Abstract
Background
Some domains of the questionnaires used to measure symptoms and quality of life (QOL) in patients with advanced cancer seem to measure similar dimensions or constructs, so it would be useful for clinicians to demonstrate the interchangeability of equivalent domains of the questionnaires in measuring the same constructs.
Objective
This study investigated the reliability and concurrent validity of the Palliative Outcome Scale (POS), the Rotterdam Symptom Checklist (RSCL), and the Brief Pain Inventory (BPI), used to measure symptom control in patients with advanced cancer.
Design
This was an evaluative study.
Setting/Subjects
Subjects were patients with advanced cancer attended by Spanish primary care physicians.
Measurements
Secondary analysis was performed of 117 outpatients who completed the POS, BPI, and RSCL at two different times, with an interval of 7 to 10 days. Bland and Altman analyses and plot, repeatability coefficient, as well as Spearman correlations were carried out.
Results
There were 117 included patients. Mean age was 69.4 (11.5) years, gender was 60% male, 37.6% completed only elementary school, diagnoses were mainly digestive and lung cancer, with a low functional rate and presence of oncologic pain. First and second questionnaire rounds showed significant correlations and agreement. Agreement was shown between pain intensity of BPI and pain and physical scales of RSCL, and between physical symptoms of RSCL and of POS, with significant correlations in equivalent dimensions.
Conclusion
BPI, POS, and RSCL have shown adequate reliability and moderate concurrent validity among them.
Introduction
The care of patients with advanced cancer implies a shift from cure and control of the disease to the management of symptoms and maintenance of quality of life (QOL). Mularski and colleagues have recommended the evaluation of interventions in palliative care (PC) using a conceptual model which includes patient condition specific, patient-reported outcomes and caregiver-reported outcomes. Symptom control and QOL are the two most relevant outcome domains recommended in the management of patients requiring PC.1
A systematic review about symptom prevalence in patients with incurable cancer found that five symptoms (fatigue, pain, lack of energy, weakness, and appetite loss) occurred in more than 50% of the patients at different times of their illness, among 37 more symptoms.2 Considering that QOL is the main objective of PC and that symptoms of cancer patients are predictors of QOL, it seems convenient to have appropriate questionnaires to help clinicians in making clinical decisions taking into account the patient's perspective.3
A large variety of QOL questionnaires are appropriate for use in PC, but at present there is no agreement on which of them are the best instrument to use.4,5 Among the recommendations proposed for measuring outcomes in randomized prospective trials in PC, especially stressed have been to use and to expand the testing of existing instruments for validity and reliability across diseases, settings, and populations.6
In a previous study performed by the authors7 to assess the clinical effectiveness of online versus traditional training in PC of primary care physicians, questionnaires were administered to patients to measure their symptom control and QOL through the short version of the Brief Pain Inventory (BPI)8 and the Rotterdam Symptom Checklist (RSCL)9 and to capture their global PC concerns though the Palliative Outcome Scale (POS).10
These questionnaires, internationally recommended because of their adequate psychometric properties to assess the impact of symptoms and QOL upon patients with advanced cancer,1,5,11 have been previously used to assess the effectiveness of the PC in Spanish clinical settings.7,8,12
The short version of the BPI (pain items) and RSCL showed, in a previous study, moderate or good correlation (mainly with psychological and global QOL scales of the RSCL).9 According to our knowledge, the current validity between BPI, RSCL, and POS has not been studied yet in patients with advanced cancer requiring PC in primary care.
Some domains of these questionnaires measure similar dimensions, BPI measures pain, RSCL measures symptoms and overall QOL, while POS also measures physical symptoms and psychological distress in addition to QOL. Taking into account that the questionnaire's comparisons in a primary care setting in a PC population are absent in the scientific literature, the evaluation of the concurrent validity among these questionnaires will increase knowledge about their psychometric properties and interchangeability of equivalent domains.
The first aim of the study was to describe the reliability and the criterion validity of the POS and also the concurrent validity with equivalent domains of the RSCL and the BPI questionnaires. A secondary objective was to study the concurrent validity between equivalent domains of the RSCL and the BPI.
Methods
Participants and setting
The methodology for the first stage of the study has been previously described.7 Briefly, during 2009 an online course on PC versus traditional training was performed in Spanish primary care physicians to assess the impact on control symptoms, QOL, and also caregiver satisfaction. Physicians from 136 primary health centers from all 17 Spanish health regions participating in that study enrolled consecutive patients with advanced cancer requiring PC. The mean number of patients recruited in each primary health center was two patients (range 1–3), who completed the questionnaires if they were able to read and write and give informed consent. Patients with Karnofsky score (PS)13 <20, cognitive impairment (>3 errors in the Pfeiffer's scale),14 and attended by a specialized palliative team were excluded. Variables included age, gender, level of education classified into six levels, type of cancer, other diseases, treatments received, oncologic pain, and time of treatment for pain, Karnofsky score, and patients' awareness of their condition (complete, partial, does not know). The data here presented are a secondary analysis focused on the concurrent validity of the questionnaires used in our previous study, which was already sent for publication.
Measurements
When enrolling patients, physicians registered patient variables and provided the patient with the POS, the BPI, and the RSCL for them to complete during the next three days. The patients again completed the three questionnaires after 7–10 days to evaluate the change of scores as a psychometric property of the questionnaires to measure change.
The POS10 is a multidimensional questionnaire with 10 items, scored in a Likert scale of 0–4 (0 reports the best score, and 4 the worst score). Its structure has shown two factors: psychological well-being and quality of care. Besides, three items are analyzed independently: pain control, family anxiety, and symptoms.15 For this study, item 9 (wasted time waiting for tests while in-hospital) was not included, as this is out of context within the primary care setting.
The BPI8 is a multidimensional questionnaire including 21 items, 11 of which are grouped into two scales: pain intensity (4 items measuring pain at its worst, pain at its least, pain on the average, and pain right now) and pain impact (7 items measuring pain impact on general activity, mood, walking ability, normal work, relations with other people, sleep, enjoyment of life). The measurement scale ranges from 0 (no pain / does not interfere with daily life) to 10 (pain as bad as you can imagine / completely interferes with daily life).
The RSCL9 is a generic cancer questionnaire including 39 items, grouped in four scales (score 0–100): physical symptoms, psychological symptoms, daily life activities, and overall QOL. It provides a score for each scale and subscale (fatigue, pain, gastrointestinal, and chemotherapy). High scores represent a worse QOL, except for activity, which is interpreted the other way around. The chemotherapy subscale was not used in this study, as it was deemed useless for patients with no active treatment.16
Statistical analyses
All patients have been considered as a single group, differentiating between first and second completions. BPI and POS scales were converted into a 0–100 scale. Means and SDs were calculated for patient characteristics. To determine test-retest reliability, the Spearman's correlation coefficient was calculated between the first and second measurements. A correlation >0.8 is described as strong, correlation <0.5 as weak. Additionally, to determine agreement, the coefficient of repeatability was calculated and Bland and Altman analyses and plots were made in which the mean difference between the first and second measurements with corresponding 95% CI and 95% limits of agreement (LOA) were presented.17,18
To determine concurrent validity among POS, BPI, and RSCL in the symptom domains, the following comparisons were made according to the objectives of the study:
The first objective: pain on POS with pain intensity on BPI; feel good and total score on POS with pain impact on BPI; pain on POS with pain on RSCL; symptoms on POS with physical on RSCL; psychological well-being on POS with psychological on RSCL; total score on POS with global on RSCL.
The second objective: pain intensity on BPI with pain and physical on RSCL; and pain impact on BPI with physical, global, and fatigue on RSCL.
Bland and Altman analyses were performed, through ANOVA, between equivalent domains of questionnaires with repeated measurements. Two different variances were estimated: within and between subjects, to found the LOA and determine whether bias occurred.18 The LOA contains the difference between measurements by the two questionnaires for 95% of pair measurements on similar individuals. The 95% CI of the mean difference between two questionnaires should contain zero in order to exclude lack of agreement.
SPSS 18.0 (SPSS Inc., Chicago, IL) was used for analysis and MedCal for Windows 12.1.4 for plots performance; p<0.05 was considered to be significant value.
Results
Participant characteristics
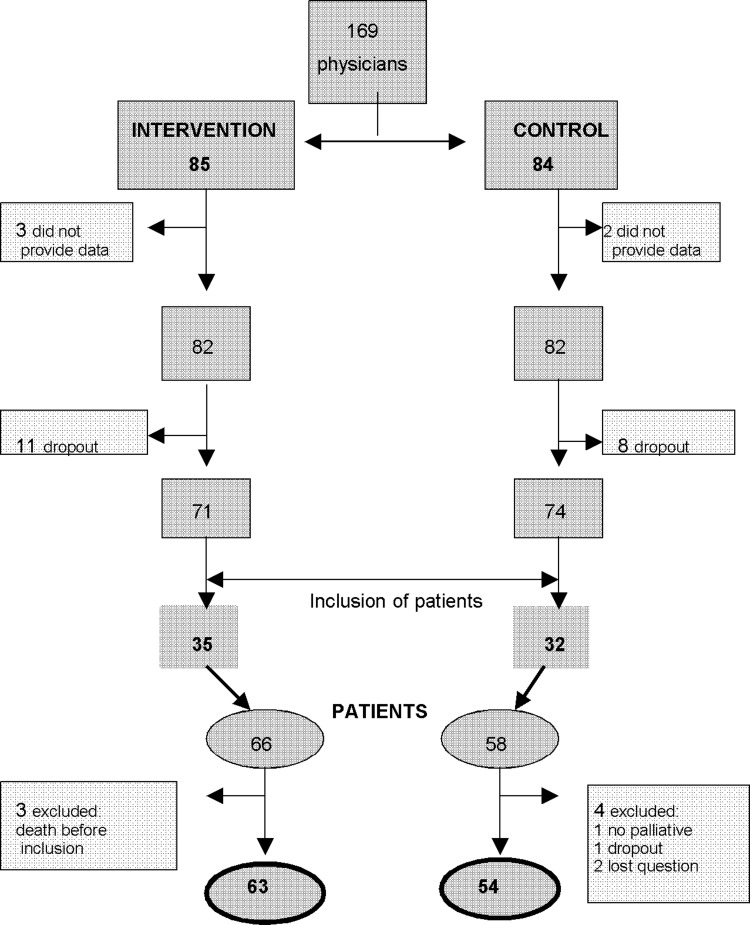
A total of 169 primary care physicians from 136 primary health centers were included in the primary study. After exclusions, 164 remained, of which 19 dropped out leaving 145 physicians. Of these, 67 from 51 primary health centers of the original 136, enrolled a total of 124 patients, from which 7 dropped out for different reasons, leaving 117 patients for analysis (see Figure 1). Out of 117 included patients, 11 (9.5%) did not complete all questionnaires. Sixty percent of patients were men, mean age 69.4 (11.5 SD) years; 37.6% had an elementary school education. Digestive and lung cancer, 56.4%; PS<50, 57.3%; presence of oncology pain, 67.5%; requiring home care, 72%; and 51.3% were fully aware of their disease diagnosis (see Table 1). Patients self-completing the questionnaires, 22.3%; needing help from a relative, 42.7% and from a physician, 35.5%.
FIG. 1.
Physicians and patients participation flowchart in the primary study.
Table 1.
Patient Characteristics
| Characteristic | Patients, N=117 (%) |
|---|---|
| Men | 70 (60.0) |
| Mean age, years (SD) | 69.4 (11.5) |
| Education | |
| - Literacy | 42 (36.0) |
| - Elementary school | 44 (37.6) |
| - Secondary education | 15 (12.8) |
| - Professional training | 5 (4.3) |
| - Undergraduate degree | 6 (5.1) |
| - High school diploma | 5 (4.3) |
| Cancer type | |
| - Digestive tract | 37 (31.6) |
| - Lung | 29 (24.8) |
| - Prostate | 10 (8.5) |
| - Larynx | 7 (6.0) |
| - Breast | 5 (4.3) |
| - Other | 29 (24.8) |
| Radiotherapy | 35 (30.0) |
| Chemotherapy | 62 (53.0) |
| Karnofsky score ≤50 | 67 (57.3) |
| Oncologic pain | 79 (67.5) |
| Time of pain treatment in days, mean (SD), range | 127 (222.0) 1–1345 |
| Opiatesa | 79 (67.5) |
| - Sustained-release morphine | 23 (19.6) |
| - Short-acting morphine | 17 (14.5) |
| - Fentanyl patch | 30 (25.6) |
| - Short-acting fentanyl | 9 (7.7) |
| - Sustained-release oxycodone | 4 (3.4) |
| - Short-acting oxycodone | 2 (3.0) |
| - Hydromorphone | 3 (2.6) |
| - Buprenorphine | 3 (2.6) |
| - Tramadol | 15 (12.8) |
| Constipation prevention in opiate treatment | 29 (36.7) |
| Analgesics (acetaminophen, metamizol) | 47 (40.2) |
| Diagnosis knowledge | |
| - Completely | 60 (51.3) |
| - Partially | 48(41.0) |
| - No knowledge | 9 (7.7) |
| Home care | 84 (71.8) |
| Diseases | |
| - Cardiovascular | 82 (70.1) |
| - Diabetes and endocrines | 54 (46.1) |
| - COPD | 23 (19.7) |
| - Osteoarthritis | 23 (19.7) |
| - Gastrointestinal | 10 (8.5) |
| - Other | 52 (44.4) |
| Other treatmentsa | |
| - Antisecretory drugs | 53 (45.3) |
| - Cardiovascular | 36 (30.8) |
| - Antidepressives/anxyolitics | 38 (32.5) |
| - Corticoids | 36 (30.8) |
| - Antianorexicos | 9 (7.7) |
| - NSAIDs | 14 (12.0) |
| - Other | 40 (35.5) |
percentage exceeds 100%.
SD, standard deviation; COPD, Chronic Obstructive Pulmonary Disease; NSAIDs, antiinflammatory nonsteroid.
Reliability
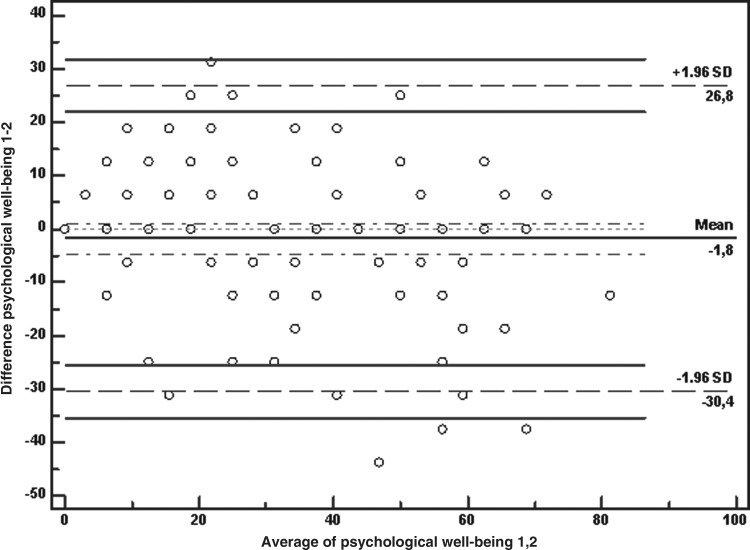
The test-retest is shown in Table 2. The Bland and Altman analyses showed that zero lies within the 95% CI of the mean difference between the first and second measurement of the POS, BPI, and RSCL except for marginal values in family anxiety of POS and pain of RSCL, indicating no bias. Total agreement with life worthwhile and feel good of POS was found. The LOA shows the limits of 95% of differences (mean of differences±1.96*SD) including the 95% CI for the lower and the upper LOA. These intervals were wide, reflecting the variation of the differences; for example, when the first measurement of psychological well-being on POS is compared with the second measurement, the 95% LOA lie between −30.4 and 26.8, with the zero into the 95% CI of the mean difference between measurements (mean difference −1.8, 95% CI −4.6, 1.1), indicating that the limits appear to fit the data well (see Figure 2).
Table 2.
Palliative Outcome Scale, Brief Pain Inventory, and Rotterdam First Measurements Agreement and Correlation with Second Measurements
| Item/scale | 95% LOA (95% CI) | Mean difference between measurements (95% CI for bias) | Repeatability coefficient | Spearman correlationa |
|---|---|---|---|---|
| Palliative Outcome Scale (POS) | ||||
| Psychological well-being | −30.4 (−25.4; −35.3) 26.8 (21.9; 31.8) | −1.8 (−4.6; 1.1) | 29.2 | 0.8 |
| Quality of care | −33.5 (−39.5; −27.4) 35.2 (29.2; 41.3) | 0.9 (−2.6; 4.4) | 35.0 | 0.7 |
| Pain | −40.4 (−47.9; −32.8) 48.4 (40.9; 55.9) | 4.0 (−0.3; 8.4) | 45.2 | 0.6 |
| Symptoms | −46.3 (−54.4; −38.1) 50.0 (41.9; 58.2) | 1.9 (−2.8; 6.6) | 49.1 | 0.5 |
| Family anxiety | −50.1 (−59.7; −50.6) 61.3 (51.7; 70.9) | 5.6 (0.0; 11.1) | 56.8 | 0.5 |
| Patient anxiety | −44.6 (−36.7; −52.4) 47.4 (39.6; 55.3) | 1.4 (−3.1; 6.1) | 46.9 | 0.7 |
| Given information | −48.2 (−39.2; −57.1) 55.0 (46.1; 64.0) | 3.4 (−1.7; 8.6) | 52.7 | 0.5 |
| Share feelings | −46.1 (−38.3; −53.8) 44.7 (36.9; 52.4) | −0.7 (−5.2; 3.8) | 46.3 | 0.6 |
| Life worthwhile | 0 | 0 | 0 | 1 |
| Feel good | 0 | 0 | 0 | 1 |
| Practical matters | −44.5 (−37.0; −52.2) 42.1 (34.6; 49.6) | −1.2 (−5.5; 3.1) | 44.2 | 0.7 |
| Total score | −21.5 (−17.5; −25.4) 22.2 (18.3; 26.1) | 0.4 (−1.9; 2.6) | 22.3 | 0.7 |
| Brief Pain Inventory (BPI) | ||||
| Pain intensity | −27.3 (−22.4; −32.2) 28.4 (23.6; 33.3) | 0.5 (−2.3; 3.4) | 28.4 | 0.8 |
| Pain impact | −35.0 (−28.7; −41.3) 35.8 (29.4; 42.1) | 0.4 (−3.2; 4.0) | 36.1 | 0.8 |
| Rotterdam Symptom Checklist (RSCL) | ||||
| Physical | −20.2 (−16.5; −23.9) 22.7 (19.1; 26.4) | 1.3 (−0.8; 3.4) | 21.9 | 0.7 |
| Psychological | −38.7 (−32.4; −45.0) 34.9 (28.5; 41.2) | −1.9 (−5.5; 1.7) | 37.5 | 0.7 |
| Activity | −31.4 (−25.1; −37.6) 41.5 (35.3; 47.0) | 5.1 (1.5; 8.7) | 37.2 | 0.8 |
| Global | −42.8 (−35.6; −50.0) 39.1 (31.9; 46.3) | −1.8 (−6.0; 2.3) | 41.8 | 0.7 |
| Fatigue | −29.5 (−24.3; −34.7) 31.1 (25.9; 36.3) | 0.8 (−2.2; 3.8) | 30.9 | 0.7 |
| Pain | −26.6 (−21.5; −31.6) 32.5 (27.3; 37.7) | 2.9 (0.0; 5.9) | 30.1 | 0.7 |
| Gastrointestinal | −27.3 (−22.3; −32.2) 30.6 (25.7; 35.6) | 1.7 (−1.2; 4.5) | 29.5 | 0.6 |
All the values are significant (p<0.01).
CI, confidence interval.
FIG. 2.
Bland-Altman plot of first and second measurements of Psychological well-being on POS. Note: The figure shows the limits of 95% of differences (mean of differences ±1.96* SD) including the 95% CI for the lower and the upper LOA.
The repeatability coefficient value of 29.2 shows that the probability of detecting a test-retest increase in the psychological well-being 29.2 scores in the test population is only 2.5%. The most stable items or scales were life worthwhile, feel good, psychological well-being, total score of POS, and physical of RSCL. All the Spearman's correlation coefficients were high and significant. The items with the higher correlations between first and second measurements were, for POS, psychological well-being (0.8), life worthwhile (1), feel good (1), practical matters (0.7), and total score (0.7); for BPI, pain intensity (0.8) and pain impact (0.8); and for RSCL, activity (0.8) and psychological (0.7).
Concurrent validity
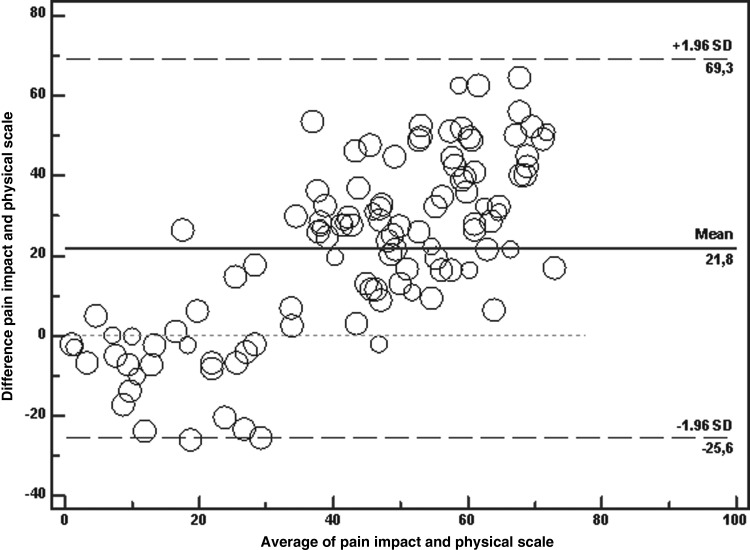
Table 3 shows comparison of items and total score of POS with equivalent domains on BPI and RSCL and repeated measurements with the difference between measurements by the questionnaires, compared two by two, for 95% of pair measurements on similar individuals. It is shown that zero lies within the 95% CI of the mean difference between symptoms on POS and physical on RSCL, and between pain intensity on BPI and pain and physical on RSCL, indicating no bias. When symptoms on POS is compared with physical domain on RSCL, the 95% LOA lie between −42.4 and 39.7. When pain intensity on the BPI is compared with pain on RSCL, the 95% LOA lie between −38.0 and 40.3. When pain intensity on BPI is compared with physical on RSCL, the 95% LOA lie between −33.5 and 37.1. In all other comparisons, zero doesn't lie within the 95% CI of the mean difference, indicating bias. Figure 3 shows that when pain impact on BPI is compared with physical on RSCL, the 95% LOA lie between −25.6 and 69.3, with zero out of the 95% CI of the mean difference between both domains (mean difference 21.8, 95% CI 17.4, 26.3), indicating lack of agreement. The limits don't appear to fit the data well.
Table 3.
Palliative Outcome Scale Agreement and Correlations with Brief Pain Inventory and Rotterdam Brief Pain Inventory Agreement and Correlation with Rotterdam
| Scale 1 | Scale 2 | 95% LOA (95% CI) | Mean difference between measurements (95% CI for bias) | Spearman correlationa |
|---|---|---|---|---|
| Pain on POS | Pain intensity on BPI | −48.7 (−42.5; −54.9) 27.0 (20.7; 33.2) | −10.9 (−14.4; −7.3) | 0.7 |
| Pain on RSCL | −59.2 (−51.5; −67.0) 36.6 (28.9; 43.8) | −11.3 (−15.7; −7.0) | 0.5 | |
| Feel good on POS | Pain impact on BPI | −37.7 (−27.7; −47.0) 82.7 (72.7; 92.7) | 22.5 (16.7; 28.3) | 0.4 |
| Symptoms on POS | Physical on RSCL | −42.4 (−35.7; −49.0) 39.7 (33.0; 40.3) | −1.3 (−5.2; 2.5) | 0.5 |
| Psychological well-being on POS | Psychological on RSCL | −27.1 (−21.0; −33.3) 48.6 (42.5; 54.7) | 10.7 (7.2; 14.3) | 0.6 |
| Total score on POS | Global on RSCL | −23.2 (−19.5; −6.9) 64.0 (57.7; 70.3) | 25.4 (21.8; 29.0) | 0.6 |
| Pain impact on BPI | −29.3 (−23.5; −35.1) 64.8 (59.0; 70.6) | 17.7 (14.4; 21.1) | 0.7 | |
| Pain intensity on BPI | Pain on RSCL | −38.0 (−31.6; −44.5) 40.3 (33.9; 46.7) | 1.1 (−2.6; 4.8) | 0.5 |
| Physical on RSCL | −33.5 (−27.7; −39.2) 37.1 (31.3; 42.9) | 1.8 (−1.5; 5.2) | 0.5 | |
| Pain impact on BPI | Physical on RSCL | −25.6 (−17.4; −32.9) 69.3 (61.1; 76.6) | 21.8 (17.4; 26.3) | 0.6 |
| Global on RSCL | −56.1 (−48.1; −64.1) 40.4 (32.4; 48.4) | −7.8 (−12.5; −3.2) | 0.6 | |
| Fatigue on RSCL | −38.3 (−31.2; −46.5) 53.3 (45.7; 60.9) | 7.2 (2.8;11.6) | 0.6 |
All the values are significant (p<0.01).
BPI, Brief Pain inventory; CI, confidence interval; POS, Palliative Outcome Scale; RSCL, Rotterdam Symptom Ckecklist.
FIG. 3.
Bland-Altman plot of Pain impact (BP) and Physical scale (RSCL) with multiple measurements per subject. Note: The figure shows that when pain impact on BPI is compared with physical on RSCL, the 95% LOA lie between −25.6 and 69.3 with zero out of the 95% CI of the mean difference between both domains.
All the Spearman's correlation coefficients were high and significant. The dimensions with the higher correlations were between pain intensity on BPI and pain on POS (0.7); pain impact on BPI and total score on POS (0.7); psychological on RSCL and psychological well-being on POS (0.6) and patient anxiety on POS (0.7).
Discussion
In this study including advanced cancer patients requiring PC provided by primary care physicians in Spain, secondary analysis of POS, BPI, and RSCL questionnaires has shown that all the questionnaires presented an adequate test-retest reliability and agreement, with marginal values in family anxiety on POS and pain on RSCL and good internal consistency. In relation to concurrent validity, agreement was only found between symptoms on POS and physical on RSCL, and between pain intensity on BPI and pain and physical domains on RSCL. However, all the correlations among questionnaires were significant and with high values. This high correlation does not mean that the questionnaires agree, due to the fact that the correlation measures the strength of a relation between two variables, not the agreement between them. The correlation is dependent on the range of the measurement and the variability among subjects—the more variable the subjects, the greater will be the correlation. These features are quite acceptable in the study of test-retest reliability, where the correlation can be regarded as an index of the information content of the measurement, but in the context of comparing two methods of measurement this approach is less convincing.19 In this study, high correlation (0.7) between pain intensity on BPI and pain on POS, for example, seems to be in poor agreement.
The questionnaires used measure different domains. In particular, POS is designed to capture all palliative concerns, not just symptoms and related domains; BPI measures pain and its impact on the patient's life; and RSCL addresses symptoms and QOL. It is unlikely that the questionnaires will agree exactly, by giving the identical result for all the individuals; so the comparison of the difference between the two questionnaires against their mean will show how much a questionnaire is likely to differ from the other. The magnitude of the difference that is acceptable is a clinical decision.18 However, the 95% CI of the mean difference between two questionnaires should contain zero in order to exclude lack of agreement. In accordance to this, symptoms on POS shows good agreement with physical on RSCL, and pain intensity on BPI shows good agreement with pain and physical on RSCL. All the other comparisons among equivalent domains of questionnaires show low agreement. To our knowledge this is the first time that the analysis of symptoms on POS shows a concurrent validity with physical on RSCL. All the other domains and items on POS are not interchangeable with RSCL or BPI.
Comparisons of these results with scientific literature data are very few. We have found concurrent validity data by means of correlation coefficients for BPI with RSCL, but not between the POS and the BPI or the RSCL. No results of questionnaire agreement have been found.
In the study by Badía and colleagues,8 the concurrent validity for both BPI dimensions was correlated with the physical, psychological, activity, and global scales of RSCL, with similar values to those obtained in our study, except for the correlation between physical and pain intensity, where the value was lower (0.28).
Although the RSCL was originally intended for use in cancer patients receiving active treatment, it was also used in advanced cancer patients, proving to be more useful as an instrument for physical and psychological symptom control than as an instrument for QOL measurement.12 The Spanish version of the RSCL has been validated by the authors of this study in patients with terminal cancer, showing good psychometric properties.9,16
Regarding BPI, pain intensity and impact are highly correlated as has been shown in previous studies.8,21 However for clinical trials in cancer patients it has been proposed to combine these two dimensions to form an overall summary score leading to a more simplified instrument with the aim of reducing the variety of existing multidimensional pain measurement tools.21 Other recommended questionnaires, translated into Spanish, to measure QOL in patients with advanced cancer are the EORTC QLQ-C30 and EORTC QLQ-C15-PAL24 questionnaires. EORTC QLQ-C30, a generic oncology questionnaire, has shown to be valid and reliable to measure QOL in patients with advanced cancer.22,23 The EORTC QLQ-C15-PAL24 questionnaire is a brief version of the QLQ-C30 for the measure of QOL in terminal patients with cancer, whose Spanish translation was published after this study was designed. In addition, there is no consensus yet on which is the best instrument to measure QOL in patients with advanced cancer.4,5
One limitation of this study comes from the secondary analysis of the basic study designed to compare symptom control for patients with advanced cancer, who were treated by physicians receiving online training or traditional training in PC. No significant differences were observed in symptom control for patients in both groups; therefore the whole set of questionnaires by all patients has been analyzed, observing the first or second completion times. Another limitation is that the number of patients included was not as expected in spite of the adequate number of physicians included; patients with cognitive impairment were excluded as well as those followed directly by the palliative team.
On the other hand, one of the strengths of the current study is that participants were patients from the whole country attended by primary care physicians, with specific characteristics (mainly men around 70 years of age, poorly educated, with low PS, treated with opiates and receiving PC at home) in whom the questionnaires have been very scarcely documented.
The practical implications of the study are that the questionnaires show an acceptable reliability in patients with advanced cancer receiving PC in a primary care setting, and that the physical scale of RSCL could be interchangeable by symptoms on POS, and pain intensity on BPI could be interchangeable by pain and physical on RSCL. Future research should expand the testing of these instruments in other patients receiving PC in different settings to confirm the results shown in this study.
Acknowledgments
The original work was supported by National Health Research Fund grant PI07051. The study sponsor had no influence on the study design, data collection, analysis, data interpretation, or decision to submit the manuscript for publication.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mularski RA. Dy SM. Shugarman LR. Wilkinson AM. Lynn J. Schekelle PG, et al. A systematic review of measures of end-of-life care and its outcomes. Health Serv Res. 2007;42:1848–1870. doi: 10.1111/j.1475-6773.2007.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teunissen SCCM. Wesker W. Kruitwagen C. de Haes H. Voest EE. de Graeff A. Symptom prevalence in patients with incurable cancer: A systematic review. J Pain Symptom Manage. 2007;34:94–104. doi: 10.1016/j.jpainsymman.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Lorenz KA. Dy SM. Naeim A. Walling AM. Sanati H. Smith P, et al. Quality measures for supportive cancer care: The cancer quality-ASSIST project. J Pain Symptom Manage. 2009;37:943–964. doi: 10.1016/j.jpainsymman.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Hjermstad MJ. Gibbins J. Haugen DF. Caraceni A. Loge JH. Kaasa S, et al. Pain assessment tools in palliative care: An urgent need for consensus. Palliat Med. 2008;22:895–903. doi: 10.1177/0269216308095701. [DOI] [PubMed] [Google Scholar]
- 5.Albers G. Echteld MA. de Vet HCW. Onwuteaka-Phlipsen BD. van der Linden MH. Deliens L. Evaluation of quality-of-life measures for use in palliative care: A systematic review. Palliat Med. 2010;24:17–37. doi: 10.1177/0269216309346593. [DOI] [PubMed] [Google Scholar]
- 6.Mularski RA. Rosenfeld K. Coons SJ. Duek A. Cella D. Fever DJ, et al. Measuring outcomes in randomized prospective trials in palliative care. J Pain Symptom Manage. 2007;34:S7–S19. doi: 10.1016/j.jpainsymman.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Pelayo M. Cebrián D. Areosa A. Agra A. Izquierdo JV. Buendía F. Effects of online palliative care training on knowledge, attitude and satisfaction of primary care physicians. www.biomedcentral.com/1471–2296/12/37. BMC Family Practice. 2011;12:37. doi: 10.1186/1471-2296-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badía X. Muriel C. Gracia A. Núñez-Olarte JM. Perulero N. Gálvez R, et al. Validation of the Spanish version of the Brief Pain Inventory in patients with oncology pain. Med Clin (Barc) 2003;120:52–59. doi: 10.1016/s0025-7753(03)73601-x. [DOI] [PubMed] [Google Scholar]
- 9.Agra Y. Badía X. Spanish version of the Rotterdam Symptom Checklist: Cross-cultural adaptation and preliminary validity in a sample of terminal cancer patients. Psychooncology. 1998;7:229–239. doi: 10.1002/(SICI)1099-1611(199805/06)7:3<229::AID-PON302>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 10.Serra-Prat M. Nabal M. Santacruz V. Picaza JM. Trellis J. Validation of the Spanish version of the Palliative Care Outcome Scale. Med Clin (Barc) 2004;123:406–412. doi: 10.1016/s0025-7753(04)74535-2. [DOI] [PubMed] [Google Scholar]
- 11.Caraceni A. Cherny N. Fainsinger R. Kaasa S. Poulain P. Radbruch L, et al. Pain measurement tools and methods in clinical research in palliative care: Recommendations of an expert working group of the European Association of Palliative Care. J Pain Symptom Manage. 2002;23:239–255. doi: 10.1016/s0885-3924(01)00409-2. [DOI] [PubMed] [Google Scholar]
- 12.Agra VY. Sacristán RA. Pelayo AM. Fernández J. Relación de la calidad de vida con diferentes modelos de atención domiciliaria en enfermos oncológicos terminales de un área sanitaria de Madrid. Rev Esp Salud Pública. 2003;77:567–579. [PubMed] [Google Scholar]
- 13.Schag CC. Heinrich RL. Ganz PA. Karnofsky performance status revisited: Reliability, validity, and guidelines. J Clin Oncology. 1984;2:187–193. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Martin P. Fernández-Mayoralas G. Frades-Payo B. Rojo-Pérez F. Petidier R. Rodriguez-Rodriguez V, et al. Validation of the Functional Independence Scale. Gac Sanit. 2009;1:49–54. doi: 10.1016/j.gaceta.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Siegert RJ. Gao W. Walkey FH. Higginson IJ. Psychological well-being and quality of care: A factor-analytic examination of the Palliative Care Outcome Scale. J Pain Symptom Manage. 2010;40:67–74. doi: 10.1016/j.jpainsymman.2009.11.326. [DOI] [PubMed] [Google Scholar]
- 16.Agra Y. Badía X. Evaluación de las propiedades psicométricas de la versión española del Rotterdam Symptom Checklist para medir calidad de vida en personas con cáncer. Rev Esp Salud Publica. 1999;73:35–44. [PubMed] [Google Scholar]
- 17.Bland JM. Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 18.Bland JM. Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17:571–582. doi: 10.1080/10543400701329422. [DOI] [PubMed] [Google Scholar]
- 19.Bland JM. Altman DG. A Note on the use of the intraclass correlation coefficient in the evaluation of agreement between two methods of measurement. Comput Biol Med. 1990;20:337–340. doi: 10.1016/0010-4825(90)90013-f. [DOI] [PubMed] [Google Scholar]
- 20.Hardy JR. Edmonds P. Turner R. Rees E. A'Hern R. The use of the Rotterdam Symptom Checklist in palliative care. J Pain Symptom Manage. 1998;18:79–84. doi: 10.1016/s0885-3924(99)00050-0. [DOI] [PubMed] [Google Scholar]
- 21.Fayers PM. Hjermstad MJ. Klepstad P. Loge JH. Caraceni A. Hanks GW, et al. The dimensionality of pain: Palliative care and chronic pain patients differ in their reports of pain intensity and pain interference. Pain. 2011;152:1608–1620. doi: 10.1016/j.pain.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 22.Arrarás JI. Illaramendi JJ. Valerdi JJ. El cuestionario de calidad de vida para cáncer de la EORTC, QOQ-C30. Estudio estadístico de validación con una muestra española. Rev Psicol Salud. 1995;7:13–34. [Google Scholar]
- 23.Godoy FMJ. Rojas TAJ. García PJL. Fiabilidad y validez de la versión española del EORTC QLQ-C30: Medida de la calidad de vida en pacientes oncológicos avanzados. Rev Psicol Salud. 1999;11:125–139. [Google Scholar]
- 24.Suárez-del-Real Y. Allende-Pérez S. Alférez-Mancera A. Rodríguez RB. Jiménez-Toxtle S. Mohar A. Oñate-Ocaña LF. Validation of the Mexican-Spanish version of the EORTC QLQ-C15-PAL questionnaire for the evaluation of health-related quality of life in patients on palliative care. Psychooncology. 2011;20(8):889–896. doi: 10.1002/pon.1801. [DOI] [PubMed] [Google Scholar]