Abstract
Significance: Both transfer RNA (tRNA) and cytochrome c are essential molecules for the survival of cells. tRNA decodes mRNA codons into amino-acid-building blocks in protein in all organisms, whereas cytochrome c functions in the electron transport chain that powers ATP synthesis in mitochondrion-containing eukaryotes. Additionally, in vertebrates, cytochrome c that is released from mitochondria is a potent inducer of apoptosis, activating apoptotic proteins (caspases) in the cytoplasm to dismantle cells. A better understanding of both tRNA and cytochrome c is essential for an insight into the regulation of cell life and death. Recent Advances: A recent study showed that the mitochondrion-released cytochrome c can be removed from the cell-death pathway by tRNA molecules. The direct binding of cytochrome c by tRNA provides a mechanism for tRNA to regulate cell death, beyond its role in gene expression. Critical Issues: The nature of the tRNA–cytochrome c binding interaction remains unknown. The questions of how this interaction affects tRNA function, cellular metabolism, and apoptotic sensitivity are unanswered. Future Directions: Investigations into the critical issues raised above will improve the understanding of tRNA in the fundamental processes of cell death and metabolism. Such knowledge will inform therapies in cell death-related diseases. Antioxid. Redox Signal. 19, 583–594.
Apoptosis and Caspases
Apoptosis is a physiological process by which unwanted or damaged cells are eliminated. It occurs extensively in developing animals, functioning in processes as diverse as sculpting organs, deleting structures that are no longer useful, eliminating nonfunctional or self-reactive lymphocytes, and matching the number of neurons with the target cells (53, 81). In adult animals, apoptosis has a fundamental role in the maintenance of homeostasis and the quality control of cells, including removal of cells infected by viruses, harboring extensive damages, or expressing oncogenes. Deregulation of apoptosis is linked to various devastating diseases. Defective apoptosis is closely linked to autoimmune disorders, viral infection, and the formation and therapeutic resistance of cancer cells, whereas excessive apoptosis is associated with various neurodegenerative diseases, myocardial infarction, and immunodeficiencies, including AIDS (105, 117).
Apoptotic cells undergo characteristic changes in their morphology, including plasma membrane blebbing, cell body shrinkage, nuclear condensation and fragmentation, and formation of membrane-bound apoptotic bodies (61). In vivo, apoptotic cells and the bodies formed by them are normally engulfed by healthy cells to prevent the release of intracellular contents. Apoptosis is also accompanied by characteristic biochemical changes, notably the appearance of discrete DNA fragments on conventional gel electrophoresis (due to cleavage between nucleosomes), the flipping of phosphatidylserine from the inner leaflet to the outer leaflet of the plasma membrane, and limited cleavage of a large number of cellular proteins.
The stereotypic changes in cell morphology and intracellular biochemistry are caused by a group of intracellular, cysteine-dependent aspartate-specific proteases or caspases (4). In healthy cells, caspases are generally kept in their inactive forms. However, caspases can also be activated to limited extents in the cells to perform diverse functions, such as proliferation, suppression of necrosis, and induction of inflammation. During apoptosis, caspases become fully active and cleave a wide array of intracellular targets. Caspase targets include other apoptotic proteins, cellular structural and survival proteins, transcriptional factors, signaling molecules, and proteins involved in DNA and RNA metabolism. These targets are cleaved by caspases in a limited manner, usually once or twice at the interdomain linker regions rather than being fully degraded. These cleavages lead to the activation of some targets and the inactivation of others (15, 70, 99).
Mechanisms of Caspase Activation
As with many other proteases, caspases are produced as latent precursors (procaspases). The activation of procaspases involves proteolytic processing at critical aspartate residues, which conform to the consensus substrate recognition sites of these enzymes. Consequently, caspases can be activated by proteolytic processing either by themselves or by another caspase. Both occur during apoptosis, where caspase activation proceeds in a cascade, with the upstream or initiator caspases (e.g., caspase-8 and 9) being activated by autoprocessing and the downstream or executioner caspases (e.g., caspase-3 and 7) being activated by initiator caspases (15, 70, 99).
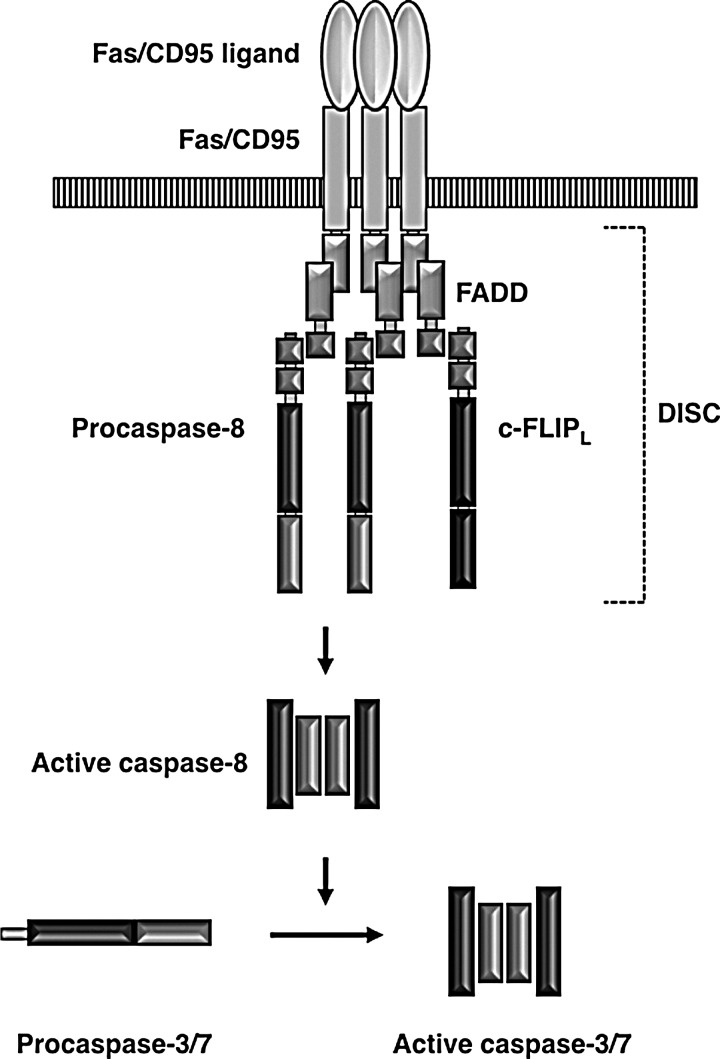
The activation of the initiator caspases, a key step in apoptosis, is induced by death adapter proteins. These adapters form caspase-activating platforms, either bound to the cell membrane or in the cytoplasm, in response to the extracellular and intracellular lethal cues, respectively. These cues activate two major apoptosis pathways in mammalian cells: the extrinsic and intrinsic pathways. The extrinsic pathway is mediated by a group of cell surface receptors, such as Fas/CD95 and tumor necrosis factor receptor. Upon binding to their cognate ligand, these receptors recruit an adaptor protein FADD. FADD then recruits an initiator procaspase, procaspase-8, to form an oligomeric death-inducing signaling complex (DISC) that leads to caspase-8 activation (6, 66) (Fig. 1). The intrinsic pathway, on the other hand, is activated by intracellular signals, including developmental lineage information, DNA damage, oncogenic stresses, and nutrient deprivation. These signals converge on mitochondria, leading to the release of cytochrome c to the cytoplasm. Cytochrome c is an essential component of the mitochondrial electron transport chain that drives ATP production. However, once in the cytoplasm, cytochrome c becomes a proapoptotic ligand. It binds to the death adapter apoptotic protease-activating factor-1 (Apaf-1), and in the presence of (d)ATP, this binding leads to the formation of an oligomeric complex known as the apoptosome. The apoptosome recruits the initiator caspase, caspase-9, leading to its activation (55, 98, 122) (Fig. 2).
FIG. 1.
The extrinsic apoptosis pathway. Engagement of death receptors (e.g., Fas, also known as CD95) by their cognate ligands (e.g., Fas/CD95 ligand) leads to the recruitment of the adaptor protein FADD. FADD in turn recruits procaspase-8, procaspase-10 (not shown), and a caspase-8/10-like molecule c-FLIP. These proteins form the DISC, in which procaspase-8 (and procaspase-10) becomes activated through an autoproteolytic cleavage. The active caspase-8 then cleaves and activates the effector caspases, caspase-3 and caspase-7. DISC, death-inducing signaling complex.
FIG. 2.
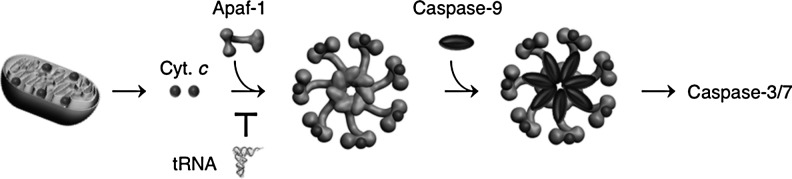
The intrinsic apoptosis pathway. Various intracellular death stimuli lead to the MOMP in a manner that is directly controlled by the Bcl-2 family proteins. This leads to the release of cytochrome c and other death inducers, including Smac/Diablo from the mitochondria. In the cytoplasm, cytochrome c binds to the death adapter Apaf-1 and promotes the formation of the oligomeric apoptosome. The apoptosome recruits procaspase-9, which becomes activated by autoproteolytic processing. Active caspase-9 then cleaves caspase-3/7. Apaf-1, apoptotic protease-activating factor-1; MOMP, mitochondrial outer membrane permeabilization; tRNA, transfer RNA.
Activation of procaspase-8 and procaspase-9 is induced by their oligomerization (12, 76, 78, 87, 113, 130, 131). Procaspase molecules such as procaspase-8 and procaspase-9 exist in healthy cells as monomers, which have no appreciable protease activity and cannot be cleaved into an active form. Upon oligomerization either in the DISC or on the apoptosome, these monomers acquire protease activity (7, 13). For caspase-8, these precursor dimers, although proteolytically active, show poor activity toward executioner caspases, and have to be first self-processed (13). A notable observation is that the dimerization also renders the caspase-8 zymogen molecules highly susceptible to cleavage to yield fully active initiator caspases (13). Thus, procaspase-8 activation likely occurs through cleavage between dimerized procaspase-8 (13). This interdimer processing mechanism provides a new paradigm for oligomerization-induced signaling, analogous to the previously established oligomerization-induced activation of receptor tyrosine kinases, in which the activation occurs through cross-phosphorylation between individual receptors. The interdimer processing mechanism minimizes caspase activation in healthy cells, yet it still permits rapid activation upon apoptosis induction. Because it requires at least four caspase-8 precursor molecules present in close proximity to initiate proteolytic processing, the interdimer processing mechanism minimizes the chance of accidental activation, as opposed to a mechanism whereby procaspase is activated by cleavage between individual caspases. At the same time, it allows for effective activation because caspases are oligomerized (not merely dimerized) during apoptosis, permitting formation of multiple dimers near one another to facilitate their cross processing. In other words, the interdimer processing mechanism enables a switch-like response of caspase activation to the apoptotic stimuli (13) (Fig. 3).
FIG. 3.
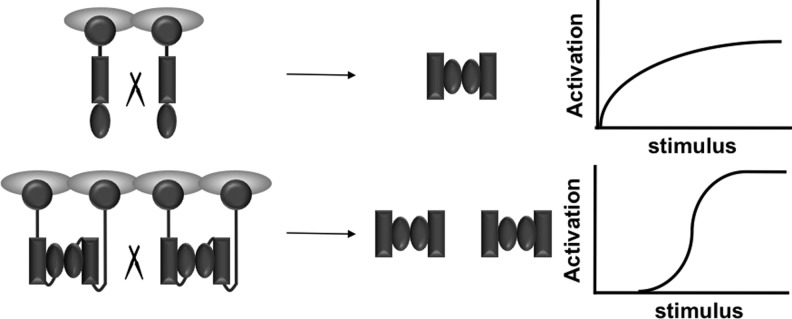
Interdimer processing mechanism of initiator caspase activation. Top: An intermonomer processing mechanism would generate graded caspase activation in response to the strength of the apoptotic stimuli. Bottom: An interdimer processing mechanism yields a switch-like activation of caspases. This would minimize accidental caspase activation while still permitting rapid caspase activation upon apoptosis induction.
The interdimer processing mechanism also engenders a new mode of regulating caspase activation. The fact that the dimerized procaspase-8, not monomeric caspase-8, is the active form permits the functional divergence of the two dimeric partners, one taking on the role of catalysis, whereas the other being a dedicated regulatory subunit. This prediction was first confirmed by an unexpected finding that c-FLIPL, a proteolytically inactive homolog of procapase-8, can promote caspase-8 activation (14). The interdimer processing mechanism was instrumental in understanding the control of caspase-8 activation during lymphocyte proliferation. Here, caspase-8 is heterodimerized with MALT1, a protease that bears similarity to caspases (paracaspase). This heterodimerization enables partial caspase-8 activation, and at the same time promotes the activity of procaspase-8 toward proliferative substrates, but not apoptotic substrates (59).
In contrast to initiator procaspases, the effector procaspases exist as dimers, although enzymatically inert. Their cleavage by active initiator caspases allows for the formation of a partially active dimer, which undergoes an autocleavage event through interdimer processing to become proteolytically fully competent (73). Thus, the interdimer processing mechanism applies to the activation of both the initiator and effect caspases. Effector caspases are the workhorse of apoptosis, responsible for the vast majority of the proteolytic events associated with apoptosis.
Regulation of Cytochrome c-Mediated Caspase-9 Activation
The intrinsic apoptosis is evolutionarily more ancient than the extrinsic pathway. It is also engaged in the extrinsic pathway in some cells to execute apoptosis. While the engagement of death receptors such as Fas sometimes can generate a proliferative signal (16), the engagement of the intrinsic pathway is almost always detrimental to a cell. In the intrinsic pathway, the release of cytochrome c from the mitochondria marks the defining step (74). The discovery that cytochrome c, a critical molecule for cell survival, is a potent death ligand came as a shock to the emerging field of apoptosis research where cell life and death were considered to be controlled by separate sets of proteins. Cytochrome c is a death inducer in vertebrates, but not in invertebrates such as Caenorhabditis elegans and Drosophila. This dichotomy of cytochrome c reflects an ingenious invention of evolution. Given the massive amount of cell death occurring normally in vertebrate cells as part of normal physiological processes, it would be challenging to ensure that, virtually, all cells retain the ability to commit suicide. By engaging cytochrome c for both cell death and survival, this provides a mechanism to coordinate the two processes by one molecule.
The release of cytochrome c follows mitochondrial outer membrane permeabilization (MOMP). MOMP is largely controlled by the pro- and antiapoptotic members of the Bcl-2 family proteins, which contain one to four Bcl-2 homology (BH) domains (1, 25). Functionally, Bcl-2 proteins are divided into three subfamilies: (i) antiapoptotic multiple-domain proteins (e.g., Bcl-2, Bcl-XL, and Mcl-1), (ii) proapoptotic multidomain proteins (mainly Bax and Bak), and (iii) proapoptotic BH3-only proteins (e.g., Bim, Puma, and Bid). Upon apoptosis induction, some BH3-only proteins (called activators) directly bind to and oligomerize the Bax/Bak protein. This leads to the formation of large channels on the outer membrane of mitochondria, allowing cytochrome c, as well as other cell death inducers, to be released from mitochondria. Other BH3-only members (called sensitizers) neutralize antiapoptotic members and sensitize cells to apoptosis stimuli. The activation of the BH3-only subfamily of proteins involves various strategies. For example, expression of Puma is induced by p53 upon severe DNA damage. Bid is activated by caspase-8-mediated proteolytic cleavage; this links the extrinsic pathway with the intrinsic one.
After MOMP, cytochrome c-mediated caspase-9 activation is also subject to intricate regulation. Effective formation of the apoptosome by Apaf-1 requires at least three additional proteins: HSP70, cellular apoptosis-susceptibility protein, and the tumor suppressor PHAPI. These proteins inhibit the aggregation of Apaf-1 into a nonfunctional complex and promote Apaf-1 assembly into the apoptosome (54, 63). The formation of apoptosome is inhibited by the oncoprotein prothymosin-α, although the underlying mechanism is unclear (54). Activation of caspase-9 and the downstream effector caspases is also regulated by inhibitors of apoptosis protein (IAPs) (38, 106). IAPs were first identified as baculovirus-encoded proteins that were able to block apoptosis of infected cells, hence their name. These cellular homologs were subsequently found in various species, each containing at least one, but often two to three, copies of the characteristic BIR sequence (baculovirus IAP repeat). In mammalian cells, the X-chromosome-linked IAP (XIAP) plays a major role in preventing caspase activation after cytochrome c release. XIAP can bind to both partially processed caspase-9 and partially processed caspase-3/7 through its BIR domains or the linker sequence, preventing the full maturation of these proteases. During apoptosis, XIAP is incapacitated through the binding of apoptotic inducers such as Smac/Diablo, which is released from mitochondria to the cytoplasm along with cytochrome c.
A notable feature of caspase-9 activation is its regulation by nucleotides. Either ATP or especially (d)ATP is required for the assembly of the apoptosome. In fact, the ability of (d)ATP to activate caspase in HeLa S100 cell lysates provided the biochemical assay that led to the identification of cytochrome c and other components (i.e., Apaf-1 and caspase-9) of the intrinsic pathway (71, 74, 133). In healthy cells, Apaf-1 binds to a (d)ATP molecule, which keeps it in an inactive conformation. Upon binding to cytochrome c, Apaf-1 hydrolyzes this (d)ATP molecule, releases the product (d)ADP, and then binds to another (d)ATP molecule. These steps are accompanied by a conformational change that permits the assembly of Apaf-1 into the apoptosome (62). In contrast, (d)NDP and (d)NMP inhibit apoptosome formation (74). In an interesting twist in the long line of research on cytochrome c-mediated caspase activation, transfer RNA (tRNA) was recently identified as a direct inhibitor of cytochrome c-mediated caspase activation.
The Structure and the Decoding Function of tRNA
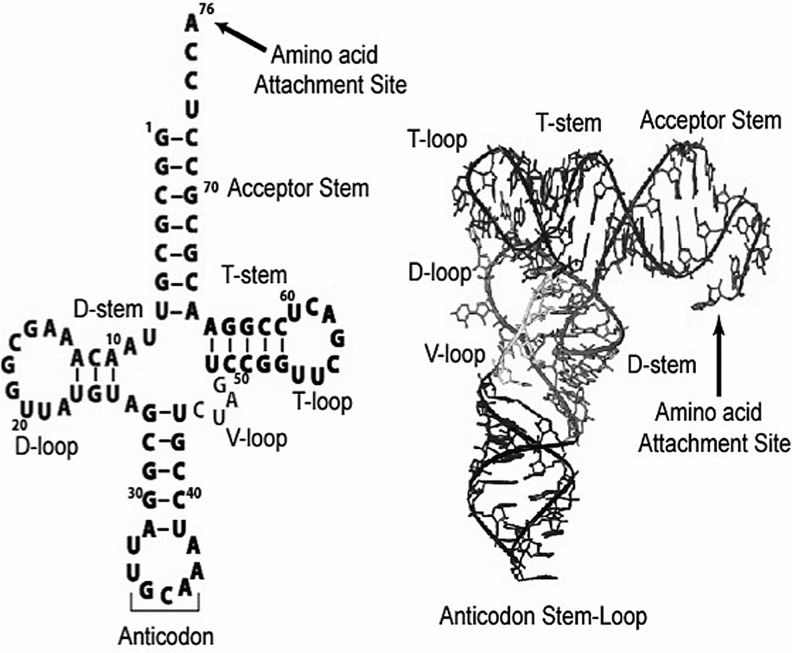
Matured tRNAs are highly differentiated nucleic acids comprised of 70–90 nucleotides that are folded into a compact cloverleaf secondary structure through base-pairing interactions within internal self-complementary regions. The cloverleaf structure is further folded into an L-shaped globular tertiary structure that brings the secondary elements dispersed in the sequence into close proximity. The folding into the L-shape is achieved by tertiary base-pairing interactions among a set of conserved nucleotides. This enables the acceptor stem to be coaxially stacked with the T stem to form the horizontal arm, whereas the dihydrouridine (D) stem and the anticodon stem to be coaxially stacked to form the vertical arm. This L-shaped arrangement places the amino acid attachment site to the conserved 3′-CCA sequence at one end of the L, while the anticodon triplet at the other end of the L (Fig. 4). This arrangement is believed to accommodate most known tRNA sequences (65), and the sequence framework in the arrangement is defined according to the sequence of yeast tRNAPhe, which was the first tRNA for which a high-resolution X-ray crystal structure was obtained (64, 100). Based on this sequence framework, the CCA sequence is at positions 74–76, while the anticodon triplet is at positions 34–36.
FIG. 4.
Secondary and tertiary structures of Escherichia coli tRNACys.
The tRNA molecules are differentiated from each other based on the amino acid that is attached (44). This attachment is determined in the aminoacylation reaction whereby an amino acid is activated by the hydrolysis of ATP, catalyzed by its cognate aminoacyl-tRNA synthetase, and is then transferred to the terminal ribose in the 3′-CCA sequence of the cognate tRNA. After this, each aminoacyl-tRNA (aa-tRNA) forms a ternary complex with a GTP-bound elongation factor (EF-Tu in bacteria and eEF-1α in eukaryotes) and is selected by a ribosome-A site for its anticodon base-pairing match with the codon of an mRNA (102). Correct pairing promotes accommodation of the aa-tRNA to the A site, along with the release of the factor and GDP. This is followed by the ribosome-catalyzed peptidyl transfer from the P site to the A site, resulting in the extension of the peptidyl group by the amino acid on the A site. After a ribosomal translocation, catalyzed by the factor EF-G and hydrolysis of GTP, the A-site tRNA is moved to the P site, and the deacylated P-site tRNA is moved to the E (exit) site (101). This cycle repeats until a stop codon enters the A site, which is followed by peptide release from the P site catalyzed by release factors.
Within the constraints of the cloverleaf and L-shaped structures, tRNA sequences can vary. The diversity in tRNA sequences provides the option to use specific sequences for peak performance in response to specific environmental demands. The diversity can arise from two mechanisms. In one, because of the degeneracy of the genetic code, there often exist several isoacceptor tRNAs for the same amino acid. Within one family of isoacceptors, while individual tRNA members differ in their anticodon sequences to read different codons; they also differ in other parts of the respective sequences. In the second, tRNAs are customized with many post-transcriptional modifications to introduce diverse chemical groups (e.g., a methyl or a sulfur group) to their bases and backbone ribose 2′-OH groups. With the exception of a few conserved modifications (e.g., s4U at position 8 before the D stem, ribothymidine at position 54 in the T loop, and Ψ [pseudouridine] at position 55 in the T loop), all other modifications are tailored to the activity of each tRNA. These modifications, each synthesized by a specific enzymatic pathway, enhance the overall structural and cellular stability of tRNA (86). Many of these modifications are concentrated at positions 34 (the wobble position of the anticodon) to expand the capacity of wobble base pairing during decoding. The modifications at position 34, alone or together with those at position 37 (on the 3′-side of the anticodon), play an important role in the overall accuracy of tRNA in the process of decoding and maintenance of the reading frame (2, 3). Free energy calculations indicate that modifications are essential for fidelity, because simple base-pairing interactions between codon and anticodon are insufficient to achieve the fidelity required for life (92, 102).
Nuclear-Encoded tRNAs
Nuclear-encoded tRNAs are synthesized by RNA polymerase III (Pol III) in the nucleus (9). Pol III is also responsible for the synthesis of 5S rRNA and several small noncoding RNAs. When yeast cells grow under a favorable growth condition, it was estimated that Pol III synthesizes 3–6×106 tRNA molecules per cell at a rate of 2–4 transcripts per gene (11). This high level of transcription is achieved in two mechanisms: (i) Pol III reinitiates many rounds of transcription on a DNA-bound initiation factor TFIIIB (29), and (ii) Pol III couples termination at the end of each round with initiation of the next round (28). The transcription activity of Pol III is tightly coordinated with the environmental growth conditions. When stress conditions occur, tRNA transcription is rapidly repressed through the action of Maf1, a negative regulator of Pol III with homologs in all eukaryotes (96). Repression of Pol III by Maf1 is the major form of transcription regulation and is initiated by adverse conditions, such as starvation or stress associated with replication, respiratory, and oxidative growth (20, 88, 126).
Recent studies have identified key aspects of Maf1 regulation. In optimal growth conditions, Maf1 is inactivated by phosphorylation, which operates at several levels; for example, phosphorylation decreases direct binding of Maf1 to Pol III, facilitates Maf1 export from the nucleus, and blocks import of cytoplasmic Maf1 to the nucleus (47, 91). By partitioning Maf1 predominantly into the cytoplasm, this reduces the probability of the factor to inhibit the Pol III elongation complex. While different protein kinases phosphorylate Maf1 for different effects (e.g., Protein kinase A and Sch9-kinase) (47, 84), two central cellular kinases, casein kinase 2 (CK2) and target of rapamycin TOR complex 1 (TORC1), are most relevant for regulation of tRNA transcription (37, 58, 83, 109, 124), based on their location directly on chromatins that contain tRNA genes. CK2 is conserved in organisms from yeast to humans as a key signaling protein in many cellular processes (34, 45, 46, 56), whereas TORC1 is the mammal-specific metabolic kinase operating on Pol III (8). Upon a shift to repressive conditions, however, CK2 is dissociated from chromatins of the tRNA genes (34), preventing Maf1 from rephosphorylation and enabling the dephosphorylated Maf1 to stay bound with Pol III. Thus, tRNA transcription is downregulated, and there is a retrograde transport of tRNAs into the nucleus (43). As shown by the recent crystal structure of Maf1 and cryo-EM structure of the Maf1-Pol III complex (121), the binding by Maf1 rearranges the subunit structure of Pol III such that the recruitment of Pol III to the promoters is impaired, and transcription initiation is inhibited. The regulation of Maf1 through phosphorylation provides a mechanism for immediate adjustment of Pol III activity according to the changes in environmental conditions.
All tRNAs are synthesized as precursors with 5′-leader and 3′-trailer sequences, and some with introns. In the nucleus, the first protein that binds to all newly synthesized pre-tRNAs is the La autoantigen, a highly abundant nuclear phosphoprotein that protects the 3′-terminus from exonuclease digestion (33, 72, 132). The La protein–pre-tRNA complex is the substrate for RNase P to remove the 5′-leader sequence. The processing of the 3′-trailer sequence in eukaryotes, where pre-tRNAs are synthesized without the CCA sequence, is achieved by a single endonucleolytic cleavage by RNase Z at position 73 (before the CCA position, known as the discrimination position) (30). The product of RNase Z is then a substrate for CCA addition. For pre-tRNAs that contain introns, the removal of the introns occurs primarily after CCA addition (132); the quality of the removal is inspected by the aminoacylation reaction inside the nucleus (75). The use of nuclear aminoacylation as a quality control is a mechanism to ensure that only properly processed tRNAs are exported to the cytoplasm. The locations where the extensive base and backbone modifications occur remain poorly understood and may be specific to each modification; for example, analysis of the location of the modification enzymes suggests that the modifications m22G26 in many tRNAs (32), m5C34 in tRNALeu (10), and Ψ55 in the conserved T loop (110) occur in end-matured, but intron-containing, pre-tRNAs, while the i6A37 modification in some tRNAs occur after intron removal (89). Some modifications, such as yW37 in tRNAPhe, may involve tRNA trafficking between the nucleus and the cytoplasm (93).
Mitochondrion-Encoded tRNAs
The mammalian mitochondrial organelles each contain a circular genome that encodes 22 tRNA genes, 2 rRNA genes, and 13 protein-coding genes. The mitochondrion-encoded tRNAs and rRNAs (mt-tRNAs and mt-rRNAs) are supplemented by nuclear-encoded protein enzymes and factors to constitute the specialized mitochondrial translation apparatus that is used to translate the 13 mitochondrion-encoded proteins, all of which are subunits of the electron transport chain (19). In contrast to the large number of nuclear-encoded tRNA genes, up to ∼270 distinct sequences (36), the rather small number of the mt-tRNA genes implies a limited capacity for decoding. Indeed, of the 22 mt-tRNAs, only mt-tRNASer and mt-tRNALeu have two isoacceptors, while each of the remaining mt-tRNAs represents a single species for one amino acid (42). In this limited decoding capacity, where one tRNA may be responsible for decoding up to four codons, the use of modified nucleotides in the tRNA anticodon and the application of wobble base pairing to read codon sequences must be extensively exploited.
The sequence and structure of mt-tRNAs have been greatly affected by the rapid evolution and genome economization of the mt-DNA sequence. To compensate for the deleterious effects caused by such pressure, mammalian mt-tRNAs appear to have created various structural motifs not present in their cytoplasmic counterparts (42, 115). Noncanonical structural features include the absence of the entire D stem–loop in mt-tRNASer (AGY) (which reads the codon AGY, where Y=C or U), the shortened D loop and V loop in mt-tRNASer (UCN) (where N=A, C, G, U), and the lack of tertiary interactions between the D and T loops in mt-tRNAPhe, mt-tRNAAsp, etc. Nonetheless, isolated mammalian mt-tRNAs having these noncanonical structural features are shown to maintain an approximate L-shape and to function in an in vitro translation system (26), indicating that they have the flexibility to accommodate the unusual features into a standard tRNA structure. However, in the case of mt-tRNALys, this accommodation is strictly dependent on the presence of an m1A9 modification located at the junction between the acceptor stem and D stem (41).
Transcription of the mt-tRNA genes is initiated by mitochondrial RNA polymerase (mt-RNAP) in complex with transcription factors Tfam and mt-TFB (35). The terminator factor mTERM recognizes both the promoter and terminator sequences to promote recycling of the transcriptional machinery (48). Transcription produces long polycistronic transcripts that are processed to generate separate mt-tRNAs, mt-rRNAs, and mt-mRNAs. Because mt-tRNAs contain no introns, the removal of the 5′-leader and 3′-trailer sequences by mt-RNaseP and mt-RNase Z, respectively, constitutes the basic steps of processing (103).
A Direct Interaction Between Cytochrome c and tRNA That Impacts Caspase Activation
From the beginning of research on cytochrome c-mediated caspase activation, a puzzling observation was that although the intracellular concentration of (d)ATP is only in a 10 μM range, up to 1 mM concentration of (d)ATP is needed to induce caspase-9 activation in cell lysates (74, 82). One possible explanation was that an inhibitor was present in the cell lysates, which decreased the effectiveness of (d)ATP. Because (d)NMP can inhibit caspase-9 activation, it seemed possible to us that RNA, which is essentially a polymer of nucleoside monophosphates, might have an inhibitory effect. To investigate the role of RNA in caspase activation, we removed RNA in HeLa S100 extracts by RNases. This strongly increased cytochrome c-induced caspase-9 activation. Conversely, the addition of RNA to the extracts impaired caspase-9 activation. These results suggested that one or more RNA species inhibit a factor required for caspase-9 activation. Systematic evaluation of the steps leading to caspase-9 activation revealed that cytochrome c is bound by an RNA species, which blocks cytochrome c from interacting with Apaf-1. To identify the RNA species, we stabilized the RNA–protein complexes inside intact cells with a low formaldehyde concentration and lysed cells in a buffer containing a strong detergent to prevent the nonspecific interaction during cell lysis. Analysis of the cross-linked complexes showed that several cytoplasmic and mitochondrial tRNAs specifically associate with cytochrome c. Microinjection of tRNA ablated the ability of cytochrome c to induce apoptosis, while treatment with Onconase, which preferentially degrades tRNA, enhanced apoptosis via the cytochrome c-dependent intrinsic pathway. Together, these findings suggested a direct role for tRNA in regulating apoptosis (80) (Fig. 2).
Both tRNA and cytochrome c are ancient molecules with fundamental roles in biology. The previously unexpected, direct interaction between them and the consequence of their interaction on caspase activation raise interesting questions as to how the interaction between tRNA and cytochrome c modulates apoptosis, metabolism, and the redox state of the cells. Below we discuss the implications of this finding in the context of apoptosis regulation, production of reactive oxygen species (ROS), and cancer pathogenesis and therapy.
Potential Regulation of the tRNA–Cytochrome c Interaction
Major inhibitors for the intrinsic apoptosis pathway appear to be counteracted upon apoptosis induction. For example, the antiapoptotic members of the Bcl-2 family proteins are inhibited by the BH3-only proteins, and IAPs by the mitochondrial death inducer Smac/Diablo. Emerging evidence suggests that tRNA metabolism is tightly linked to cellular responses. A conserved response to a variety of cellular stress conditions is cleavage of tRNAs near the anticodon by endonucleolytic ribonucleases (120). While this cleavage is not limited to specific tRNAs, it appears to target a small fraction of tRNAs, because full-length tRNA levels do not decline significantly (68, 118, 129). The cleavage generates approximately tRNA half-fragments, which have been found in several large-scale small RNA-sequencing projects (21, 40, 69). The existence of tRNA fragments in cells indicates their stability and argues that they are not produced by a mechanism to degrade misprocessed tRNA, which would have been degraded rapidly (17, 18, 23). Rather, the stable existence of tRNA fragments suggests that they may have cellular activities. Indeed, a recent study has shown that some tRNA fragments can inhibit protein synthesis by displacing eukaryotic initiation factors eIF4G/eIF4A from capped mRNAs and by displacing eIF4F from an isolated cap (52), thus inducing the formation of stress granules, which are essential components of the stress response program. Others show that tRNA fragments can guide RNase Z to target sequences (31), suggesting a mechanism to target specific mRNAs for degradation. In a third function, tRNA fragments are found in the argonaute and piwi complexes (60, 67), suggesting that they could have functions similar to siRNAs or miRNAs.
The endonucleases that generate tRNA fragments, Rny1 in yeast and angiogenin in mammals, are normally secreted or sequestered, but are released to the cytosol during stress (119, 129). As mentioned above, although mature nuclear-encoded tRNA functions predominantly in the cytoplasm, some mature tRNAs may return to the nucleus in response to particular stresses (108). It would be of great interest to investigate whether stress-induced tRNA processing or retrograde transport to the nucleus impacts cellular sensitivity to apoptosis.
tRNA–Cytochrome c Interaction and Post-MOMP Survival
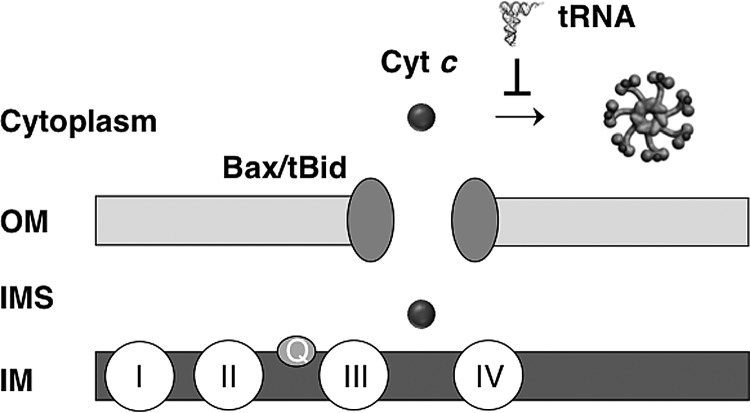
Although the release of cytochrome c after MOMP is the defining event in the intrinsic apoptosis pathway, cells sometimes recover from MOMP if caspase activation can be blocked (116). Both sympathetic neurons and cardiac myocytes are capable of recovery after MOMP in a physiological setting and are resistant to both endogenous cytochrome c release as well as exogenous cytochrome c injection (27, 79, 97, 128). The survival of these postmitotic cells after MOMP is highly advantageous, as these cells are essentially irreplaceable. Proliferating cells are also able to recover from MOMP. A genetic screen in the HeLa cells identified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as being capable of protecting cells from death after MOMP when caspase activation is prevented by chemical inhibitors (22). GAPDH increases glycolysis in HeLa cells. Unexpectedly, GADPH also has a nuclear function in stimulating the expression of the genes involved in autophagy (22), which also contributes to the post-MOMP survival of HeLa cells. The inhibition of caspase activation in both postmitotic and tumor cells would be made possible in part through the downregulation of Apaf-1 or the upregulation of XIAP, as suggested by some experiments (111). We speculate that tRNA in these cells may contribute to caspase inhibition through the binding to cytochrome c (Fig. 5).
FIG. 5.
Possible role of tRNA in cell survival after MOMP. The release of cytochrome c from the mitochondrial IMS is facilitated by proapoptotic multidomain Bcl-2 proteins (e.g., Bax) and BH3-only proteins (e.g., truncated Bid or tBid). The inhibitory effect of tRNA on the apoptotic function of cytochrome c may promote cell survival after the release of cytochrome c. BH, Bcl-2 homology; I–IV, complex I–IV of the electron transport chain; IM: mitochondrial inner membrane; IMS, inner membrane space; OM, mitochondrial outer membrane;.
Potential Effects of tRNA on Cytochrome c's Function in the Electron Transport Chain
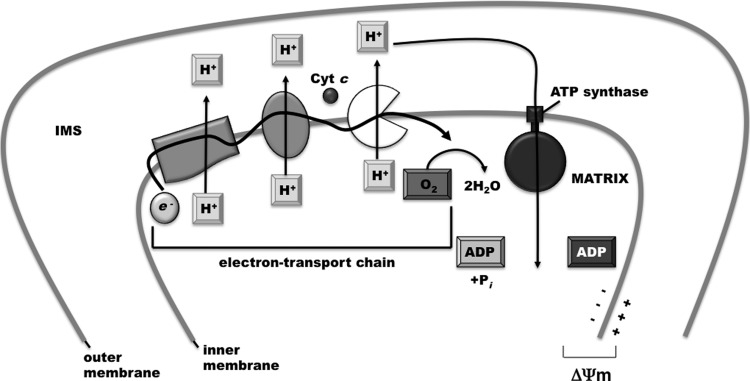
In most mitochondrion-containing eukaryotes, cytochrome c has an ancient and essential function in the electron transport chains. Cytochrome c resides in the mitochondrial inter membrane space carrying electrons from the coenzyme Q: cytochrome c-oxidoreductase (cytochrome bc1 complex/complex III) to cytochrome c oxidase (COX or complex IV). The proton gradient built by the electron transport chain creates the mitochondrial transmembrane potential (ΔΨm), which powers the synthesis of ATP via ATP synthase (Fig. 6). COX uses oxygen as the electron receptor for reduced cytochrome c, generating water as the end product. COX accounts for the vast majority of oxygen usage in our body, and is also a major source of ROS. Although highly speculative, we envision below two scenarios where the tRNA–cytochrome c interaction could influence the electron transport chain and ROS production.
FIG. 6.
Role of cytochrome c in the electron transport chain. Cytochrome c is normally located to the mitochondrial IMS. During electron transport, it carries electrons from the coenzyme Q: cytochrome c oxidoreductase (cytochrome bc1 complex/complex III) to COX or complex IV. The proton gradient built by this and the other steps of the electron transport chain creates the ΔΨm, which powers the synthesis of ATP through ATP synthase. COX or complex IV, cytochrome c oxidase; ΔΨm, mitochondrial transmembrane potential.
In the first scenario, mitochondrial function has been shown to be compromised after MOMP and decrease of ΔΨm. The mechanism for ΔΨm decline is still unclear, but the loss of cytochrome c from the mitochondria is likely a main contributing factor. The addition of exogenous cytochrome c alone to post-MOMP mitochondria could restore electron transport and maintain ΔΨm (85, 123). It is possible that upon MOMP, cytoplasmic tRNA gains access to the intermembrane space, where it may inhibit the function of cytochrome c. This might contribute to the decline in ΔΨm and influence ROS production.
In the second scenario, the interaction between cytochrome c and tRNA may occur in healthy cells. This is suggested by our previous work. To capture the cytochrome c–tRNA interaction in cells and to prevent their association after cell lysates, we fixed the HeLa cells in low concentrations of formaldehyde, which fixes the tRNA–protein interaction, and then the lysed cells were in a buffer containing zwitterionic detergent Empigen BB, which disrupts the noncovalent RNA–protein interactions. Under these conditions, we detected the cytochrome c–tRNA interaction in healthy cells. This association needs to be further verified. If it indeed occurs, the function of cytochrome c inside mitochondria could be regulated by tRNA. Of note, although the mammalian mitochondrial genome encodes tRNAs for all amino acids, nuclear-encoded tRNAs are still imported into mitochondria (104). This raises the possibility that the cytochrome c–nuclear tRNA interaction in healthy cells, if it occurs, may represent a way of communication between mitochondria and the rest of the cells.
tRNA–Cytochrome c Interaction in Cancer Biology and Therapy
Evasion of apoptosis is a hallmark of cancer cells (39). It contributes to both the formation and therapeutic resistance of cancer cells. Relative to normal cells, cancer cells increase proliferation and elevate protein synthesis levels by altering the regulation of individual components of the translation machinery (125, 127). Deregulation of Pol III and its products is observed in a wide range of transformed cells, and this deregulation itself can also drive transformation. For example, moderate overexpression of initiator tRNA drives cell proliferation, resulting in malignant transformation of immortalized mouse fibroblasts (77). While initiator tRNA is important for controlling the initiation phase of protein synthesis, elongator tRNAs have the ability to regulate the speed of translation during the elongation phase, suggesting that both initiator and elongator tRNAs at elevated levels are relevant to cancer development and progression. Indeed, a genome-wide analysis of tRNA expression has shown an increase by up to threefold for nuclear-encoded tRNA and by up to fivefold for mitochondrion-encoded tRNA in cancer-derived versus noncancer-derived breast cell lines, and an increase by up to 13–20-fold for both types of tRNAs in tumors versus normal breast tissues (95).
Further analysis in the above study has shown that the tRNA overexpression in cancer cells is not random (95); certain individual tRNAs are more strongly overexpressed than others. For example, among the nuclear-encoded tRNAs, those specific for Ser, Thr, and Tyr are the most strongly overexpressed. These amino acids are targets for protein kinases, suggesting that tRNA overexpression can provide a mechanism to regulate the proteins involved in signal transduction. Additionally, tRNA overexpression is specific to certain isoacceptors. For example, while tRNAArg, tRNALeu, tRNASer are known to have a greater number of isoacceptors than other tRNAs, only two isoacceptors of tRNAArg (those with the anticodon UCU and CCU), one isoacceptor of tRNALeu (with the anticodon UAA), and one isoacceptor of tRNASer (with the anticodon CGA) are among those most highly expressed. The selectivity among isoacceptors suggests a correlation between tRNA overexpression and codon usage. This correlation has been confirmed in the cancer-related genes, such as those involved in cell cycle control, in extracellular matrix formation, and in transcription regulation, but not found in cell-line-specific or in house-keeping genes (95). These data suggest that the elevated tRNA expression in breast cancer cells can fine-tune the translation efficiency of specific codons in specific genes that are important for cancer. This notion is consistent with the general concept developed from studies of bacteria and yeast, in that the amount of tRNA isoacceptors during active cell growth is correlated with the codon usage of the most highly expressed genes (49, 50, 57, 90).
New work also suggests that stress-induced tRNA cleavage and release of RNases into the cytosol may be involved in cancer, as well as other diseases. For example, tRNA fragments are found in the serum and urine of humans and mice with certain tumors (112), suggesting that tRNA cleavage occurs in tumors in situ. Further studies are necessary to elucidate the functional consequence of elevated tRNA expression and apoptosis resistance of cancer cells.
The effects of current chemo- and radiation therapies are largely directed at DNA in tumor cells. In contrast, few therapeutics, either being developed or being used in the clinic, target RNA. Of note, a few RNase drugs have a specific antitumor activity dependent on their ribonucleolytic cleavage activities (5, 24). Onconase/Ranpirnase, the furthest developed, is in clinical trials for various cancers (5, 24). Onconase preferentially cleaves tRNA in a manner consistent with apoptotic sensitivity (51, 107, 114). Compared to traditional chemotherapy, Onconase has a low systemic toxicity. Because Onconase potentially disables an apoptosis resistance mechanism downstream of cytochrome c release, it kills tumors cells independently of the p53 status. Similarly, inhibition of the cleavage by antagonists of angiogenin (a member of the RNase A family) has been shown to delay or to prevent tumor development in vivo (94). A clearer understanding of the advantage tRNA provides tumor cells beyond supporting protein synthesis, and why tumor cells particularly are sensitive to tRNA hydrolysis may lead to the improvement of Onconase and other RNase therapeutics.
Summary
tRNA is present in all known forms of life. While its role in mRNA translation has been extensively investigated, the functions of tRNA beyond gene expression are rarely explored. Cytochrome c is similarly evolutionarily ancient. It is an essential component of the mitochondrial electron transport chain that powers ATP synthesis, and is required for life in mitochondrion-containing eukaryotes. Cytochrome c also has a critical role in cell death in vertebrates, and its release from mitochondria is a defining step in the intrinsic apoptosis pathway that leads to the formation of the apoptosome and the activation of caspase-9. The finding that tRNA directly binds to cytochrome c and diminishes its proapoptotic activity presents a unique paradigm for the innate and dynamic connections of the RNA and protein worlds. Further investigation of the structural basis of this interaction, its regulation, and its role in apoptosis, metabolism, and tumorigenesis will likely enrich our understanding of tRNA biology and life and death in both dividing cells and in postmitotic cells. This understanding will provide a basis for therapies of a range of cell death-related diseases.
Abbreviations Used
- ΔΨm
mitochondrial transmembrane potential
- Ψ
pseudouridine
- aa-tRNA
aminoacyl-tRNA
- Apaf-1
apoptotic protease-activating factor-1
- BH
Bcl-2 homology
- CK2
casein kinase 2
- COX or complex IV
cytochrome c oxidase
- DISC
death-inducing signaling complex
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IAPs
inhibitors of apoptosis protein
- I–IV
complex I–IV of the electron-transport chain
- IMS
inner membrane space
- MOMP
mitochondrial outer membrane permeabilization
- mt-RNAP
mitochondrial RNA polymerase
- mt-tRNA and mt-mRNA
mitochondrion-encoded tRNA and mRNA
- Pol III
RNA polymerase
- ROS
reactive oxygen species
- TORC1
Tor complex 1
- tRNA
transfer RNA
- XIAP
X-chromosome-linked IAP
Acknowledgments
This work was supported in part by NIH grants (GM081601 and AG042169) and by an MDA grant (157681) to Y.-M. H., and by GM060911 to X.Y. The authors thank C. O'Neill for help with manuscript preparation.
References
- 1.Adams JM. Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Agris PF. Decoding the genome: a modified view. Nucleic Acids Res. 2004;32:223–238. doi: 10.1093/nar/gkh185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agris PF. Vendeix FA. Graham WD. tRNA's wobble decoding of the genome: 40 years of modification. J Mol Biol. 2007;366:1–13. doi: 10.1016/j.jmb.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Alnemri ES. Livingston DJ. Nicholson DW. Salvesen G. Thornberry NA. Wong WW. Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 5.Ardelt W. Shogen K. Darzynkiewicz Z. Onconase and amphinase, the antitumor ribonucleases from Rana pipiens oocytes. Curr Pharm Biotechnol. 2008;9:215–225. doi: 10.2174/138920108784567245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashkenazi A. Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 7.Boatright KM. Renatus M. Scott FL. Sperandio S. Shin H. Pedersen IM. Ricci JE. Edris WA. Sutherlin DP. Green DR. Salvesen GS. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 8.Boguta M. Control of RNA polymerases I and III by the TOR signaling pathway. Cell Cycle. 2009;8:4023–4024. doi: 10.4161/cc.8.24.10534. [DOI] [PubMed] [Google Scholar]
- 9.Boguta M. Graczyk D. RNA polymerase III under control: repression and de-repression. Trends Biochem Sci. 2011;36:451–456. doi: 10.1016/j.tibs.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Brzezicha B. Schmidt M. Makalowska I. Jarmolowski A. Pienkowska J. Szweykowska-Kulinska Z. Identification of human tRNA:m5C methyltransferase catalysing intron-dependent m5C formation in the first position of the anticodon of the pre-tRNA Leu (CAA) Nucleic Acids Res. 2006;34:6034–6043. doi: 10.1093/nar/gkl765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabart P. Lee J. Willis IM. Facilitated recycling protects human RNA polymerase III from repression by Maf1 in vitro. J Biol Chem. 2008;283:36108–36117. doi: 10.1074/jbc.M807538200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang DW. Ditsworth D. Liu H. Srinivasula SM. Alnemri ES. Yang X. Oligomerization is a general mechanism for the activation of apoptosis initiator and inflammatory procaspases. J Biol Chem. 2003;278:16466–16469. doi: 10.1074/jbc.C300089200. [DOI] [PubMed] [Google Scholar]
- 13.Chang DW. Xing Z. Capacio VL. Peter ME. Yang X. Interdimer processing mechanism of procaspase-8 activation. EMBO J. 2003;22:4132–4142. doi: 10.1093/emboj/cdg414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang DW. Xing Z. Pan Y. Algeciras-Schimnich A. Barnhart BC. Yaish-Ohad S. Peter ME. Yang X. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002;21:3704–3714. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang HY. Yang X. Proteases for cell suicide: functions and regulation of caspases. Microbiol Mol Biol Rev. 2000;64:821–846. doi: 10.1128/mmbr.64.4.821-846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L. Park SM. Tumanov AV. Hau A. Sawada K. Feig C. Turner JR. Fu YX. Romero IL. Lengyel E. Peter ME. CD95 promotes tumour growth. Nature. 2010;465:492–496. doi: 10.1038/nature09075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chernyakov I. Baker MA. Grayhack EJ. Phizicky EM. Chapter 11. Identification and analysis of tRNAs that are degraded in Saccharomyces cerevisiae due to lack of modifications. Methods Enzymol. 2008;449:221–237. doi: 10.1016/S0076-6879(08)02411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chernyakov I. Whipple JM. Kotelawala L. Grayhack EJ. Phizicky EM. Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5′-3′ exonucleases Rat1 and Xrn1. Genes Dev. 2008;22:1369–1380. doi: 10.1101/gad.1654308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christian BE. Spremulli LL. Mechanism of protein biosynthesis in mammalian mitochondria. Biochim Biophys Acta. 2012;1819:1035–1054. doi: 10.1016/j.bbagrm.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciesla M. Towpik J. Graczyk D. Oficjalska-Pham D. Harismendy O. Suleau A. Balicki K. Conesa C. Lefebvre O. Boguta M. Maf1 is involved in coupling carbon metabolism to RNA polymerase III transcription. Mol Cell Biol. 2007;27:7693–7702. doi: 10.1128/MCB.01051-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole C. Sobala A. Lu C. Thatcher SR. Bowman A. Brown JW. Green PJ. Barton GJ. Hutvagner G. Filtering of deep sequencing data reveals the existence of abundant Dicer-dependent small RNAs derived from tRNAs. RNA. 2009;15:2147–2160. doi: 10.1261/rna.1738409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colell A. Ricci JE. Tait S. Milasta S. Maurer U. Bouchier-Hayes L. Fitzgerald P. Guio-Carrion A. Waterhouse NJ. Li CW. Mari B. Barbry P. Newmeyer DD. Beere HM. Green DR. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–997. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 23.Copela LA. Fernandez CF. Sherrer RL. Wolin SL. Competition between the Rex1 exonuclease and the La protein affects both Trf4p-mediated RNA quality control and pre-tRNA maturation. RNA. 2008;14:1214–1227. doi: 10.1261/rna.1050408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costanzi J. Sidransky D. Navon A. Goldsweig H. Ribonucleases as a novel pro-apoptotic anticancer strategy: review of the preclinical and clinical data for ranpirnase. Cancer Invest. 2005;23:643–650. doi: 10.1080/07357900500283143. [DOI] [PubMed] [Google Scholar]
- 25.Danial NN. Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 26.de Bruijn MH. Schreier PH. Eperon IC. Barrell BG. Chen EY. Armstrong PW. Wong JF. Roe BA. A mammalian mitochondrial serine transfer RNA lacking the “dihydrouridine” loop and stem. Nucleic Acids Res. 1980;8:5213–5222. doi: 10.1093/nar/8.22.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deshmukh M. Johnson EM., Jr Evidence of a novel event during neuronal death: development of competence-to-die in response to cytoplasmic cytochrome c. Neuron. 1998;21:695–705. doi: 10.1016/s0896-6273(00)80587-5. [DOI] [PubMed] [Google Scholar]
- 28.Dieci G. Sentenac A. Facilitated recycling pathway for RNA polymerase III. Cell. 1996;84:245–252. doi: 10.1016/s0092-8674(00)80979-4. [DOI] [PubMed] [Google Scholar]
- 29.Dieci G. Sentenac A. Detours and shortcuts to transcription reinitiation. Trends Biochem Sci. 2003;28:202–209. doi: 10.1016/S0968-0004(03)00054-9. [DOI] [PubMed] [Google Scholar]
- 30.Dubrovsky EB. Dubrovskaya VA. Levinger L. Schiffer S. Marchfelder A. Drosophila RNase Z processes mitochondrial and nuclear pre-tRNA 3′ ends in vivo. Nucleic Acids Res. 2004;32:255–262. doi: 10.1093/nar/gkh182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elbarbary RA. Takaku H. Uchiumi N. Tamiya H. Abe M. Takahashi M. Nishida H. Nashimoto M. Modulation of gene expression by human cytosolic tRNase Z(L) through 5′-half-tRNA. PLoS One. 2009;4:e5908. doi: 10.1371/journal.pone.0005908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellis SR. Morales MJ. Li JM. Hopper AK. Martin NC. Isolation and characterization of the TRM1 locus, a gene essential for the N2,N2-dimethylguanosine modification of both mitochondrial and cytoplasmic tRNA in Saccharomyces cerevisiae. J Biol Chem. 1986;261:9703–9709. [PubMed] [Google Scholar]
- 33.Fan H. Goodier JL. Chamberlain JR. Engelke DR. Maraia RJ. 5′ Processing of tRNA precursors can be modulated by the human La antigen phosphoprotein. Mol Cell Biol. 1998;18:3201–3211. doi: 10.1128/mcb.18.6.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghavidel A. Schultz MC. TATA binding protein-associated CK2 transduces DNA damage signals to the RNA polymerase III transcriptional machinery. Cell. 2001;106:575–584. doi: 10.1016/s0092-8674(01)00473-1. [DOI] [PubMed] [Google Scholar]
- 35.Gleyzer N. Vercauteren K. Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol. 2005;25:1354–1366. doi: 10.1128/MCB.25.4.1354-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodenbour JM. Pan T. Diversity of tRNA genes in eukaryotes. Nucleic Acids Res. 2006;34:6137–6146. doi: 10.1093/nar/gkl725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graczyk D. Debski J. Muszynska G. Bretner M. Lefebvre O. Boguta M. Casein kinase II-mediated phosphorylation of general repressor Maf1 triggers RNA polymerase III activation. Proc Natl Acad Sci U S A. 2011;108:4926–4931. doi: 10.1073/pnas.1010010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gyrd-Hansen M. Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer. 2010;10:561–574. doi: 10.1038/nrc2889. [DOI] [PubMed] [Google Scholar]
- 39.Hanahan D. Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Haussecker D. Huang Y. Lau A. Parameswaran P. Fire AZ. Kay MA. Human tRNA-derived small RNAs in the global regulation of RNA silencing. RNA. 2010;16:673–695. doi: 10.1261/rna.2000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helm M. Brule H. Degoul F. Cepanec C. Leroux JP. Giege R. Florentz C. The presence of modified nucleotides is required for cloverleaf folding of a human mitochondrial tRNA. Nucleic Acids Res. 1998;26:1636–1643. doi: 10.1093/nar/26.7.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helm M. Brule H. Friede D. Giege R. Putz D. Florentz C. Search for characteristic structural features of mammalian mitochondrial tRNAs. RNA. 2000;6:1356–1379. doi: 10.1017/s1355838200001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hopper AK. Shaheen HH. A decade of surprises for tRNA nuclear-cytoplasmic dynamics. Trends Cell Biol. 2008;18:98–104. doi: 10.1016/j.tcb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Hou YM. Francklyn C. Schimmel P. Molecular dissection of a transfer RNA and the basis for its identity. Trends Biochem Sci. 1989;14:233–237. doi: 10.1016/0968-0004(89)90033-9. [DOI] [PubMed] [Google Scholar]
- 45.Hu P. Samudre K. Wu S. Sun Y. Hernandez N. CK2 phosphorylation of Bdp1 executes cell cycle-specific RNA polymerase III transcription repression. Mol Cell. 2004;16:81–92. doi: 10.1016/j.molcel.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Hu P. Wu S. Hernandez N. A minimal RNA polymerase III transcription system from human cells reveals positive and negative regulatory roles for CK2. Mol Cell. 2003;12:699–709. doi: 10.1016/j.molcel.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 47.Huber A. Bodenmiller B. Uotila A. Stahl M. Wanka S. Gerrits B. Aebersold R. Loewith R. Characterization of the rapamycin-sensitive phosphoproteome reveals that Sch9 is a central coordinator of protein synthesis. Genes Dev. 2009;23:1929–1943. doi: 10.1101/gad.532109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hyvarinen AK. Pohjoismaki JL. Reyes A. Wanrooij S. Yasukawa T. Karhunen PJ. Spelbrink JN. Holt IJ. Jacobs HT. The mitochondrial transcription termination factor mTERF modulates replication pausing in human mitochondrial DNA. Nucleic Acids Res. 2007;35:6458–6474. doi: 10.1093/nar/gkm676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981;151:389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- 50.Ikemura T. Correlation between the abundance of yeast transfer RNAs and the occurrence of the respective codons in protein genes. Differences in synonymous codon choice patterns of yeast and Escherichia coli with reference to the abundance of isoaccepting transfer RNAs. J Mol Biol. 1982;158:573–597. doi: 10.1016/0022-2836(82)90250-9. [DOI] [PubMed] [Google Scholar]
- 51.Iordanov MS. Ryabinina OP. Wong J. Dinh TH. Newton DL. Rybak SM. Magun BE. Molecular determinants of apoptosis induced by the cytotoxic ribonuclease onconase: evidence for cytotoxic mechanisms different from inhibition of protein synthesis. Cancer Res. 2000;60:1983–1994. [PubMed] [Google Scholar]
- 52.Ivanov P. Emara MM. Villen J. Gygi SP. Anderson P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell. 2011;43:613–623. doi: 10.1016/j.molcel.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacobson MD. Weil M. Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 54.Jiang X. Kim HE. Shu H. Zhao Y. Zhang H. Kofron J. Donnelly J. Burns D. Ng SC. Rosenberg S. Wang X. Distinctive roles of PHAP proteins and prothymosin-alpha in a death regulatory pathway. Science. 2003;299:223–226. doi: 10.1126/science.1076807. [DOI] [PubMed] [Google Scholar]
- 55.Jiang X. Wang X. Cytochrome C-mediated apoptosis. Annu Rev Biochem. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- 56.Johnston IM. Allison SJ. Morton JP. Schramm L. Scott PH. White RJ. CK2 forms a stable complex with TFIIIB and activates RNA polymerase III transcription in human cells. Mol Cell Biol. 2002;22:3757–3768. doi: 10.1128/MCB.22.11.3757-3768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanaya S. Yamada Y. Kudo Y. Ikemura T. Studies of codon usage and tRNA genes of 18 unicellular organisms and quantification of Bacillus subtilis tRNAs: gene expression level and species-specific diversity of codon usage based on multivariate analysis. Gene. 1999;238:143–155. doi: 10.1016/s0378-1119(99)00225-5. [DOI] [PubMed] [Google Scholar]
- 58.Kantidakis T. Ramsbottom BA. Birch JL. Dowding SN. White RJ. mTOR associates with TFIIIC, is found at tRNA and 5S rRNA genes, and targets their repressor Maf1. Proc Natl Acad Sci U S A. 2010;107:11823–11828. doi: 10.1073/pnas.1005188107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawadler H. Gantz MA. Riley JL. Yang X. The paracaspase MALT1 controls caspase-8 activation during lymphocyte proliferation. Mol Cell. 2008;31:415–421. doi: 10.1016/j.molcel.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawamura Y. Saito K. Kin T. Ono Y. Asai K. Sunohara T. Okada TN. Siomi MC. Siomi H. Drosophila endogenous small RNAs bind to Argonaute 2 in somatic cells. Nature. 2008;453:793–797. doi: 10.1038/nature06938. [DOI] [PubMed] [Google Scholar]
- 61.Kerr JFR. Wyllie AH. Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim HE. Du F. Fang M. Wang X. Formation of apoptosome is initiated by cytochrome c-induced dATP hydrolysis and subsequent nucleotide exchange on Apaf-1. Proc Natl Acad Sci U S A. 2005;102:17545–17550. doi: 10.1073/pnas.0507900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim HE. Jiang X. Du F. Wang X. PHAPI, CAS, and Hsp70 promote apoptosome formation by preventing Apaf-1 aggregation and enhancing nucleotide exchange on Apaf-1. Mol Cell. 2008;30:239–247. doi: 10.1016/j.molcel.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 64.Kim SH. Suddath FL. Quigley GJ. McPherson A. Sussman JL. Wang AH. Seeman NC. Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974;185:435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- 65.Kim SH. Sussman JL. Suddath FL. Quigley GJ. McPherson A. Wang AH. Seeman NC. Rich A. The general structure of transfer RNA molecules. Proc Natl Acad Sci U S A. 1974;71:4970–4974. doi: 10.1073/pnas.71.12.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krammer PH. Arnold R. Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 67.Lau NC. Seto AG. Kim J. Kuramochi-Miyagawa S. Nakano T. Bartel DP. Kingston RE. Characterization of the piRNA complex from rat testes. Science. 2006;313:363–367. doi: 10.1126/science.1130164. [DOI] [PubMed] [Google Scholar]
- 68.Lee SR. Collins K. Starvation-induced cleavage of the tRNA anticodon loop in Tetrahymena thermophila. J Biol Chem. 2005;280:42744–42749. doi: 10.1074/jbc.M510356200. [DOI] [PubMed] [Google Scholar]
- 69.Lee YS. Shibata Y. Malhotra A. Dutta A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs) Genes Dev. 2009;23:2639–2649. doi: 10.1101/gad.1837609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li J. Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–6206. doi: 10.1038/onc.2008.297. [DOI] [PubMed] [Google Scholar]
- 71.Li P. Nijhawan D. Budihardjo I. Srinivasula SM. Ahmad M. Alnemri ES. Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 72.Lin-Marq N. Clarkson SG. Efficient synthesis, termination and release of RNA polymerase III transcripts in Xenopus extracts depleted of La protein. EMBO J. 1998;17:2033–2041. doi: 10.1093/emboj/17.7.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu H. Chang DW. Yang X. Interdimer processing and linearity of procaspase-3 activation. A unifying mechanism for the activation of initiator and effector caspases. J Biol Chem. 2005;280:11578–11582. doi: 10.1074/jbc.M414385200. [DOI] [PubMed] [Google Scholar]
- 74.Liu X. Kim CN. Yang J. Jemmerson R. Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 75.Lund E. Dahlberg JE. Proofreading and aminoacylation of tRNAs before export from the nucleus. Science. 1998;282:2082–2085. doi: 10.1126/science.282.5396.2082. [DOI] [PubMed] [Google Scholar]
- 76.MacCorkle RA. Freeman KW. Spencer DM. Synthetic activation of caspases: artificial death switches. Proc Natl Acad Sci U S A. 1998;95:3655–3660. doi: 10.1073/pnas.95.7.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marshall L. Kenneth NS. White RJ. Elevated tRNA(iMet) synthesis can drive cell proliferation and oncogenic transformation. Cell. 2008;133:78–89. doi: 10.1016/j.cell.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 78.Martin DA. Siegel RM. Zheng L. Lenardo MJ. Membrane oligomerization and cleavage activates the caspase-8 (FLICE/MACHalpha1) death signal. J Biol Chem. 1998;273:4345–4349. doi: 10.1074/jbc.273.8.4345. [DOI] [PubMed] [Google Scholar]
- 79.Martinou I. Desagher S. Eskes R. Antonsson B. Andre E. Fakan S. Martinou JC. The release of cytochrome c from mitochondria during apoptosis of NGF-deprived sympathetic neurons is a reversible event. J Cell Biol. 1999;144:883–889. doi: 10.1083/jcb.144.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mei Y. Yong J. Liu H. Shi Y. Meinkoth J. Dreyfuss G. Yang X. tRNA binds to cytochrome c and inhibits caspase activation. Mol Cell. 2010;37:668–678. doi: 10.1016/j.molcel.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meier P. Finch A. Evan G. Apoptosis in development. Nature. 2000;407:796–801. doi: 10.1038/35037734. [DOI] [PubMed] [Google Scholar]
- 82.Mesner PW., Jr. Bible KC. Martins LM. Kottke TJ. Srinivasula SM. Svingen PA. Chilcote TJ. Basi GS. Tung JS. Krajewski S. Reed JC. Alnemri ES. Earnshaw WC. Kaufmann SH. Characterization of caspase processing and activation in HL-60 cell cytosol under cell-free conditions. Nucleotide requirement and inhibitor profile. J Biol Chem. 1999;274:22635–22645. doi: 10.1074/jbc.274.32.22635. [DOI] [PubMed] [Google Scholar]
- 83.Michels AA. Robitaille AM. Buczynski-Ruchonnet D. Hodroj W. Reina JH. Hall MN. Hernandez N. mTORC1 directly phosphorylates and regulates human MAF1. Mol Cell Biol. 2010;30:3749–3757. doi: 10.1128/MCB.00319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moir RD. Lee J. Haeusler RA. Desai N. Engelke DR. Willis IM. Protein kinase A regulates RNA polymerase III transcription through the nuclear localization of Maf1. Proc Natl Acad Sci U S A. 2006;103:15044–15049. doi: 10.1073/pnas.0607129103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mootha VK. Wei MC. Buttle KF. Scorrano L. Panoutsakopoulou V. Mannella CA. Korsmeyer SJ. A reversible component of mitochondrial respiratory dysfunction in apoptosis can be rescued by exogenous cytochrome c. EMBO J. 2001;20:661–671. doi: 10.1093/emboj/20.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Motorin Y. Helm M. tRNA stabilization by modified nucleotides. Biochemistry. 2010;49:4934–4944. doi: 10.1021/bi100408z. [DOI] [PubMed] [Google Scholar]
- 87.Muzio M. Stockwell BR. Stennicke HR. Salvesen GS. Dixit VM. An induced proximity model of caspase-8 activation. J Biol Chem. 1998;273:2926–2930. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- 88.Nguyen VC. Clelland BW. Hockman DJ. Kujat-Choy SL. Mewhort HE. Schultz MC. Replication stress checkpoint signaling controls tRNA gene transcription. Nat Struct Mol Biol. 2010;17:976–981. doi: 10.1038/nsmb.1857. [DOI] [PubMed] [Google Scholar]
- 89.Nishikura K. De Robertis EM. RNA processing in microinjected Xenopus oocytes. Sequential addition of base modifications in the spliced transfer RNA. J Mol Biol. 1981;145:405–420. doi: 10.1016/0022-2836(81)90212-6. [DOI] [PubMed] [Google Scholar]
- 90.Novoa EM. Ribas de Pouplana L. Speeding with control: codon usage, tRNAs, and ribosomes. Trends Genet. 2012;28:574–581. doi: 10.1016/j.tig.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 91.Oficjalska-Pham D. Harismendy O. Smagowicz WJ. Gonzalez de Peredo A. Boguta M. Sentenac A. Lefebvre O. General repression of RNA polymerase III transcription is triggered by protein phosphatase type 2A-mediated dephosphorylation of Maf1. Mol Cell. 2006;22:623–632. doi: 10.1016/j.molcel.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 92.Ogle JM. Ramakrishnan V. Structural insights into translational fidelity. Annu Rev Biochem. 2005;74:129–177. doi: 10.1146/annurev.biochem.74.061903.155440. [DOI] [PubMed] [Google Scholar]
- 93.Ohira T. Suzuki T. Retrograde nuclear import of tRNA precursors is required for modified base biogenesis in yeast. Proc Natl Acad Sci U S A. 2011;108:10502–10507. doi: 10.1073/pnas.1105645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Olson KA. Fett JW. French TC. Key ME. Vallee BL. Angiogenin antagonists prevent tumor growth in vivo. Proc Natl Acad Sci U S A. 1995;92:442–446. doi: 10.1073/pnas.92.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pavon-Eternod M. Gomes S. Geslain R. Dai Q. Rosner MR. Pan T. tRNA over-expression in breast cancer and functional consequences. Nucleic Acids Res. 2009;37:7268–7280. doi: 10.1093/nar/gkp787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pluta K. Lefebvre O. Martin NC. Smagowicz WJ. Stanford DR. Ellis SR. Hopper AK. Sentenac A. Boguta M. Maf1p, a negative effector of RNA polymerase III in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:5031–5040. doi: 10.1128/MCB.21.15.5031-5040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Potts MB. Vaughn AE. McDonough H. Patterson C. Deshmukh M. Reduced Apaf-1 levels in cardiomyocytes engage strict regulation of apoptosis by endogenous XIAP. J Cell Biol. 2005;171:925–930. doi: 10.1083/jcb.200504082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Riedl SJ. Salvesen GS. The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- 99.Riedl SJ. Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 100.Robertus JD. Ladner JE. Finch JT. Rhodes D. Brown RS. Clark BF. Klug A. Structure of yeast phenylalanine tRNA at 3 A resolution. Nature. 1974;250:546–551. doi: 10.1038/250546a0. [DOI] [PubMed] [Google Scholar]
- 101.Rodnina MV. Beringer M. Wintermeyer W. How ribosomes make peptide bonds. Trends Biochem Sci. 2007;32:20–26. doi: 10.1016/j.tibs.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 102.Rodnina MV. Wintermeyer W. Ribosome fidelity: tRNA discrimination, proofreading and induced fit. Trends Biochem Sci. 2001;26:124–130. doi: 10.1016/s0968-0004(00)01737-0. [DOI] [PubMed] [Google Scholar]
- 103.Rossmanith W. Tullo A. Potuschak T. Karwan R. Sbisa E. Human mitochondrial tRNA processing. J Biol Chem. 1995;270:12885–12891. doi: 10.1074/jbc.270.21.12885. [DOI] [PubMed] [Google Scholar]
- 104.Rubio MA. Rinehart JJ. Krett B. Duvezin-Caubet S. Reichert AS. Soll D. Alfonzo JD. Mammalian mitochondria have the innate ability to import tRNAs by a mechanism distinct from protein import. Proc Natl Acad Sci U S A. 2008;105:9186–9191. doi: 10.1073/pnas.0804283105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rudin CM. Thompson CB. Apoptosis and disease: regulation and clinical relevance of programmed cell death. Annu Rev Med. 1997;48:267–281. doi: 10.1146/annurev.med.48.1.267. [DOI] [PubMed] [Google Scholar]
- 106.Salvesen GS. Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 107.Saxena SK. Sirdeshmukh R. Ardelt W. Mikulski SM. Shogen K. Youle RJ. Entry into cells and selective degradation of tRNAs by a cytotoxic member of the RNase A family. J Biol Chem. 2002;277:15142–15146. doi: 10.1074/jbc.M108115200. [DOI] [PubMed] [Google Scholar]
- 108.Shaheen HH. Horetsky RL. Kimball SR. Murthi A. Jefferson LS. Hopper AK. Retrograde nuclear accumulation of cytoplasmic tRNA in rat hepatoma cells in response to amino acid deprivation. Proc Natl Acad Sci U S A. 2007;104:8845–8850. doi: 10.1073/pnas.0700765104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shor B. Wu J. Shakey Q. Toral-Barza L. Shi C. Follettie M. Yu K. Requirement of the mTOR kinase for the regulation of Maf1 phosphorylation and control of RNA polymerase III-dependent transcription in cancer cells. J Biol Chem. 2010;285:15380–15392. doi: 10.1074/jbc.M109.071639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Simos G. Tekotte H. Grosjean H. Segref A. Sharma K. Tollervey D. Hurt EC. Nuclear pore proteins are involved in the biogenesis of functional tRNA. EMBO J. 1996;15:2270–2284. [PMC free article] [PubMed] [Google Scholar]
- 111.Soengas MS. Alarcon RM. Yoshida H. Giaccia AJ. Hakem R. Mak TW. Lowe SW. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science. 1999;284:156–159. doi: 10.1126/science.284.5411.156. [DOI] [PubMed] [Google Scholar]
- 112.Speer J. Gehrke CW. Kuo KC. Waalkes TP. Borek E. tRNA breakdown products as markers for cancer. Cancer. 1979;44:2120–2123. doi: 10.1002/1097-0142(197912)44:6<2120::aid-cncr2820440623>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 113.Srinivasula SM. Ahmad M. Fernandes-Alnemri T. Alnemri ES. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol Cell. 1998;1:949–957. doi: 10.1016/s1097-2765(00)80095-7. [DOI] [PubMed] [Google Scholar]
- 114.Suhasini AN. Sirdeshmukh R. Transfer RNA cleavages by onconase reveal unusual cleavage sites. J Biol Chem. 2006;281:12201–12209. doi: 10.1074/jbc.M504488200. [DOI] [PubMed] [Google Scholar]
- 115.Suzuki T. Nagao A. Human mitochondrial tRNAs: biogenesis, function, structural aspects, and diseases. Annu Rev Genet. 2011;45:299–329. doi: 10.1146/annurev-genet-110410-132531. [DOI] [PubMed] [Google Scholar]
- 116.Tait SW. Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 117.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 118.Thompson DM. Lu C. Green PJ. Parker R. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA. 2008;14:2095–2103. doi: 10.1261/rna.1232808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thompson DM. Parker R. The RNase Rny1p cleaves tRNAs and promotes cell death during oxidative stress in Saccharomyces cerevisiae. J Cell Biol. 2009;185:43–50. doi: 10.1083/jcb.200811119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thompson DM. Parker R. Stressing out over tRNA cleavage. Cell. 2009;138:215–219. doi: 10.1016/j.cell.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 121.Vannini A. Ringel R. Kusser AG. Berninghausen O. Kassavetis GA. Cramer P. Molecular basis of RNA polymerase III transcription repression by Maf1. Cell. 2010;143:59–70. doi: 10.1016/j.cell.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 122.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 123.Waterhouse NJ. Goldstein JC. von Ahsen O. Schuler M. Newmeyer DD. Green DR. Cytochrome c maintains mitochondrial transmembrane potential and ATP generation after outer mitochondrial membrane permeabilization during the apoptotic process. J Cell Biol. 2001;153:319–328. doi: 10.1083/jcb.153.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wei Y. Tsang CK. Zheng XF. Mechanisms of regulation of RNA polymerase III-dependent transcription by TORC1. EMBO J. 2009;28:2220–2230. doi: 10.1038/emboj.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.White RJ. RNA polymerase III transcription and cancer. Oncogene. 2004;23:3208–3216. doi: 10.1038/sj.onc.1207547. [DOI] [PubMed] [Google Scholar]
- 126.Willis IM. Moir RD. Integration of nutritional and stress signaling pathways by Maf1. Trends Biochem Sci. 2007;32:51–53. doi: 10.1016/j.tibs.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 127.Winter AG. Sourvinos G. Allison SJ. Tosh K. Scott PH. Spandidos DA. White RJ. RNA polymerase III transcription factor TFIIIC2 is overexpressed in ovarian tumors. Proc Natl Acad Sci U S A. 2000;97:12619–12624. doi: 10.1073/pnas.230224097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wright KM. Linhoff MW. Potts PR. Deshmukh M. Decreased apoptosome activity with neuronal differentiation sets the threshold for strict IAP regulation of apoptosis. J Cell Biol. 2004;167:303–313. doi: 10.1083/jcb.200406073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yamasaki S. Ivanov P. Hu GF. Anderson P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol. 2009;185:35–42. doi: 10.1083/jcb.200811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang X. Chang HY. Baltimore D. Autoproteolytic activation of pro-caspases by oligomerization. Mol Cell. 1998;1:319–325. doi: 10.1016/s1097-2765(00)80032-5. [DOI] [PubMed] [Google Scholar]
- 131.Yang X. Chang HY. Baltimore D. Essential role of CED-4 oligomerization in CED-3 activation and apoptosis. Science. 1998;281:1355–1357. doi: 10.1126/science.281.5381.1355. [DOI] [PubMed] [Google Scholar]
- 132.Yoo CJ. Wolin SL. The yeast La protein is required for the 3′ endonucleolytic cleavage that matures tRNA precursors. Cell. 1997;89:393–402. doi: 10.1016/s0092-8674(00)80220-2. [DOI] [PubMed] [Google Scholar]
- 133.Zou H. Henzel WJ. Liu X. Lutschg A. Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]