Improved availability of antiretroviral therapy (ART) has transformed pediatric HIV infection in sub-Saharan Africa from a rapidly fatal to a treatable disease; saving tens of thousands of lives.1
Clinical trials have been conducted to determine optimal ART regimens with careful attention being given to evaluating the efficacy of protease inhibitors (PI) versus non-nucleoside reverse transcriptase inhibitors (NNRTI) for paediatric first line regimens.2–4 Relatively little attention has been given to the nucleoside reverse transcriptase inhibitor (NRTI) “backbone” of the regimens. South African guidelines recommend treatment with ritonavir-boosted lopinavir (LPV/r)-based regimens for children under 3 years of age and efavirenz (EFV)-based regimens for children older than 3 years of age. Lamivudine (3TC) and stavudine (d4T) were initially used as standard first-line NRTI backbone. In 2010, following WHO recommendations,5,6 South African guidelines replaced d4T with abacavir (ABC) due to concerns around toxicity. We were unable to find evidence comparing ABC-containing to other NRTI-containing regimens in similar pediatric populations. The closest comparison comes from the PENTA-5 trial demonstrating better virological suppression on a nelfinavir-based regimen for ABC relative to zidovudine (AZT) in older children.7,8 Suppression rates with d4T/3TC and NNRTI (88–92% at six months; 87–90% at twelve months) or PI-based therapy (63–78% at six months; 78–91% at twelve months) have been documented in South African cohorts.9–11
This analysis was undertaken in response to clinicians raising concerns about viral suppression after the introduction of ABC for first-line ART. We investigated virological outcomes among children receiving different starting regimens using routinely-collected program data.
METHODS
Empilweni clinic at Rahima Moosa Mother and Child Hospital (RMMCH) is a large pediatric HIV treatment centre in Johannesburg, South Africa, and has provided HIV services since 1995: over 1600 HIV-infected children are in active care. National guidelines are followed which require HIV viral load (VL) monitoring at 6 months and 12 months following ART initiation. VL monitoring was recommended 6 monthly during therapy but since 2010 only annual monitoring after the first year of treatment is recommended.6 No fixed dose combinations have been used at RMMCH. The formulations available during the period of observation included: d4T syrup and capsules; 3TC syrup and tablets; ABC syrup and tablets; EFV capsules and tablets and LPV/r syrup, soft gel capsules and tablets. Children starting ART before age four to five years were given only liquid formulations while older children able to swallow tablets/capsules were offered either formulation. Neither the formulation dispensed nor the exact dosages are currently electronically recorded.
Demographic and clinical details, laboratory results and ART initiation data are routinely recorded by clinicians in paper records and then captured electronically daily onto a Microsoft Access database. Data collection is a supervised process with daily and weekly data verification. Data are kept securely and may only be used for the purpose of research with appropriate IRB approval. De-identified data were analyzed using SAS (Version 9.3, SAS Institute Inc., Cary, NC, USA) software.
Data were extracted on the 28th June 2012 and all children initiating either d4T/3TC or ABC/3TC first-line regimens in combination with either EFV or LPV/r since the start of the program were included in the analysis provided they had initiated ART at least 6 months before the extraction date, allowing for at least 6 months of follow-up. Analyses were repeated restricting to children initiated from 2008 to limit the effects of temporal trends related to calendar time, e.g. smaller size of the clinic during 2004–2008. Variables used included first-line regimen as well as the following variables at ART initiation: sex, age, CD4 count and percentage, VL, weight- and height-for-age z-scores (WAZ, HAZ). Follow-up data included date and result of each VL test, defined as being at “6 months” and “12 months” if performed within windows of 4–7 and 8–14 months post ART initiation respectively. Loss to follow-up was defined as no clinic visit within 180 days of the last visit in the absence of death or transfer-out. The primary outcomes were virological suppression (<400 RNA copies/ml) at 6 and 12 months after treatment initiation.
VL measurements were with NucliSENS EasyQ HIV-1 version 1.2 (bioMérieu, Boxtel, Netherlands) from April 2004 to November 2009, then NucliSENS EasyQ HIV-1 version 2.0 (bioMérieu, Marcy L’Etoile, France) until September 2010, thereafter COBAS AmpliPrep/COBAS TaqMan HIV-1 test (Roche Molecular Systems, Inc., Branchburg, NJ, USA – personal communication, Dr Sergio Carmona, National Health Laboratory Service). These have a reported precision that lies within the reference window of 0.3 log10 copies/ml.
Categorical variables and the proportions suppressed at different time points were compared using Chi-squared tests. Continuous variables were compared across groups using t-tests if normally-distributed or otherwise Wilcoxon tests. Time to viral suppression (<400 copies/ml) within the first year of ART and time to rebound to >1000 copies/ml in the first year after initial suppression (defined as having occurred by the end of the “6 Month” window, i.e. by 8 months on ART) were described using Kaplan-Meier methods. Logistic regression was used to compare viral suppression rates at 6 and 12 months after ART adjusting for a priori-defined potential confounders, including calendar time, pre-treatment VL (stratified as ≥ 100,000 or < 100,000 copies/ml), CD4 percentage, WAZ, age, sex and missed visits (used as a proxy for adherence and defined as at least one episode of >100 days between consecutive visits within the first 12 months on ART). Missing data for CD4 percentage and WAZ were imputed using the mean values while dummy variables were created for missing pre-treatment VL and missed visits data.
Permission for the collection and analysis of routine clinic data has been obtained from the Human Research Ethics Committee of the University of the Witwatersrand (M090501).
RESULTS
Among 2423 children who initiated ART at RMMCH from April 2004 until 28 December 2011, 2036 (84%) were included and had initiated d4T/3TC+LPV/r (n=672); ABC/3TC+LPV/r (n=192); d4T/3TC+EFV (n=962) or ABC/3TC+EFV (n=210). The children excluded had initiated other regimens (n=387, including nevirapine, ritonavir, didanosine, zidovudine and ‘super-boosted’ LPV/r for concurrent rifampicin usage).
Pre-treatment characteristics of the children stratified by starting regimen are tabulated in Supplemental Digital Content 1. The data reflects the ABC-containing regimens being the more recent regimens. There were no differences in gender distribution but age at initiation was different in the EFV-based group with children initiating ABC-containing regimens being a median of 8 months older than those initiating d4T-containing regimens (p=0.005). Children who initiated ABC-containing regimens had marginally higher pre-treatment WAZ and HAZ scores (p=0.02 and 0.93 respectively) in the EFV-based group but significantly higher scores in the LPV/r group (p<0.0001). CD4 percentages were higher in children commenced on ABC-containing regimens but a difference in absolute CD4 counts was only evident in the EFV group (p=0.04). Pre-treatment VL was slightly higher in the d4T/LPV/r group (p=0.03) but not significantly different within the EFV and LPV/r groups in terms of proportion above 100,000 copies/ml. Children on EFV had lower VLs than those on LPV/r-based regimens (50 vs. 81% above 100,000 copies/ml; p<0.0001). Mortality, loss to follow-up and transfer out rates were similar between children given ABC and d4T stratified by LPV/r- or EFV-based treatment (Supplemental Digital Content 1, Table of Pre-treatment characteristics stratified by starting regimen).
At both 6 and 12 months, fewer children reached virological suppression and median VL logs were higher in children receiving ABC compared to d4T, in both the EFV and LPV/r-treated children (Table 1). In children treated with LPV/r-based regimens, 71% receiving d4T versus 40% receiving ABC had VL<400 copies/ml at 6 months (p<0.0001). Similarly, in those on EFV, 91% versus 67% had VL<400 copies/ml at 6 months when receiving d4T- versus ABC (p<0.0001). No significant changes to these findings were noted when using only data from 2008 onwards.
Sixty percent of all children who were eligible for VL measurement at 6 months had a VL done and 81% at 12 months. There were no significant differences in compliance with testing guidelines for the 6 month window between those receiving ABC or d4T. More ABC-treated children had yet to reach the 12 month testing window, but among those on ABC who had reached the 12 month window, proportionately more underwent the 12 month test (p=0.02 in EFV-based group, p=0.13 in LPV/r group – Table 1) suggesting a trend towards increased testing at 12 months in ABC-based, i.e. more recent regimens.
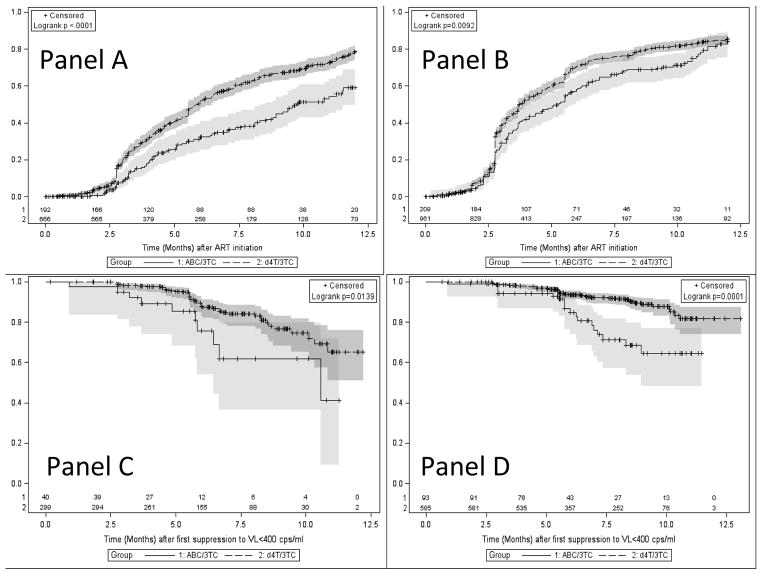
Time to viral suppression was significantly longer and time to viral rebound (>1000 copies/ml) after suppression shorter in the ABC-treated children for both LPV/r and EFV-based regimens (Figure 1). A stronger association was seen in the LPV/r-based regimens, where children on ABC had an almost 2-fold increased risk of failure to suppress (41% versus 21%, log-rank p<0.0001) by 12 months. Children receiving EFV had an almost 2-fold higher risk of rebound by 12 months after first suppression (35% versus 18%, log-rank p=0.0001) if they were on ABC compared to d4T (Figure 1).
Figure 1. Probability of achieving Viral suppression (<400 copies/ml) by 12 months stratifying by whether stavudine/lamivudine (d4T/3TC) or abacavir/lamivudine (ABC/3TC) was used among children initiating ritonavir-boosted lopinavir (LPV/r)-based therapy (Panel A) or efavirenz (EFV)-based therapy (Panel B) and Probability of maintaining suppression (<1000 copies/ml) stratifying by whether d4T/3TC or ABC/3TC was used among children initiating LPV/r-based therapy (Panel C) or EFV-based therapy (Panel D).
The shaded areas represent 95% confidence limits. Abbreviation: Viral Load (VL)
Adjusted multiple logistic regression analysis (Table 2) was performed using all data and then only data from 2008 onwards in order to limit the calendar effect but this did not significantly affect the results and therefore the complete model is shown. The strongest predictor of viral suppression at 12 months remained receiving d4T instead of ABC. Missed visits, a proxy for poorer adherence, showed a significant independent effect towards not suppressing at 12 months (stronger within LPV/r group p=0.0021 than within EFV group p=0.057).
DISCUSSION
These data demonstrate that children treated with ABC/3TC had a lower probability of viral suppression at 6 and 12 months and a higher probability of virological rebound than those treated with d4T/3TC in both LPV/r- and EFV-based regimens, even after adjustment for calendar time and other potential confounders. These results raise concern that the shift to ABC regimens may not be as virologically efficacious as d4T regimens in this pediatric HIV service.
The poorer virological outcomes in children on ABC/3TC were seen despite higher pre-treatment WAZ, HAZ and CD4 percentages. The differences were found with LPV/r- and EFV-based regimens. Within the LPV/r group of mostly younger children, the probable explanation for the improvement in pre-treatment characteristics is earlier diagnosis within an expanding and improving prevention-of-mother-to-child-transmission (PMTCT) service including early infant diagnosis in South Africa. This would lead us to expect better, not worse virological outcomes in the ABC group.10 However, earlier treatment may pose other challenges including those related to adherence.12
It is difficult to determine the reasons for the poorer performance of ABC-containing regimens in our setting. Since the change in regimen is almost entirely related to calendar time, we cannot exclude secular trends in the population characteristics of children. In particular, guideline changes on when to initiate therapy have occurred. ART initiation for infants is recommended upon diagnosis and initiation of older children earlier i.e. at higher pre-treatment CD4 counts and/or percentages than in earlier guidelines.6 Changes have also occurred in the recommended PMTCT regimens as well as improvements in coverage of these programs. In addition, programmatic changes over time such as increasing program size accompanied by less intensive follow-up and possibly less intensive adherence counseling cannot be excluded as reasons for poorer virological outcomes, yet the effect remains when limiting to data from 2008 onwards and adjusting for missed patient visits.
Intermittent brief interruptions of ABC supply occurred particularly towards the end of 2011 due to national procurement and supply problems. Drug stock-outs were managed on a case by case basis with drug or formulation substitutions, for example ABC syrup was used to replace tablets when these went out of stock. These changes may have resulted in confusion for caregivers leading to treatment interruptions and adherence challenges. Stock-outs of d4T have not been recorded at RMMCH since program inception. In addition, administering LPV/r to young children can be challenging due to poor palatability. Since d4T has a comparatively higher genetic barrier to resistance than ABC, d4T is theoretically better able to “tolerate” suboptimal adherence than ABC.13 While ABC and 3TC share the cross-resistant mutation, M184V, d4T and 3TC do not. In fact, d4T resistance may be delayed by 3TC resistance.13 For these reasons, children with treatment interruptions or suboptimal adherence on ABC/3TC may fare worse compared to those on d4T/3TC and potential M184V mutations selected for by PMTCT regimens may further compound this.14 This is especially of concern as a slightly stronger effect size was seen in the LPV/r-treated group, which represents the youngest children, possibly also exposed to the more recent multi-drug PMTCT regimens. The greater effect seen with LPV/r seems contrary to the superiority of LPV/r over NNRTI (nevirapine based) regimens reported recently in young children on AZT/3TC, though this trial compared the two regimens in younger children, while our data separates LPV/r in younger from EFV in older children.15 A possible explanation of the greater effect in LPV/r based regimens may be a potential drug interaction between ABC and LPV/r of as yet uncertain clinical significance.16
Given the short follow-up period presented in our analysis, it is not possible to determine whether the worse virological outcomes in the first year of treatment indicate lower absolute rates of suppression or simply slower time to suppression. Nevertheless, viral suppression is expected by 12 months on ART, even in infants. Failure to suppress within the first year of treatment in such a proportion of children is of concern and may influence longer term outcomes with reduced regimen options at later time points. More rapid evolution of resistance in a failing or non-suppressed ABC/3TC regimen described in the PENTA-5 trial is a further concern.17 More rapid rebound in the EFV-group (with lower genetic resistance threshold compared to LPV/r) may theoretically predispose children to higher rates of regimen failure and increased need for regimen switches.
Our analysis does not directly corroborate or refute findings from recently-published data showing poorer virological performance of ABC/3TC versus tenofovir/emtricitabine regimens among adults with higher pre-treatment VL (>100,000 copies/ml).18,19 It is important to note that 82% of children on LPV/r had pre-treatment VL ≥100,000 copies/ml. Other adult studies have found suboptimal performance of an ABC-containing triple NRTI regimen at higher pre-treatment VL levels when compared to AZT/3TC and a PI.20,21
A limitation and important concerning finding, was the low rate of VL testing in all treatment groups during the six month window, although rates improved by the twelve month window. With sub-optimal rates of VL testing we cannot rule out selection bias as a potential explanation for the findings. Other limitations include that these findings are from a single center with limited follow-up time related to the relatively recent introduction of ABC as well as an almost concurrent change in the technology used to determine VL. Nevertheless the consistency of the association in children treated with both EFV and LPV/r within a large public sector pediatric treatment centre in South Africa is notable. Formulations and exact doses issued were not captured and therefore the impact of formulation changes cannot be further assessed. Data on PMTCT exposure is incomplete and stratification by presence/absence of PMTCT and details of drug exposure is important but not possible in this study. Previous analyses in our setting have shown that 30–50% of infants starting ART did not have any previous PMTCT ART exposure.22
This study is a retrospective analysis of data from a non-trial setting with inherent limitations and it cannot be taken as a definitive comparison between ABC and d4T containing NRTI backbones. Children initiating ART in the same clinic at two different time periods are compared and we are unable to control for other ecological factors and unanticipated confounders. Electronic data capture has been ongoing since 2006 and data cleaning and verification systems are in place, but data quality is not that of a clinical trial given the resource constraints of the public health sector.
These results highlight the importance of routine VL measurements and close monitoring of children initiating ART. We recommend a return to 6 monthly monitoring after the first year of treatment particularly for children on ABC/3TC. We strongly recommend urgent review of data from other pediatric treatment sites to establish whether the lower virological effectiveness seen in our cohort exists elsewhere. We advise close monitoring of outcomes at clinical sites following any guideline changes. Further, a central surveillance system that tracks trends in virological suppression rates across multiple sites should be considered.
Supplementary Material
Acknowledgments
We would like to acknowledge all the children and their caregivers, whose data were used in this analysis. We acknowledge the work of Mr. Vincent Kgakgdi and his administrative team. The Empilweni Services and Research Unit receives support from the National Institutes of Health (National Institute of Allergy and Infectious Diseases and the Eunice Kennedy Shriver National Institute of Child Health and Human Development) through the IeDEA-Southern Africa collaboration (grant number U01AI069924) and also receives support from the President’s Emergency Plan for AIDS Relief (PEPFAR-RFA-A-00-08-00005-00). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Source of funding: KT – Institution receives NIH funding (IeDEA-Southern Africa, Grant Number 2U01 AI069924-06) and support by the President’s Emergency Plan for AIDS Relief (PEPFAR-RFA-A-00-08-00005-00). LK – Institution receives funding from NIH. GR – received honoraria from the South African Medical Association, Sanofi and Pfizer for activities unrelated to the submitted work. MD – Institution receives NIH funding (IeDEA-Southern Africa, Grant Number 2U01 AI069924-06) and WHO funding (APW: HQHIV1206639). AC – Institution receives funding from the NIH (IeDEA-Southern Africa, Grant Number 2U01 AI069924-06) and in the past from Abbott.
Footnotes
Supplemental Digital Content 1 Table of Pre-treatment characteristics stratified by starting regimen.doc
Conflict of interest: No conflict of interest declared for the remaining authors. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization, UNAIDS, UNICEF. Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report 2011. Geneva: World Health Organization; 2011. [Google Scholar]
- 2.Babiker A, Castro nee Green H, Compagnucci A, et al. First-line antiretroviral therapy with a protease inhibitor versus non-nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. Lancet Infect Dis. 2011;11:273–83. doi: 10.1016/S1473-3099(10)70313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coovadia A, Abrams EJ, Stehlau R, et al. Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial. JAMA. 2010;304:1082–90. doi: 10.1001/jama.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuhn L, Coovadia A, Strehlau R, et al. Switching children previously exposed to nevirapine to nevirapine-based treatment after initial suppression with a protease-inhibitor-based regimen: long-term follow-up of a randomised, open-label trial. Lancet Infect Dis. 2012;12:521–30. doi: 10.1016/S1473-3099(12)70051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Antiretroviral therapy for HIV infection in infants and children: towards universal access - 2010 revision. 2010. Geneva: World Health Organization; 2010. rev. ed. [PubMed] [Google Scholar]
- 6.National Department of Health. Guidelines for the Management of HIV in Children -2nd Edition 2010. 2010. Pretoria: National Department of Health; 2010. rev. ed. [Google Scholar]
- 7.Green H, Gibb DM, Walker AS, et al. Lamivudine/abacavir maintains virological superiority over zidovudine/lamivudine and zidovudine/abacavir beyond 5 years in children. AIDS. 2007;21:947–55. doi: 10.1097/QAD.0b013e3280e087e7. [DOI] [PubMed] [Google Scholar]
- 8.Welch S, Sharland M, Lyall EG, et al. PENTA 2009 guidelines for the use of antiretroviral therapy in paediatric HIV-1 infection. HIV Med. 2009;10:591–613. doi: 10.1111/j.1468-1293.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 9.Meyers TM, Yotebieng M, Kuhn L, Moultrie H. Antiretroviral therapy responses among children attending a large public clinic in Soweto, South Africa. Pediatr Infect Dis J. 2011;30:974–9. doi: 10.1097/INF.0b013e31822539f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies MA, Moultrie H, Eley B, et al. Virologic failure and second-line antiretroviral therapy in children in South Africa--the IeDEA Southern Africa collaboration. J Acquir Immune Defic Syndr. 2011;56:270–8. doi: 10.1097/QAI.0b013e3182060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zanoni BC, Phungula T, Zanoni HM, France H, Feeney ME. Impact of tuberculosis cotreatment on viral suppression rates among HIV-positive children initiating HAART. AIDS. 2011;25:49–55. doi: 10.1097/QAD.0b013e32833f9e04. [DOI] [PubMed] [Google Scholar]
- 12.Davies MA, Boulle A, Fakir T, Nuttall J, Eley B. Adherence to antiretroviral therapy in young children in Cape Town, South Africa, measured by medication return and caregiver self-report: a prospective cohort study. BMC Pediatr. 2008;8:34. doi: 10.1186/1471-2431-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson VA, Calvez V, Gunthard HF, et al. 2011 update of the drug resistance mutations in HIV-1. Top Antivir Med. 2011;19:156–64. [PMC free article] [PubMed] [Google Scholar]
- 14.Zeh C, Weidle PJ, Nafisa L, et al. HIV-1 drug resistance emergence among breastfeeding infants born to HIV-infected mothers during a single-arm trial of triple-antiretroviral prophylaxis for prevention of mother-to-child transmission: a secondary analysis. PLoS medicine. 2011;8:e1000430. doi: 10.1371/journal.pmed.1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Violari A, Lindsey JC, Hughes MD, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med. 2012;366:2380–9. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waters LJ, Moyle G, Bonora S, et al. Abacavir plasma pharmacokinetics in the absence and presence of atazanavir/ritonavir or lopinavir/ritonavir and vice versa in HIV-infected patients. Antivir Ther. 2007;12:825–30. [PubMed] [Google Scholar]
- 17.Gibb DM, Walker AS, Kaye S, et al. Evolution of antiretroviral phenotypic and genotypic drug resistance in antiretroviral-naive HIV-1-infected children treated with abacavir/lamivudine, zidovudine/lamivudine or abacavir/zidovudine, with or without nelfinavir (the PENTA 5 trial) Antivir Ther. 2002;7:293–303. [PubMed] [Google Scholar]
- 18.Sax PE, Tierney C, Collier AC, et al. Abacavir/lamivudine versus tenofovir DF/emtricitabine as part of combination regimens for initial treatment of HIV: final results. J Infect Dis. 2011;204:1191–201. doi: 10.1093/infdis/jir505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill A, Sawyer W. Effects of nucleoside reverse transcriptase inhibitor backbone on the efficacy of first-line boosted highly active antiretroviral therapy based on protease inhibitors: meta-regression analysis of 12 clinical trials in 5168 patients. HIV Med. 2009;10:527–35. doi: 10.1111/j.1468-1293.2009.00724.x. [DOI] [PubMed] [Google Scholar]
- 20.Staszewski S, Keiser P, Montaner J, et al. Abacavir-lamivudine-zidovudine vs indinavir-lamivudine-zidovudine in antiretroviral-naive HIV-infected adults: A randomized equivalence trial. JAMA. 2001;285:1155–63. doi: 10.1001/jama.285.9.1155. [DOI] [PubMed] [Google Scholar]
- 21.Kumar PN, Salvato P, Lamarca A, et al. A randomized, controlled trial of initial anti-retroviral therapy with abacavir/lamivudine/zidovudine twice-daily compared to atazanavir once-daily with lamivudine/zidovudine twice-daily in HIV-infected patients over 48 weeks (ESS100327, the ACTION Study) AIDS Res Ther. 2009;6:3. doi: 10.1186/1742-6405-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Technau K, Kalk E, Sherman GG, et al. Influx of Infants to HIV Treatment Services in the Context of Improvements in PMTCT. 15th International Workshop on HIV Observational Databases; 2011 24th to 26th March; Prague, Czech Republic. 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.