcinoma-infiltrating natural killer cells express differential repertoires of activating and inhibitory receptors and are inhibited by specific HLA class I allotypes. Int J Cancer. 2003;106(6):905–912. doi: 10.1002/ijc.11321. [DOI] [PubMed] [Google Scholar]
- 109.Esendagli G, Bruderek K, Goldmann T, Busche A, Branscheid D, Vollmer E, Brandau S. Malignant and non-malignant lung tissue areas are differentially populated by natural killer cells and regulatory T cells in non-small cell lung cancer. Lung Cancer. 2008;59(1):32–40. doi: 10.1016/j.lungcan.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 110.Sandel MH, Speetjens FM, Menon AG, Albertsson PA, Basse PH, Hokland M, Nagelkerke JF, Tollenaar RA, van de Velde CJ, Kuppen PJ. Natural killer cells infiltrating colorectal cancer and MHC class I expression. Mol Immunol. 2005;42(4):541–546. doi: 10.1016/j.molimm.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 111.Albertsson PA, Basse PH, Hokland M, Goldfarb RH, Nagelkerke JF, Nannmark U, Kuppen PJ. NK cells and the tumour microenvironment: implications for NK-cell function and anti-tumour activity. Trends Immunol. 2003;24(11):603–609. doi: 10.1016/j.it.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 112.Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol Rev. 2006;214:47–55. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 113.Lavergne E, Combadiere B, Bonduelle O, Iga M, Gao JL, Maho M, Boissonnas A, Murphy PM, Debre P, Combadiere C. Fractalkine mediates natural killer-dependent antitumor responses in vivo. Cancer Res. 2003;63(21):7468–7474. [PubMed] [Google Scholar]
- 114.Pachynski RK, Zabel BA, Kohrt HE, Tejeda NM, Monnier J, Swanson CD, Holzer AK, Gentles AJ, Sperinde GV, Edalati A, Hadeiba HA, Alizadeh AA, Butcher EC. The chemoattractant chemerin suppresses melanoma by recruiting natural killer cell antitumor defenses. J Exp Med. 2012;209(8):1427–1435. doi: 10.1084/jem.20112124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6(1):32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 116.Reiss K, Ludwig A, Saftig P. Breaking up the tie: disintegrin-like metalloproteinases as regulators of cell migration in inflammation and invasion. Pharmacol Ther. 2006;111(3):985–1006. doi: 10.1016/j.pharmthera.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 117.White JM. ADAMs: modulators of cell-cell and cell-matrix interactions. Curr Opin Cell Biol. 2003;15(5):598–606. doi: 10.1016/j.ceb.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 118.Waldhauer I, Goehlsdorf D, Gieseke F, Weinschenk T, Wittenbrink M, Ludwig A, Stevanovic S, Rammensee HG, Steinle A. Tumor-associated MICA is shed by ADAM proteases. Cancer Res. 2008;68(15):6368–6376. doi: 10.1158/0008-5472.CAN-07-6768. [DOI] [PubMed] [Google Scholar]
- 119.Boutet P, Aguera-Gonzalez S, Atkinson S, Pennington CJ, Edwards DR, Murphy G, Reyburn HT, Vales-Gomez M. Cutting edge: the metalloproteinase ADAM17/TNF-alpha-converting enzyme regulates proteolytic shedding of the MHC class I-related chain B protein. J Immunol. 2009;182(1):49–53. doi: 10.4049/jimmunol.182.1.49. [DOI] [PubMed] [Google Scholar]
- 120.Grzywacz B, Kataria N, Verneris MR. CD56(dim)CD16(+) NK cells downregulate CD16 following target cell induced activation of matrix metalloproteinases. Leukemia. 2007;21(2):356–359. doi: 10.1038/sj.leu.2404499. [DOI] [PubMed] [Google Scholar]
- 121.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356(9244):1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]