Abstract
Significance: The intrinsic apoptosis pathway is conserved from worms to humans and plays a critical role in the normal development and homeostatic control of adult tissues. As a result, numerous diseases from cancer to neurodegeneration are associated with either too little or too much apoptosis. Recent Advances: B cell lymphoma-2 (BCL-2) family members regulate cell death, primarily via their effects on mitochondria. In stressed cells, proapoptotic BCL-2 family members promote mitochondrial outer membrane permeabilization (MOMP) and cytochrome c (cyt c) release into the cytoplasm, where it stimulates formation of the “apoptosome.” This large, multimeric complex is composed of the adapter protein, apoptotic protease-activating factor-1, and the cysteine protease, caspase-9. Recent studies suggest that proteins involved in the processes leading up to (and including) formation of the apoptosome are subject to various forms of post-translational modification, including proteolysis, phosphorylation, and in some cases, direct oxidative modification. Critical Issues: Despite intense investigation of the intrinsic pathway, significant questions remain regarding how cyt c is released from mitochondria, how the apoptosome is formed and regulated, and how caspase-9 is activated within the complex. Future Directions: Further studies on the biochemistry of MOMP and apoptosome formation are needed to understand the mechanisms that underpin these critical processes, and novel animal models will be necessary in the future to ascertain the importance of the many posttranslational modifications reported for BCL-2 family members and components of the apoptosome. Antioxid. Redox Signal. 19, 546–558.
Introduction
Apoptosis is a programmed form of cell death, characterized by the activation of cysteinyl aspartate-specific proteases (caspases) and the systematic breakdown of dying cells into easily phagocytized apoptotic bodies. Depending upon the stimulus, apoptosis is generally executed through activation of either the extrinsic (death receptor) pathway or the intrinsic (mitochondrial) pathway. Both pathways initially activate an apical caspase (i.e., caspase-8 or caspase-9), which in turn activates the downstream effector caspases-3 and -7, resulting in cell death. The intrinsic pathway is activated in response to intracellular stressors, induced by a litany of stimuli including DNA damage and growth factor withdrawal. These stress signals ultimately trigger mitochondrial outer membrane permeabilization (MOMP), wherein the outer mitochondrial membrane (OMM) undergoes permeabilization, generally as a result of the activation of certain proapoptotic BCL-2 family members (142). MOMP then facilitates the release of several proapoptotic factors, including cytochrome c (cyt c), from the mitochondrial intermembrane space (IMS) into the cytoplasm, where they promote cell death. In particular, cyt c interacts with apoptotic protease-activating factor-1 (Apaf-1) and stimulates (d)ATP-dependent oligomerization of Apaf-1 into a caspase-activating complex known as the “Apaf-1 apoptosome.” The apoptosome subsequently recruits the initiator procasapase-9 through caspase recruitment domains (CARDs) present in the N-termini of both Apaf-1 and procaspase-9. Once bound, active caspase-9 then processes the effector caspase-3 and induces death (16).
The goal of this review is to cover recent discoveries related to the apoptosome, and more generally, the impact of reactive oxygen species (ROS) on the intrinsic pathway. However, as one might expect, ROS can potentially impact this pathway in many ways. For those familiar with pop culture, there is a trivia game known as the “Six Degrees of Kevin Bacon,” in which players attempt to connect any person in Hollywood with the actor, Kevin Bacon, in less than six steps. Similarly, there are numerous ways, both direct and indirect, to connect oxidative stress with the intrinsic pathway, far more than can be adequately addressed here. Thus, we shall focus primarily on those events that immediately impact cyt c (or its release), Apaf-1, or caspase-9—or to keep with the analogy, we will not stray more than two degrees from the apoptosome.
BCL-2 Family Members and MOMP
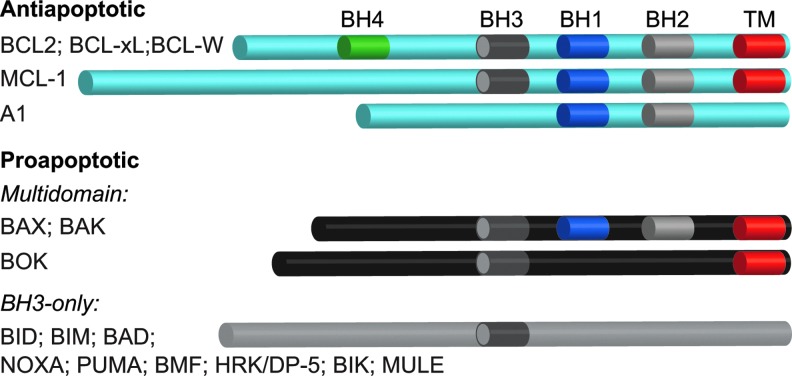
Arguably, the key step in the intrinsic pathway is permeabilization of the OMM, an event that is regulated by members of the BCL-2 family (Fig. 1). BCL-2 proteins are characterized by the presence of one to four BCL-2 homology (BH) domains and the ability to regulate apoptosis, at least in part, through the regulation of MOMP. The proapoptotic family members include a BAX-like subfamily (BAX, BAK, and BOK), as well as a “BH3-only” subfamily (BIM, BID, PUMA, BAD, NOXA, etc.). BAX and BAK proteins contain BH 1-3 domains, but lack a BH4 domain, and when activated, are thought to stimulate MOMP and the release of cyt c by forming pores in the OMM (Figs. 1 and 2). BH3-only proteins such as truncated BID (tBID), on the other hand, promote apoptosis by antagonizing antiapoptotic BCL-2 family members and/or by directly activating BAX-like family members (Figs. 1 and 2). Antiapoptotic family members (BCL-2, BCL-XL, MCL-1, BCL-W, and A1) contain all four BH domains (with the exception of MCL-1 and A1) and neutralize BAX-like and/or BH3-only family members, thereby preventing BAX/BAK oligomerization and pore formation (128, 142) (Figs. 1 and 2).
FIG. 1.
Anti- and proapoptotic BCL2 family members. Antiapoptotic BCL-2 family members (BCL-2, BCL-xL, BCL-W, MCL-1, and A1) keep the multidomain proapoptotic family members (BAX, BAK, and BOK) in check through heterodimerization. BH3-only family members stimulate MOMP and cell death, either by directly activating BAX, BAK, and/or BOK, or by binding to and neutralizing the antiapoptotic family members, although these mechanisms are not mutually exclusive. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
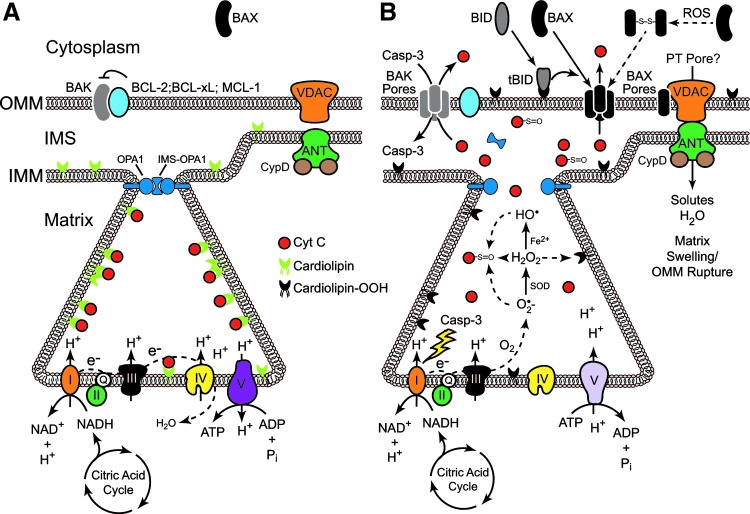
FIG. 2.
Regulation of MOMP by BCL-2 family members and ROS. (A) Cartoon of a mitochondrion prior to MOMP. In resting cells, BAX is located in the cytoplasm in an inactive state, while BAK is located in the OMM, where it is directly inhibited by antiapoptotic BCL-2 family members. The convoluted IMM forms functional cristae that meet at crista junctions through the action of OPA1 complexes. Cyt c is bound to cardiolipin within the cristae and engages the ETC, transferring electrons between complexes III and IV. The proton gradient generated from the ETC drives ATP production via the F1F0-ATP synthase (complex V). (B) Cartoon of a mitochondrion during MOMP. BH3-only family members (e.g., tBID), antagonize antiapoptotic family members and/or directly activate BAX and BAK, resulting in the formation of BAX/BAK pores in the OMM through which cyt c may pass. There is some evidence that ROS may also oxidize BAX, resulting in the formation of BAX dimers (connected via a disulfide bond), which subsequently translocate to the OMM. At the crista junctions, tBID and non-oligomerized BAX/BAK induce OPA1-dependent opening of the junction through mechanisms that remain unresolved. Within the cristae, ROS disrupt the interaction of cyt c with cardiolipin by directly oxidizing cardiolipin. Cyt c then freely passes out of the intra-cristae space into the IMS and subsequently into the cytoplasm through BAX/BAK pores, where it can activate the Apaf-1 apoptosome. Loss of cyt c from the ETC then triggers the production of O2•− as electrons are transferred to O2, rather than complex IV. Notably, cyt c can also be directly oxidized, particularly at Met-80, which disrupts its interaction with heme iron. The absence of heme alters the conformational shape of cyt c and inhibits its ability to stimulate activation of the apoptosome. Once caspases are activated, they can cross the permeabilized OMM and target subunits in complexes I and II, further disrupting the ETC, and forcing complex V to run in reverse in a futile effort to re-establish a proton gradient and membrane potential. Finally, in some cases, particularly following exposure to ROS and Ca2+, mitochondria can undergo the permeability transition, wherein the IMM becomes permeable to water and solutes (perhaps through activation of a PT pore). In this case, the matrix begins to swell, and because the surface area of the IMM vastly exceeds that of the OMM, the OMM eventually ruptures and cyt c escapes into the cytoplasm. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
In terms of the direct effects of ROS on BCL-2 family members, hydrogen peroxide (H2O2) reportedly induces BAX dimerization through direct formation of a Cys-62/Cys-126 disulfide bond that promotes its translocation from the cytoplasm (where it normally resides) to the OMM (30) (Fig. 2B). However, in most cases, oxidative stress alters the function of BCL-2 family members by regulating the kinases that phosphorylate them. Broadly speaking, ROS are capable of activating p38 mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and Akt pathways, depending upon the context (132). However, a detailed discussion of all the mechanisms whereby ROS alter kinase function, and consequently BCL-2 function, are beyond the scope of the current review. Suffice it to say, these kinases have established roles in the regulation of many pro- and antiapoptotic BCL-2 family members, often converging upon them and regulating their expression or function in highly complex ways, as in the case of MCL-1 regulation by ERKs, JNKs, and glycogen synthase kinase-3β (GSK-3β) (32, 53, 83, 86).
Cyt c and ROS
Oxidants are thought to play important roles in the release of cyt c from mitochondria following MOMP and in the ability of cyt c to stimulate formation of the apoptosome. However, cyt c release also disrupts the electron transport chain (ETC) and stimulates the production of ROS. In the next section, we discuss these mechanisms in greater detail.
Role of ROS in cyt c release
Cardiolipin is a unique phospholipid found in the inner mitochondrial membrane (IMM), where it associates with several enzymes required for oxidative phosphorylation, including cyt c (108, 109). Since mitochondria are the main intracellular source of ROS, especially at Complexes I and III, cardiolipin is directly exposed to relatively high concentrations of ROS in stressed mitochondria. Moreover, cardiolipin contains four typically unsaturated fatty acyl chains (compared with two in most phospholipids) and is therefore more vulnerable to oxidation. Cardiolipin-bound cyt c also serves as a cardiolipin-specific peroxidase that can oxidize cardiolipin in an H2O2-dependent manner (54). Once oxidized, cardiolipin dissociates from enzymes in the IMM, including cyt c, and becomes enriched in the OMM (91, 117). tBID appears to target the OMM, at least in part, due to its strong binding affinity for cardiolipin and then promotes BAX oligomerization, which ultimately leads to pore formation and cyt c release (43, 44, 66, 72, 73). Thus, in short, cyt c release is a cardiolipin-dependent process in which cardiolipin oxidation plays an important role (Fig. 2).
Notably, the vast majority of cyt c (>85%) is present within cristae—folded structures formed by the IMM—and it appears that crista junction opening (CJO) is required to liberate oxidized cyt c from the intra-cristae space into the IMS (115). Optic atrophy 1 (OPA1), a dynamin-related GTPase located on the IMM, regulates mitochondrial fusion and CJO. OPA1 is proteolytically cleaved, most likely by OMA1 and Yme1 (and possibly PARL), and the truncated form of OPA1 reportedly oligomerizes with noncleaved OPA1 to form a complex at the crista junction that regulates its “tightness” (29, 33, 36, 48, 97, 121). Remarkably, CJO and MOMP are separable but required events for cyt c release and apoptosis. Indeed, while tBID and other BH3-only proteins induce the disassembly of OPA1 complexes in a BAX/BAK-dependent manner, unlike MOMP, CJO does not require oligomerization of BAK. Moreover, cells expressing a disassembly-resistant OPA1 mutant (Q297V) undergo normal MOMP, but fail to release significant amounts of cyt c (140). Finally, it is worth noting that some antiapoptotic BCL-2 family members, once thought to reside only in the OMM, have recently been observed in the IMM and appear to regulate mitochondrial fission/fusion dynamics, cristae ultrastructure, membrane potential and/or F1F0-ATP synthase activity (3, 25, 90). Thus, collectively, it appears that cyt c release occurs through a two-step process: in the first step, CJO, along with cardiolipin oxidation, allows for cyt c to be released from the intra-cristae space into the IMS; and in the second step, cyt c is released into the cytoplasm through an oligomerized BAX/BAK pore in the OMM (88, 140) (Fig. 2B).
While formation of a BAX/BAK pore in the OMM is the most widely accepted model for MOMP, an alternative model involves BAX/BAK-dependent activation of a permeability transition pore (PT pore), comprised of the voltage-dependent anion channel (VDAC), the adenine nucleotide transporter (ANT), and cyclophilin D (Fig. 2). Unlike the OMM, the IMM is charged and impermeable to water and solutes, but in response to certain proapoptotic stimuli, the PT pore opens, resulting in catastrophic matrix swelling, rupture of the OMM, and ultimately cyt c release (40) (Fig. 2B). Importantly, most well-known inducers of the permeability transition involve oxidative stress and/or the influx of Ca2+ (70). While there is little debate regarding the permeability transition per se, there is significant disagreement as to the existence of the PT pore (as defined) and its role in cyt c release, as well as the mechanisms whereby pro- and antiapoptotic BCL-2 family members regulate the permeability transition. Indeed, many studies invoke a role for the PT pore in oxidative stress-induced apoptosis, and both BID and BAX reportedly interact with the PT pore, promote its formation, and/or regulate its opening (74, 82, 107, 118, 119, 145). However, other studies have demonstrated that cells deficient in all isoforms of VDAC, ANT or cyclophilin D are still capable of releasing cyt c and undergoing apoptosis in response to most stimuli, though some do show defects in the permeability transition (7, 65). Thus, the specific mechanisms underpinning the permeability transition and its role in MOMP remain unresolved.
Direct effects of ROS on cyt c
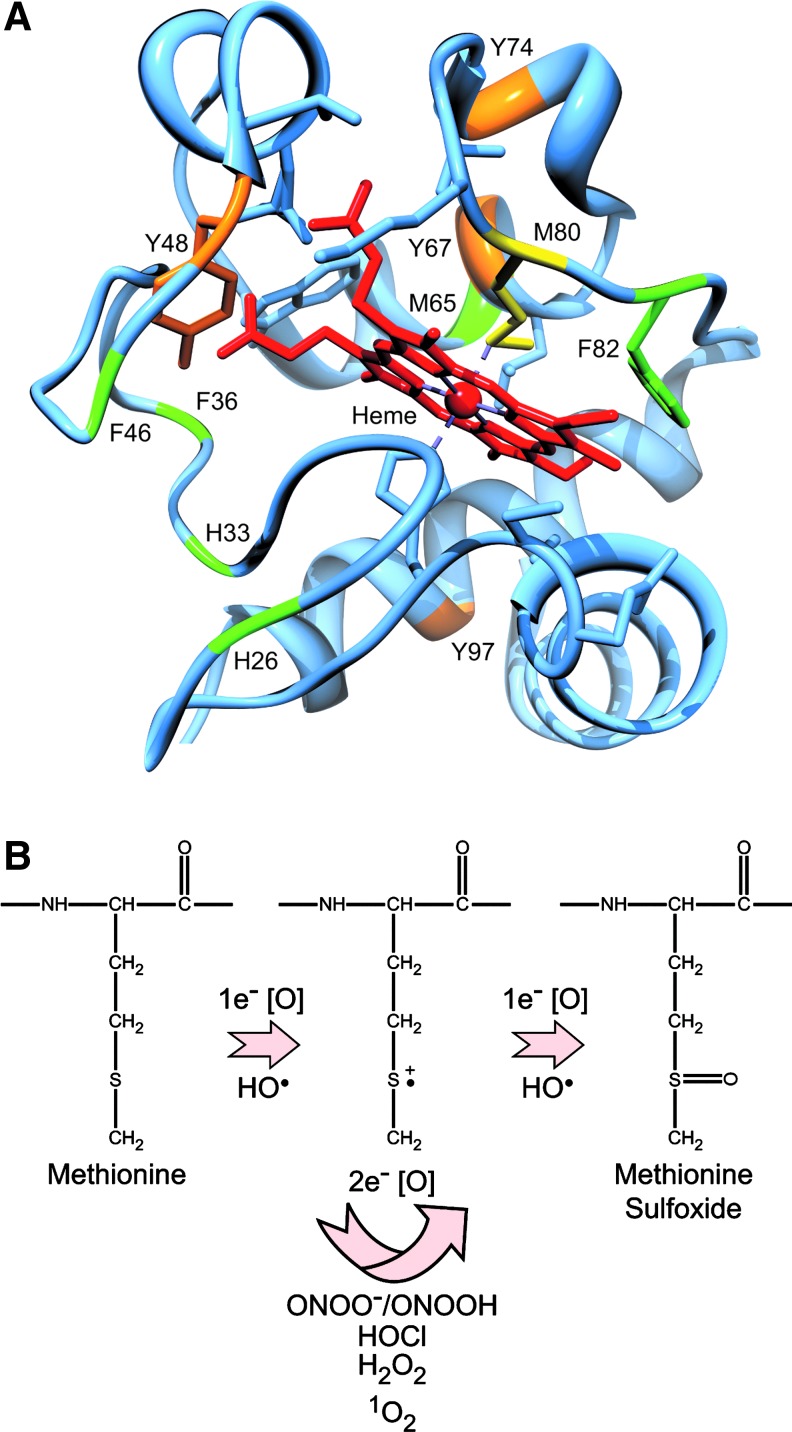
The notion that the redox state of cyt c affects apoptosis is also controversial. On the one hand, replacing iron in the heme group of cyt c with copper or zinc dramatically inhibits its capacity to transfer electrons, but only partially inhibits its ability to induce apoptosome formation in vitro (63). Cyt c is also rapidly reduced in cytosolic extracts and in the cytoplasm of stressed cells following MOMP (46, 104), implying that the redox state of cyt c has no major impact on apoptosome formation or apoptosis. Other studies, however, suggest that only oxidized cyt c (Fe3+) possesses pro-apoptotic activity (13, 89, 127). This controversy notwithstanding, it is clear that ROS can directly modify specific amino acids in cyt c (Fig. 3A). Superoxide anion (O2•−), H2O2, hydroxyl radicals (HO•), and peroxynitrite (ONOO−) oxidize methionine residues, particularly Met-80 (26, 61, 87, 129) (Fig. 3B), and singlet oxygen (1O2) not only oxidizes the ferrous form of cyt c, but also modifies several residues including His-26, His-33, Met-65, Met-80, and Phe-82 (60) (Fig. 3A). Importantly, oxidative modification of Met-80 dissociates it from its heme group and inhibits cyt c-dependent apoptosome formation (42, 78, 79, 127). Cyt c is also susceptible to nitration by ONOO- at Tyr-67, Tyr-74, and Tyr-97 (Fig. 3A), resulting in disruption of the heme iron-Met-80 bond (2, 21, 42), and NO-dependent nitration of Tyr-46 (human), and Tyr-48 (Fig. 3A) reportedly causes cyt c to assemble into a nonfunctional apoptosome (39). Finally, in addition to generating ROS, some redox-cycling quinones have been shown to directly arylate key lysines in cyt c, including Lys-72, thereby disrupting its interaction with Apaf-1 and preventing formation of the apoptosome (34, 35, 47, 62).
FIG. 3.
Oxidative modifications in cyt c. (A) Crystal structure of cyt c with the coordinated heme group (PDB 2B4Z). Shown in green are residues oxidized by 1O2, O2•−, H2O2, and/or •OH; whereas those shown in orange are susceptible to nitration by ONOO−. (B) Met-80 (shown in yellow in the crystal structure) is particularly susceptible to either 1e− or 2e− oxidations to methionine sulfoxide (114). (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Impact of cyt c release on ROS production
In general, when cyt c is released, electron transport is disrupted, and free electrons are donated to oxygen to produce O2•− (18) (Fig. 2B). Studies have shown, however, that generation of O2•− precedes the loss in IMM potential (ΔΨm) (18), and that even following cyt c release, a fraction of cyt c can diffuse back across the permeabilized OMM, reengage the ETC, and maintain some level of ETC function (138). Mitochondria may also utilize ATP, generated through glycolysis, to help maintain a near normal ΔΨm by allowing the F1F0-ATP synthase (complex V) to operate in reverse, hydrolyzing ATP to help generate a proton gradient (137) (Fig. 2B). Indeed, in many cellular systems, ΔΨm can be maintained for many hours, so long as caspases are inhibited and not allowed to attack the ETC. If caspase-3 gains access to the ETC, it can cleave the p75 NDUFS1 subunit in complex I, which in turn disrupts electron transport and promotes ROS production (102) (Fig. 2B). Other more recent studies argue that caspases-9 and -3 may have opposite effects on the ETC, in that caspase-9 activity promotes ROS production, whereas caspase-3 activity inhibits it (22). These studies underscore an important question, as it relates to the intrinsic pathway: at what point can a cell no longer survive, or more succinctly, when is the point of no return? Is it MOMP, loss of ETC function, loss of ΔΨm, apoptosome formation, or effector caspase activation? Antiapoptotic BCL-2 family members inhibit all of these events and promote clonal growth, implying that one of these downstream events is likely the final lynch pin to guarantee cell death.
The Apoptosome and ROS
In this last section, we will provide basic information on the structure and function of Apaf-1, caspase-9, and the apoptosome. Where applicable, we will also discuss how this complex and its individual components are regulated directly and/or indirectly by ROS.
Structure and function of Apaf-1
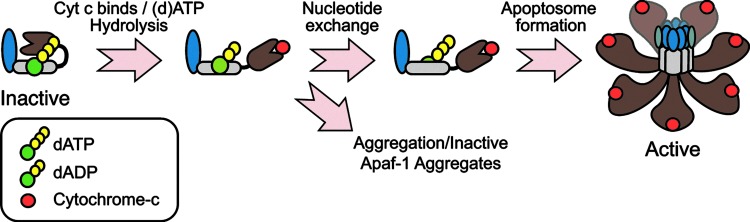
The adapter protein Apaf-1 possesses an N-terminal CARD domain, through which it binds to caspase-9; a nucleotide binding and oligomerization domain (NBD/NOD) that contains a AAA+ ATPase cassette, with Walker's A and B boxes that bind to (d)ATP and Mg2+, respectively; and a series of C-terminal WD40 repeats that form seven and eight-blade ß-propellers (101, 103, 149) (Fig. 4). In normal cells, monomeric Apaf-1 is likely present in an autoinhibited state, bound to (d)ATP (50, 101, 103). Cyt c, following its release from mitochondria, is thought to bind Apaf-1 between its ß-propellers and induce (d)ATP hydrolysis, providing the energy necessary to adopt a semi-open conformation (10, 49, 57). At this stage, in the absence of nucleotide exchange, cyt c-bound Apaf-1 is prone to aggregation that results in the formation of inactive apoptosome complexes (57) (Fig. 4). Thus, nucleotide exchange is critical, and a nucleotide exchange factor complex, composed of PHAPI, Hsp70, and cellular apoptosis susceptibility (CAS), has recently been identified for Apaf-1 (58). Following nucleotide exchange, the NBDs are exposed, and Apaf-1 undergoes proper oligomerization to form the functional complex (19, 111, 150). Based upon cryo-electron microscopy and structural modeling techniques, the functional complex appears to contain seven Apaf-1 molecules, arranged in a wheel-like structure with the NBDs forming a central hub and the CARDs forming a ring that is situated directly above the hub (143, 144). The WD-40 repeats then form spokes that radiate outward from the hub and end in a two-pronged fork that stabs cyt c (143, 144) (Fig. 4). Once formed, the Apaf-1 apoptosome then sequentially recruits and activates the initiator caspase-9 and the effector caspase-3 (17).
FIG. 4.
Cyt c/dATP-dependent formation of the Apaf-1 apoptosome. Apaf-1 is initially present in an inactive form in the cytoplasm, bound with (d)ATP/ATP. Upon its interaction with cyt c, most likely through its WD-40 repeats, (d)ATP is hydrolyzed to (d)ADP, and Apaf-1 assumes a semi-open conformation. At this stage, if (d)ADP is replaced with (d)ATP, Apaf-1 undergoes oligomerization through its NBD/NOD domain to form a fully functional apoptosome. If nucleotide exchange does not occur, Apaf-1 instead undergoes aggregation into a nonfunctional complex. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Direct and indirect effects of ROS on Apaf-1
How or if ROS directly regulate Apaf-1 remains unclear. However, in one study, ROS were found to be required for Fas-mediated apoptosome formation. Using in vitro translated Apaf-1 and caspase-9 (and purified cyt c), the authors reported that apoptosome formation was inhibited by the addition of antioxidants or reducing agents, and that oxidation of Apaf-1 in particular was essential for the activation of caspase-9 in their reconstituted system (112). In complete contrast, others report that ROS can indirectly inhibit apoptosome function through the activation of certain kinases. For example, one study found that JNKs, activated in response to ROS, bound to Apaf-1 and cyt c in a catalytically inactive ∼1.4–2.0 MDa complex, which the authors referred to as a “preapoptosome complex” (133). Interestingly, we previously reported the formation of active ∼700 kDa and inactive ∼1.4 MDa apoptosome complexes following cyt c/dATP activation of lysates (19). Given that similar inactive complexes are formed as a result of inadequate nucleotide exchange (57), it will be interesting to determine if JNK binding (or perhaps JNK-dependent phosphorylation of Apaf-1) interferes with nucleotide exchange. More recently, 90-kDa ribosomal S6 kinase (Rsk) has been reported to phosphorylate Apaf-1 at Ser-268 and Ser-357 (59). Phosphorylation at these sites results in Apaf-1 sequestration by 14-3-3ɛ and decreases cellular responsiveness to cyt c. Though not examined in the study, H2O2 reportedly activates Rsk (1), raising the possibility that ROS might suppress apoptosis in certain contexts via RSK-mediated phosphorylation of Apaf-1.
Structure and function of caspase-9
Procaspase-9 is generated as a single-chain 46 kDa protein with an N-terminal prodomain (CARD), followed by large (∼20 kDa) and small (∼12 kDa) subunits, connected via an intersubunit linker (16) (Figs. 5 and 6). Following apoptosome formation, procaspase-9 is recruited to the complex through CARD–CARD interactions with Apaf-1 (95). Procaspase-9 then undergoes autocatalytic processing within its intersubunit linker (Asp-315) to generate a two-chain (p35/p12) enzyme (122) (Fig. 5A). Importantly, unlike other initiator caspases, the prodomain in caspase-9 is generally not removed at any step in its activation. Thus, caspase-9 is unique in that it must remain bound to its caspase-activating complex, the apoptosome, in order to sustain significant catalytic activity (17, 105, 125). Once formed, the Apaf-1-caspase-9 complex activates the downstream effector procaspases-3 and -7 (17, 105, 141). In some cases, active caspase-3 can then feedback on and cleave unbound procaspase-9 or p35/p12 caspase-9 at Asp-330 to generate p37/12 or p35/p10 caspase-9 proteins, respectively (15, 123, 124, 151).
FIG. 5.
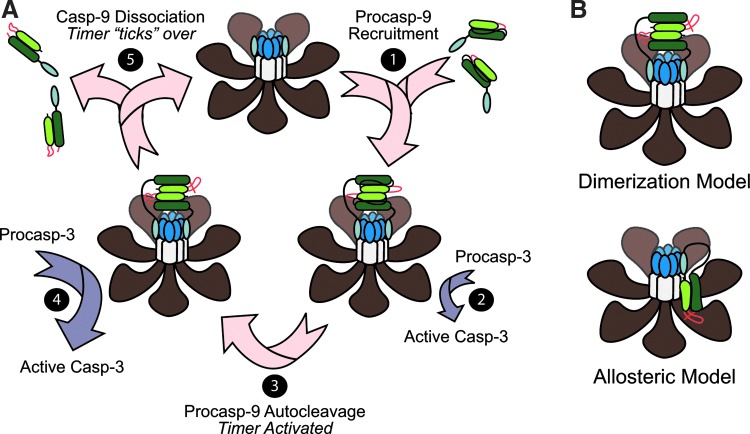
Proposed mechanisms of caspase-9 activation within the Apaf-1 apoptosome. (A) The Apaf-1-caspase-9 apoptosome functions as a “molecular timer.” Once the apoptosome is formed, it can recruit and activate procaspase-9 through dimerization (shown) and/or induced conformational changes in the enzyme (step 1). Importantly, caspase-9 must be bound and remain bound in order to exhibit significant catalytic activity. While procaspase-9 is capable of activating the downstream effector caspase-3 (step 2), its rate of autocatalytic cleavage is so fast (step 3) that processed caspase-9 (p35/p12) activates the vast majority of caspase-3 (step 4). This is important, because unlike procaspase-9, which has high affinity for the apoptosome, the processed form of caspase-9 has low affinity for the complex and dissociates over time to be replaced by a new procaspase-9 molecule (step 5). Thus, the Apaf-1 apoptosome functions like a proteolytic-based molecular timer, wherein the intracellular concentration of procaspase-9 sets the overall duration of the timer, the autoprocessing of procaspase-9 activates the timer, and the rate at which p35/p12 caspase-9 dissociates from the complex (and thus loses its capacity to activate procaspase-3) dictates how fast the timer “ticks” over (75). (B) Is active caspase-9 a dimer or a monomer? The apoptosome is thought to activate caspase-9, either by increasing its local concentration and facilitating dimerization (top), or by inducing a conformational change in monomeric caspase-9 that promotes its activation (bottom). (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
FIG. 6.
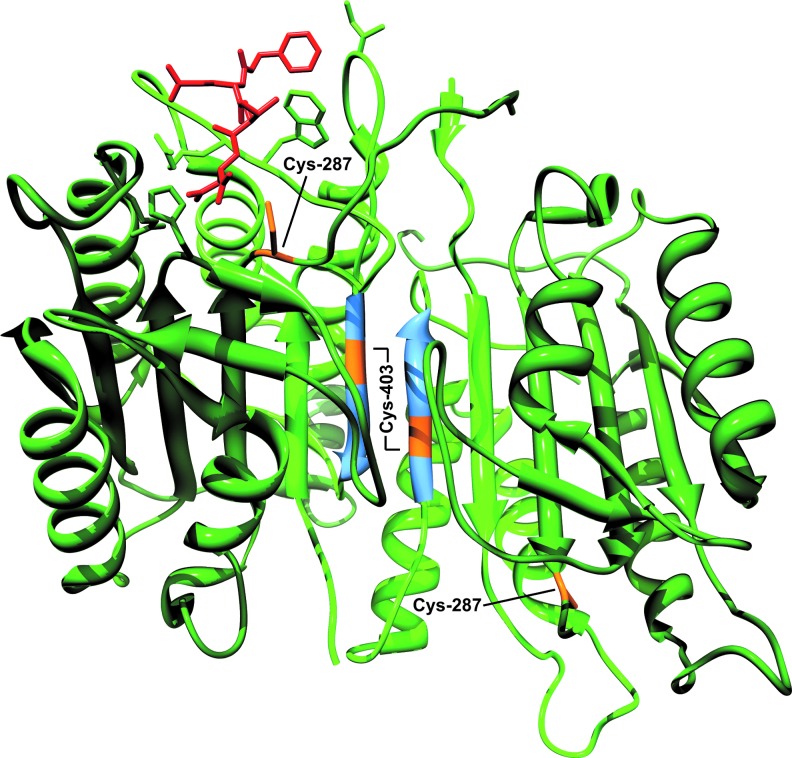
Oxidative modifications in caspase-9. In this crystal structure of dimerized caspase-9 (PDB code 1JXQ), the enzyme contains two large subunits (dark green) and two small subunits (light green) that contain the dimerization interface (blue). This dimerized caspase-9 possesses only one active site, as indicated by the presence of inhibitor (red). ROS are proposed to activate caspase-9 through oxidation of Cys-403 and formation of a stable dimer, resulting from disulfide bond formation. Conversely, ROS may also inactivate the enzyme through oxidation of its catalytic cysteine, Cys-287. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Apoptosome-bound caspase-9: an active monomer or a dimer?
One of the more highly debated topics in caspase biology has been the question of how caspase-9 is activated following its interaction with the apoptosome? Two models have been proposed: in the currently more well-accepted dimerization model, the apoptosome serves merely as a structural platform to recruit caspase-9 and increase its local concentration, thereby promoting its dimerization and subsequent activation (12, 92) (Fig. 5B). Indeed, unlike effector caspases, which are constitutive dimers, caspase-9 possesses a relatively low affinity for itself and is normally present as a monomer in solution. However, crystal structures of caspase-9 strongly suggest that it is a dimer, and mutations along the proposed dimerization interface kill the enzyme's activity (100) (Fig. 6). In addition, caspase-9 can be activated by artificially enforcing its dimerization (12, 92), and if one swaps the prodomain of caspase-8 for the CARD in caspase-9, the apoptosome then activates caspase-8, arguing that there is nothing special about the apoptosome, other than its ability to concentrate caspases (12, 92).
Proponents of the competing holoenzyme model argue instead that the apoptosome functions as a positive allosteric regulator of caspase-9, inducing conformational changes in the enzyme that are necessary for its activation (24, 105) (Fig. 5B). They insist that the seven-fold (rather than eight-fold) symmetry of the apoptosome is inconsistent with the idea that caspase-9 would form dimers and point out that caspase-9 has never been shown to dimerize in a wild-type Apaf-1-caspase-9 apoptosome complex. Moreover, while enforced dimerization of truncated caspase-9 (artificially lacking its prodomain) activates the enzyme, the observed activity appears to be significantly lower than that observed in a complex reconstituted with full-length Apaf-1 and caspase-9 (24). It is also notable that swapping the putative dimerization domain in caspase-9, with that found in caspase-3, produces a constitutive caspase-9 dimer, which is structurally indistinguishable from the wild-type enzyme. However, this caspase-9 dimer still exhibits significantly less activity than apoptosome-bound caspase-9 (24). Finally, a very recent cryo-electron microscopy structure of the apoptosome suggests that a single catalytic subunit of caspase-9 is bound near the hub of the apoptosome (143) (Fig. 5B). Thus, future studies are needed to assess not only the stoichiometry and dimerization status of caspase-9 within the apoptosome, but their requirements for activity.
The apoptosome as a molecular timer for caspase activation
We have recently demonstrated that procaspase-9 possesses higher affinity for the apoptosome and can displace processed p35/p12 caspase-9 from the complex, thereby facilitating a continuous cycle of procaspase-9 recruitment/ activation, processing, and release from the complex (75) (Fig. 5A). Importantly, we found that procaspase-9 undergoes autoprocessing so rapidly upon its recruitment to the complex that the proform of the enzyme contributes little to the activation of procaspase-3. Consequently, our results led us to the conclusion that the apoptosome functions as a proteolytic-based “molecular timer” (Fig. 5A). In our proposed model, the intracellular concentration of procaspase-9 sets the overall duration of the timer, the autoprocessing of procaspase-9 activates the timer, and the rate at which p35/p12 caspase-9 dissociates from the complex (and thus loses its capacity to activate procaspase-3) dictates how fast the timer “ticks” over (75). Our in vitro studies suggest that noncleavable caspase-9 should disengage the timer and result in an apoptosome complex that, once formed, cannot be “turned off” (75). However, in cellular systems it is important to note that processing of procaspase-9 to p35/p12 caspase-9 also produces a form of the enzyme that can be inhibited by X-linked IAP (XIAP), a potent endogenous inhibitor of caspases-9, -3, and -7 (15, 124). Indeed, XIAP specifically binds to a neo-epitope on the N-terminus of the small p12 subunit of processed caspase-9. Thus, in future studies, it will be important to determine the interplay between XIAP and the molecular timer, as well as the relative importance of these two mechanisms for regulating apoptosome function in vivo.
Direct and indirect effects of ROS on caspase-9
Regarding the direct effects of oxidative stress on caspases, several studies have shown that caspase-3 activation and/or activity can be negatively regulated by oxidants. In fact, several cysteines in caspase-3, including Cys-73, Cys-220, and its catalytic cysteine, Cys-163, undergo oxidation, nitrosylation, and/or glutathionylation (11, 51, 69, 76, 84, 106, 147). The reported effects of ROS on caspase-9, however, are more inconsistent. One study found that exposure of caspase-9 to H2O2 promoted its interaction with Apaf-1, through a disulfide bond involving Cys-403 (152). Another study reported the presence of active homodimerized caspase-9 within H2O2-treated mitochondria and suggested that multiple cysteines (including Cys-403) were sites of disulfide bond formation (55) (Fig. 6). While these studies are intriguing and warrant further investigation, some aspects are surprising. For example, a few studies have reported the presence of caspase-9 in mitochondria (67, 126, 148), but procaspase-9 does not possess a typical mitochondrial targeting sequence and others find that most of the caspase-9 that localizes to mitochondria does so following MOMP (23). The purpose of mitochondrial caspase-9 is also unclear, as it would presumably be released from the IMS along with cyt c during apoptosis. Given the consensus that far more procaspase-9 exists in the cytoplasm than in mitochondria, one might expect that apoptosome-driven activation of cytoplasmic caspase-9 would be far more active and plentiful. Finally, Cys-403 is one of the five amino acids that constitute the dimerization domain in caspase-9 (Fig. 6), and mutations along this interface often kill enzyme activity (100, 120). By contrast, other studies suggest that ROS inhibit caspase-9 activation. H2O2 reportedly blocks caspase-9 activation through oxidation of cysteine residues, including its catalytic cysteine (Cys-287) (Fig. 6), and this process appears to be iron-dependent, implying that Fenton chemistry and the production of HO• is involved (8). Similar to caspase-3, the caspase-9 active site cysteine is also susceptible to S-nitrosylation (131), and nitric oxide donors have been shown to block formation of the Apaf-1-caspase-9 complex by disrupting normal CARD-CARD interactions (146).
In addition to the direct effects of oxidative stress on caspase-9, there are also a few potential indirect effects, many of which involve the activation of kinases. Over the last decade, there have been several reports that phosphorylation of caspase-9 regulates its activity. Most prominently, human caspase-9 is phosphorylated at Thr-125 by ERK2, DYRK1A, and CDK1/cyclin B1 kinases during survival signaling and mitosis, and phosphorylation at this site directly inhibits the activity of procaspase-9 following its recruitment to the apoptosome (4, 5, 80, 81, 116). PKCζ and Akt also phosphorylate human caspase-9 at Ser-144 and Ser-196, respectively, leading to its inhibition (14, 20), but interestingly, neither of these sites is conserved in the mouse. Finally, and perhaps most surprising, c-Abl appears to activate caspase-9 through phosphorylation at Tyr-153 (98). It remains unclear how any of the aforementioned phosphorylation sites regulate caspase-9 activity, since none are located in the active site or putative dimerization domain. Thus, in the coming years, it will be exciting to determine, mechanistically, how phosphorylation impacts caspase-9 structure and function, as well as to test the importance of phosphorylation in vivo. For additional insight into ROS-independent regulation of the Apaf-1-caspase-9 apoptosome, the reader is directed to the following reviews (16, 113).
Other effects of ROS on apoptosis in vitro and in vivo
As previously noted, in light of the pleiotropic effects of ROS on apoptosis, we have chosen to focus the majority of this review on the more immediate effects of ROS on the intrinsic pathway. However, we wish to emphasize that oxidative modification of DNA, proteins, and lipids can impact a variety of signaling pathways, both upstream and downstream of the intrinsic pathway. For example, ROS can activate the kinase ATM, either through direct modification or as a consequence of DNA damage, resulting in the activation of p53, a transcription factor that drives expression of proapoptotic BCL-2 family members, including NOXA, PUMA, and BAX (27, 45, 68, 85, 136). ROS also regulate the upstream production of sphingolipids through direct oxidative modification of the acidic sphingomyelinase at Cys-629, or through oxidation of glutathione (GSH), which normally serves as an endogenous inhibitor of neutral sphingomyelinases (71, 96). In an elegant biochemical study, Green and colleagues have recently shown that two sphingolipid metabolites, sphingosine-1-phosphate and hexadecenal, directly promote BAK and BAX-induced MOMP, respectively (28). ROS may also promote apoptosis via the extrinsic pathway through upregulation of death receptors or by serving as intermediates in the activation of kinases, such as JNKs, which promote MOMP through direct phosphorylation of BCL-2 family members (52, 53, 56, 93, 110, 130, 139).
How ROS mediate apoptosis in vivo has been more challenging, in part due to difficulties in monitoring cell and tissue-specific generation of ROS, as well as isolating oxidatively-modified macromolecules, which can be short-lived or artificially introduced from the isolation procedure. There is little doubt, however, that ROS play important roles in apoptosis in vivo, as transgenic and knockout mice for various antioxidant genes, including superoxide dismutases (SOD1 and SOD2), catalase, and glutathione peroxidases, exhibit the expected decreases or increases in apoptosis in response to ischemic injury and other pro-death stimuli (37, 38, 41, 64, 94, 99, 134, 135). Moreover, the recent development of various genetically-encoded fluorescent probes and imaging techniques can now be used to monitor the production of ROS in a spatiotemporal manner (6, 9, 31, 77). Thus, it should be possible to establish the relative importance of ROS-induced apoptosis versus inflammation, mutagenesis, etc. in the development of disease and to determine the relative importance of a given oxidative modification.
Concluding Remarks
After many years of research, the intrinsic pathway remains an important area of investigation. Each step in the pathway is regulated by various modulators and posttranslational modifications. However, while the importance of many of the genes in this pathway (e.g., BCL-2, cyt c, Apaf-1, caspase-9, and XIAP) have been verified in transgenic and knockout animal models, particularly during development, many of these gene products and their post-translational modifications, including oxidative modifications, have not been thoroughly vetted in animal models of disease. This is certainly true in cancer where most studies have been limited to hematopoietic malignancies. Thus, in the future, it will be critical to fully characterize in detail not only how the apoptosome is formed and regulated at the biochemical level, but to assess its importance in vivo.
Abbreviations Used
- ANT
adenine nucleotide transporter
- APAF-1
apoptotic protease-activating factor-1
- BCL-2
B cell lymphoma-2
- BH
BCL-2 homology domains
- CARD
caspase recruitment domain
- Caspases
cysteinyl aspartate-specific proteases
- CJO
crista junction opening
- Cyt-c
cytochrome c
- ERK
extracellular signal-regulated kinase
- GSK-3β
glycogen synthase kinase-3β
- H2O2
hydrogen peroxide
- HO•
hydroxyl radical
- IMM
inner mitochondrial membrane
- IMS
intermembrane space
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MOMP
mitochondrial outer membrane permeabilization
- NBD/NOD
nucleotide binding and oligomerization domain
- O2•−
superoxide anion
- 1O2
singlet oxygen
- OPA1
optic atrophy 1
- OMM
outer mitochondrial membrane
- ONOO-
peroxynitrite
- PT pore
permeability transition pore
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- tBID
truncated BID
- VDAC
voltage-dependent anion channel
- XIAP
X-linked IAP
Acknowledgments
This work was supported in part by NIH Grants R01CA129521 and R01GM096101.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Abe J. Okuda M. Huang Q. Yoshizumi M. Berk BC. Reactive oxygen species activate p90 ribosomal S6 kinase via Fyn and Ras. J Biol Chem. 2000;275:1739–1748. doi: 10.1074/jbc.275.3.1739. [DOI] [PubMed] [Google Scholar]
- 2.Abriata LA. Cassina A. Tortora V. Marin M. Souza JM. Castro L. Vila AJ. Radi R. Nitration of solvent-exposed tyrosine 74 on cytochrome c triggers heme iron-methionine 80 bond disruption. Nuclear magnetic resonance and optical spectroscopy studies. J Biol Chem. 2009;284:17–26. doi: 10.1074/jbc.M807203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alavian KN. Li H. Collis L. Bonanni L. Zeng L. Sacchetti S. Lazrove E. Nabili P. Flaherty B. Graham M. Chen Y. Messerli SM. Mariggio MA. Rahner C. McNay E. Shore GC. Smith PJ. Hardwick JM. Jonas EA. Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat Cell Biol. 2011;13:1224–1233. doi: 10.1038/ncb2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allan LA. Clarke PR. Phosphorylation of caspase-9 by CDK1/cyclin B1 protects mitotic cells against apoptosis. Mol Cell. 2007;26:301–310. doi: 10.1016/j.molcel.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Allan LA. Morrice N. Brady S. Magee G. Pathak S. Clarke PR. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol. 2003;5:647–654. doi: 10.1038/ncb1005. [DOI] [PubMed] [Google Scholar]
- 6.Back P. De Vos WH. Depuydt GG. Matthijssens F. Vanfleteren JR. Braeckman BP. Exploring real-time in vivo redox biology of developing and aging Caenorhabditis elegans. Free Rad Biol Med. 2012;52:850–859. doi: 10.1016/j.freeradbiomed.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 7.Baines CP. Kaiser RA. Sheiko T. Craigen WJ. Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbouti A. Amorgianiotis C. Kolettas E. Kanavaros P. Galaris D. Hydrogen peroxide inhibits caspase-dependent apoptosis by inactivating procaspase-9 in an iron-dependent manner. Free Radic Biol Med. 2007;43:1377–1387. doi: 10.1016/j.freeradbiomed.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Belousov VV. Fradkov AF. Lukyanov KA. Staroverov DB. Shakhbazov KS. Terskikh AV. Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 10.Benedict MA. Hu Y. Inohara N. Nunez G. Expression and functional analysis of Apaf-1 isoforms. Extra WD-40 repeat is required for cytochrome c binding and regulated activation of procaspase-9. J Biol Chem. 2000;275:8461–8468. doi: 10.1074/jbc.275.12.8461. [DOI] [PubMed] [Google Scholar]
- 11.Benhar M. Forrester MT. Hess DT. Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boatright KM. Renatus M. Scott FL. Sperandio S. Shin H. Pedersen IM. Ricci JE. Edris WA. Sutherlin DP. Green DR. Salvesen GS. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 13.Borutaite V. Brown GC. Mitochondrial regulation of caspase activation by cytochrome oxidase and tetramethylphenylenediamine via cytosolic cytochrome c redox state. J Biol Chem. 2007;282:31124–31130. doi: 10.1074/jbc.M700322200. [DOI] [PubMed] [Google Scholar]
- 14.Brady SC. Allan LA. Clarke PR. Regulation of caspase 9 through phosphorylation by protein kinase C zeta in response to hyperosmotic stress. Mol Cell Biol. 2005;25:10543–10555. doi: 10.1128/MCB.25.23.10543-10555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bratton SB. Lewis J. Butterworth M. Duckett CS. Cohen GM. XIAP inhibition of caspase-3 preserves its association with the Apaf-1 apoptosome and prevents CD95- and Bax-induced apoptosis. Cell Death Differ. 2002;9:881–892. doi: 10.1038/sj.cdd.4401069. [DOI] [PubMed] [Google Scholar]
- 16.Bratton SB. Salvesen GS. Regulation of the Apaf-1-caspase-9 apoptosome. J Cell Sci. 2010;123:3209–3214. doi: 10.1242/jcs.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bratton SB. Walker G. Srinivasula SM. Sun XM. Butterworth M. Alnemri ES. Cohen GM. Recruitment, activation and retention of caspases-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J. 2001;20:998–1009. doi: 10.1093/emboj/20.5.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai J. Jones DP. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J Biol Chem. 1998;273:11401–11404. doi: 10.1074/jbc.273.19.11401. [DOI] [PubMed] [Google Scholar]
- 19.Cain K. Bratton SB. Langlais C. Walker G. Brown DG. Sun XM. Cohen GM. Apaf-1 oligomerizes into biologically active approximately 700-kDa and inactive approximately 1.4-MDa apoptosome complexes. J Biol Chem. 2000;275:6067–6070. doi: 10.1074/jbc.275.9.6067. [DOI] [PubMed] [Google Scholar]
- 20.Cardone MH. Roy N. Stennicke HR. Salvesen GS. Franke TF. Stanbridge E. Frisch S. Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 21.Cassina AM. Hodara R. Souza JM. Thomson L. Castro L. Ischiropoulos H. Freeman BA. Radi R. Cytochrome c nitration by peroxynitrite. J Biol Chem. 2000;275:21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 22.Cepero E. King AM. Coffey LM. Perez RG. Boise LH. Caspase-9 and effector caspases have sequential and distinct effects on mitochondria. Oncogene. 2005;24:6354–6366. doi: 10.1038/sj.onc.1208793. [DOI] [PubMed] [Google Scholar]
- 23.Chandra D. Tang DG. Mitochondrially localized active caspase-9 and caspase-3 result mostly from translocation from the cytosol and partly from caspase-mediated activation in the organelle. Lack of evidence for Apaf-1-mediated procaspase-9 activation in the mitochondria. J Biol Chem. 2003;278:17408–17420. doi: 10.1074/jbc.M300750200. [DOI] [PubMed] [Google Scholar]
- 24.Chao Y. Shiozaki EN. Srinivasula SM. Rigotti DJ. Fairman R. Shi Y. Engineering a dimeric caspase-9: a re-evaluation of the induced proximity model for caspase activation. PLoS Biol. 2005;3:1079–1087. doi: 10.1371/journal.pbio.0030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen YB. Aon MA. Hsu YT. Soane L. Teng X. McCaffery JM. Cheng WC. Qi B. Li H. Alavian KN. Dayhoff-Brannigan M. Zou S. Pineda FJ. O'Rourke B. Ko YH. Pedersen PL. Kaczmarek LK. Jonas EA. Hardwick JM. Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J Cell Biol. 2011;195:263–276. doi: 10.1083/jcb.201108059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen YR. Deterding LJ. Sturgeon BE. Tomer KB. Mason RP. Protein oxidation of cytochrome C by reactive halogen species enhances its peroxidase activity. J Biol Chem. 2002;277:29781–29791. doi: 10.1074/jbc.M200709200. [DOI] [PubMed] [Google Scholar]
- 27.Chipuk JE. Bouchier-Hayes L. Kuwana T. Newmeyer DD. Green DR. PUMA couples the nuclear and cytoplasmic proapoptotic function of p53. Science. 2005;309:1732–1735. doi: 10.1126/science.1114297. [DOI] [PubMed] [Google Scholar]
- 28.Chipuk JE. McStay GP. Bharti A. Kuwana T. Clarke CJ. Siskind LJ. Obeid LM. Green DR. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012;148:988–1000. doi: 10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cipolat S. Rudka T. Hartmann D. Costa V. Serneels L. Craessaerts K. Metzger K. Frezza C. Annaert W. D'Adamio L. Derks C. Dejaegere T. Pellegrini L. D'Hooge R. Scorrano L. De Strooper B. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 30.D'Alessio M. De Nicola M. Coppola S. Gualandi G. Pugliese L. Cerella C. Cristofanon S. Civitareale P. Ciriolo MR. Bergamaschi A. Magrini A. Ghibelli L. Oxidative Bax dimerization promotes its translocation to mitochondria independently of apoptosis. FASEB J. 2005;19:1504–1506. doi: 10.1096/fj.04-3329fje. [DOI] [PubMed] [Google Scholar]
- 31.Dickinson BC. Tang Y. Chang Z. Chang CJ. A nuclear-localized fluorescent hydrogen peroxide probe for monitoring sirtuin-mediated oxidative stress responses in vivo. Chem Biol. 2011;18:943–948. doi: 10.1016/j.chembiol.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Domina AM. Vrana JA. Gregory MA. Hann SR. Craig RW. MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene. 2004;23:5301–5315. doi: 10.1038/sj.onc.1207692. [DOI] [PubMed] [Google Scholar]
- 33.Ehses S. Raschke I. Mancuso G. Bernacchia A. Geimer S. Tondera D. Martinou JC. Westermann B. Rugarli EI. Langer T. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol. 2009;187:1023–1036. doi: 10.1083/jcb.200906084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher AA. Labenski MT. Malladi S. Chapman JD. Bratton SB. Monks TJ. Lau SS. The frequency of 1,4-benzoquinone-lysine adducts in cytochrome c correlate with defects in apoptosome activation. Tox Sci. 2011;122:64–72. doi: 10.1093/toxsci/kfr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher AA. Labenski MT. Malladi S. Gokhale V. Bowen ME. Milleron RS. Bratton SB. Monks TJ. Lau SS. Quinone electrophiles selectively adduct “electrophile binding motifs” within cytochrome c. Biochemistry. 2007;46:11090–11100. doi: 10.1021/bi700613w. [DOI] [PubMed] [Google Scholar]
- 36.Frezza C. Cipolat S. Martins de Brito O. Micaroni M. Beznoussenko GV. Rudka T. Bartoli D. Polishuck RS. Danial NN. De Strooper B. Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 37.Fujimura M. Morita-Fujimura Y. Kawase M. Copin JC. Calagui B. Epstein CJ. Chan PH. Manganese superoxide dismutase mediates the early release of mitochondrial cytochrome C and subsequent DNA fragmentation after permanent focal cerebral ischemia in mice. J Neurosci. 1999;19:3414–3422. doi: 10.1523/JNEUROSCI.19-09-03414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujimura M. Morita-Fujimura Y. Noshita N. Sugawara T. Kawase M. Chan PH. The cytosolic antioxidant copper/zinc-superoxide dismutase prevents the early release of mitochondrial cytochrome c in ischemic brain after transient focal cerebral ischemia in mice. J Neurosci. 2000;20:2817–2824. doi: 10.1523/JNEUROSCI.20-08-02817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Heredia JM. Diaz-Moreno I. Diaz-Quintana A. Orzaez M. Navarro JA. Hervas M. De la Rosa MA. Specific nitration of tyrosines 46 and 48 makes cytochrome c assemble a non-functional apoptosome. FEBS Lett. 2011;586:154–158. doi: 10.1016/j.febslet.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Garrido C. Galluzzi L. Brunet M. Puig PE. Didelot C. Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–1433. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- 41.Godin N. Liu F. Lau GJ. Brezniceanu ML. Chenier I. Filep JG. Ingelfinger JR. Zhang SL. Chan JS. Catalase overexpression prevents hypertension and tubular apoptosis in angiotensinogen transgenic mice. Kidney Int. 2010;77:1086–1097. doi: 10.1038/ki.2010.63. [DOI] [PubMed] [Google Scholar]
- 42.Godoy LC. Munoz-Pinedo C. Castro L. Cardaci S. Schonhoff CM. King M. Tortora V. Marin M. Miao Q. Jiang JF. Kapralov A. Jemmerson R. Silkstone GG. Patel JN. Evans JE. Wilson MT. Green DR. Kagan VE. Radi R. Mannick JB. Disruption of the M80-Fe ligation stimulates the translocation of cytochrome c to the cytoplasm and nucleus in nonapoptotic cells. Proc Natl Acad Sci USA. 2009;106:2653–2658. doi: 10.1073/pnas.0809279106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalvez F. Bessoule JJ. Rocchiccioli F. Manon S. Petit PX. Role of cardiolipin on tBid and tBid/Bax synergistic effects on yeast mitochondria. Cell Death Differ. 2005;12:659–667. doi: 10.1038/sj.cdd.4401585. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalvez F. Pariselli F. Dupaigne P. Budihardjo I. Lutter M. Antonsson B. Diolez P. Manon S. Martinou JC. Goubern M. Wang X. Bernard S. Petit PX. tBid interaction with cardiolipin primarily orchestrates mitochondrial dysfunctions and subsequently activates Bax and Bak. Cell Death Differ. 2005;12:614–626. doi: 10.1038/sj.cdd.4401571. [DOI] [PubMed] [Google Scholar]
- 45.Guo Z. Kozlov S. Lavin MF. Person MD. Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 46.Hampton MB. Zhivotovsky B. Slater AF. Burgess DH. Orrenius S. Importance of the redox state of cytochrome c during caspase activation in cytosolic extracts. Biochem J. 1998;329:95–99. doi: 10.1042/bj3290095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hao Z. Duncan GS. Chang CC. Elia A. Fang M. Wakeham A. Okada H. Calzascia T. Jang Y. You-Ten A. Yeh WC. Ohashi P. Wang X. Mak TW. Specific ablation of the apoptotic functions of cytochrome C reveals a differential requirement for cytochrome C and Apaf-1 in apoptosis. Cell. 2005;121:579–591. doi: 10.1016/j.cell.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 48.Head B. Griparic L. Amiri M. Gandre-Babbe S. van der Bliek AM. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol. 2009;187:959–966. doi: 10.1083/jcb.200906083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu Y. Benedict MA. Ding L. Nunez G. Role of cytochrome c and dATP/ATP hydrolysis in Apaf-1-mediated caspase-9 activation and apoptosis. EMBO J. 1999;18:3586–3595. doi: 10.1093/emboj/18.13.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu Y. Ding L. Spencer DM. Nunez G. WD-40 repeat region regulates Apaf-1 self-association and procaspase-9 activation. J Biol Chem. 1998;273:33489–33494. doi: 10.1074/jbc.273.50.33489. [DOI] [PubMed] [Google Scholar]
- 51.Huang Z. Pinto JT. Deng H. Richie JP., Jr. Inhibition of caspase-3 activity and activation by protein glutathionylation. Biochem Pharmacol. 2008;75:2234–2244. doi: 10.1016/j.bcp.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ichijo H. Nishida E. Irie K. ten Dijke P. Saitoh M. Moriguchi T. Takagi M. Matsumoto K. Miyazono K. Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 53.Inoshita S. Takeda K. Hatai T. Terada Y. Sano M. Hata J. Umezawa A. Ichijo H. Phosphorylation and inactivation of myeloid cell leukemia 1 by JNK in response to oxidative stress. J Biol Chem. 2002;277:43730–43734. doi: 10.1074/jbc.M207951200. [DOI] [PubMed] [Google Scholar]
- 54.Kagan VE. Tyurin VA. Jiang J. Tyurina YY. Ritov VB. Amoscato AA. Osipov AN. Belikova NA. Kapralov AA. Kini V. Vlasova , II Zhao Q. Zou M. Di P. Svistunenko DA. Kurnikov IV. Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 55.Katoh I. Tomimori Y. Ikawa Y. Kurata S. Dimerization and processing of procaspase-9 by redox stress in mitochondria. J Biol Chem. 2004;279:15515–15523. doi: 10.1074/jbc.M311819200. [DOI] [PubMed] [Google Scholar]
- 56.Kharbanda S. Saxena S. Yoshida K. Pandey P. Kaneki M. Wang Q. Cheng K. Chen YN. Campbell A. Sudha T. Yuan ZM. Narula J. Weichselbaum R. Nalin C. Kufe D. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J Biol Chem. 2000;275:322–327. doi: 10.1074/jbc.275.1.322. [DOI] [PubMed] [Google Scholar]
- 57.Kim HE. Du F. Fang M. Wang X. Formation of apoptosome is initiated by cytochrome c-induced dATP hydrolysis and subsequent nucleotide exchange on Apaf-1. Proc Natl Acad Sci USA. 2005;102:17545–17550. doi: 10.1073/pnas.0507900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim HE. Jiang X. Du F. Wang X. PHAPI, CAS, and Hsp70 promote apoptosome formation by preventing Apaf-1 aggregation and enhancing nucleotide exchange on Apaf-1. Mol Cell. 2008;30:239–247. doi: 10.1016/j.molcel.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 59.Kim J. Parrish AB. Kurokawa M. Matsuura K. Freel CD. Andersen JL. Johnson CE. Kornbluth S. Rsk-mediated phosphorylation and 14-3-3varepsilon binding of Apaf-1 suppresses cytochrome c-induced apoptosis. EMBO J. 2012;31:1279–1292. doi: 10.1038/emboj.2011.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim J. Rodriguez ME. Guo M. Kenney ME. Oleinick NL. Anderson VE. Oxidative modification of cytochrome c by singlet oxygen. Free Radic Biol Med. 2008;44:1700–1711. doi: 10.1016/j.freeradbiomed.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim NH. Jeong MS. Choi SY. Kang JH. Oxidative modification of cytochrome c by hydrogen peroxide. Mol Cells. 2006;22:220–227. [PubMed] [Google Scholar]
- 62.Kluck RM. Ellerby LM. Ellerby HM. Naiem S. Yaffe MP. Margoliash E. Bredesen D. Mauk AG. Sherman F. Newmeyer DD. Determinants of cytochrome c pro-apoptotic activity. The role of lysine 72 trimethylation. J Biol Chem. 2000;275:16127–16133. doi: 10.1074/jbc.275.21.16127. [DOI] [PubMed] [Google Scholar]
- 63.Kluck RM. Martin SJ. Hoffman BM. Zhou JS. Green DR. Newmeyer DD. Cytochrome c activation of CPP32-like proteolysis plays a critical role in a Xenopus cell-free apoptosis system. EMBO J. 1997;16:4639–4649. doi: 10.1093/emboj/16.15.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kokoszka JE. Coskun P. Esposito LA. Wallace DC. Increased mitochondrial oxidative stress in the Sod2 (+/−) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci USA. 2001;98:2278–2283. doi: 10.1073/pnas.051627098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kokoszka JE. Waymire KG. Levy SE. Sligh JE. Cai J. Jones DP. MacGregor GR. Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Korytowski W. Basova LV. Pilat A. Kernstock RM. Girotti AW. Permeabilization of the mitochondrial outer membrane by Bax/truncated Bid (tBid) proteins as sensitized by cardiolipin hydroperoxide translocation: Mechanistic implications for the intrinsic pathway of oxidative apoptosis. J Biol Chem. 2011;286:26334–26343. doi: 10.1074/jbc.M110.188516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krajewski S. Krajewska M. Ellerby LM. Welsh K. Xie Z. Deveraux QL. Salvesen GS. Bredesen DE. Rosenthal RE. Fiskum G. Reed JC. Release of caspase-9 from mitochondria during neuronal apoptosis and cerebral ischemia. Proc Natl Acad Sci USA. 1999;96:5752–5757. doi: 10.1073/pnas.96.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee JH. Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 69.Lee YJ. Shacter E. Hydrogen peroxide inhibits activation, not activity, of cellular caspase-3 in vivo. Free Radic Biol Med. 2000;29:684–692. doi: 10.1016/s0891-5849(00)00366-x. [DOI] [PubMed] [Google Scholar]
- 70.Lemasters JJ. Theruvath TP. Zhong Z. Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu B. Hannun YA. Inhibition of the neutral magnesium-dependent sphingomyelinase by glutathione. J Biol Chem. 1997;272:16281–16287. doi: 10.1074/jbc.272.26.16281. [DOI] [PubMed] [Google Scholar]
- 72.Lutter M. Fang M. Luo X. Nishijima M. Xie X. Wang X. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat Cell Biol. 2000;2:754–761. doi: 10.1038/35036395. [DOI] [PubMed] [Google Scholar]
- 73.Lutter M. Perkins GA. Wang X. The pro-apoptotic Bcl-2 family member tBid localizes to mitochondrial contact sites. BMC Cell Biol. 2001;2:22. doi: 10.1186/1471-2121-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Madesh M. Hajnoczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol. 2001;155:1003–1015. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malladi S. Challa-Malladi M. Fearnhead HO. Bratton SB. The Apaf-1*procaspase-9 apoptosome complex functions as a proteolytic-based molecular timer. EMBO J. 2009;28:1916–1925. doi: 10.1038/emboj.2009.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mannick JB. Hausladen A. Liu L. Hess DT. Zeng M. Miao QX. Kane LS. Gow AJ. Stamler JS. Fas-induced caspase denitrosylation. Science. 1999;284:651–654. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- 77.Markvicheva KN. Bilan DS. Mishina NM. Gorokhovatsky AY. Vinokurov LM. Lukyanov S. Belousov VV. A genetically encoded sensor for H2O2 with expanded dynamic range. Bioorg Med Chem. 2011;19:1079–1084. doi: 10.1016/j.bmc.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 78.Martin AG. Fearnhead HO. Apocytochrome c blocks caspase-9 activation and Bax-induced apoptosis. J Biol Chem. 2002;277:50834–50841. doi: 10.1074/jbc.M209369200. [DOI] [PubMed] [Google Scholar]
- 79.Martin AG. Nguyen J. Wells JA. Fearnhead HO. Apo cytochrome c inhibits caspases by preventing apoptosome formation. Biochem Biophys Res Commun. 2004;319:944–950. doi: 10.1016/j.bbrc.2004.05.084. [DOI] [PubMed] [Google Scholar]
- 80.Martin MC. Allan LA. Lickrish M. Sampson C. Morrice N. Clarke PR. Protein kinase A regulates caspase-9 activation by Apaf-1 downstream of cytochrome c. J Biol Chem. 2005;280:15449–15455. doi: 10.1074/jbc.M414325200. [DOI] [PubMed] [Google Scholar]
- 81.Martin MC. Allan LA. Mancini EJ. Clarke PR. The docking interaction of caspase-9 with ERK2 provides a mechanism for the selective inhibitory phosphorylation of caspase-9 at threonine 125. J Biol Chem. 2008;283:3854–3865. doi: 10.1074/jbc.M705647200. [DOI] [PubMed] [Google Scholar]
- 82.Marzo I. Brenner C. Zamzami N. Jurgensmeier JM. Susin SA. Vieira HL. Prevost MC. Xie Z. Matsuyama S. Reed JC. Kroemer G. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- 83.Maurer U. Charvet C. Wagman AS. Dejardin E. Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 84.Mitchell DA. Marletta MA. Thioredoxin catalyzes the S-nitrosation of the caspase-3 active site cysteine. Nat Chem Biol. 2005;1:154–158. doi: 10.1038/nchembio720. [DOI] [PubMed] [Google Scholar]
- 85.Miyashita T. Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 86.Morel C. Carlson SM. White FM. Davis RJ. Mcl-1 integrates the opposing actions of signaling pathways that mediate survival and apoptosis. Mol Cell Biol. 2009;29:3845–3852. doi: 10.1128/MCB.00279-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nukuna BN. Sun G. Anderson VE. Hydroxyl radical oxidation of cytochrome c by aerobic radiolysis. Free Radic Biol Med. 2004;37:1203–1213. doi: 10.1016/j.freeradbiomed.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 88.Ott M. Robertson JD. Gogvadze V. Zhivotovsky B. Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci USA. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pan Z. Voehringer DW. Meyn RE. Analysis of redox regulation of cytochrome c-induced apoptosis in a cell-free system. Cell Death Differ. 1999;6:683–688. doi: 10.1038/sj.cdd.4400544. [DOI] [PubMed] [Google Scholar]
- 90.Perciavalle RM. Stewart DP. Koss B. Lynch J. Milasta S. Bathina M. Temirov J. Cleland MM. Pelletier S. Schuetz JD. Youle RJ. Green DR. Opferman JT. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat Cell Biol. 2012;14:575–583. doi: 10.1038/ncb2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Petrosillo G. Ruggiero FM. Pistolese M. Paradies G. Reactive oxygen species generated from the mitochondrial electron transport chain induce cytochrome c dissociation from beef-heart submitochondrial particles via cardiolipin peroxidation. Possible role in the apoptosis. FEBS Lett. 2001;509:435–438. doi: 10.1016/s0014-5793(01)03206-9. [DOI] [PubMed] [Google Scholar]
- 92.Pop C. Timmer J. Sperandio S. Salvesen GS. The apoptosome activates caspase-9 by dimerization. Mol Cell. 2006;22:269–275. doi: 10.1016/j.molcel.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 93.Putcha GV. Le S. Frank S. Besirli CG. Clark K. Chu B. Alix S. Youle RJ. LaMarche A. Maroney AC. Johnson EM., Jr. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 94.Qin F. Lennon-Edwards S. Lancel S. Biolo A. Siwik DA. Pimentel DR. Dorn GW. Kang YJ. Colucci WS. Cardiac-specific overexpression of catalase identifies hydrogen peroxide-dependent and -independent phases of myocardial remodeling and prevents the progression to overt heart failure in G(alpha)q-overexpressing transgenic mice. Circ Heart Fail. 2010;3:306–313. doi: 10.1161/CIRCHEARTFAILURE.109.864785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qin H. Srinivasula SM. Wu G. Fernandes-Alnemri T. Alnemri ES. Shi Y. Structural basis of procaspase-9 recruitment by the apoptotic protease-activating factor 1. Nature. 1999;399:549–557. doi: 10.1038/21124. [DOI] [PubMed] [Google Scholar]
- 96.Qiu H. Edmunds T. Baker-Malcolm J. Karey KP. Estes S. Schwarz C. Hughes H. Van Patten SM. Activation of human acid sphingomyelinase through modification or deletion of C-terminal cysteine. J Biol Chem. 2003;278:32744–32752. doi: 10.1074/jbc.M303022200. [DOI] [PubMed] [Google Scholar]
- 97.Quiros PM. Ramsay AJ. Sala D. Fernandez-Vizarra E. Rodriguez F. Peinado JR. Fernandez-Garcia MS. Vega JA. Enriquez JA. Zorzano A. Lopez-Otin C. Loss of mitochondrial protease OMA1 alters processing of the GTPase OPA1 and causes obesity and defective thermogenesis in mice. EMBO J. 2012;31:2117–2133. doi: 10.1038/emboj.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raina D. Pandey P. Ahmad R. Bharti A. Ren J. Kharbanda S. Weichselbaum R. Kufe D. c-Abl tyrosine kinase regulates caspase-9 autocleavage in the apoptotic response to DNA damage. J Biol Chem. 2005;280:11147–11151. doi: 10.1074/jbc.M413787200. [DOI] [PubMed] [Google Scholar]
- 99.Ran Q. Liang H. Gu M. Qi W. Walter CA. Roberts LJ., 2nd Herman B. Richardson A. Van Remmen H. Transgenic mice overexpressing glutathione peroxidase 4 are protected against oxidative stress-induced apoptosis. J Biol Chem. 2004;279:55137–55146. doi: 10.1074/jbc.M410387200. [DOI] [PubMed] [Google Scholar]
- 100.Renatus M. Stennicke HR. Scott FL. Liddington RC. Salvesen GS. Dimer formation drives the activation of the cell death protease caspase 9. Proc Natl Acad Sci USA. 2001;98:14250–14255. doi: 10.1073/pnas.231465798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reubold TF. Wohlgemuth S. Eschenburg S. Crystal structure of full-length Apaf-1: How the death signal is relayed in the mitochondrial pathway of apoptosis. Structure. 2011;19:1074–1083. doi: 10.1016/j.str.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 102.Ricci JE. Munoz-Pinedo C. Fitzgerald P. Bailly-Maitre B. Perkins GA. Yadava N. Scheffler IE. Ellisman MH. Green DR. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004;117:773–786. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 103.Riedl SJ. Li W. Chao Y. Schwarzenbacher R. Shi Y. Structure of the apoptotic protease-activating factor 1 bound to ADP. Nature. 2005;434:926–933. doi: 10.1038/nature03465. [DOI] [PubMed] [Google Scholar]
- 104.Ripple MO. Abajian M. Springett R. Cytochrome c is rapidly reduced in the cytosol after mitochondrial outer membrane permeabilization. Apoptosis. 2010;15:563–573. doi: 10.1007/s10495-010-0455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rodriguez J. Lazebnik Y. Caspase-9 and APAF-1 form an active holoenzyme. Genes Dev. 1999;13:3179–3184. doi: 10.1101/gad.13.24.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rossig L. Fichtlscherer B. Breitschopf K. Haendeler J. Zeiher AM. Mulsch A. Dimmeler S. Nitric oxide inhibits caspase-3 by S-nitrosation in vivo. J Biol Chem. 1999;274:6823–6826. doi: 10.1074/jbc.274.11.6823. [DOI] [PubMed] [Google Scholar]
- 107.Roy SS. Ehrlich AM. Craigen WJ. Hajnoczky G. VDAC2 is required for truncated BID-induced mitochondrial apoptosis by recruiting BAK to the mitochondria. EMBO Rep. 2009;10:1341–1347. doi: 10.1038/embor.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rytomaa M. Kinnunen PK. Reversibility of the binding of cytochrome c to liposomes. Implications for lipid–protein interactions. J Biol Chem. 1995;270:3197–3202. doi: 10.1074/jbc.270.7.3197. [DOI] [PubMed] [Google Scholar]
- 109.Rytomaa M. Mustonen P. Kinnunen PK. Reversible, nonionic, and pH-dependent association of cytochrome c with cardiolipin-phosphatidylcholine liposomes. J Biol Chem. 1992;267:22243–22248. [PubMed] [Google Scholar]
- 110.Saitoh M. Nishitoh H. Fujii M. Takeda K. Tobiume K. Sawada Y. Kawabata M. Miyazono K. Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal- regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saleh A. Srinivasula SM. Acharya S. Fishel R. Alnemri ES. Cytochrome c and dATP-mediated oligomerization of Apaf-1 is a prerequisite for procaspase-9 activation. J Biol Chem. 1999;274:17941–17945. doi: 10.1074/jbc.274.25.17941. [DOI] [PubMed] [Google Scholar]
- 112.Sato T. Machida T. Takahashi S. Iyama S. Sato Y. Kuribayashi K. Takada K. Oku T. Kawano Y. Okamoto T. Takimoto R. Matsunaga T. Takayama T. Takahashi M. Kato J. Niitsu Y. Fas-mediated apoptosome formation is dependent on reactive oxygen species derived from mitochondrial permeability transition in Jurkat cells. J Immunol. 2004;173:285–296. doi: 10.4049/jimmunol.173.1.285. [DOI] [PubMed] [Google Scholar]
- 113.Schafer ZT. Kornbluth S. The apoptosome: physiological, developmental, and pathological modes of regulation. Dev Cell. 2006;10:549–561. doi: 10.1016/j.devcel.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 114.Schoneich C. Methionine oxidation by reactive oxygen species: Reaction mechanisms and relevance to Alzheimer's disease. Biochim Biophys Acta. 2005;1703:111–119. doi: 10.1016/j.bbapap.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 115.Scorrano L. Ashiya M. Buttle K. Weiler S. Oakes SA. Mannella CA. Korsmeyer SJ. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 116.Seifert A. Allan LA. Clarke PR. DYRK1A phosphorylates caspase-9 at an inhibitory site and is potently inhibited in human cells by harmine. FEBS J. 2008;275:6268–6280. doi: 10.1111/j.1742-4658.2008.06751.x. [DOI] [PubMed] [Google Scholar]
- 117.Shidoji Y. Hayashi K. Komura S. Ohishi N. Yagi K. Loss of molecular interaction between cytochrome c and cardiolipin due to lipid peroxidation. Biochem Biophys Res Commun. 1999;264:343–347. doi: 10.1006/bbrc.1999.1410. [DOI] [PubMed] [Google Scholar]
- 118.Shimizu S. Matsuoka Y. Shinohara Y. Yoneda Y. Tsujimoto Y. Essential role of voltage-dependent anion channel in various forms of apoptosis in mammalian cells. J Cell Biol. 2001;152:237–250. doi: 10.1083/jcb.152.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shimizu S. Narita M. Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 120.Shiozaki EN. Chai J. Rigotti DJ. Riedl SJ. Li P. Srinivasula SM. Alnemri ES. Fairman R. Shi Y. Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell. 2003;11:519–527. doi: 10.1016/s1097-2765(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 121.Song Z. Chen H. Fiket M. Alexander C. Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178:749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Srinivasula SM. Ahmad M. Fernandes-Alnemri T. Alnemri ES. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol Cell. 1998;1:949–957. doi: 10.1016/s1097-2765(00)80095-7. [DOI] [PubMed] [Google Scholar]
- 123.Srinivasula SM. Fernandes-Alnemri T. Zangrilli J. Robertson N. Armstrong RC. Wang L. Trapani JA. Tomaselli KJ. Litwack G. Alnemri ES. The Ced-3/interleukin 1beta converting enzyme-like homolog Mch6 and the lamin-cleaving enzyme Mch2alpha are substrates for the apoptotic mediator CPP32. J Biol Chem. 1996;271:27099–27106. doi: 10.1074/jbc.271.43.27099. [DOI] [PubMed] [Google Scholar]
- 124.Srinivasula SM. Hegde R. Saleh A. Datta P. Shiozaki E. Chai J. Lee RA. Robbins PD. Fernandes-Alnemri T. Shi Y. Alnemri ES. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001;410:112–116. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]
- 125.Stennicke HR. Deveraux QL. Humke EW. Reed JC. Dixit VM. Salvesen GS. Caspase-9 can be activated without proteolytic processing. J Biol Chem. 1999;274:8359–8362. doi: 10.1074/jbc.274.13.8359. [DOI] [PubMed] [Google Scholar]
- 126.Susin SA. Lorenzo HK. Zamzami N. Marzo I. Brenner C. Larochette N. Prevost MC. Alzari PM. Kroemer G. Mitochondrial release of caspase-2 and −9 during the apoptotic process. J Exp Med. 1999;189:381–394. doi: 10.1084/jem.189.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Suto D. Sato K. Ohba Y. Yoshimura T. Fujii J. Suppression of the pro-apoptotic function of cytochrome c by singlet oxygen via a haem redox state-independent mechanism. Biochem J. 2005;392:399–406. doi: 10.1042/BJ20050580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tait SW. Green DR. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 129.Thariat J. Collin F. Marchetti C. Ahmed-Adrar NS. Vitrac H. Jore D. Gardes-Albert M. Marked difference in cytochrome c oxidation mediated by HO(*) and/or O(2)(*-) free radicals in vitro. Biochimie. 2008;90:1442–1451. doi: 10.1016/j.biochi.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 130.Tobiume K. Matsuzawa A. Takahashi T. Nishitoh H. Morita K. Takeda K. Minowa O. Miyazono K. Noda T. Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Torok NJ. Higuchi H. Bronk S. Gores GJ. Nitric oxide inhibits apoptosis downstream of cytochrome C release by nitrosylating caspase 9. Cancer Res. 2002;62:1648–1653. [PubMed] [Google Scholar]
- 132.Torres M. Mitogen-activated protein kinase pathways in redox signaling. Front Biosci. 2003;8:369–391. doi: 10.2741/999. [DOI] [PubMed] [Google Scholar]
- 133.Tran TH. Andreka P. Rodrigues CO. Webster KA. Bishopric NH. Jun kinase delays caspase-9 activation by interaction with the apoptosome. J Biol Chem. 2007;282:20340–20350. doi: 10.1074/jbc.M702210200. [DOI] [PubMed] [Google Scholar]
- 134.Van Remmen H. Qi W. Sabia M. Freeman G. Estlack L. Yang H. Mao Guo Z. Huang TT. Strong R. Lee S. Epstein CJ. Richardson A. Multiple deficiencies in antioxidant enzymes in mice result in a compound increase in sensitivity to oxidative stress. Free Rad Biol Med. 2004;36:1625–1634. doi: 10.1016/j.freeradbiomed.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 135.Van Remmen H. Williams MD. Guo Z. Estlack L. Yang H. Carlson EJ. Epstein CJ. Huang TT. Richardson A. Knockout mice heterozygous for Sod2 show alterations in cardiac mitochondrial function and apoptosis. Am J Phys Heart Circ Physiol. 2001;281:H1422–1432. doi: 10.1152/ajpheart.2001.281.3.H1422. [DOI] [PubMed] [Google Scholar]
- 136.Villunger A. Michalak EM. Coultas L. Mullauer F. Bock G. Ausserlechner MJ. Adams JM. Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 137.von Ballmoos C. Wiedenmann A. Dimroth P. Essentials for ATP synthesis by F1F0 ATP synthases. Annu Rev Biochem. 2009;78:649–672. doi: 10.1146/annurev.biochem.78.081307.104803. [DOI] [PubMed] [Google Scholar]
- 138.Waterhouse NJ. Goldstein JC. von Ahsen O. Schuler M. Newmeyer DD. Green DR. Cytochrome c maintains mitochondrial transmembrane potential and ATP generation after outer mitochondrial membrane permeabilization during the apoptotic process. J Cell Biol. 2001;153:319–328. doi: 10.1083/jcb.153.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu GS. Kim K. el-Deiry WS. KILLER/DR5, a novel DNA-damage inducible death receptor gene, links the p53-tumor suppressor to caspase activation and apoptotic death. Adv Exp Med Biol. 2000;465:143–151. doi: 10.1007/0-306-46817-4_13. [DOI] [PubMed] [Google Scholar]
- 140.Yamaguchi R. Lartigue L. Perkins G. Scott RT. Dixit A. Kushnareva Y. Kuwana T. Ellisman MH. Newmeyer DD. Opa1-mediated cristae opening is Bax/Bak and BH3 dependent, required for apoptosis, and independent of Bak oligomerization. Mol Cell. 2008;31:557–569. doi: 10.1016/j.molcel.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yin Q. Park HH. Chung JY. Lin SC. Lo YC. da Graca LS. Jiang X. Wu H. Caspase-9 holoenzyme is a specific and optimal procaspase-3 processing machine. Mol Cell. 2006;22:259–268. doi: 10.1016/j.molcel.2006.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Youle RJ. Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 143.Yuan S. Yu X. Asara JM. Heuser JE. Ludtke SJ. Akey CW. The holo-apoptosome: Activation of procaspase-9 and interactions with caspase-3. Structure. 2011;19:1084–1096. doi: 10.1016/j.str.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yuan S. Yu X. Topf M. Ludtke SJ. Wang X. Akey CW. Structure of an apoptosome-procaspase-9 CARD complex. Structure. 2010;18:571–583. doi: 10.1016/j.str.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zamzami N. El Hamel C. Maisse C. Brenner C. Munoz-Pinedo C. Belzacq AS. Costantini P. Vieira H. Loeffler M. Molle G. Kroemer G. Bid acts on the permeability transition pore complex to induce apoptosis. Oncogene. 2000;19:6342–6350. doi: 10.1038/sj.onc.1204030. [DOI] [PubMed] [Google Scholar]
- 146.Zech B. Kohl R. von Knethen A. Brune B. Nitric oxide donors inhibit formation of the Apaf-1/caspase-9 apoptosome and activation of caspases. Biochem J. 2003;371:1055–1064. doi: 10.1042/BJ20021720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zech B. Wilm M. van Eldik R. Brune B. Mass spectrometric analysis of nitric oxide-modified caspase-3. J Biol Chem. 1999;274:20931–20936. doi: 10.1074/jbc.274.30.20931. [DOI] [PubMed] [Google Scholar]
- 148.Zhivotovsky B. Samali A. Gahm A. Orrenius S. Caspases: Their intracellular localization and translocation during apoptosis. Cell Death Differ. 1999;6:644–651. doi: 10.1038/sj.cdd.4400536. [DOI] [PubMed] [Google Scholar]
- 149.Zou H. Henzel WJ. Liu X. Lutschg A. Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 150.Zou H. Li Y. Liu X. Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 151.Zou H. Yang R. Hao J. Wang J. Sun C. Fesik SW. Wu JC. Tomaselli KJ. Armstrong RC. Regulation of the Apaf-1/caspase-9 apoptosome by caspase-3 and XIAP. J Biol Chem. 2003;278:8091–8098. doi: 10.1074/jbc.M204783200. [DOI] [PubMed] [Google Scholar]
- 152.Zuo Y. Xiang B. Yang J. Sun X. Wang Y. Cang H. Yi J. Oxidative modification of caspase-9 facilitates its activation via disulfide-mediated interaction with Apaf-1. Cell Res. 2009;19:449–457. doi: 10.1038/cr.2009.19. [DOI] [PubMed] [Google Scholar]