Abstract
The presence of iduronic acid in chondroitin/dermatan sulfate changes the properties of the polysaccharides because it generates a more flexible chain with increased binding potentials. Iduronic acid in chondroitin/dermatan sulfate influences multiple cellular properties, such as migration, proliferation, differentiation, angiogenesis and the regulation of cytokine/growth factor activities. Under pathological conditions such as wound healing, inflammation and cancer, iduronic acid has diverse regulatory functions. Iduronic acid is formed by two epimerases (i.e. dermatan sulfate epimerase 1 and 2) that have different tissue distribution and properties. The role of iduronic acid in chondroitin/dermatan sulfate is highlighted by the vast changes in connective tissue features in patients with a new type of Ehler–Danlos syndrome: adducted thumb-clubfoot syndrome. Future research aims to understand the roles of the two epimerases and their interplay with the sulfotransferases involved in chondroitin sulfate/dermatan sulfate biosynthesis. Furthermore, a better definition of chondroitin/dermatan sulfate functions using different knockout models is needed. In this review, we focus on the two enzymes responsible for iduronic acid formation, as well as the role of iduronic acid in health and disease.
Keywords: cancer, dermatan sulfate epimerase, DSE, DSEL, iduronic acid, inflammation, proteoglycans
Introduction
Dermatan sulfate (DS) is a glycosaminoglycan (GAG) that is distinguished from chondroitin sulfate (CS) by the presence of iduronic acid (IdoA), the C-5 epimer of d-glucuronic acid (GlcA). IdoA occurs in variable proportions in DS (Fig. 1A) and, as a result of the different position of the carboxyl moiety (Fig. 1B), it generates a more flexible polysaccharide chain, allowing specific interactions with several proteins and polysaccharides. To form CS/DS, three specific enzymes, dermatan sulfate epimerase 1 (DS-epi1), dermatan sulfate epimerase 2 (DS-epi2) and dermatan 4-O-sulfotransferse 1 (D4ST1), are required 1. These enzymes are differently organized in various tissues and, under different physiological conditions, they generate CS/DS of a very different structure. DS is found relatively late in the evolutionary tree and first appears in molluscs, sea urchins and sea cucumbers. It is then found in ascidians and in the whole vertebrate phyla 2. However, it is absent in Caenorhabditis elegans and Drosophila melanogaster. The present review presents the structure, function and biosynthesis of these structurally different CS/DS polymers and explains how they are modified in response to different physiological and pathological processes.
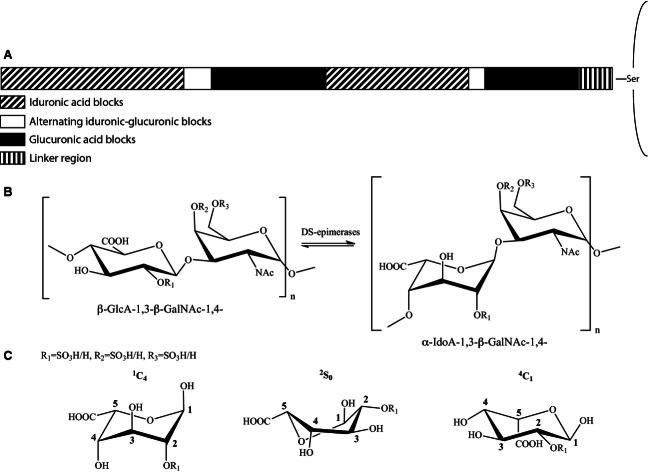
Fig. 1.
Structure of CS/DS and conformations of IdoA. (A) The domains of variable length containing blocks of IdoA, alternating IdoA and GlcA or blocks of GlcA. (B) The epimerase reaction. (C) Conformations of IdoA.
Structure of CS/DS
CS/DS chains are found on at least 32 different core proteins forming proteoglycans (Table 1). Six of these are also substituted with heparan sulfate. Some of these proteoglycans, such as CD44, α5β1 integrin and collagen XV, are only part-time proteoglycans.
Table 1.
CS/DS PGs and functions of the CS/DS chain. NA, not analyzed
| PG | Presence of IdoA | Functions of PG | CS/DS binding proteins and CS/DS functions |
|---|---|---|---|
| Extracellular matrix | |||
| Aggrecan | NA | Chondroskeletal morphogenesis, chondrocyte–matrix adhesion, cartilage hydration, neuronal cell aggregation 78 | Water retention |
| Versican | IdoA+ | Increases differentiation, motility, proliferation and metastasis 79,80. ECM assembly 81 | FGF family, L- and P-selectin, chemokines |
| Decorin | IdoA+ | TGF-β interaction 82, self-association 83, modulation of proliferation, survival, migration and angiogenesis 84, coagulation 60, LDL interaction 54, Borrelia invasion 65, α-defensin targeting 66, progeroid and Ehlers–Danlos syndromes 85 | FGF2, FGF7, HGF, HCII, α2β1integrin, tenascin-X, fibril formation, DS:DS self-association 86 |
| Biglycan | IdoA+ | Interactions withTGF-β 87, BMP4/chordin 88, collagen I 89, associated with tumour in gastric tissue 90 and endothelial cells 91, involved in inflammation and development 92,93, neuronal survival 94, bone development and osteoporosis 95,96 | HCII, FGF family |
| Epiphycan | IdoA+ | Chondrocyte differentiation 97 and matrix organization in the growth plate 98 | NA |
| Collagen IX | NA | Organization of cartilage 99, associated with fibroblasts in colon cancer | NA |
| Collagen XII | NA | Organization of cartilage and skin 100 | NA |
| Collagen XIV | NA | Organization of cartilage and skin 101,102 | NA |
| Cell surface | |||
| Betaglycan | NA | TGF-β presentation 103,104 and suppression of cancer progression and metastasis 105, binds inhibin and suppresses activin signalling 106 | NA |
| Syndecan-1 | IdoA+ | Regulation of tumour cell survival and proliferation, growth factor and cytokine binding, adhesion 107–109 | Midkine, pleiotropin, FGF |
| Syndecan-3 | NA | Role in human labour 110,111, adhesion, growth factors co-receptor, neurite outgrowth 112, expressed in tumour stromal vessels 113 | NA |
| Syndecan-4 | NA | Interaction with Frizzled7 and Dishevelled, regulates noncanonical Wnt signalling and convergent extension movements in Xenopus 114, regulates neural crest cells migration 115 and neural induction via extracellular signal-regulated kinase and protein kinase C pathways 116, adhesion, growth factors co-receptor 109, wound healing and angiogenesis 117, up-regulated in cancer and mediator of cell spreading 118 | Midkine, pleiothropin, bFGF 109 |
| CD44 | IdoA+ | Tumour growth, angiogenesis, metastasis, migration, HGF binding 119 | Migration, HGF |
| NG2 | NA | Regulates tumour cell growth, motility and survival 120 | Differentiation, proliferation and motility, PDGF-AA and FGF2, adhesion 121 |
| α5β1 integrin | NA | Fibronectin binding, regulation of adhesion and migration 122 | NA |
| Nervous system | |||
| Neuropilin-1 | NA | Metastasis, neuronal guidance, regulation of cell migration 123 | VEGF signalling |
| Neurocan | NA | Up-regulated in astrocytoma 124, neurite outgrowth, growth factors binding, brain ECM organization 125 | N-CAM, HB-GAM, amphoterin |
| Phosphacan | IdoA+ | Mediates migration and adhesion, differentiation of neuro stem cells 125–128 | HB-GAM, amphoterin, midkine |
| Brevican | NA | Promotes glioma invasion 129,130, regulation of synaptic plasticity 131 | Neuritogenic activity |
| Appican (AβPP isofom) | NA | Neuronal cell adhesion and migration, neurite outgrowth 132 | Midkine, pleiotrophin |
| Neuroglycan C | NA | Cerebral development and neuritogenesis | NA |
| Basal membranes | |||
| Perlecan | NA | Basal membrane stability, embryogenesis, cytokine interaction 133 interaction with FGFs, angiogenis 134 | NA |
| Bamacan | NA | Basal membrane, regulator of angiogenesis 135, anchorage-independent growth 136 | NA |
| Leprecan | NA | Kidney development, fibrillar collagen regulator 137 | NA |
| Collagen XV | NA | Suppresses tumour growth 138 | NA |
| Intracellular | |||
| Serglycin | IdoA+ | Inflammatory process 139 | Cytokine binding and coagulation, granulocyte maturation |
| Other proteoglycans | |||
| SRPX2 | IdoA+ | Overexpressed in gastrointestinal cancer, increases endothelial proliferation, cell signalling modulation, endothelial cell migration and angiogenesis 140 | HGF |
| Endocan | IdoA+ | Promotes tumour formation 141,142, mitogenic regulator, inflammation | HGF |
| Testican-1 | NA | Inhibition of proteases, neurite extension 143 | NA |
| Testican-2 | NA | Promotes invasion and abrogates proteases inhibition of other proteins of the testican family 144 | NA |
| Testican-3 | NA | Inhibits invasion, regulates neurite development 145 | NA |
| Bikunin | NA | Stabilization of ECM, activity in cumuli oophori, modulation of antiproteases 14,146 | |
CS is a long polysaccharide consisting of the repeating disaccharide units GlcA and N-acetyl-galactosamine (GalNAc), attached to serine residues of core proteins. The chains from eukaryotic organisms are extensively modified by sulfation, yielding six different disaccharides: GlcA-GalNac residues (O unit), GlcA-GalNAc-4-sulfate (A unit), GlcA-GalNAc-6-sulfate (C unit), GlcA-GalNAc-4,6-disulfated (E unit). The GlcA residue can also be sulfated at the 2-position giving rise to B units (GlcA-2-sulfated-GalNAc-4-sulfated) and D units (GlcA-2-sulfated-GalNAc-6-sulfated) 3. Even more complex sulfation patterns have been described in the invertebrate phyla 2.
An important modification is the epimerization of GlcA residues to IdoA residues by C-5 inversion at the polymer level of a (β-GlcA-1,3-β-GalNAc-1,4-)n substrate (Fig. 1B) 4. Individual saccharide units in CS/DS can exist in different conformations depending on their structural arrangement. IdoA residues allow flexibility given their ability to switch between 1C4 (chair), 2S0 (skew boat) and 4C1 (chair) conformations (Fig. 1C), whereas GlcA residues are less flexible and exist in the 4C1 (chair) conformation 5. IdoA can occur in three different arrangements: (a) as a single IdoA-containing disaccharide surrounded by GlcA containing disaccharides; (b) in structures where they alternate with GlcA containing disaccharides or (c) in long blocks of adjacent IdoA-containing disaccharides (Fig. 1A). The sulfation pattern differs according to the IdoA distribution because IdoA blocks are mainly 4-sulfated with some adjacent sulfated IdoA residues (iB) close to the nonreducing terminal of the blocks 6,7. The short GlcA blocks are mostly 4-sulfated, whereas longer blocks also contain 6-sulfated GalNac residues 8. The resulting CS/DS chains therefore contain different domains that enrich their functional properties. The presence of alternating IdoA-GlcA or isolated IdoA has been overlooked in many cases. Furthermore, the content of IdoA varies within the same proteoglycan depending on the tissue of expression 6 and physiological conditions 9. This is the case for decorin, which is highly iduronated in skin. In bone decorin, however, IdoA is virtually absent 6. Given the fact that a chain containing IdoA always contains GlcA, the name CS/DS indicates the hybrid nature of the chain.
The structural characterization of CS/DS takes advantage of specific lyases such as chondroitinase ABC, AC and B, which specifically degrade galactosaminoglycans depending on the presence of IdoA or GlcA. The development of high-resolution HPLC systems with pre- or post-column fluorescent derivatization has enabled the separation and quantitation of the various building blocks 10,11. These methods can only determine the degree of sulfation and the occurrence of IdoA- and GlcA-blocks. However, detailed sequence analysis is not possible. The advent of sensitive MS with different fragmentation procedures has lead to promising results 12,13. Recently, the complete sequence determination of the chondroitin sulfate in bikunin has been accomplished 14.
Biosynthesis of DS
DS-epi1 and DS-epi2 catalyze the formation of IdoA, the stereoisomeric form of GlcA, by repositioning the C5 carboxyl group in space (Fig. 1B). DS-epi1 (coded by the gene DSE) and DS-epi2 (coded by the gene DSEL) are both ubiquitously expressed and have common structural features 15,16.
DS-epi1 and 2 share a common N-terminal epimerase domain (Fig. 2A) with 51% amino acid sequence identity between the two enzymes. The secondary and tertiary structures of this domain in the two enzymes are very similar. DS-epi1 has a C-terminal domain of unknown function and three-dimensional structure. There is a similarly positioned domain in DS-epi2 with unknown function and structure. These two domains in the two epimerases do not have significant homology. In addition, in DS-epi2, there is a C-terminal domain, which has 16% amino acid identity with chondroitin-O-sulfotransferase 1, recognized in the database as a CS/DS–O-sulfotransferase domain (Fig. 2A), suggesting that DS-epi2 is an enzyme with dual epimerase and O-sulfotransferase activity. Other enzymes for GAG biosynthesis have been shown to accommodate dual activities 17,18. The functional epimerase domain of the DS epimerases comprises two structural domains: one mainly composed of α-helices and one of β-sheets (Fig. 2B). These two domains of DS-epi1 were modelled on the crystal structure of heparinase II 19. At their boundary, they form a groove, where the substrate is positioned. Some amino acids that are essential for enzyme activity have been identified and a catalytic mechanism has been proposed. Histidine 450 abstracts the C5 proton from one side of the sugar plane of GlcA. This is followed by cleavage or glycosidic linkage between GalNAc and GlcA to generate a C4–C5 double bond containing hexuronic acid intermediate. This structure is finally protonated by histidine 205 adding a hydrogen at the side of the sugar plane that is opposite to the abstraction side. Finally, the glycosidic link is recreated. As a result of the reaction, the carboxyl group has a different spacial orientation in the IdoA epimer than in the starting GlcA. A prerequisite for activity is the presence of at least three of the four N-glycans.
Fig. 2.
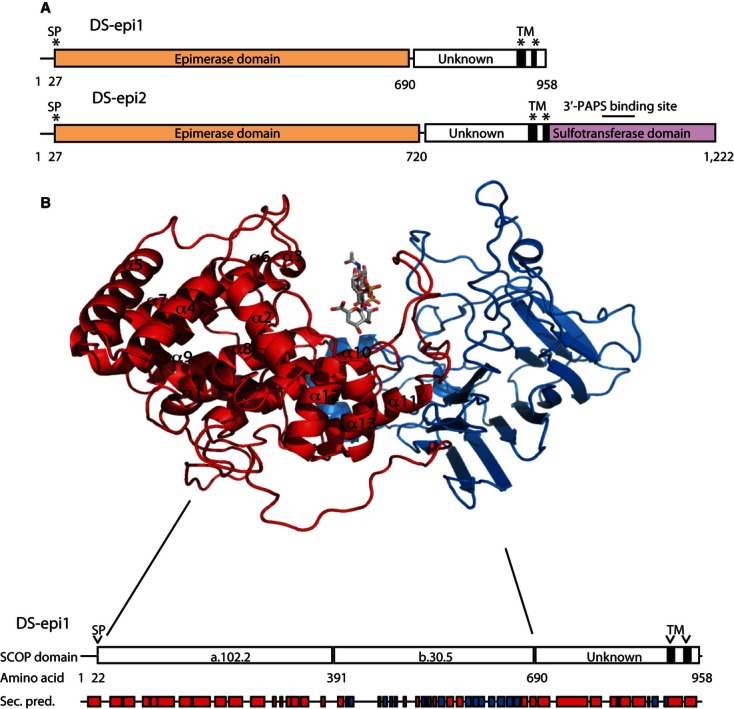
(A) DS-epi1 and DS-epi2 domain structures. (B) Three-dimensional modelling of the DS-epi1 epimerase domain based on the crystal structure of heparinase II. A chondroitin sulfate tetrasaccharide is positioned in the groove containing the active site.
DSE and DSEL are on chromosomes 6 and 18, respectively 15,20. The exon/intron organization of the two enzymes is very different because DSE has six exons and the coding sequence spans five exons, whereas DSEL has only two exons (being the whole ORF present in the single exon 2).
The epimerase activity is highly expressed in the spleen, stomach, uterus, ovary, kidney and lung. In the brain, the activity is low and no activity is found in serum 21. By analyzing the total activity in tissues and mouse embryonic fibroblasts of DS-epi1−/− and DS-epi2−/− mice, it is possible to show that DS-epi1 is the predominant epimerase in most tissues, whereas DS-epi2 is the main epimerase in the brain 21,22. DS-epi2 also has a relatively high expression in the kidney.
The epimerase reaction is reversible, with an equilibrium of 9 : 1 (GlcA to IdoA) under in vitro conditions when the biosynthetic complex has been solubilized with detergent 4. On the other hand, CS/DS chains in vivo can contain a higher proportion of IdoA. This is assumed to be achieved through functional collaboration between DS-epi1 and D4ST1 (Fig. 3) 23. In support of this, transient down-regulation of D4ST1 results in a reduced IdoA content 24. Genetic mutations in D4ST1 found in a new type of Ehlers–Danlos syndrome (i.e. adducted thumb-clubfoot syndrome) also result in CS/DS of low IdoA content 25.
Fig. 3.
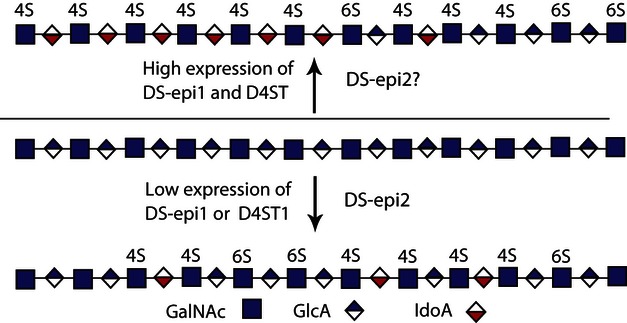
Formation of IdoA in CS/DS. The amount and distribution of IdoA depends upon the expression level of the DS epimerases and D4ST1.
Little is known about the regulation of epimerase activity. Transforming growth factor (TGF)-β-stimulated fibroblasts have reduced levels of epimerase activity, a reduced expression of D4ST1 and an increased expression of C4ST1, resulting in CS/DS with a considerably lower amount of IdoA 26. This effect is further increased by combined treatment with TGF-β, epidermal growth factor and platelet-derived growth factor (PDGF) (9). In another study, PDGF promoted the migration of fibroblasts, comprising a mechanism that is proposed to involve the up-regulation of IdoA in the proteoglycan CD44 27.
The products of DS-epi1 and 2 are difficult to assess as a result of the complex interaction with D4ST1. DS-epi1 can generate long blocks of IdoA together with D4ST1 (Fig. 3). Down-regulation of D4ST1 resulted in the abrogation of IdoA-containing blocks without affecting overall epimerase activity 24. The role of DS-epi2 has been more difficult to assess. Overexpression of DS-epi2 increased IdoA in hybrid structures (Fig. 3). No increase of IdoA blocks was recorded upon overexpression of DS-epi2, whereas overexpression of DS-epi1 resulted in enhanced block formation 16. By contrast, down-regulation of DS-epi2 in fibroblasts decreased the proportion of IdoA blocks, although to a smaller degree than that obtained by down-regulation of DS-epi1. Data obtained from DS-epi1 knockout mice show that DS-epi2 mainly forms alternating structures 28. These data indicate that DS-epi2 might be primarily involved in the formation of isolated or alternating IdoA structures (Fig. 3).
Different proteoglycans produced by the same cell can vary greatly with respect to their IdoA content and distribution. For example, decorin and biglycan have been found to contain blocks of IdoA, whereas versican only has isolated IdoA. Other studies have suggested that the core protein regulates the activity of the DS epimerases. This was demonstrated by the generation of chimeric proteins of decorin, which has a high content of IdoA, and colony-stimulating factor, a part-time proteoglycan with a low content of IdoA. The chimeric decorin–colony-stimulating factor contained less IdoA than the unmodified decorin 29. This suggests that core proteins carry information that may direct the proteoglycan cores to compartments within the Golgi complex with different amounts of DS epimerase activity 30.
Functions of IdoA as indicated by targeting of the two epimerases
The phenotype observed in DS-epi1 knockout mice is dependent upon the genetic background. Using mice with a pure C57BL6 genetic background, all pups die perinatally, whereas, when using mice with a pure NFR background, approximately half of the pups die. The NFR pups have a retarded growth rate in the late embryological stages of development and, furthermore, ∼ 20% of the pups display gastroschisis, an abdominal wall-closure defect that presents intestines outside the body (R. Gustafsson, unpublished data). DS-epi1 depleted mice in a mixed 129Sv/C57BL6 genetic background have been investigated in more detail. The pups were born at a normal Mendelian frequency 28. At birth, they are smaller and have a crooked tail. Because decorin is a major proteoglycan involved in the organization of collagen fibrils in skin, this tissue was studied in more detail. DS-epi1−/− skin was more fragile than the skin of wild-type mice. DS-epi1−/− collagen fibrils were more heterogeneous in denaturation profiles and in vitro experiments showed that, in DS-epi1−/− skin, decorin was the proteoglycan that was responsible for altered collagen structure (Fig. 4A). Electron microscopy showed that the diameter of DS-epi1−/− fibrils was 85 nm compared to 62 nm for wild-type mice 28. In summary, iduronic acid in the CS/DS chain and particularly of IdoA blocks participates in skin collagen fibril maturation.
Fig. 4.
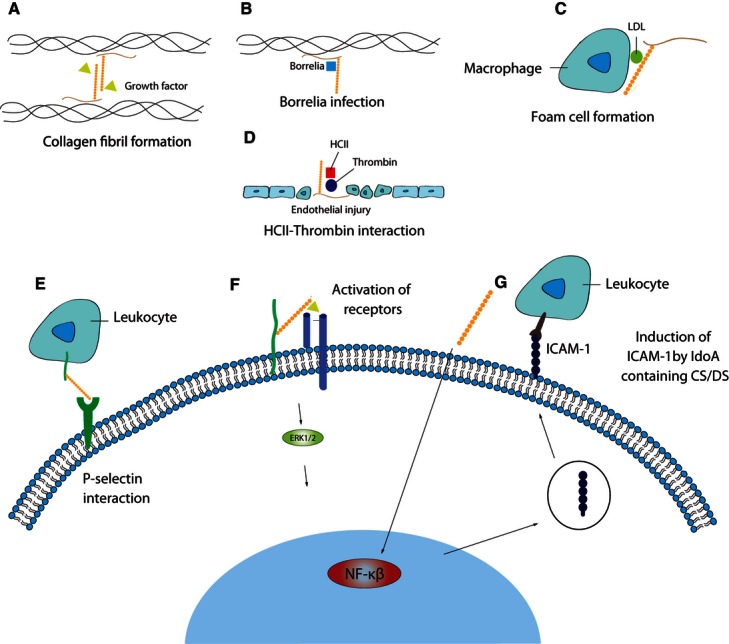
Overview of the functions of IdoA in CS/DS. Role of IdoA in the storage of cytokines growth factors and collagen fibril formation (A), Borrelia infection (B), atherosclerosis (C), coagulation (D), P-selectin-dependent leukocyte recruitment (E), activation of cytokine and growth factor receptors (F) and leukocyte recruitment by ICAM (G).
DS-epi2−/− mice do not show any evident phenotype 22. The brain was analyzed because DS-epi2 is the predominant epimerase in this tissue 22,31. Accordingly, DS-epi2−/− brains had a 90% reduction in epimerase activity. The brains of newborn mice contain little IdoA (2% of the total chain), and this was further reduced in DS-epi2−/− mice. However, the brain extracellular matrix (ECM) architecture was unaltered. It would be interesting to determine whether more subtle phenotypes such as behavioural changes are present in DS-epi2−/− mice.
Mice deficient in DS-epi1 and 2 were recently obtained in a mixed 129Sv/C57BL6 genetic background. A large proportion of the pups die perinatally, although a few survive until 7 weeks of age. Double knockout mice are dwarf and have approximately half the size and weight of their wild-type littermates.
Down-regulation of DS-epi1 has been achieved in the frog Xenopus laevis using morpholino injections (E. Pera, unpublished data). Several abnormalities were observed, such as the absence of the dorsal fin, which could be explained by the altered migration of neural crest cells into that anatomical structure.
Genetic alterations affecting IdoA formation in humans
There are no mutations in DS-epi1 associated with human diseases. However, mutations in D4ST1, which functionally collaborates with DS-epi1 to make IdoA blocks (Fig. 3), result in adducted thumb-clubfoot syndrome 32. Mutations of D4ST1 result in reduced amount of IdoA in CS/DS 25, also resulting in a defect in collagen fibril maturation and reduced collagen strength 28. This autosomal recessive syndrome 33 is characterized by facial changes, contractures of thumbs and fingers, joint instability, skin hyperextensibility, and heart and kidney defects. Additionally, myopathy has been described in these patients 34.
DS-epi2 has been genetically associated with bipolar disorder, which is a disease affecting ∼ 1% of mankind 15. Interestingly, two single nucleotide polymorphisms predicted to change the amino acid sequence were present in the bipolar disorder group and not in the control group.
The role of IdoA in stem cell development
Embryonic stem cells are obtained from embryos and can be maintained in cell cultures as pluripotent stem cell lines with a capacity to differentiate into whole embryos. Studies have shown a four- to six-fold increase of CS/DS during the differentiation of murine embryonic stem cells to embroid bodies and to extra embryonic endodermal cells. The formation of embroid bodies and extra embryonic endodermal cells was accompanied by a two- and four-fold increase of IdoA, respectively 35, suggesting a role for IdoA. The biosynthetic genes DSE, DSEL and CHST14, coding for D4ST1, were expressed at all stages. CHST14 was also expressed in the extra embryonic endodermal cells. However, the detailed structure of CS/DS, as well as its functions, still needs to be determined.
CS/DS is enriched in the neural stem cell niche and has been shown to play important role in the differentiation of neural progenitor cells 36. Its importance has been demonstrated in progenitor cells from mice with ablations of C4ST1 (a 4-O-sulfotransferase acting on GlcA-containing sequences) and D4ST1. Down-regulation of D4ST1 resulted in the abrogation of IdoA blocks, as well as decreased neurogenesis and proliferation and a change in the expression of cell surface receptors for fibroblast growth factor (FGF)-2 and epidermal growth factor, whereas C4ST1 deficiency did not affect these processes 37. The importance of IdoA motifs was further underlined by the fact that mRNA expression of the DS epimerases was higher in differentiated neurones than in precursor stem cells 38.
IdoA-containing structures in brain development
CS/DS structures are implicated in brain development 39 and injury to the central nervous system 40. During development, IdoA-containing structures (iA, iB, iE and iD) are ubiquitous in different parts of the brain 31,41, although at low concentrations. Indeed, CS/DS brains of newborn mice comprise only 2% iduronic acid 22. The CS/DS bioenzymatic machinery is carefully regulated during brain development, resulting in a large variation of IdoA-containing structures. For example, in the cerebellum, iD decreases and iB increases from newborn to adult age 31. Interestingly, the embryo-derived CS/DS shows a greater binding of FGFs (FGF-2, -10 and -18), pleiotrophin, midkine, vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) than CS/DS from the brains of adult animals 42.
The role of IdoA in CS/DS under pathological conditions
Inflammation
The involvement of CS/DS in inflammation has been extensively explored, whereas the role of IdoA is not well defined 43,44. The inflammatory response initiated by infection or injury results in diverse processes, involving cell recruitment, extravasation and cell/pathogen clearance. For example, during wound healing, CS/DS is reported to be the dominating GAG in wound fluid 45,46. FGF2 and FGF7 are two important growth factors during wound repair and they have been shown to preferentially bind to IdoA-containing motifs in CS/DS, promoting proliferative processes (Fig. 4A,F). CS/DS has been proposed, in combination with FGF-10, as a pharmacological accelerator of wound closure as a result of its capacity to stimulate re-epithelialization 47. CS/DS can potentially affect several steps during cell recruitment. For example, CS/DS has been shown to interact with P-selectin, which is expressed on endothelial cells and platelets 48 (Fig. 4E). CS/DS is reported to influence the recruitment of polymorphonuclear cells in a thioglycollate-induced inflammatory model in a supposedly P-selectin manner 49. RANTES, a leukocyte-recruiting chemokine, also interacts with IdoA-containing segments in CS/DS 50. An essential step during extravasation is the increased expression of intercellular adhesion molecule-1 (ICAM-1) on endothelial cells. IdoA in CS/DS induces endothelial expression of ICAM-1 mediated by nuclear factor-kβ 45 (Fig. 4G). Interestingly, macrophages are reported to produce CS/DS containing up to 70% of IdoA 51. After lipopolysaccharide stimulation, macrophages predominantly secrete CS/DS, either as free chains or bound to the serglycin core protein.
Immune response
Autoimmunity is a result of a disarray in the immune response, which becomes directed towards its own tissue and cells. B cells participate in autoimmunity by the production of antibodies and presentation of self-antigens to T cells. IdoA motifs in CS/DS are reported to augment the proliferation of B1-a cells and increase their autoantibody production 52. IdoA in CS/DS interacts with components from apoptotic and dead cells and forms complexes that enhance autoantibody production. IdoA-containing structures in CS/DS bind autoantigens, which were enriched after CS/DS-affinity chromatography of cellular lysates. Two hundred autoantigens were identified by MS and could be used in western blot experiments to detect different autoantibody patterns of diagnostic value in patient sera 52,53. Further studies are needed to clarify the physiological role of CS/DS in the generation of natural autoantibodies.
Atherosclerosis
Atherosclerosis, an inflammatory-driven disease, is characterized by the accumulation of cholesterol in arterial blood vessels, resulting in thicker and more fragile artery vessels. Binding of low-density lipoprotein (LDL) to GAGs is considered to be one of the steps in the onset of this disease 54. The GAG interaction enhances LDL uptake by macrophages, leading to the formation of foam cells (Fig. 4C). IdoA both in CS/DS and heparan sulfate is reported to enhance the binding of VLDL and LDL 55,56. Recently, it was reported that an antibody against CS/DS inhibited the LDL–CS/DS interaction and inhibited LDL oxidation in vitro 57. Furthermore, the injection of anti-CS/DS antibody in an atherosclerosis model of ApoE−/− mice resulted in decreased arteriosclerotic lesions 58.
Coagulation
Coagulation is essential under normal physiological conditions and several pathological conditions (e.g. cancer, atherosclerosis and sepsis) have enhanced coagulation. Thrombin, a serine protease, catalyzes the conversion of fibrinogen to fibrin, which forms blood clots in conjunction with platelets. Heparin cofactor II (HCII) is a thrombin inhibitor and the only known serpin to be activated by IdoA-containing CS/DS (Fig. 4D). The HCII binding site to CS/DS differs from that to HS 59. The HCII binding structures in CS/DS contain IdoA-2S-GalNAc-4S 60 or GlcA-GalNAc-4,6-disulfated 61 in hexa- and octasaccharides as minimal binding motifs. The complex CS/DS-HCII is considered to be the major anticoagulant system after injury of the vessel wall 60,62,63. CS/DS containing 2-O-sulfated IdoA also controls coagulation by activating protein C 64.
Infection
CS/DS is involved in bacterial infections. Borrelia (causing Lyme disease) was shown to use the core protein of decorin, as well as its CS/DS side chain, as a binding target in the initial phase of infection 65 (Fig. 4B). CS/DS released from decorin by proteases produced by Pseudomonas, Enterococcus and Streptococcus 66 targets α-defensin and inhibits its bactericidal activity. The optimal structure for interaction to α-defensin is a motif containing a mix of IdoA and GlcA, which is found in decorin present in fibrous connective tissue 66.
IdoA motifs in cancer
CS/DS is implicated in several cancer-promoting processes, such as cell proliferation and metastasis 3. DS-epi1, previously named SART2 (squamous cell carcinoma antigen recognized by T cell 2), is highly expressed in many tumours and cell lines 20. DS-epi1 expressed by cancer cells was recognized by HLA-A24-restricted and tumour-specific cytotoxic lymphocytes. Peptides from DS-epi1 were used in peptide-based immunotherapy phase I clinical trials for prostate cancer 67, glioblastoma multiforme 68 and hepatocellular carcinoma 69 with moderate success. We have established that DS-epi1 is not tumour specific because DS-epi1 is ubiquitously expressed in normal tissues 21. Squamous cell carcinoma from oesophagus contains epimerase activity that is increased four- to five-fold compared to normal oesophagus 13. DS-epi1 is localized both in stroma surrounding the tumour and in cancer cells. To investigate the role of IdoA, DS-epi1 was stably down-regulated in oesophagus squamous carcinoma cell lines using shRNA sequences. IdoA was shown to facilitate the binding of HGF to its receptor and was essential for cMET-dependent signalling 13 (Fig. 4F). In addition, DS-epi1 down-regulated cells displayed fewer cytoplasmic stress fibres than control cells. Furthermore, the focal adhesion complexes were evenly distributed at the cell surface in DS-epi1 down-regulated cells compared to control cells, which displayed focal adhesion complexes predominantly at the leading edge. This resulted in less migration and invasion of DS-epi1 down-regulated cells compared to control cells 13.
Different CS/DS structures mediate diverse function during cancer development. The sulfation pattern of CS/DS in cancer differs from normal tissue. For example, 6-O-mono-sulfated disaccharides are accumulated in tumours compared to normal tissues, whereas 4-O-mono-sulfated disaccharides are reduced 70. During metastasis, CS/DS disaccharides sulfated at positions 4 and 6 (E units) present on the surface of cancer cells facilitate colonization of the lung and liver 71,72. The process might be mediated by the receptor RAGE, which is highly expressed in the lung 73. Another pro-metastatic activity of the E units on cancer cells could be a result of the capability to bind platelet P-selectin 49, resulting in the formation of tumour microemboli. These cell–platelet aggregates protect cancer cells against elimination by the immune system. IdoA in CS/DS is also known to mediate P-selectin binding. Two CS/DS structures containing IdoA (iB units or iD units), as isolated from marine animals, inhibit metastasis in a P-selectin-dependent manner in a metastatic tumour model 49. Several studies report that CS/DS structures mediate growth factor and chemokine binding. IdoA is essential for HGF-mediated binding and an IdoA-containing tetrasaccharide is the minimum structure required to confer affinity 74. Exogenously added IdoA-containing motifs inhibit the proliferation of normal and malignant cells 75. Elimination of CS/DS on the cancer cell membrane by chondroitinase B inhibits the migration and invasion of tumour cells 76.
Future perspectives in research and clinical therapy
Still largely unknown is how the complex structure of CS/DS is formed and how it is regulated. A key question is the organization of the biosynthetic enzymes in the Golgi and how this organization is modulated in different cells and tissues. The role of the two different epimerases, DS-epi1 and 2, as well as that of D4ST1, needs to be clarified.
Different functions of IdoA have been found both in vitro and in vivo. The human situations where DS-epi1 expression is changed in tumours and where D4ST1 mutations lead to deranged connective tissue have been highlighted. The importance of IdoA is evident from observations of DS-epi1 KO mice, which die perinatally and/or present gastroschisis. Furthermore, a decrease of IdoA leads to an altered collagen structure, resulting in a decreased tensile strength. Provocation of mice with targeted DS-epi1 and 2 will most likely provide more information about other biological functions of IdoA. Other data indicate the importance of IdoA in cytokine activity and storage, cell proliferation and migration, the control of coagulation, the formation of autoantibodies, the control of stem cell stability and differentiation.
In disease, IdoA contributes to cancer progression and infection. New avenues for future therapies have been tested, such as vaccination against cancer 67–69, or are warranted to control infection 65,66 and cancer 13,76,77. DS epimerases inhibitors could be used in cancer and fibrosis, as well as to guide stem cell differentiation 3.
Acknowledgments
This work was supported by grants received from the Swedish Science Research Council; the Medical Faculty of Lund University; the Mizutani Foundation for Glycoscience (to M.M. and A.M.); the Albert Österlund Foundation; the Greta and Johan Kock Foundation; and the Anna-Greta Crafoord Foundation.
Glossary
- CS
chondroitin sulfate
- D4ST1
dermatan sulfate 4-O-sulfotransferase 1
- DS
dermatan sulfate
- DS-epi1
dermatan sulfate epimerase 1
- DS-epi2
dermatan sulfate epimerase 2
- ECM
extracellular matrix
- FGF
fibroblast growth factor
- GAG
glycosaminoglycan
- GalNAc
N-acetyl-galactosamine
- GlcA
d-glucuronic acid
- HCII
heparin cofactor II
- HGF
hepatocyte growth factor
- IdoA
iduronic acid
- INF-γ
interferon-γ
- PDGF
platelet-derived growth factor
- TGF
transforming growth factor
- VEGF
vascular endothelial growth factor
References
- 1.Malmstrom A, Bartolini B, Thelin MA, Pacheco B, Maccarana M. Iduronic acid in chondroitin/dermatan sulfate: biosynthesis and biological function. J Histochem Cytochem. 2012;60:916–925. doi: 10.1369/0022155412459857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamada S, Sugahara K, Ozbek S. Evolution of glycosaminoglycans: comparative biochemical study. Commun Integr Biol. 2011;4:150–158. doi: 10.4161/cib.4.2.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada S, Sugahara K. Potential therapeutic application of chondroitin sulfate/dermatan sulfate. Curr Drug Discov Technol. 2008;5:289–301. doi: 10.2174/157016308786733564. [DOI] [PubMed] [Google Scholar]
- 4.Malmstrom A. Biosynthesis of dermatan sulfate. II. Substrate specificity of the C-5 uronosyl epimerase. J Biol Chem. 1984;259:161–165. [PubMed] [Google Scholar]
- 5.Ferro DR, Provasoli A, Ragazzi M, Casu B, Torri G, Bossennec V, Perly B, Sinay P, Petitou M, Choay J. Conformer populations of L-iduronic acid residues in glycosaminoglycan sequences. Carbohydr Res. 1990;195:157–167. doi: 10.1016/0008-6215(90)84164-p. [DOI] [PubMed] [Google Scholar]
- 6.Cheng F, Heinegard D, Malmstrom A, Schmidtchen A, Yoshida K, Fransson LA. Patterns of uronosyl epimerization and 4-/6-O-sulphation in chondroitin/dermatan sulphate from decorin and biglycan of various bovine tissues. Glycobiology. 1994;4:685–696. doi: 10.1093/glycob/4.5.685. [DOI] [PubMed] [Google Scholar]
- 7.Fransson LA, Coster L, Havasmark B, Malmstrom A, Sjoberg I. The copolymeric structure of pig skin dermatan sulphate. Isolation and characterization of L-idurono-sulphate-containing oligosaccharides from copolymeric chains. Biochem J. 1974;143:379–389. doi: 10.1042/bj1430379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fransson LA, Coster L, Malmstrom A, Sjoberg I. The copolymeric structure of pig skin dermatan suplhate. Characterization of D-glucuronic acid-containing oligosaccharides isolated after controlled degradation of oxydermatan sulphate. Biochem J. 1974;143:369–378. doi: 10.1042/bj1430369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiedemann K, Olander B, Eklund E, Todorova L, Bengtsson M, Maccarana M, Westergren-Thorsson G, Malmstrom A. Regulation of the chondroitin/dermatan fine structure by transforming growth factor-beta1 through effects on polymer-modifying enzymes. Glycobiology. 2005;15:1277–1285. doi: 10.1093/glycob/cwj027. [DOI] [PubMed] [Google Scholar]
- 10.Ambrosius M, Kleesiek K, Gotting C. Quantitative determination and comparison of the glycosaminoglycan delta-disaccharide composition in 22 different human cell lines. Cell Biol Int. 2009;33:848–852. doi: 10.1016/j.cellbi.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Mizumoto S, Sugahara K. Glycosaminoglycan chain analysis and characterization (glycosylation/epimerization) Methods Mol Biol. 2012;836:99–115. doi: 10.1007/978-1-61779-498-8_7. [DOI] [PubMed] [Google Scholar]
- 12.Miller MJ, Costello CE, Malmstrom A, Zaia J. A tandem mass spectrometric approach to determination of chondroitin/dermatan sulfate oligosaccharide glycoforms. Glycobiology. 2006;16:502–513. doi: 10.1093/glycob/cwj093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thelin MA, Svensson KJ, Shi X, Bagher M, Axelsson J, Isinger-Ekstrand A, van Kuppevelt TH, Johansson J, Nilbert M, Zaia J, et al. Dermatan sulfate is involved in the tumorigenic properties of esophagus squamous cell carcinoma. Cancer Res. 2012;72:1943–1952. doi: 10.1158/0008-5472.CAN-11-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ly M, Leach FE, III, Laremore TN, Toida T, Amster IJ, Linhardt RJ. The proteoglycan bikunin has a defined sequence. Nat Chem Biol. 2011;7:827–833. doi: 10.1038/nchembio.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goossens D, Van Gestel S, Claes S, De Rijk P, Souery D, Massat I, Van den BD, Backhovens H, Mendlewicz J, Van Broeckhoven C, et al. A novel CpG-associated brain-expressed candidate gene for chromosome 18q-linked bipolar disorder. Mol Psychiatry. 2003;8:83–89. doi: 10.1038/sj.mp.4001190. [DOI] [PubMed] [Google Scholar]
- 16.Pacheco B, Malmstrom A, Maccarana M. Two dermatan sulfate epimerases form iduronic acid domains in dermatan sulfate. J Biol Chem. 2009;284:9788–9795. doi: 10.1074/jbc.M809339200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinhal MA, Smith B, Olson S, Aikawa J, Kimata K, Esko JD. Enzyme interactions in heparan sulfate biosynthesis: uronosyl 5-epimerase and 2-O-sulfotransferase interact in vivo. Proc Natl Acad Sci USA. 2001;98:12984–12989. doi: 10.1073/pnas.241175798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Presto J, Thuveson M, Carlsson P, Busse M, Wilen M, Eriksson I, Kusche-Gullberg M, Kjellen L. Heparan sulfate biosynthesis enzymes EXT1 and EXT2 affect NDST1 expression and heparan sulfate sulfation. Proc Natl Acad Sci USA. 2008;105:4751–4756. doi: 10.1073/pnas.0705807105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaya D, Tocilj A, Li Y, Myette J, Venkataraman G, Sasisekharan R, Cygler M. Crystal structure of heparinase II from Pedobacter heparinus and its complex with a disaccharide product. J Biol Chem. 2006;281:15525–15535. doi: 10.1074/jbc.M512055200. [DOI] [PubMed] [Google Scholar]
- 20.Nakao M, Shichijo S, Imaizumi T, Inoue Y, Matsunaga K, Yamada A, Kikuchi M, Tsuda N, Ohta K, Takamori S, et al. Identification of a gene coding for a new squamous cell carcinoma antigen recognized by the CTL. J Immunol. 2000;164:2565–2574. doi: 10.4049/jimmunol.164.5.2565. [DOI] [PubMed] [Google Scholar]
- 21.Maccarana M, Olander B, Malmstrom J, Tiedemann K, Aebersold R, Lindahl U, Li JP, Malmstrom A. Biosynthesis of dermatan sulfate: chondroitin-glucuronate C5-epimerase is identical to SART2. J Biol Chem. 2006;281:11560–11568. doi: 10.1074/jbc.M513373200. [DOI] [PubMed] [Google Scholar]
- 22.Bartolini B, Thelin MA, Rauch U, Feinstein R, Oldberg A, Malmstrom A, Maccarana M. Mouse development is not obviously affected by the absence of dermatan sulfate epimerase 2 in spite of a modified brain dermatan sulfate composition. Glycobiology. 2012;22:1007–1016. doi: 10.1093/glycob/cws065. [DOI] [PubMed] [Google Scholar]
- 23.Malmstrom A, Fransson LA. Biosynthesis of dermatan sulfate. I. Formation of L-iduronic acid residues. J Biol Chem. 1975;250:3419–3425. [PubMed] [Google Scholar]
- 24.Pacheco B, Maccarana M, Malmstrom A. Dermatan 4-O-sulfotransferase 1 is pivotal in the formation of iduronic acid blocks in dermatan sulfate. Glycobiology. 2009;19:1197–1203. doi: 10.1093/glycob/cwp110. [DOI] [PubMed] [Google Scholar]
- 25.Miyake N, Kosho T, Mizumoto S, Furuichi T, Hatamochi A, Nagashima Y, Arai E, Takahashi K, Kawamura R, Wakui K, et al. Loss-of-function mutations of CHST14 in a new type of Ehlers–Danlos syndrome. Hum Mutat. 2010;31:966–974. doi: 10.1002/humu.21300. [DOI] [PubMed] [Google Scholar]
- 26.Tiedemann K, Malmstrom A, Westergren-Thorsson G. Cytokine regulation of proteoglycan production in fibroblasts: separate and synergistic effects. Matrix Biol. 1997;15:469–478. doi: 10.1016/s0945-053x(97)90020-2. [DOI] [PubMed] [Google Scholar]
- 27.Lin F, Ren XD, Pan Z, Macri L, Zong WX, Tonnesen MG, Rafailovich M, Bar-Sagi D, Clark RA. Fibronectin growth factor-binding domains are required for fibroblast survival. J Invest Dermatol. 2011;131:84–98. doi: 10.1038/jid.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maccarana M, Kalamajski S, Kongsgaard M, Magnusson SP, Oldberg A, Malmstrom A. Dermatan sulfate epimerase 1-deficient mice have reduced content and changed distribution of iduronic acids in dermatan sulfate and an altered collagen structure in skin. Mol Cell Biol. 2009;29:5517–5528. doi: 10.1128/MCB.00430-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seidler DG, Breuer E, Grande-Allen KJ, Hascall VC, Kresse H. Core protein dependence of epimerization of glucuronosyl residues in galactosaminoglycans. J Biol Chem. 2002;277:42409–42416. doi: 10.1074/jbc.M208442200. [DOI] [PubMed] [Google Scholar]
- 30.Herzog C, Lippmann I, Grobe K, Zamfir AD, Echtermeyer F, Seidler DG. The amino acid tryptophan prevents the biosynthesis of dermatan sulfate. Mol BioSyst. 2011;7:2872–2881. doi: 10.1039/c1mb05139c. [DOI] [PubMed] [Google Scholar]
- 31.Akatsu C, Mizumoto S, Kaneiwa T, Maccarana M, Malmstrom A, Yamada S, Sugahara K. Dermatan sulfate epimerase 2 is the predominant isozyme in the formation of the chondroitin sulfate/dermatan sulfate hybrid structure in postnatal developing mouse brain. Glycobiology. 2011;21:565–574. doi: 10.1093/glycob/cwq208. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu K, Okamoto N, Miyake N, Taira K, Sato Y, Matsuda K, Akimaru N, Ohashi H, Wakui K, Fukushima Y, et al. Delineation of dermatan 4-O-sulfotransferase 1 deficient Ehlers–Danlos syndrome: observation of two additional patients and comprehensive review of 20 reported patients. Am J Med Genet A. 2011;155A:1949–1958. doi: 10.1002/ajmg.a.34115. [DOI] [PubMed] [Google Scholar]
- 33.Dundar M, Muller T, Zhang Q, Pan J, Steinmann B, Vodopiutz J, Gruber R, Sonoda T, Krabichler B, Utermann G, et al. Loss of dermatan-4-sulfotransferase 1 function results in adducted thumb-clubfoot syndrome. Am J Hum Genet. 2009;85:873–882. doi: 10.1016/j.ajhg.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voermans NC, Kempers M, Lammens M, van Alfen N, Janssen MC, Bonnemann C, van Engelen BG, Hamel BC. Myopathy in a 20-year-old female patient with D4ST-1 deficient Ehlers–Danlos syndrome due to a homozygous CHST14 mutation. Am J Med Genet A. 2012;158A:850–855. doi: 10.1002/ajmg.a.35232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nairn AV, Kinoshita-Toyoda A, Toyoda H, Xie J, Harris K, Dalton S, Kulik M, Pierce JM, Toida T, Moremen KW, et al. Glycomics of proteoglycan biosynthesis in murine embryonic stem cell differentiation. J Proteome Res. 2007;6:4374–4387. doi: 10.1021/pr070446f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purushothaman A, Sugahara K, Faissner A. Chondroitin sulfate ‘wobble motifs’ modulate maintenance and differentiation of neural stem cells and their progeny. J Biol Chem. 2012;287:2935–2942. doi: 10.1074/jbc.R111.298430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bian S, Akyuz N, Bernreuther C, Loers G, Laczynska E, Jakovcevski I, Schachner M. Dermatan sulfotransferase Chst14/D4st1, but not chondroitin sulfotransferase Chst11/C4st1, regulates proliferation and neurogenesis of neural progenitor cells. J Cell Sci. 2011;124:4051–4063. doi: 10.1242/jcs.088120. [DOI] [PubMed] [Google Scholar]
- 38.Yamauchi S, Kurosu A, Hitosugi M, Nagai T, Oohira A, Tokudome S. Differential gene expression of multiple chondroitin sulfate modification enzymes among neural stem cells, neurons and astrocytes. Neurosci Lett. 2011;493:107–111. doi: 10.1016/j.neulet.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 39.Maeda N, Fukazawa N, Ishii M. Chondroitin sulfate proteoglycans in neural development and plasticity. Front Biosci. 2010;15:626–644. doi: 10.2741/3637. [DOI] [PubMed] [Google Scholar]
- 40.Crespo D, Asher RA, Lin R, Rhodes KE, Fawcett JW. How does chondroitinase promote functional recovery in the damaged CNS? Exp Neurol. 2007;206:159–171. doi: 10.1016/j.expneurol.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Mitsunaga C, Mikami T, Mizumoto S, Fukuda J, Sugahara K. Chondroitin sulfate/dermatan sulfate hybrid chains in the development of cerebellum. Spatiotemporal regulation of the expression of critical disulfated disaccharides by specific sulfotransferases. J Biol Chem. 2006;281:18942–18952. doi: 10.1074/jbc.M510870200. [DOI] [PubMed] [Google Scholar]
- 42.Sugahara K, Mikami T. Chondroitin/dermatan sulfate in the central nervous system. Curr Opin Struct Biol. 2007;17:536–545. doi: 10.1016/j.sbi.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 43.Malavaki C, Mizumoto S, Karamanos N, Sugahara K. Recent advances in the structural study of functional chondroitin sulfate and dermatan sulfate in health and disease. Connect Tissue Res. 2008;49:133–139. doi: 10.1080/03008200802148546. [DOI] [PubMed] [Google Scholar]
- 44.Trowbridge JM, Gallo RL. Dermatan sulfate: new functions from an old glycosaminoglycan. Glycobiology. 2002;12:117R–125R. doi: 10.1093/glycob/cwf066. [DOI] [PubMed] [Google Scholar]
- 45.Penc SF, Pomahac B, Eriksson E, Detmar M, Gallo RL. Dermatan sulfate activates nuclear factor-kappab and induces endothelial and circulating intercellular adhesion molecule-1. J Clin Invest. 1999;103:1329–1335. doi: 10.1172/JCI4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Penc SF, Pomahac B, Winkler T, Dorschner RA, Eriksson E, Herndon M, Gallo RL. Dermatan sulfate released after injury is a potent promoter of fibroblast growth factor-2 function. J Biol Chem. 1998;273:28116–28121. doi: 10.1074/jbc.273.43.28116. [DOI] [PubMed] [Google Scholar]
- 47.Plichta JK, Radek KA. Sugar-coating wound repair: a review of FGF-10 and dermatan sulfate in wound healing and their potential application in burn wounds. J Burn Care Res. 2012;33:299–310. doi: 10.1097/BCR.0b013e318240540a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawashima H, Hirose M, Hirose J, Nagakubo D, Plaas AH, Miyasaka M. Binding of a large chondroitin sulfate/dermatan sulfate proteoglycan, versican, to L-selectin, P-selectin, and CD44. J Biol Chem. 2000;275:35448–35456. doi: 10.1074/jbc.M003387200. [DOI] [PubMed] [Google Scholar]
- 49.Kozlowski EO, Pavao MS, Borsig L. Ascidian dermatan sulfates attenuate metastasis, inflammation and thrombosis by inhibition of P-selectin. J Thromb Haemost. 2011;9:1807–1815. doi: 10.1111/j.1538-7836.2011.04401.x. [DOI] [PubMed] [Google Scholar]
- 50.Kuschert GS, Coulin F, Power CA, Proudfoot AE, Hubbard RE, Hoogewerf AJ, Wells TN. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry. 1999;38:12959–12968. doi: 10.1021/bi990711d. [DOI] [PubMed] [Google Scholar]
- 51.Petricevich VL, Michelacci YM. Proteoglycans synthesized in vitro by nude and normal mouse peritoneal macrophages. Biochim Biophys Acta. 1990;1053:135–143. doi: 10.1016/0167-4889(90)90005-x. [DOI] [PubMed] [Google Scholar]
- 52.Rho JH, Zhang W, Murali M, Roehrl MH, Wang JY. Human proteins with affinity for dermatan sulfate have the propensity to become autoantigens. Am J Pathol. 2011;178:2177–2190. doi: 10.1016/j.ajpath.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang JY, Lee J, Yan M, Rho JH, Roehrl MH. Dermatan sulfate interacts with dead cells and regulates CD5(+) B-cell fate: implications for a key role in autoimmunity. Am J Pathol. 2011;178:2168–2176. doi: 10.1016/j.ajpath.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Camejo G, Hurt-Camejo E, Wiklund O, Bondjers G. Association of apo B lipoproteins with arterial proteoglycans: pathological significance and molecular basis. Atherosclerosis. 1998;139:205–222. doi: 10.1016/s0021-9150(98)00107-5. [DOI] [PubMed] [Google Scholar]
- 55.Iverius PH. The interaction between human plasma lipoproteins and connective tissue glycosaminoglycans. J Biol Chem. 1972;247:2607–2613. [PubMed] [Google Scholar]
- 56.Theocharis AD, Theocharis DA, De Luca G, Hjerpe A, Karamanos NK. Compositional and structural alterations of chondroitin and dermatan sulfates during the progression of atherosclerosis and aneurysmal dilatation of the human abdominal aorta. Biochimie. 2002;84:667–674. doi: 10.1016/s0300-9084(02)01428-1. [DOI] [PubMed] [Google Scholar]
- 57.Soto Y, Acosta E, Delgado L, Perez A, Falcon V, Becquer MA, Fraga A, Brito V, Alvarez I, Grinan T, et al. Antiatherosclerotic effect of an antibody that binds to extracellular matrix glycosaminoglycans. Arterioscler Thromb Vasc Biol. 2012;32:595–604. doi: 10.1161/ATVBAHA.111.238659. [DOI] [PubMed] [Google Scholar]
- 58.Brito V, Mellal K, Portelance SG, Perez A, Soto Y, Deblois D, Ong H, Marleau S, Vazquez AM. Induction of anti-anti-idiotype antibodies against sulfated glycosaminoglycans reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:2847–2853. doi: 10.1161/ATVBAHA.112.300444. [DOI] [PubMed] [Google Scholar]
- 59.Blinder MA, Andersson TR, Abildgaard U, Tollefsen DM. Heparin cofactor IIOslo. Mutation of Arg-189 to His decreases the affinity for dermatan sulfate. J Biol Chem. 1989;264:5128–5133. [PubMed] [Google Scholar]
- 60.Maimone MM, Tollefsen DM. Structure of a dermatan sulfate hexasaccharide that binds to heparin cofactor II with high affinity. J Biol Chem. 1990;265:18263–18271. [PubMed] [Google Scholar]
- 61.Halldorsdottir AM, Zhang L, Tollefsen DM. N-Acetylgalactosamine 4,6-O-sulfate residues mediate binding and activation of heparin cofactor II by porcine mucosal dermatan sulfate. Glycobiology. 2006;16:693–701. doi: 10.1093/glycob/cwj117. [DOI] [PubMed] [Google Scholar]
- 62.Tollefsen DM. Vascular dermatan sulfate and heparin cofactor II. Prog Mol Biol Transl Sci. 2010;93:351–372. doi: 10.1016/S1877-1173(10)93015-9. [DOI] [PubMed] [Google Scholar]
- 63.He L, Giri TK, Vicente CP, Tollefsen DM. Vascular dermatan sulfate regulates the antithrombotic activity of heparin cofactor II. Blood. 2008;111:4118–4125. doi: 10.1182/blood-2007-12-127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernandez JA, Petaja J, Griffin JH. Dermatan sulfate and LMW heparin enhance the anticoagulant action of activated protein C. Thromb Haemost. 1999;82:1462–1468. [PubMed] [Google Scholar]
- 65.Brown EL, Wooten RM, Johnson BJ, Iozzo RV, Smith A, Dolan MC, Guo BP, Weis JJ, Hook M. Resistance to Lyme disease in decorin-deficient mice. J Clin Invest. 2001;107:845–852. doi: 10.1172/JCI11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmidtchen A, Frick IM, Bjorck L. Dermatan sulphate is released by proteinases of common pathogenic bacteria and inactivates antibacterial alpha-defensin. Mol Microbiol. 2001;39:708–713. doi: 10.1046/j.1365-2958.2001.02251.x. [DOI] [PubMed] [Google Scholar]
- 67.Noguchi M, Kobayashi K, Suetsugu N, Tomiyasu K, Suekane S, Yamada A, Itoh K, Noda S. Induction of cellular and humoral immune responses to tumor cells and peptides in HLA-A24 positive hormone-refractory prostate cancer patients by peptide vaccination. Prostate. 2003;57:80–92. doi: 10.1002/pros.10276. [DOI] [PubMed] [Google Scholar]
- 68.Terasaki M, Shibui S, Narita Y, Fujimaki T, Aoki T, Kajiwara K, Sawamura Y, Kurisu K, Mineta T, Yamada A, et al. Phase I trial of a personalized peptide vaccine for patients positive for human leukocyte antigen–A24 with recurrent or progressive glioblastoma multiforme. J Clin Oncol. 2011;29:337–344. doi: 10.1200/JCO.2010.29.7499. [DOI] [PubMed] [Google Scholar]
- 69.Mizukoshi E, Fushimi K, Arai K, Yamashita T, Honda M, Kaneko S. Expression of chondroitin-glucuronate C5-epimerase and cellular immune responses in patients with hepatocellular carcinoma. Liver Int. 2012;32:1516–1526. doi: 10.1111/j.1478-3231.2012.02853.x. [DOI] [PubMed] [Google Scholar]
- 70.Theocharis AD, Vynios DH, Papageorgakopoulou N, Skandalis SS, Theocharis DA. Altered content composition and structure of glycosaminoglycans and proteoglycans in gastric carcinoma. Int J Biochem Cell Biol. 2003;35:376–390. doi: 10.1016/s1357-2725(02)00264-9. [DOI] [PubMed] [Google Scholar]
- 71.Basappa Murugan S, Sugahara KN, Lee CM, ten Dam GB, van Kuppevelt TH, Miyasaka M, Yamada S, Sugahara K. Involvement of chondroitin sulfate E in the liver tumor focal formation of murine osteosarcoma cells. Glycobiology. 2009;19:735–742. doi: 10.1093/glycob/cwp041. [DOI] [PubMed] [Google Scholar]
- 72.Li F, Ten Dam GB, Murugan S, Yamada S, Hashiguchi T, Mizumoto S, Oguri K, Okayama M, van Kuppevelt TH, Sugahara K. Involvement of highly sulfated chondroitin sulfate in the metastasis of the Lewis lung carcinoma cells. J Biol Chem. 2008;283:34294–34304. doi: 10.1074/jbc.M806015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mizumoto S, Takahashi J, Sugahara K. Receptor for advanced glycation end products (RAGE) functions as receptor for specific sulfated glycosaminoglycans, and anti-RAGE antibody or sulfated glycosaminoglycans delivered in vivo inhibit pulmonary metastasis of tumor cells. J Biol Chem. 2012;287:18985–18994. doi: 10.1074/jbc.M111.313437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deakin JA, Blaum BS, Gallagher JT, Uhrin D, Lyon M. The binding properties of minimal oligosaccharides reveal a common heparan sulfate/dermatan sulfate-binding site in hepatocyte growth factor/scatter factor that can accommodate a wide variety of sulfation patterns. J Biol Chem. 2009;284:6311–6321. doi: 10.1074/jbc.M807671200. [DOI] [PubMed] [Google Scholar]
- 75.Nikitovic D, Zafiropoulos A, Tzanakakis GN, Karamanos NK, Tsatsakis AM. Effects of glycosaminoglycans on cell proliferation of normal osteoblasts and human osteosarcoma cells depend on their type and fine chemical compositions. Anticancer Res. 2005;25:2851–2856. [PubMed] [Google Scholar]
- 76.Denholm EM, Lin YQ, Silver PJ. Anti-tumor activities of chondroitinase AC and chondroitinase B: inhibition of angiogenesis, proliferation and invasion. Eur J Pharmacol. 2001;416:213–221. doi: 10.1016/s0014-2999(01)00884-6. [DOI] [PubMed] [Google Scholar]
- 77.Westergren-Thorsson G, Persson S, Isaksson A, Onnervik PO, Malmstrom A, Fransson LA. L-iduronate-rich glycosaminoglycans inhibit growth of normal fibroblasts independently of serum or added growth factors. Exp Cell Res. 1993;206:93–99. doi: 10.1006/excr.1993.1124. [DOI] [PubMed] [Google Scholar]
- 78.Heinegard D. Proteoglycans and more – from molecules to biology. Int J Exp Pathol. 2009;90:575–586. doi: 10.1111/j.1365-2613.2009.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ricciardelli C, Sakko AJ, Ween MP, Russell DL, Horsfall DJ. The biological role and regulation of versican levels in cancer. Cancer Metastasis Rev. 2009;28:233–245. doi: 10.1007/s10555-009-9182-y. [DOI] [PubMed] [Google Scholar]
- 80.Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- 81.Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- 82.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 83.Coster L, Fransson LA, Sheehan J, Nieduszynski IA, Phelps CF. Self-association of dermatan sulphate proteoglycans from bovine sclera. Biochem J. 1981;197:483–490. doi: 10.1042/bj1970483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neill T, Schaefer L, Iozzo RV. Decorin: a guardian from the matrix. Am J Pathol. 2012;181:380–387. doi: 10.1016/j.ajpath.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quentin E, Gladen A, Roden L, Kresse H. A genetic defect in the biosynthesis of dermatan sulfate proteoglycan: galactosyltransferase I deficiency in fibroblasts from a patient with a progeroid syndrome. Proc Natl Acad Sci USA. 1990;87:1342–1346. doi: 10.1073/pnas.87.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fransson LA, Coster L, Malmstrom A, Sheehan JK. Self-association of scleral proteodermatan sulfate. Evidence for interaction via the dermatan sulfate side chains. J Biol Chem. 1982;257:6333–6338. [PubMed] [Google Scholar]
- 87.Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302:527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moreno M, Munoz R, Aroca F, Labarca M, Brandan E, Larrain J. Biglycan is a new extracellular component of the chordin-BMP4 signaling pathway. EMBO J. 2005;24:1397–1405. doi: 10.1038/sj.emboj.7600615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schonherr E, Hausser H, Beavan L, Kresse H. Decorin-type I collagen interaction. Presence of separate core protein-binding domains. J Biol Chem. 1995;270:8877–8883. doi: 10.1074/jbc.270.15.8877. [DOI] [PubMed] [Google Scholar]
- 90.Wang B, Li GX, Zhang SG, Wang Q, Wen YG, Tang HM, Zhou CZ, Xing AY, Fan JW, Yan DW, et al. Biglycan expression correlates with aggressiveness and poor prognosis of gastric cancer. Exp Biol Med (Maywood) 2011;236:1247–1253. doi: 10.1258/ebm.2011.011124. [DOI] [PubMed] [Google Scholar]
- 91.Yamamoto K, Ohga N, Hida Y, Maishi N, Kawamoto T, Kitayama K, Akiyama K, Osawa T, Kondoh M, Matsuda K, et al. Biglycan is a specific marker and an autocrine angiogenic factor of tumour endothelial cells. Br J Cancer. 2012;106:1214–1223. doi: 10.1038/bjc.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Westergren-Thorsson G, Hernnas J, Sarnstrand B, Oldberg A, Heinegard D, Malmstrom A. Altered expression of small proteoglycans, collagen, and transforming growth factor-beta 1 in developing bleomycin-induced pulmonary fibrosis in rats. J Clin Invest. 1993;92:632–637. doi: 10.1172/JCI116631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hou S, Maccarana M, Min TH, Strate I, Pera EM. The secreted serine protease xHtrA1 stimulates long-range FGF signaling in the early Xenopus embryo. Dev Cell. 2007;13:226–241. doi: 10.1016/j.devcel.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 94.Kappler J, Junghans U, Koops A, Stichel CC, Hausser HJ, Kresse H, Muller HW. Chondroitin/dermatan sulphate promotes the survival of neurons from rat embryonic neocortex. Eur J Neurosci. 1997;9:306–318. doi: 10.1111/j.1460-9568.1997.tb01401.x. [DOI] [PubMed] [Google Scholar]
- 95.Young MF, Bi Y, Ameye L, Chen XD. Biglycan knockout mice: new models for musculoskeletal diseases. Glycoconj J. 2002;19:257–262. doi: 10.1023/A:1025336114352. [DOI] [PubMed] [Google Scholar]
- 96.Xu T, Bianco P, Fisher LW, Longenecker G, Smith E, Goldstein S, Bonadio J, Boskey A, Heegaard AM, Sommer B, et al. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet. 1998;20:78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- 97.Johnson HJ, Rosenberg L, Choi HU, Garza S, Hook M, Neame PJ. Characterization of epiphycan, a small proteoglycan with a leucine-rich repeat core protein. J Biol Chem. 1997;272:18709–18717. doi: 10.1074/jbc.272.30.18709. [DOI] [PubMed] [Google Scholar]
- 98.Johnson J, Shinomura T, Eberspaecher H, Pinero G, Decrombrugghe B, Hook M. Expression and localization of PG-Lb/epiphycan during mouse development. Dev Dyn. 1999;216:499–510. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<499::AID-DVDY18>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 99.McCormick D, van der Rest M, Goodship J, Lozano G, Ninomiya Y, Olsen BR. Structure of the glycosaminoglycan domain in the type IX collagen-proteoglycan. Proc Natl Acad Sci USA. 1987;84:4044–4048. doi: 10.1073/pnas.84.12.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koch M, Bohrmann B, Matthison M, Hagios C, Trueb B, Chiquet M. Large and small splice variants of collagen XII: differential expression and ligand binding. J Cell Biol. 1995;130:1005–1014. doi: 10.1083/jcb.130.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Watt SL, Lunstrum GP, McDonough AM, Keene DR, Burgeson RE, Morris NP. Characterization of collagen types XII and XIV from fetal bovine cartilage. J Biol Chem. 1992;267:20093–20099. [PubMed] [Google Scholar]
- 102.Agarwal P, Zwolanek D, Keene DR, Schulz JN, Blumbach K, Heinegard D, Zaucke F, Paulsson M, Krieg T, Koch M, et al. Collagen XII and XIV, new partners of cartilage oligomeric matrix protein in the skin extracellular matrix suprastructure. J Biol Chem. 2012;287:22549–22559. doi: 10.1074/jbc.M111.335935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bilandzic M, Stenvers KL. Reprint of: betaglycan: a multifunctional accessory. Mol Cell Endocrinol. 2012;359:13–22. doi: 10.1016/j.mce.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 104.Andres JL, Ronnstrand L, Cheifetz S, Massague J. Purification of the transforming growth factor-beta (TGF-beta) binding proteoglycan betaglycan. J Biol Chem. 1991;266:23282–23287. [PubMed] [Google Scholar]
- 105.Gatza CE, Oh SY, Blobe GC. Roles for the type III TGF-beta receptor in human cancer. Cell Signal. 2010;22:1163–1174. doi: 10.1016/j.cellsig.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, Vale W. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature. 2000;404:411–414. doi: 10.1038/35006129. [DOI] [PubMed] [Google Scholar]
- 107.Teng YH, Aquino RS, Park PW. Molecular functions of syndecan-1 in disease. Matrix Biol. 2012;31:3–16. doi: 10.1016/j.matbio.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee PH, Trowbridge JM, Taylor KR, Morhenn VB, Gallo RL. Dermatan sulfate proteoglycan and glycosaminoglycan synthesis is induced in fibroblasts by transfer to a three-dimensional extracellular environment. J Biol Chem. 2004;279:48640–48646. doi: 10.1074/jbc.M407241200. [DOI] [PubMed] [Google Scholar]
- 109.Deepa SS, Yamada S, Zako M, Goldberger O, Sugahara K. Chondroitin sulfate chains on syndecan-1 and syndecan-4 from normal murine mammary gland epithelial cells are structurally and functionally distinct and cooperate with heparan sulfate chains to bind growth factors. A novel function to control binding of midkine, pleiotrophin, and basic fibroblast growth factor. J Biol Chem. 2004;279:37368–37376. doi: 10.1074/jbc.M403031200. [DOI] [PubMed] [Google Scholar]
- 110.Cluff AH, Bystrom B, Klimaviciute A, Dahlqvist C, Cebers G, Malmstrom A, Ekman-Ordeberg G. Prolonged labour associated with lower expression of syndecan 3 and connexin 43 in human uterine tissue. Reprod Biol Endocrinol. 2006;4:24. doi: 10.1186/1477-7827-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hjelm CA, Malmstrom A, Tingaker B, David G, Ekman-Ordeberg G. Normal labor associated with changes in uterine heparan sulfate proteoglycan expression and localization. Acta Obstet Gynecol Scand. 2005;84:217–224. doi: 10.1111/j.0001-6349.2005.00484.x. [DOI] [PubMed] [Google Scholar]
- 112.Nakanishi T, Kadomatsu K, Okamoto T, Ichihara-Tanaka K, Kojima T, Saito H, Tomoda Y, Muramatsu T. Expression of syndecan-1 and -3 during embryogenesis of the central nervous system in relation to binding with midkine. J Biochem. 1997;121:197–205. [PubMed] [Google Scholar]
- 113.Roskams T, De Vos R, David G, Van Damme B, Desmet V. Heparan sulphate proteoglycan expression in human primary liver tumours. J Pathol. 1998;185:290–297. doi: 10.1002/(SICI)1096-9896(199807)185:3<290::AID-PATH91>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 114.Munoz R, Moreno M, Oliva C, Orbenes C, Larrain J. Syndecan-4 regulates non-canonical Wnt signalling and is essential for convergent and extension movements in Xenopus embryos. Nat Cell Biol. 2006;8:492–500. doi: 10.1038/ncb1399. [DOI] [PubMed] [Google Scholar]
- 115.Matthews HK, Marchant L, Carmona-Fontaine C, Kuriyama S, Larrain J, Holt MR, Parsons M, Mayor R. Directional migration of neural crest cells in vivo is regulated by syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development. 2008;135:1771–1780. doi: 10.1242/dev.017350. [DOI] [PubMed] [Google Scholar]
- 116.Kuriyama S, Mayor R. A role for syndecan-4 in neural induction involving ERK- and PKC-dependent pathways. Development. 2009;136:575–584. doi: 10.1242/dev.027334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, Goetinck P. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest. 2001;107:R9–R14. doi: 10.1172/JCI10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Beauvais DM, Rapraeger AC. Syndecans in tumor cell adhesion and signaling. Reprod Biol Endocrinol. 2004;2:3. doi: 10.1186/1477-7827-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zoller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 120.Price MA, Colvin Wanshura LE, Yang J, Carlson J, Xiang B, Li G, Ferrone S, Dudek AZ, Turley EA, McCarthy JB. CSPG4, a potential therapeutic target, facilitates malignant progression of melanoma. Pigment Cell Melanoma Res. 2011;24:1148–1157. doi: 10.1111/j.1755-148X.2011.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stallcup WB. The NG2 proteoglycan: past insights and future prospects. J Neurocytol. 2002;31:423–435. doi: 10.1023/a:1025731428581. [DOI] [PubMed] [Google Scholar]
- 122.Franco CR, Trindade ES, Rocha HA, da Silveira RB, Paludo KS, Chammas R, Veiga SS, Nader HB, Dietrich CP. Glycosaminoglycan chains from alpha5beta1 integrin are involved in fibronectin-dependent cell migration. Biochem Cell Biol. 2009;87:677–686. doi: 10.1139/o09-047. [DOI] [PubMed] [Google Scholar]
- 123.Zachary IC. How neuropilin-1 regulates receptor tyrosine kinase signalling: the knowns and known unknowns. Biochem Soc Trans. 2011;39:1583–1591. doi: 10.1042/BST20110697. [DOI] [PubMed] [Google Scholar]
- 124.Varga I, Hutoczki G, Szemcsak CD, Zahuczky G, Toth J, Adamecz Z, Kenyeres A, Bognar L, Hanzely Z, Klekner A. Brevican, neurocan, tenascin-C and versican are mainly responsible for the invasiveness of low-grade astrocytoma. Pathol Oncol Res. 2012;18:413–420. doi: 10.1007/s12253-011-9461-0. [DOI] [PubMed] [Google Scholar]
- 125.Ida M, Shuo T, Hirano K, Tokita Y, Nakanishi K, Matsui F, Aono S, Fujita H, Fujiwara Y, Kaji T, et al. Identification and functions of chondroitin sulfate in the milieu of neural stem cells. J Biol Chem. 2006;281:5982–5991. doi: 10.1074/jbc.M507130200. [DOI] [PubMed] [Google Scholar]
- 126.Muller S, Kunkel P, Lamszus K, Ulbricht U, Lorente GA, Nelson AM, von Schack D, Chin DJ, Lohr SC, Westphal M, et al. A role for receptor tyrosine phosphatase zeta in glioma cell migration. Oncogene. 2003;22:6661–6668. doi: 10.1038/sj.onc.1206763. [DOI] [PubMed] [Google Scholar]
- 127.Adamsky K, Schilling J, Garwood J, Faissner A, Peles E. Glial tumor cell adhesion is mediated by binding of the FNIII domain of receptor protein tyrosine phosphatase beta (RPTPbeta) to tenascin C. Oncogene. 2001;20:609–618. doi: 10.1038/sj.onc.1204119. [DOI] [PubMed] [Google Scholar]
- 128.Feng ZJ, Gao SB, Wu Y, Xu XF, Hua X, Jin GH. Lung cancer cell migration is regulated via repressing growth factor PTN/RPTP beta/zeta signaling by menin. Oncogene. 2010;29:5416–5426. doi: 10.1038/onc.2010.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hu B, Kong LL, Matthews RT, Viapiano MS. The proteoglycan brevican binds to fibronectin after proteolytic cleavage and promotes glioma cell motility. J Biol Chem. 2008;283:24848–24859. doi: 10.1074/jbc.M801433200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Viapiano MS, Hockfield S, Matthews RT. BEHAB/brevican requires ADAMTS-mediated proteolytic cleavage to promote glioma invasion. J Neurooncol. 2008;88:261–272. doi: 10.1007/s11060-008-9575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Frischknecht R, Seidenbecher CI. Brevican: a key proteoglycan in the perisynaptic extracellular matrix of the brain. Int J Biochem Cell Biol. 2012;44:1051–1054. doi: 10.1016/j.biocel.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 132.Thinakaran G, Slunt HH, Sisodia SS. Novel regulation of chondroitin sulfate glycosaminoglycan modification of amyloid precursor protein and its homologue, APLP2. J Biol Chem. 1995;270:16522–16525. doi: 10.1074/jbc.270.28.16522. [DOI] [PubMed] [Google Scholar]
- 133.Iozzo RV, Zoeller JJ, Nystrom A. Basement membrane proteoglycans: modulators par excellence of cancer growth and angiogenesis. Mol Cells. 2009;27:503–513. doi: 10.1007/s10059-009-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aviezer D, Hecht D, Safran M, Eisinger M, David G, Yayon A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. 1994;79:1005–1013. doi: 10.1016/0092-8674(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 135.Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med. 2011;15:1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ghiselli G, Iozzo RV. Overexpression of bamacan/SMC3 causes transformation. J Biol Chem. 2000;275:20235–20238. doi: 10.1074/jbc.C000213200. [DOI] [PubMed] [Google Scholar]
- 137.Lauer M, Scruggs B, Chen S, Wassenhove-McCarthy D, McCarthy KJ. Leprecan distribution in the developing and adult kidney. Kidney Int. 2007;72:82–91. doi: 10.1038/sj.ki.5002269. [DOI] [PubMed] [Google Scholar]
- 138.Harris A, Harris H, Hollingsworth MA. Complete suppression of tumor formation by high levels of basement membrane collagen. Mol Cancer Res. 2007;5:1241–1245. doi: 10.1158/1541-7786.MCR-07-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chang MY, Chan CK, Braun KR, Green PS, O'Brien KD, Chait A, Day AJ, Wight TN. Monocyte-to-macrophage differentiation: synthesis and secretion of a complex extracellular matrix. J Biol Chem. 2012;287:14122–14135. doi: 10.1074/jbc.M111.324988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tanaka K, Arao T, Tamura D, Aomatsu K, Furuta K, Matsumoto K, Kaneda H, Kudo K, Fujita Y, Kimura H, et al. SRPX2 is a novel chondroitin sulfate proteoglycan that is overexpressed in gastrointestinal cancer. PLoS ONE. 2012;7:e27922. doi: 10.1371/journal.pone.0027922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bechard D, Scherpereel A, Hammad H, Gentina T, Tsicopoulos A, Aumercier M, Pestel J, Dessaint JP, Tonnel AB, Lassalle P. Human endothelial-cell specific molecule-1 binds directly to the integrin CD11a/CD18 (LFA-1) and blocks binding to intercellular adhesion molecule-1. J Immunol. 2001;167:3099–3106. doi: 10.4049/jimmunol.167.6.3099. [DOI] [PubMed] [Google Scholar]
- 142.Scherpereel A, Gentina T, Grigoriu B, Senechal S, Janin A, Tsicopoulos A, Plenat F, Bechard D, Tonnel AB, Lassalle P. Overexpression of endocan induces tumor formation. Cancer Res. 2003;63:6084–6089. [PubMed] [Google Scholar]
- 143.Roll S, Seul J, Paulsson M, Hartmann U. Testican-1 is dispensable for mouse development. Matrix Biol. 2006;25:373–381. doi: 10.1016/j.matbio.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 144.Nakada M, Miyamori H, Yamashita J, Sato H. Testican 2 abrogates inhibition of membrane-type matrix metalloproteinases by other testican family proteins. Cancer Res. 2003;63:3364–3369. [PubMed] [Google Scholar]
- 145.Nakada M, Yamada A, Takino T, Miyamori H, Takahashi T, Yamashita J, Sato H. Suppression of membrane-type 1 matrix metalloproteinase (MMP)-mediated MMP-2 activation and tumor invasion by testican 3 and its splicing variant gene product, N-Tes. Cancer Res. 2001;61:8896–8902. [PubMed] [Google Scholar]
- 146.Zhuo L, Salustri A, Kimata K. A physiological function of serum proteoglycan bikunin: the chondroitin sulfate moiety plays a central role. Glycoconj J. 2002;19:241–247. doi: 10.1023/A:1025331929373. [DOI] [PubMed] [Google Scholar]