Abstract
Aims: Ischemia/reperfusion (I/R) injury is a common clinical problem. Although the pathophysiological mechanisms underlying I/R injury are unclear, oxidative damage is considered a key factor in the initiation of I/R injury. Findings from preclinical studies consistently show that quenching reactive oxygen and nitrogen species (RONS), thus limiting oxidative damage, alleviates I/R injury. Results from clinical intervention studies on the other hand are largely inconclusive. In this study, we systematically evaluated the release of established biomarkers of oxidative and nitrosative damage during planned I/R of the kidney and heart in a wide range of clinical conditions. Results: Sequential arteriovenous concentration differences allowed specific measurements over the reperfused organ in time. None of the biomarkers of oxidative and nitrosative damage (i.e., malondialdehyde, 15(S)-8-iso-prostaglandin F2α, nitrite, nitrate, and nitrotyrosine) were released upon reperfusion. Cumulative urinary measurements confirmed plasma findings. As of these negative findings, we tested for oxidative stress during I/R and found activation of the nuclear factor erythroid 2-related factor 2 (Nrf2), the master regulator of oxidative stress signaling. Innovation: This comprehensive, clinical study evaluates the role of RONS in I/R injury in two different human organs (kidney and heart). Results show oxidative stress, but do not provide evidence for oxidative damage during early reperfusion, thereby challenging the prevailing paradigm on RONS-mediated I/R injury. Conclusion: Findings from this study suggest that the contribution of oxidative damage to human I/R may be less than commonly thought and propose a re-evaluation of the mechanism of I/R. Antioxid. Redox Signal. 19, 535–545.
Introduction
Ischemia/reperfusion injury (I/R) is the paradoxical increase of tissue damage upon reperfusion of ischemic tissue. I/R is considered a major contributor to tissue damage in multiple clinical situations such as myocardial infarction, stroke, and organ transplantation. The pathophysiology of I/R injury is complex and incompletely understood, and effective treatment is currently lacking.
Reactive oxygen and nitrogen species (RONS) are considered key initiators of I/R injury. Ischemia-related metabolic adaptations and dysregulated mitochondrial homeostasis are thought to result in substantial RONS release upon reintroduction of oxygen. This RONS overload can overwhelm the endogenous antioxidant system, resulting in oxidative damage. This may trigger secondary processes such as a proinflammatory response (7, 18, 32, 44).
It has long been supposed that antioxidant therapy mitigates I/R injury. The validity of this concept has been proven in numerous animal studies, which all clearly demonstrate that antioxidant therapy ameliorates I/R injury (2, 26, 46). Despite these findings, studies in humans consistently fail to show any clinically relevant effect (3, 15, 28, 39, 46). The basis for this discrepancy between human and animal studies is still unclear, yet it may suggest that the contribution of RONS to I/R injury in humans may be less than commonly thought.
Innovation.
Ischemia/reperfusion (I/R) injury is a common clinical problem, complicating myocardial infarction, cardiovascular surgery, and organ transplantation. As such, it is of great importance to unravel the exact pathophysiological mechanisms leading to I/R injury. This comprehensive clinical study systematically assessed the putative role of oxidative stress in human I/R injury in two diverse clinical settings. Findings for all biomarkers studied were highly consistent and did not indicate release ofmarkers of oxidative damage from the reperfused organs nor did we observe upregulation of redox-response genes. As such, this study questions the involvement of reactive oxygen and nitrogen species in the pathophysiology of I/R injury in humans.
In this study, a double approach was used to evaluate the role of RONS in clinical I/R injury. First, release of established biomarkers of oxidative damage from the human kidney and heart was assessed during planned I/R. By cannulation of the efferent vein, that is, the renal vein during kidney transplantation and the coronary sinus during cardiac valve surgery, sensitive and organ-specific measurements of net changes in oxidative damage biomarkers over the organ were conducted. Next, as none of the biomarkers were released during the reperfusion phase, we tested for the presence of oxidative stress during early reperfusion, which did show activation of the nuclear factor erythroid 2-related factor 2 (Nrf2), a critical transcription factor in oxidative stress signaling. All together, results from these studies, comprising different organs, clinical settings, and durations of ischemia, are highly consistent and do indicate oxidative stress, but no RONS-related damage during the acute reperfusion phase in humans, suggesting that the endogenous antioxidant system is able to cope with I/R-related oxidative stress.
Results
Patient characteristics and outcome
Patient characteristics for kidney transplantation and cardiac valve surgery are summarized in Tables 1 and 2, respectively. One-year patient and graft survival was 100% for the patients who underwent kidney transplantation. One-year survival was also 100% for all patients who underwent cardiac valve surgery.
Table 1.
Transplantation and Outcome Characteristics in Living Donor and Deceased Donor Kidney Transplantations
| LD | DD | p-value | |
|---|---|---|---|
| n | 8 | 16 | |
| Recipient age (years), mean±SD | 41.1±10.5 | 54.6±12.2 | 0.02 |
| Recipient gender, (% male) | 38 (n=3) | 56 (n=9) | 0.39 |
| Cause renal failure | |||
| ADPKD | 2 (25%) | 4 (25%) | |
| Renal fibrosis | 2 (25%) | 1 (6%) | |
| MPG | 1 (13%) | 1 (6%) | |
| IgA nephropathy | 1 (13%) | 1 (6%) | |
| BM nephropathy | 0 | 2 (13%) | |
| Hypertension | 0 | 2 (13%) | |
| FSG | 0 | 1 (6%) | |
| Unknown | 2 (25%) | 4 (25%) | |
| Donor age (years), mean±SD | 43.9±10.6 | 53.5±16.1 | 0.14 |
| Donor gender, (% male) | 75 (n=6) | 44 (n=7) | 0.15 |
| Cause of death donor | N/A | ||
| BDD: trauma | 3 (19%) | ||
| BDD: SAH | 2 (13%) | ||
| BDD: hypoxic | 3 (19%) | ||
| CDD: traumatic brain injury | 2 (13%) | ||
| CDD: intracranial hemorrhage | 4 (25%) | ||
| CDD: SAH | 2 (13%) | ||
| Preservation fluids | HTK (n=8) | UW (n=11) | 0.001 |
| HTK (n=5) | |||
| CIT (min), mean±SD | 179.1±18.6 | 1117.7±299.1 | <0.001 |
| WIT (min), mean±SD | 34.0±6.3 | 33.5±6.1 | 0.85 |
ADPKD, autosomal dominant polycystic kidney disease; BDD, brain-dead donor; BM, basement membrane; CDD, cardiac-dead donor; CIT, cold ischemia time; FSG, focal segmental glomerulosclerosis; HTK, histidine–tryptophan–ketoglutarate; MPG, membranous glomerulonephritis; SAH, subarachnoid hemorrhage; UW, University of Wisconsin; WIT, warm ischemia time; DD, deceased donor; LD, living donor; SD, standard deviation.
Table 2.
Characteristics of Patients With and Without Preexisting Heart Failure
| Heart failure | No heart failure | p-value | |
|---|---|---|---|
| n | 12 | 12 | |
| Gender, (% male) | 75 (n=9) | 42 (n=5) | 0.11 |
| Age (years) | 66.75±8.91 | 62.68±11.65 | 0.20 |
| Mean±SD | |||
| Ischemia time (min) | 128.25±50.66 | 127.00±32.62 | 0.89 |
| Mean±SD | |||
| Duration ICU stay (h)a | 62.50±40.75 | 31.58±23.90 | 0.02 |
| Mean±SD | |||
Duration ICU stay: the postoperative period that patients were admitted to the intensive care unit.
Validation of arteriovenous measurements
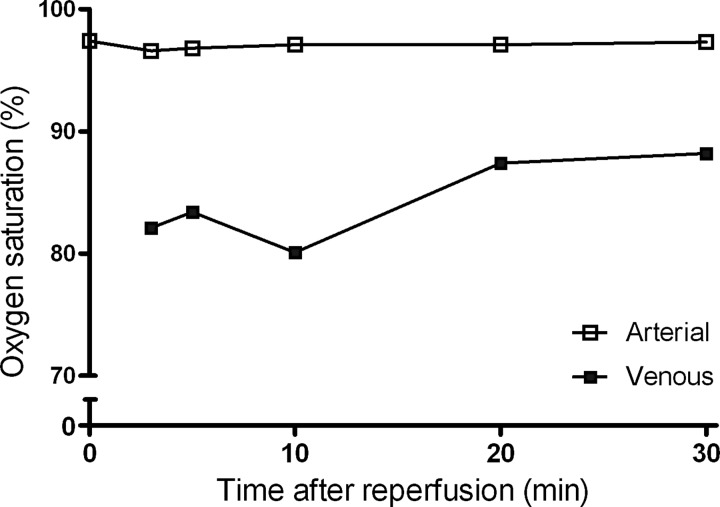
The methodology of the arteriovenous sampling is schematically shown in Figure 1. The principle of the method is illustrated by arterial and venous oxygen saturation levels measured during early reperfusion, showing an effective and consistent consumption of oxygen (Fig. 2).
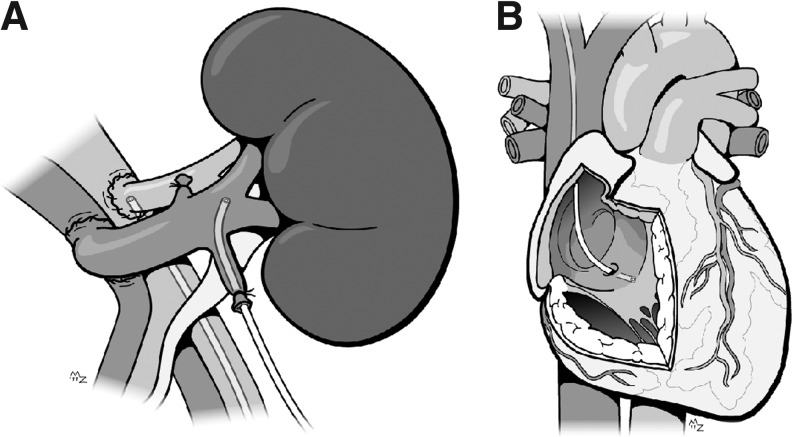
FIG. 1.
Schematic representation of the arteriovenous measurements of the kidney (A) and heart (B). The figure shows the position of the arterial and renal venous catheter in the kidney, and the coronary sinus catheter in the heart. ©ManonProject.com. Printed with permission of the artist.
FIG. 2.
A typical example of arteriovenous oxygen saturation differences during reperfusion of the kidney. Arterial and renal venous oxygen saturation was measured during the first 30 min of reperfusion. The consistently lower venous oxygen saturation level indicates the uptake of oxygen by the reperfused kidney, demonstrating its immediate metabolic activity.
Biomarkers of oxidative damage
Renal I/R is not associated with oxidative damage
Malondialdehyde (MDA) and 15(S)-8-iso-prostaglandin F2α (15(S)-8-iso-PGF2α) were measured as established biomarkers of RONS-mediated damage. Arteriovenous measurements of MDA did not indicate its release from the kidney during the first 30 min of reperfusion of living donor (LD) kidneys by either gas chromatography–mass spectrometry (GC-MS) (p=0.17; Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ars) or the thiobarbituric acid-reactive substances (TBARS) method (p=0.06). Urinary MDA levels (TBARS method) were also assessed in the first urine produced immediately after reperfusion (LDs only) as an aggregate measure of total MDA formation in the kidney. The MDA levels in this first urine collected after reperfusion were similar to the control urine, that is, urine produced by the kidney before its removal from the donor (p=0.40).
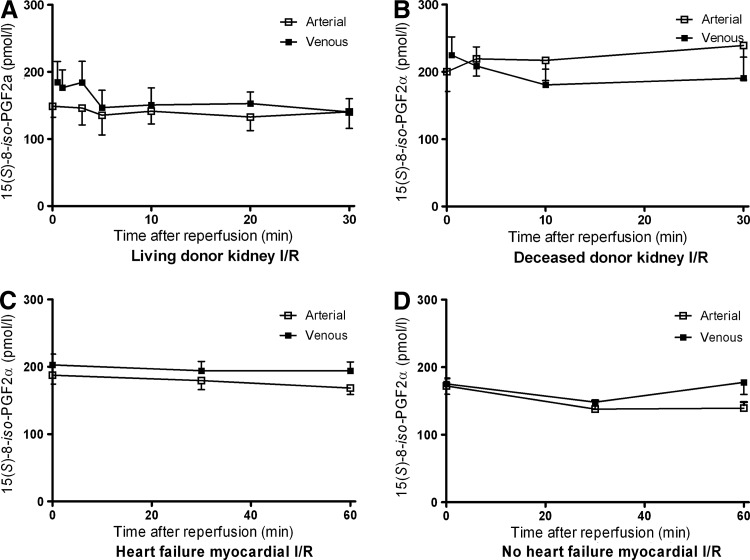
Minimal release was observed for 15(S)-8-iso-PGF2α from reperfused LD kidney grafts (p=0.03). However, clinically more vulnerable deceased donor (DD) kidneys did not show any 15(S)-8-iso-PGF2α release upon reperfusion (p=0.16; Fig. 3). Arteriovenous measurements over the nonischemic kidney in LDs, that is, before donor nephrectomy, revealed no release of 15(S)-8-iso-PGF2α (p=0.69), demonstrating that its postreperfusion release is specific for I/R. Urinary 15(S)-8-iso-PGF2α levels were not influenced by reperfusion (p=0.35; Table 3).
FIG. 3.
Arterial and venous plasma concentrations of 15(S)-8-iso-prostaglandin F2α. (A) Arterial and renal venous plasma 15(S)-8-iso-PGF2α concentrations during the first 30 min of reperfusion in living donor (LD) kidney transplantation showed a small, but significant release from the kidney (p=0.03); (B) In deceased donor (DD) kidney transplantation, 15(S)-8-iso-PGF2α was not released from the reperfused kidney during the first 30 min of reperfusion (p=0.16); (C) Arterial and myocardial venous plasma 15(S)-8-iso-PGF2α concentrations during the first 60 min of reperfusion in patients with preexisting heart failure showed a small, but significant release from the myocardium (p=0.02); (D) In patients without preexisting heart failure, 15(S)-8-iso-PGF2α was not released from the reperfused heart during the first 60 min of reperfusion (p=0.18).
Table 3.
AUC Venous Minus AUC Arterial (Delta AUC) of the Measured Plasma Biomarkers for Oxidative and Nitrosative Damage During the Early Reperfusion Phase (p Values)
| |
Kidney transplantation |
Cardiac valve surgery |
|||
|---|---|---|---|---|---|
| Delta AUCa | LD | DD | LD urineb | Heart failure | No heart failure |
| Oxidative damage | |||||
| Malondialdehyde (nM×min) | 138.2 | Not measured | −4544 | 1758 | |
| (p value) | (0.17) | (0.40) | (0.05) | (0.43) | |
| 15(S)-8-iso-PGF2α (pM×min) | 356 | −1306 | 1056 | 934 | |
| (p value) | (0.03c) | (0.16) | (0.35) | (0.02c) | (0.18) |
| Nitrosative stress and damage | |||||
| Nitrite (nM×min) | 308 | −910 | −6949 | −5878 | |
| (p value) | (0.50) | (0.87) | (0.06) | (0.002) | (0.03) |
| Nitrate (μM×min) | −36 | 77 | −14 | 75 | |
| (p value) | (0.50) | (0.98) | (0.69) | (0.53) | (0.81) |
| Nitrotyrosine (nM×min) | −113 | Not measured | Not measured | Not measured | Not measured |
| (p value) | (0.93) | ||||
Delta AUC: AUC venous minus AUC arterial (p value) of the measured plasma markers during the early reperfusion phase. A positive number indicates a release from the reperfused organ, whereas a negative number indicates an uptake of the biomarker.
Results in urine indicate p-values of the difference of the pre- and postreperfusion measure.
Significant venous release (p<0.05).
AUC, area under the curve.
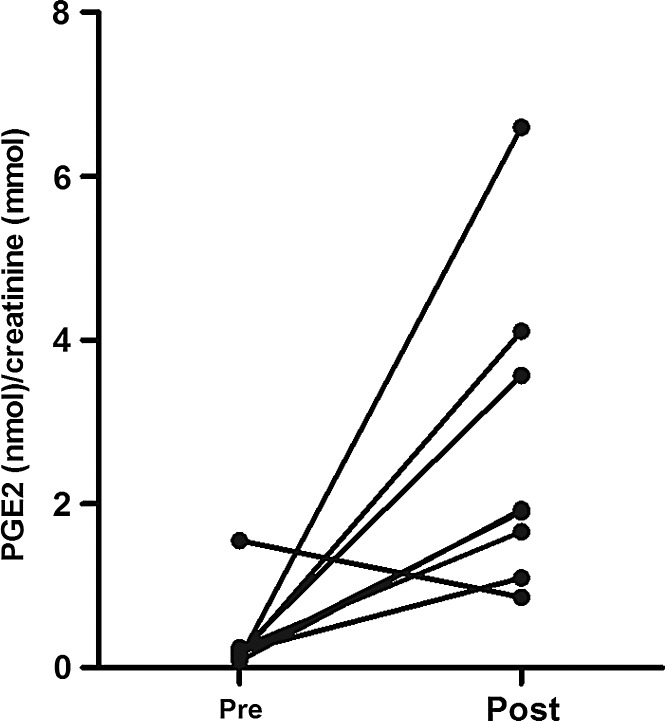
Urinary prostaglandin E2 (PGE2) was measured as a marker of the cyclooxygenase-2 (COX-2) activity. PGE2 levels in the first urine collected after reperfusion were higher than those in the control urine, that is, urine produced by the kidney before its removal from the donor (p=0.02; Fig. 4). This shows that in contrast to the absence of apparent oxidative damage, oxidative stress appears to be increased after renal reperfusion.
FIG. 4.
Prostaglandin E2 (PGE2) excretion in urine. Creatinine-corrected levels of PGE2 in the first urine collected after reperfusion (post) were higher than those of control urine (pre), that is, urine produced by the kidney before its removal from the donor (p=0.02).
Myocardial I/R is not associated with oxidative damage
Results of myocardial I/R resembled those of the kidney. There was no myocardial MDA release during the first hour after reperfusion in both patients with and without heart failure (p=0.05 and p=0.43, respectively; Table 3 and Supplementary Fig. S1). In fact, venous MDA levels tended to be lower than arterial levels in patients with preexisting heart failure.
15(S)-8-iso-PGF2α showed a small local release early after reperfusion in patients with preexisting heart failure (p=0.02; Fig. 3). In contrast, arteriovenous measurements of 15(S)-8-iso-PGF2α in patients without preexisting heart failure did not indicate a local release during the first hour after reperfusion (p=0.18; Table 3).
Biomarkers of nitrosative damage
No evidence for nitrosative damage in human kidney transplantation
Nitrite concentrations were equal in renal arterial and venous blood samples (LDs p=0.50, DDs p=0.87; Supplementary Fig. S2). Likewise, nitrate was not released from the reperfused graft either (LDs p=0.50, DDs p=0.98; Supplementary Fig. S3). These findings were confirmed by the additional measurement of plasma nitrotyrosine in LD kidney grafts, indicating no such release (p=0.93, Supplementary Fig. S4). The integral urinary measurement of nitrite and nitrate remained similar after reperfusion in LD kidney transplantations (p=0.06, p=0.69, respectively; Table 3).
No evidence for nitrosative damage after reperfusion of the myocardium
Arterial nitrite concentrations were higher than venous concentrations in both patient groups during the early reperfusion phase (p=0.002 preexisting heart failure, p=0.03 no preexisting heart failure; Supplementary Fig. S2), excluding myocardial nitrite release. Nitrate did not change over the reperfused myocardium in both patient groups (p=0.53 preexisting heart failure, p=0.81 no preexisting heart failure; Table 3 and Supplementary Fig. S3).
Oxidative stress
Kidney
Since the above-mentioned results indicated no extracellular evidence for RONS-mediated damage, we tested for the occurrence of oxidative stress during I/R by assessing activation of Nrf2, the master regulator of the antioxidant stress response.
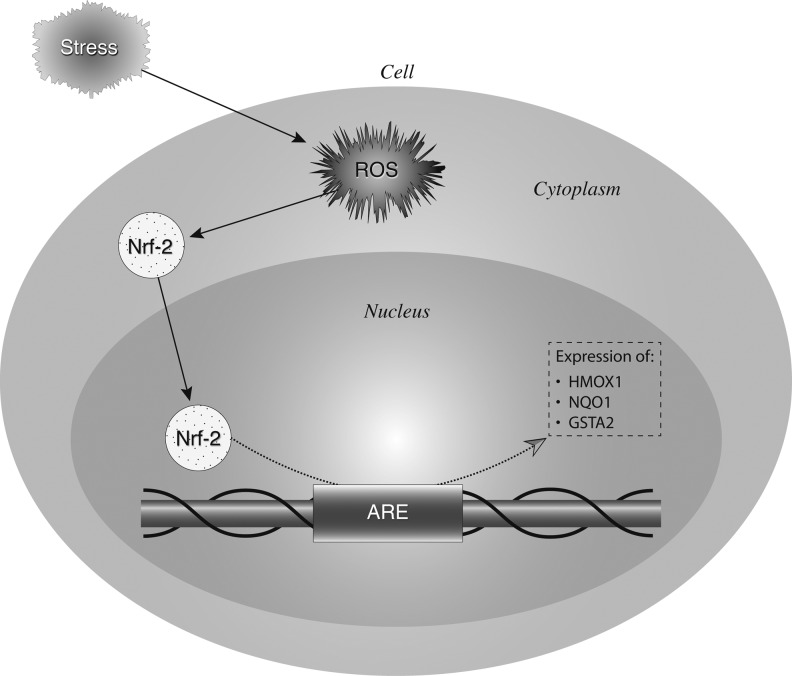
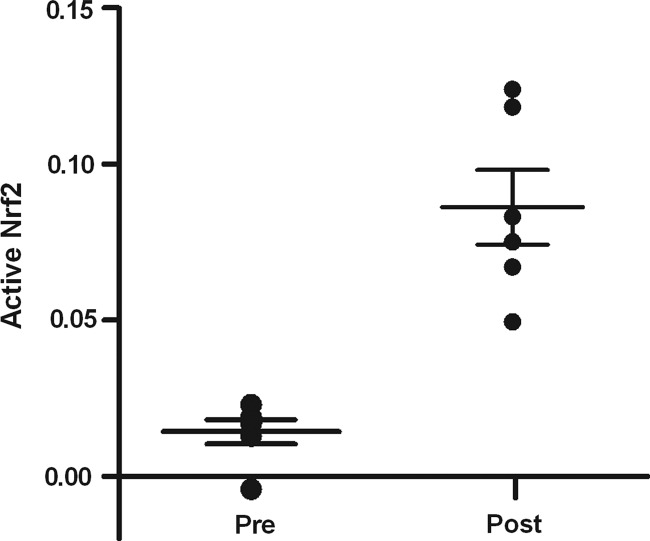
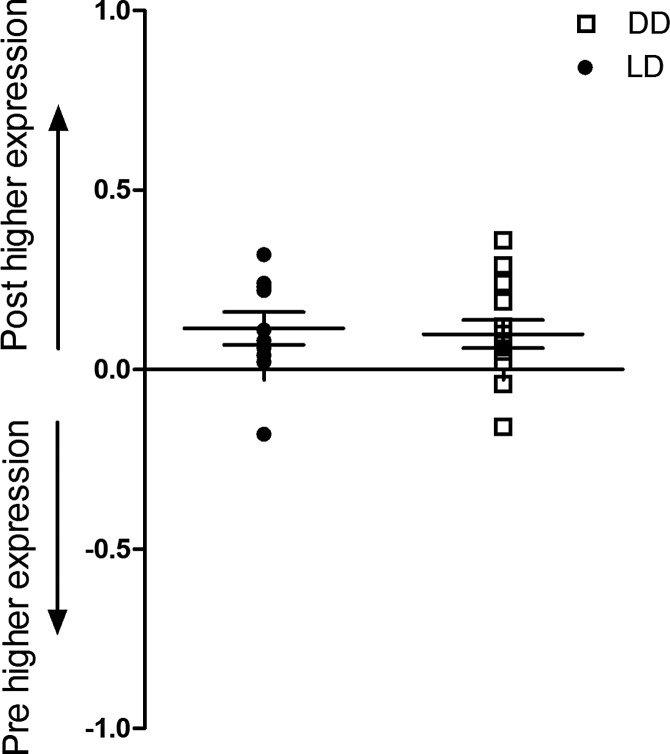
Activation of Nrf2 was quantified, as well as the upregulation of Nrf2 responsive genes, heme oxygenase-1 (HMOX1), NAD(P)H quinone oxidoreductase (NQO1), and glutathione-S-transferase A2 (GSTA2) in pre- and postbiopsies collected from both the kidney and heart (Fig. 5). Using a DNA binding ELISA for activated Nrf2, we found clear activation of this transcription factor upon reperfusion (p=0.01; Fig. 6), while Nrf2 gene expression increased as well in both the LD and DD kidney grafts (p=0.03, p=0.04, respectively; Fig. 7). Pathway-based analysis of Nrf2 downstream genes (Ingenuity Pathway Analysis suite, Supplementary Fig. S5) showed highly significant (p=7.06×10−12) upregulation of the canonical antioxidant responsive element (ARE) pathway in kidneys from LDs.
FIG. 5.
Schematic representation of the nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant responsive element (ARE) pathway and ARE-induced gene expression. Environmental stress exerts oxidative stress on cells through production of reactive oxygen and nitrogen species (RONS). The oxidative stress response is largely coordinated by a transcription factor, Nrf2. Under homeostatic conditions, there is only an inactive minimal basal level of Nrf2-directed gene expression under tight control of the protein Keap 1. RONS lead to the activation of mitogen-activated protein kinases (MAPK, ERK, p38), protein kinase C (PKC), phosphatidylinositol 3 kinase (PI3K), and others, which phosphorylate Keap1 and Nrf2. The Keap1-Nrf2 complex is then disrupted and the transcriptionally active Nrf2 is translocated to the nucleus where it binds to AREs in association with Maf proteins. This results in an increase of the transcriptional expression of Nrf2-inducible genes such as those encoding heme oxygenase-1 (HMOX1), NAD(P)H quinone oxidoreductase (NQO1), and glutathione-S-transferase A2 (GSTA2). Together, these proteins scavenge RONS and limit oxidative damage.
FIG. 6.
Nrf2 activation upon renal reperfusion. Nrf2 in renal tissue was significantly converted to its activated form after reperfusion (p=0.01) during LD kidney transplantation.
FIG. 7.
Changes in renal expression of Nrf2 gene. Renal expression of Nrf2/ARE gene in biopsies collected before (pre) and 45 min after (post) reperfusion during LD and DD kidney transplantation. Y-axis shows the relative change in gene expression as calculated by log(1/2ddCt), where the dCt is the Ct of studied gene minus the Ct of GAPDH, and ddCt is dCt of postbiopsy minus dCt of prebiopsy. A positive number indicates an increase in expression after reperfusion, whereas a negative number indicates a downregulation after reperfusion. Expression of the transcription factor Nrf2 gene NFE2L2 was significantly upregulated after reperfusion in both LD (p=0.03) and DD (p=0.04) kidney grafts.
Conversion of the xanthine reductase to xanthine oxidase (XO) and accumulation of hypoxanthine during ischemia has been proposed as a major source of oxygen radicals during I/R injury. Histochemical analysis of kidney biopsies collected before and after reperfusion did not indicate the XO activity in renal tissue, whereas ample XO activity and abundant hypoxanthine were observed in the positive control (rat liver, Supplementary Fig. S6).
Antioxidant enzyme activities
Superoxide dismutase (SOD) and peroxidase activities (catalase and glutathione peroxidase) were evaluated by histochemical analyses of kidney biopsies taken before and 45 min after reperfusion. Results of this analysis show abundant and similar antioxidant activities before and after reperfusion (Supplementary Fig. S7).
Discussion
Oxidative damage is long considered a key factor in the initiation of I/R injury. Although animal studies repeatedly showed the involvement of free radicals in I/R injury, studies in humans are scarce, and results of clinical intervention studies using antioxidants are inconclusive. Our study shows minimal release of markers of oxidative and nitrosative damage, and did not indicate upregulation of the classical oxidative response genes in both heart and kidney I/R. Results of our study are highly consistent and suggest that the role of oxidative and nitrosative damage in the initiation of clinical I/R injury may be less than commonly thought.
RONS have long been held responsible for the initiation and propagation of I/R injury in many different tissue types, including the kidney, liver, and heart (31). Initial studies date back to 20 years ago when an increase in lipid peroxidation was shown after experimental reperfusion of rat kidneys (34).
Since then, further animal studies have supported a role for oxidative damage in I/R (33, 38, 45). In contrast, studies in humans are scarce and findings are inconclusive. In a clinical study of Grech et al., it was confirmed that RONS are formed after I/R (19). By using direct spin trapping methods, they demonstrated an immediate free radical production during the first 4 h after myocardial reperfusion. However, whether RONS actually lead to tissue damage was not studied. Further studies on oxidative damage in I/R injury in humans show conflicting results. Zahmatkesh et al. showed that after kidney transplantation, circulating lipid peroxidation biomarkers rise in peripheral blood (47). Whether this can be attributed to oxidative damage in the kidney itself or in any other part of the body cannot be distinguished by peripheral measurements. Some studies involving myocardial I/R used more specific methods, that is, arteriovenous measurements over the reperfused heart, after percutaneous transluminal coronary angioplasty (PTCA). F2-isoprostanes were released from the reperfused myocardium after PTCA (4, 22). However, results on MDA release were contradictory, showing no myocardial release after PTCA (6) and low extent MDA release during coronary artery bypass grafting (29).
Although available antioxidant intervention studies are limited and results are inconclusive, antioxidant treatment appears a potential therapy to study in humans. Somehow, only few trials using antioxidants in human I/R injury are known, and it seems reasonable that a certain amount of publication bias has been introduced.
El-Hamamsy et al. showed that administration of the antioxidant N-acetylcysteine around human coronary artery bypass surgery did not lead to improvement in clinical endpoints or decreased release of biochemical markers (15). Administration of tirilazad mesylate, a nonglucocorticoid 21-aminosteroid inhibiting lipid peroxidation, to patients with acute ischemic stroke even increased the endpoint of death instead of having beneficial effects (3). In kidney I/R, only two clinical trials have been published using antioxidants in renal transplantation. Pollak et al. administered SOD intravenously before and 1 h after reperfusion, but no beneficial effects were observed with respect to early graft function (35). One year later, Land et al. administered a single, higher intravenous dose of SOD, immediately before reperfusion. The results demonstrated a significant reduction in first acute rejection (27). However, up to now, none of both observations has been confirmed in other studies or have led to application in daily practice.
Thus, despite encouraging results of antioxidant I/R therapy in animals, published clinical studies lack an appreciable, clinically relevant effect thus far (3, 15, 28, 39, 46). Let alone minor differences in a clinical setup, there appears to be a mechanistic disparity in the role of oxidative damage and endogenous antioxidant capacity in I/R injury between animals and humans. An explanation could be derived from studies on ageing that have shown that organisms with a large mass-specific metabolic rate, such as mice, have a relative high RONS production and a weak capacity to maintain homeostasis compared to humans (11). This difference in homeostatic capacity may explain differences in the role of oxidative damage between species as well. Thus, it can be suggested that endogenous antioxidant systems in humans are sufficiently equipped to handle the excess RONS load during clinical reperfusion, thus preventing damage. A finding that is supported by abundant SOD and peroxidase activities before and after I/R.
Whether oxidative damage is indeed prevented in human I/R injury was assessed in this study. RONS have a transient nature and are difficult to assess in vitro and in vivo (13). Consequently, we measured more stable oxidation products caused by RONS to determine the extent of oxidative damage in I/R injury. By combining multiple biomarkers, a footprint of oxidative and nitrosative damage could be reconstructed. Arteriovenous concentration differences of various established oxidative and nitrosative damage biomarkers were sequentially measured during reperfusion in kidney transplantation. To exclude organ- and procedure-specific findings, kidney results were compared to a different clinical setting, that is, myocardial I/R.
Arteriovenous measurements did not indicate release of oxidative or nitrosative damage biomarkers upon reperfusion of both the kidney and heart with the sole exception of a minimal and transient release of 15(S)-8-iso-PGF2α in a selection of patients. Although the arteriovenous measurements provide important information on local processes in the reperfused organ, sensitivity may be restricted by dilution of released biomarkers because of high flow rates. Therefore, accumulation of oxidative damage markers was measured in the urine produced by the transplanted kidney (LD) during the first minutes of reperfusion. Urinary measurements were in accordance to plasma findings, showing no release of oxidative or nitrosative damage biomarkers, including 15(S)-8-iso-PGF2α.
In the absence of other markers, it can be questioned whether venous 15(S)-8-iso-PGF2α release observed in subgroups of patients in this study reflects oxidative damage or alternatively reflects activation of inflammatory pathways. Since all other markers refute oxidative damage, it is highly unlikely that 15(S)-8-iso-PGF2α is the sole reflection of oxidative damage. Altogether, the multiple biomarkers of oxidative and nitrosative damage showed no consistent release upon reperfusion, denying RONS-mediated tissue damage.
Absence of venous release of oxidative and nitrosative damage markers may suggest that oxidative and nitrosative stress during I/R is less pronounced than commonly thought or may even be absent. We evaluated activation of Nrf2, the master regulator in cellular oxidative defense pathways, in a DNA-binding ELISA that exclusively measures the activated form of this transcription factor. Moreover, the activation of the conical downstream pathway of Nrf2 was evaluated through pathway analysis. Clear increases in Nrf2 activation and its downstream pathway show that oxidative stress does occur during I/R.
Evaluation of the XO activity, an often referred source of oxygen radicals in I/R injury, did not indicate the XO activity in the human kidney, although abundant XO was found in the rat liver. These observations are in line with previous reports showing abundant XO activities in rodent tissue, but not in human tissue (5). XO therefore appears not to be a prominent source of RONS in human tissue.
Limitations
This is a clinical study, and as such, procedures and timing were dictated by the operative procedures. We did include two clinically distinct situations of I/R to identify universal mechanisms of free radical-mediated damage after reperfusion. In human kidney transplantation, I/R included extremes in both short and long cold ischemia duration, the use of antioxidant preservation fluids, corticosteroids, and other immunosuppressive drugs. In cardiac surgery, I/R involved a normothermic ischemic period, and no antioxidants, corticosteroids, or other immunosuppressive drugs were administered. Throughout this whole spectrum of clinical conditions, independent of antioxidant use or immunosuppression, results unequivocally showed no dominant role of oxidative and nitrosative damage in human I/R injury.
Sampling in our study was restricted to less than an hour postreperfusion. This time window should be adequate as it is generally assumed that I/R injury is an acute process, initiated directly after reperfusion (20). This notion is supported by spin trap experiments indicating free radical formation within 15 min after reperfusion (19). Yet, we cannot exclude that I/R injury is less acute than commonly thought and that oxidative damage occurs after an hour of reperfusion.
A second limitation of the study is that it largely relies on repetitive measurement of arteriovenous concentration differences. Although this methodology has the advantage of specificity, and has been proven effective in elaborating the inflammatory response after I/R (9, 10), the high flow rates over the organs may compromise the sensitivity. To compensate for this limitation, we considered an evaluation of the first urine produced after I/R and measurement of the classical redox response genes relevant.
In conclusion, the findings from this study do not indicate a dominant role for RONS-induced damage in the pathophysiology of early I/R injury. The production of RONS during I/R is explicitly not questioned by our findings. In fact, Nrf2 activation and the higher PGE2 levels in post-transplantation urine indicate an increased COX activity after reperfusion, suggesting that oxidative stress does occur during early reperfusion. Yet, the generated RONS apparently do not induce tissue damage in human I/R. These observations suggest an efficient antioxidant system, providing an explanation for the limited efficacy of antioxidant therapy in human I/R. Altogether, this study challenges the prevailing paradigm of prominent involvement of oxidative damage in the initiation of human I/R injury.
Methods
Patients and surgical procedures
This prospective observational study involved patients undergoing renal I/R during kidney transplantation with LD and DD grafts, and patients undergoing myocardial I/R (i.e., during cardiac valve surgery) with and without preexisting heart failure. The study protocols were approved by the local Ethics Committee, and written informed consent was obtained from each patient.
Kidney transplantation
Twenty-four patients undergoing renal allograft transplantation were included: 8 patients receiving a kidney from a LD and 16 patients receiving a kidney from a DD (9 brain-dead donors and 7 cardiac-dead donors). LD and DD kidney transplantations were separately analyzed and compared because of large differences in ischemia times, pretransplantation graft handling, and clinical outcome between these groups. Within groups, variability of patient and graft characteristics was small for both donor types, rendering the comparison more reliable. LD kidney transplantations allowed for measurements in urine produced by the graft directly after reperfusion, providing a more cumulative measure than the arteriovenous measurements.
Kidney transplantations were performed according to the local standardized protocol. For technical reasons (renal vein sampling), only patients receiving a left kidney were included. In LDs, a minimally invasive open nephrectomy was performed. The immunosuppressive regimen was based on induction therapy with basiliximab followed by maintenance therapy with tacrolimus or cyclosporine A in addition to mycophenolate mofetil and steroids.
Cardiac valve surgery
Myocardial I/R was studied in patients undergoing cardiac valve surgery with aortic cross-clamping (i.e., cessation of blood flow in the coronary arteries). Twenty-four patients scheduled for elective mitral valve annuloplasty with use of cardiopulmonary bypass were included: 12 patients with preexisting heart failure and 12 patients without heart failure. Heart failure was defined as an inadequate pump function with an echocardiographically estimated ejection fraction biplane below 35% (21) and the presence of one or more clinical symptoms of heart failure (New York Heart Association classification) (12). Exclusion criteria were perioperative corticosteroid therapy, minimal invasive surgical procedures, emergency cardiac operations, and previous cardiac surgery.
Cardiac surgery was performed according to the local standardized protocol. All surgical procedures were performed via a midline sternotomy under normothermic cardiopulmonary bypass (Jostra Maquet, Maquet, Hirrlingen, Germany) with intermittent potassium-enriched antegrade warm-blood cardioplegia. This solution was administered every 15 to 20 min throughout the entire ischemic period. The cardiopulmonary bypass system was coated with a heparin softline coating. No antioxidants or other preservational or therapeutical substances were added.
During cardioplegia-induced cardiac arrest (myocardial ischemia), valve surgery was executed. Eventually, the aortic cross-clamp was removed to restore the blood flow through the heart (start of reperfusion).
Arteriovenous measurements
Arterial and venous blood samples were collected directly over the reperfused organ, that is, from the afferent and efferent blood vessel. Comparison of multiple biomarkers in these samples allowed for accurate and specific assessment of locally ongoing processes in the reperfusion phase (10). This method was used to determine the release of biomarkers of oxidative damage in both kidney and heart I/R.
Kidney transplantation
Arterial and renal venous blood samples were obtained as described before (10). Via an umbilical vein catheter placed in the renal vein, blood aliquots were sampled at 30 s, 3, 10, and 30 min after reperfusion. Paired arterial blood samples were simultaneously obtained via the iliac artery at 0, 3, 10, and 30 min after reperfusion (Fig. 1A). The endpoint of sampling was reached 30 min after reperfusion by closing the abdominal wall. The initial urine produced by the graft in the first 10 min after reperfusion was collected directly from the graft's ureter. Control urine was collected from the urethral catheter of LDs during the nephrectomy procedure before kidney ischemia was induced. All samples were collected in precooled tubes containing EDTA (BD Vacutainer, Plymouth, United Kingdom) and placed on melting ice immediately. Blood and urine samples were centrifuged (1.550 g, 20 min, 4°C) within 1 h after collection and the derived plasma or supernatant was recentrifuged (1.550 g, 20 min, 4°C) to deplete it from remaining leukocytes and platelets. Material was aliquoted and stored at −70°C until analysis.
Cardiac valve surgery
A 5 French indwelling jugular vein catheter (PICC, Arrow International Inc., REF PS-01651, PA USA) was inserted in the right atrium and placed in the coronary sinus during cardiac surgery (Fig. 1B). As all patients underwent mitral valve surgery using a vertical trans-septal incision, the coronary sinus could be easily cannulated during the surgical procedure. An arterial catheter was routinely placed in the radial artery. Arterial and paired myocardial venous blood (coronary sinus) samples were obtained at 0, 15, 30, and 60 min after reperfusion as described recently (25). All samples were collected in precooled tubes containing EDTA (BD Vacutainer) and placed on melting ice immediately. Blood samples were centrifuged (1.550 g, 10 min, 4°C) within 1 h after collection and the derived plasma was recentrifuged (10.000 g, 4 min, 4°C) to obtain leukocyte- and platelet-poor plasma. Aliquots were stored at −70°C until analysis.
Biopsy collection
A pretransplantation renal cortical biopsy was obtained just before transplantation, when the graft was still on ice. A paired needle biopsy of the same kidney was collected 45 min after reperfusion. Myocardial biopsies were collected at the start of cardioplegia-induced cardiac arrest and a paired, second biopsy was obtained at the end of the ischemic period, just before reperfusion of the heart. Because of technical reasons, a postreperfusion biopsy could not be collected since most of the included heart failure patients were hemodynamically instable in the early reperfusion phase. Lifting the heart, to take a postreperfusion biopsy, was considered unsafe. All biopsies were snap-frozen in liquid nitrogen and stored at −70°C until analysis.
Laboratory plasma measurements
To validate the method of arteriovenous measurements and to assess whether there is oxygen consumption by the reperfused organ, the oxygen saturation level was assessed in arterial as well as venous blood samples of a LD by means of a routinely used validated blood gas analyzer. The extent of RONS-mediated damage was evaluated by measuring different well-established biomarkers of oxidative and nitrosative damage by state of the art stable isotope dilution GC-MS and gas chromatography–tandem mass spectrometry (GC-MS/MS) methods. MDA and 15(S)-8-iso-PGF2α are widely recognized biomarkers of lipid peroxidation (17). In humans, nitrite and nitrate are reliable indicators of nitric oxide synthesis and frequently measured breakdown products of RONS (17, 20, 41).
MDA and total 15(S)-8-iso-PGF2α, that is, free and esterified 15(S)-8-iso-PGF2α, were assessed with an extensively validated GC-MS/MS method as described previously (40, 42). In parallel, the TBARS method was used to detect MDA in plasma as well as in urine samples (30). Free 15(S)-8-iso-PGF2α in urine was measured by GC-MS/MS (42). Nitrite and nitrate were measured simultaneously by GC-MS in plasma and urine aliquots as described elsewhere in detail (13, 40). The PGE2 concentration in urine was measured by GC-MS/MS after immunoaffinity column chromatography extraction (42). Urinary parameters were corrected for creatinine excretion (43). Study samples were analyzed alongside quality control (QC) samples as described previously (13, 40, 43). The MDA plasma concentration in the QC samples was determined to be 57.3±7.8 nM (mean±standard deviation [SD], n=25) corresponding to an imprecision (relative SD) of 13.7%. The total 15(S)-8-iso-PGF2α plasma concentration in the QC samples was determined to be 157±6.4 pM (mean±SD, n=10) corresponding to an imprecision of 4.1%. In the QC samples for plasma nitrite and nitrate (n=30), accuracy and imprecision were 90% to 112% and below 6%, respectively. 3-Nitrotyrosine in proteins, a biomarker of nitrosative damage (17), was measured by ELISA in accordance with the manufacturer's instructions (Biotech nitrotyrosine EIA; Oxis).
Nrf2/ARE pathway
Nrf2 activation was assessed in whole-cell lysate. Snap-frozen renal biopsies were homogenized and whole-cell extracts were prepared according to the manufacturers' instructions (Nuclear extract kit; Active Motif). Next, activated Nrf2 in cell lysates was quantified by DNA-binding ELISA according to the manufacturers' instructions (TransAM Nrf2; Active Motif). Total RNA was extracted from renal or myocardial tissues as described earlier, using GAPDH as internal control (1). For expression profiling, the integrity of each RNA sample was examined by Agilent Lab-on-a-chip technology using the RNA 6000 Nano LabChip kit and a bioanalyzer 2100 (Agilent Technologies). RNA preparations were considered suitable for array hybridization only if samples showed intact 18S and 28S rRNA bands, and displayed no chromosomal peaks or RNA degradation products (RNA Integrity Number >8.0) (23). Subsequent microarray analysis was performed using Illumina whole-genome gene expression BeadChips (Illumina BeadArray®) according to instructions of the manufacturer at Service XS.
Selected probes from the canonical Nrf2 pathway were used as an input for pathway analysis through the Ingenuity Pathway Analysis suite (www.ingenuity.com). Hierarchical clustering and heatmap visualization were carried out using Hierarchical Clustering Viewer and Heatmap Viewer modules within the GenePattern analysis suite (8, 14, 37).
Histological analyses
In kidney biopsies collected before and after reperfusion, presence of various antioxidant enzymes and RONS generation by XO was assessed. Antioxidant enzymes SOD and peroxidases (catalase and glutathione peroxidase) were measured by histochemical methods as described previously in detail (16). The XO activity was measured in renal tissue, based on the generation of superoxide anions from hypoxanthine, as described earlier (24). Specificity of the assay was demonstrated by inhibition of XO by allopurinol. Rat liver tissue was included as a positive control.
Statistical analysis
Statistical analysis was performed with the Statistical Package for the Social Sciences 16.0 (SPSS, Inc.). All data in the text and tables are expressed as mean±SD. Patient characteristics were compared by the paired t test. The area under the curve (AUC) was calculated for the arterial and venous curve. AUC's and urinary measurements were compared by using the Wilcoxon signed rank test. Differential expression of probes was assessed using the unpaired moderated t-test (LIMMA) through the Remote Analysis Computation for gene Expression data (RACE) suite at http://race.unil.ch (36). A p-value less than 0.05 was considered significant. Graph error bars indicate the standard error of the mean.
Supplementary Material
Abbreviations Used
- 15(S)-8-iso-PGF2α
15(S)-8-iso-prostaglandin F2α
- ADPKD
autosomal dominant polycystic kidney disease
- ARE
antioxidant responsive element
- AUC
area under the curve
- BDD
brain-dead donor
- BM
basement membrane
- CDD
cardiac-dead donor
- CIT
cold ischemia time
- COX-2
cyclooxygenase-2
- DD
deceased donor
- FSG
focal segmental glomerulosclerosis
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GC-MS
gas chromatography–mass spectrometry
- GC-MS/MS
gas chromatography–tandem mass spectrometry
- HMOX-1
heme oxygenase-1
- HTK
histidine–tryptophan–ketoglutarate
- ICU
intensive care unit
- I/R
ischemia/reperfusion
- LD
living donor
- MDA
malondialdehyde
- MPG
membranous glomerulonephritis
- NQO1
NAD(P)H quinone oxidoreductase
- Nrf2
nuclear factor erythroid 2-related factor 2
- PGE2
prostaglandin E2
- PI3K
phosphatidylinositol 3 kinase
- PKC
protein kinase C
- PTCA
percutaneous transluminal coronary angioplasty
- QC
quality control
- RONS
reactive oxygen and nitrogen species
- ROS
reactive oxygen species
- SAH
subarachnoid hemorrhage
- SD
standard deviation
- SEM
standard error of the mean
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid-reactive substances
- UW
University of Wisconsin
- WIT
warm ischemia time
- XO
xanthine oxidase
Acknowledgments
Robert J.M. Klautz received a grant from The Netherlands Heart Foundation (2007B150) and Dorottya K. de Vries received support from The Netherlands Organization for Health Research and Development (project 92003525). We thank Lars Verschuren for his help with the pathway analysis. Manon Zuurmond and Gerrit Kracht are gratefully acknowledged for providing, respectively, the image in Figure 1 (©ManonProject.com) and Figure 4. Frank-Mathias Gutzki is thanked for performing GC-MS and GC-MS/MS analyses. Professor Anton K. Raap is gratefully acknowledged for the critical reading of the manuscript.
Author Disclosure Statement
The authors state no conflict of interests in this study.
References
- 1.Abdul-Hussien H. Soekhoe RG. Weber E. Von der Thusen JH. Kleemann R. Mulder A. Van Bockel JH. Hanemaaijer R. Lindeman JH. Collagen degradation in the abdominal aneurysm: a conspiracy of matrix metalloproteinase and cysteine collagenases. Am J Pathol. 2007;170:809–817. doi: 10.2353/ajpath.2007.060522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosio G. Flaherty JT. Duilio C. Tritto I. Santoro G. Elia PP. Condorelli M. Chiariello M. Oxygen radicals generated at reflow induce peroxidation of membrane lipids in reperfused hearts. J Clin Invest. 1991;87:2056–2066. doi: 10.1172/JCI115236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bath PM. Iddenden R. Bath FJ. Orgogozo JM. Tirilazad for acute ischaemic stroke. Cochrane Database Syst Rev. 2001:CD002087. doi: 10.1002/14651858.CD002087. [DOI] [PubMed] [Google Scholar]
- 4.Berg K. Jynge P. Bjerve K. Skarra S. Basu S. Wiseth R. Oxidative stress and inflammatory response during and following coronary interventions for acute myocardial infarction. Free Radic Res. 2005;39:629–636. doi: 10.1080/10715760400028027. [DOI] [PubMed] [Google Scholar]
- 5.Bianciardi P. Scorza R. Ghilardi G. Samaja M. Xanthine oxido-reductase activity in ischemic human and rat intestine. Free Radic Res. 2004;38:919–925. doi: 10.1080/10715760412331273430. [DOI] [PubMed] [Google Scholar]
- 6.Cedro K. Marczak E. Czerwosz L. Herbaczynska-Cedro K. Ruzyllo W. Elective coronary angioplasty with 60 s balloon inflation does not cause peroxidative injury. Eur J Clin Invest. 2002;32:148–152. doi: 10.1046/j.1365-2362.2002.00967.x. [DOI] [PubMed] [Google Scholar]
- 7.Crimi E. Sica V. Williams-Ignarro S. Zhang H. Slutsky AS. Ignarro LJ. Napoli C. The role of oxidative stress in adult critical care. Free Radic Biol Med. 2006;40:398–406. doi: 10.1016/j.freeradbiomed.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 8.de Hoon MJ. Imoto S. Nolan J. Miyano S. Open source clustering software. Bioinformatics. 2004;20:1453–1454. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- 9.de Vries DK. Lindeman JH. Ringers J. Reinders ME. Rabelink TJ. Schaapherder AF. Donor brain death predisposes human kidney grafts to a proinflammatory reaction after transplantation. Am J Transplant. 2011;11:1064–1070. doi: 10.1111/j.1600-6143.2011.03466.x. [DOI] [PubMed] [Google Scholar]
- 10.de Vries DK. Lindeman JH. Tsikas D. de Heer E. Roos A. de Fijter JW. Baranski AG. van Pelt J. Schaapherder AF. Early renal ischemia-reperfusion injury in humans is dominated by IL-6 release from the allograft. Am J Transplant. 2009;9:1574–1584. doi: 10.1111/j.1600-6143.2009.02675.x. [DOI] [PubMed] [Google Scholar]
- 11.Demetrius L. Of mice and men. When it comes to studying ageing and the means to slow it down, mice are not just small humans. EMBO Rep. 2005;6 doi: 10.1038/sj.embor.7400422. Spec No: S39–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickstein K. Cohen-Solal A. Filippatos G. McMurray JJ. Ponikowski P. Poole-Wilson PA. Stromberg A. van Veldhuisen DJ. Atar D. Hoes AW. Keren A. Mebazaa A. Nieminen M. Priori SG. Swedberg K. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Dreissigacker U. Suchy MT. Maassen N. Tsikas D. Human plasma concentrations of malondialdehyde (MDA) and the F2-isoprostane 15(S)-8-iso-PGF(2alpha) may be markedly compromised by hemolysis: evidence by GC-MS/MS and potential analytical and biological ramifications. Clin Biochem. 2010;43:159–167. doi: 10.1016/j.clinbiochem.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Eisen MB. Spellman PT. Brown PO. Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Hamamsy I. Stevens LM. Carrier M. Pellerin M. Bouchard D. Demers P. Cartier R. Page P. Perrault LP. Effect of intravenous N-acetylcysteine on outcomes after coronary artery bypass surgery: a randomized, double-blind, placebo-controlled clinical trial. J Thorac Cardiovasc Surg. 2007;133:7–12. doi: 10.1016/j.jtcvs.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 16.Frederiks WM. Bosch KS. Localization of superoxide dismutase activity in rat tissues. Free Radic Biol Med. 1997;22:241–248. doi: 10.1016/s0891-5849(96)00328-0. [DOI] [PubMed] [Google Scholar]
- 17.Giustarini D. Dalle-Donne I. Tsikas D. Rossi R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci. 2009;46:241–281. doi: 10.3109/10408360903142326. [DOI] [PubMed] [Google Scholar]
- 18.Gourdin MJ. Bree B. De Kock M. The impact of ischaemia-reperfusion on the blood vessel. Eur J Anaesthesiol. 2009;26:537–547. doi: 10.1097/EJA.0b013e328324b7c2. [DOI] [PubMed] [Google Scholar]
- 19.Grech ED. Dodd NJ. Jackson MJ. Morrison WL. Faragher EB. Ramsdale DR. Evidence for free radical generation after primary percutaneous transluminal coronary angioplasty recanalization in acute myocardial infarction. Am J Cardiol. 1996;77:122–127. doi: 10.1016/s0002-9149(96)90580-9. [DOI] [PubMed] [Google Scholar]
- 20.Halliwell B. Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford: Oxford Science Publications; 2004. pp. 645–661. [Google Scholar]
- 21.Hoffmann R. von Bardeleben S. ten Cate F. Borges AC. Kasprzak J. Firschke C. Lafitte S. Al-Saadi N. Kuntz-Hehner S. Engelhardt M. Becher H. Vanoverschelde JL. Assessment of systolic left ventricular function: a multi-centre comparison of cineventriculography, cardiac magnetic resonance imaging, unenhanced and contrast-enhanced echocardiography. Eur Heart J. 2005;26:607–616. doi: 10.1093/eurheartj/ehi083. [DOI] [PubMed] [Google Scholar]
- 22.Iuliano L. Pratico D. Greco C. Mangieri E. Scibilia G. FitzGerald GA. Violi F. Angioplasty increases coronary sinus F2-isoprostane formation: evidence for in vivo oxidative stress during PTCA. J Am Coll Cardiol. 2001;37:76–80. doi: 10.1016/s0735-1097(00)01040-8. [DOI] [PubMed] [Google Scholar]
- 23.Kleemann R. Verschuren L. van Erk MJ. Nikolsky Y. Cnubben NH. Verheij ER. Smilde AK. Hendriks HF. Zadelaar S. Smith GJ. Kaznacheev V. Nikolskaya T. Melnikov A. Hurt-Camejo E. van der Greef J. van Ommen B. Kooistra T. Atherosclerosis and liver inflammation induced by increased dietary cholesterol intake: a combined transcriptomics and metabolomics analysis. Genome Biol. 2007;8:R200–R216. doi: 10.1186/gb-2007-8-9-r200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kooij A. Frederiks WM. Gossrau R. Van Noorden CJ. Localization of xanthine oxidoreductase activity using the tissue protectant polyvinyl alcohol and final electron acceptor Tetranitro BT. J Histochem Cytochem. 1991;39:87–93. doi: 10.1177/39.1.1983876. [DOI] [PubMed] [Google Scholar]
- 25.Kortekaas KA. Lindeman JH. Versteegh MI. van Beelen E. Kleemann R. Klautz RJ. Heart failure determines the myocardial inflammatory response to injury. Eur J Heart Fail. 2012 doi: 10.1093/eurjhf/hfs183. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Lakhan SE. Kirchgessner A. Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97–107. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Land W. Schneeberger H. Schleibner S. Illner WD. Abendroth D. Rutili G. Arfors KE. Messmer K. The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation. 1994;57:211–217. doi: 10.1097/00007890-199401001-00010. [DOI] [PubMed] [Google Scholar]
- 28.Land W. Zweler JL. Prevention of reperfusion-induced, free radical-mediated acute endothelial injury by superoxide dismutase as an effective tool to delay/prevent chronic renal allograft failure: a review. Transplant Proc. 1997;29:2567–2568. doi: 10.1016/s0041-1345(97)00509-5. [DOI] [PubMed] [Google Scholar]
- 29.Lazzarino G. Raatikainen P. Nuutinen M. Nissinen J. Tavazzi B. Di Pierro D. Giardina B. Peuhkurinen K. Myocardial release of malondialdehyde and purine compounds during coronary bypass surgery. Circulation. 1994;90:291–297. doi: 10.1161/01.cir.90.1.291. [DOI] [PubMed] [Google Scholar]
- 30.Londero D. Lo Greco P. Automated high-performance liquid chromatographic separation with spectrofluorometric detection of a malondialdehyde-thiobarbituric acid adduct in plasma. J Chromatogr A. 1996;729:207–210. doi: 10.1016/0021-9673(95)00959-0. [DOI] [PubMed] [Google Scholar]
- 31.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 32.Misra MK. Sarwat M. Bhakuni P. Tuteja R. Tuteja N. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15:RA209–RA219. [PubMed] [Google Scholar]
- 33.Nath KA. Paller MS. Dietary deficiency of antioxidants exacerbates ischemic injury in the rat kidney. Kidney Int. 1990;38:1109–1117. doi: 10.1038/ki.1990.320. [DOI] [PubMed] [Google Scholar]
- 34.Paller MS. Hoidal JR. Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollak R. Andrisevic JH. Maddux MS. Gruber SA. Paller MS. A randomized double-blind trial of the use of human recombinant superoxide dismutase in renal transplantation. Transplantation. 1993;55:57–60. doi: 10.1097/00007890-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Psarros M. Heber S. Sick M. Thoppae G. Harshman K. Sick B. RACE: remote analysis computation for gene expression data. Nucleic Acids Res. 2005;33:W638–W643. doi: 10.1093/nar/gki490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reich M. Liefeld T. Gould J. Lerner J. Tamayo P. Mesirov JP. GenePattern 2.0. Nat Genet. 2006;38:500–501. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- 38.Schneider MP. Sullivan JC. Wach PF. Boesen EI. Yamamoto T. Fukai T. Harrison DG. Pollock DM. Pollock JS. Protective role of extracellular superoxide dismutase in renal ischemia/reperfusion injury. Kidney Int. 2010;78:374–381. doi: 10.1038/ki.2010.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki K. Anti-oxidants for therapeutic use: why are only a few drugs in clinical use? Adv Drug Deliv Rev. 2009;61:287–289. doi: 10.1016/j.addr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Tsikas D. Simultaneous derivatization and quantification of the nitric oxide metabolites nitrite and nitrate in biological fluids by gas chromatography/mass spectrometry. Anal Chem. 2000;72:4064–4072. doi: 10.1021/ac9913255. [DOI] [PubMed] [Google Scholar]
- 41.Tsikas D. A critical review and discussion of analytical methods in the L-arginine/nitric oxide area of basic and clinical research. Anal Biochem. 2008;379:139–163. doi: 10.1016/j.ab.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 42.Tsikas D. Schwedhelm E. Suchy MT. Niemann J. Gutzki FM. Erpenbeck VJ. Hohlfeld JM. Surdacki A. Frolich JC. Divergence in urinary 8-iso-PGF(2alpha) (iPF(2alpha)-III, 15-F(2t)-IsoP) levels from gas chromatography-tandem mass spectrometry quantification after thin-layer chromatography and immunoaffinity column chromatography reveals heterogeneity of 8-iso-PGF(2alpha). Possible methodological, mechanistic and clinical implications. J Chromatogr B. 2003;794:237–255. doi: 10.1016/s1570-0232(03)00457-4. [DOI] [PubMed] [Google Scholar]
- 43.Tsikas D. Wolf A. Mitschke A. Gutzki FM. Will W. Bader M. GC-MS determination of creatinine in human biological fluids as pentafluorobenzyl derivative in clinical studies and biomonitoring: inter-laboratory comparison in urine with Jaffe, HPLC and enzymatic assays. J Chromatogr B. 2010;878:2582–2592. doi: 10.1016/j.jchromb.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 44.Valko M. Leibfritz D. Moncol J. Cronin MT. Mazur M. Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Weight SC. Furness PN. Nicholson ML. Nitric oxide generation is increased in experimental renal warm ischaemia-reperfusion injury. Br J Surg. 1998;85:1663–1668. doi: 10.1046/j.1365-2168.1998.00960.x. [DOI] [PubMed] [Google Scholar]
- 46.Yellon DM. Hausenloy DJ. Mechanisms of disease: myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 47.Zahmatkesh M. Kadkhodaee M. Mahdavi-Mazdeh M. Ghaznavi R. Hemati M. Seifi B. Golab F. Hasani K. Lessan-Pezeshki M. Einollahi B. Oxidative stress status in renal transplant recipients. Exp Clin Transplant. 2010;8:38–44. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.