Abstract
Urothelium covers the inner surfaces of the renal pelvis, ureter, bladder and prostatic urethra. Although morphologically indistinguishable, the urothelia in these anatomic locations differ in their embryonic origin and lineages of cellular differentiation, as reflected in their different uroplakin content, expandability during micturition and susceptibility to chemical carcinogens. Previously thought to be an inert tissue forming a passive barrier between the urine and blood, urothelia have recently been shown to have a secretory activity that actively modifies the urine composition. Urothelial cells express a number of ion channels, receptors and ligands, enabling them to receive and send signals and communicate with adjoining cells and their broader environment. The urothelial surface bears specific receptors that not only allow uropathogenic E. coli to attach to and invade into the bladder mucosa, but also provide a route by which the bacteria ascend via the ureters to the kidney to cause pyelonephritis. Genetic ablation of one or more uroplakin genes in mice causes severe retrograde vesicoureteral reflux, hydronephrosis and renal failure, conditions that mirror certain human congenital diseases. Clearly, abnormalities of the lower urinary tract can impact on the upper tract, and vice versa, through the urothelial connection. In this review, we highlight recent advances in the field of urothelial biology by focusing on the uroplakins, a group of urothelium-specific and differentiation-dependent integral membrane proteins. We discuss these proteins’ biochemistry, structure, assembly, intracellular trafficking and their emerging roles in urothelial biology, function and pathological processes. We also call attention to important areas where greater investigative efforts are warranted.
Keywords: uroplakin, urothelium, differentiation, tetraspanin, urinary tract infection, tumorigenesis, permeability barrier, vesicular transport
INTRODUCTION
Urothelium is also known as “transitional epithelium” in histology textbooks and scientific literature, largely based on the erroneous assumption that, although it appears as a stratified epithelium, all cell layers are in direct contact with the basement membrane, a characteristic of simple epithelia. This notion has been contradicted, however, by electron microscopic and immunohistochemical studies, which have failed to show any upper urothelial layer/basement-membrane connection but instead delineate distinct urothelial layers. In rodents, for example, there are at least three discernable cell layers (basal, intermediate and superficial/umbrella), whereas in higher mammals such as cattle and humans more than one intermediate layer is present. Thus, the term “transitional epithelium” is evidently a misnomer and should be replaced by “urothelium”, as has been previously proposed 1, but never universally adopted. Similarly, the term “transitional cell carcinoma” should be replaced by “urothelial carcinoma” 2.
Urothelium is one of the slowest cycling epithelia in the body with a turnover rate of ~200 days and a tritium-thymidine labeling index of ~0.01% 3,4. Such a remarkable durability is functionally desirable as the urothelium needs to act as a constant permeability barrier to protect the blood from toxic urinary substances. In fact, the urothelium is among the most effective barriers of any biomembrane, with a transepithelial electric resistance of up to 75,000 Ω/cm2, making it a far more effective barrier than the epidermis 5,6. Urothelium also needs to remain highly flexible throughout the micturition cycle so that it can accommodate significant changes in surface area 4,7,8. How does urothelium achieve a high degree of impermeability and flexibility all at once? The answer seems to rest with a membrane specialization called the urothelial plaque elaborated by the urothelium during an advanced stage of differentiation.
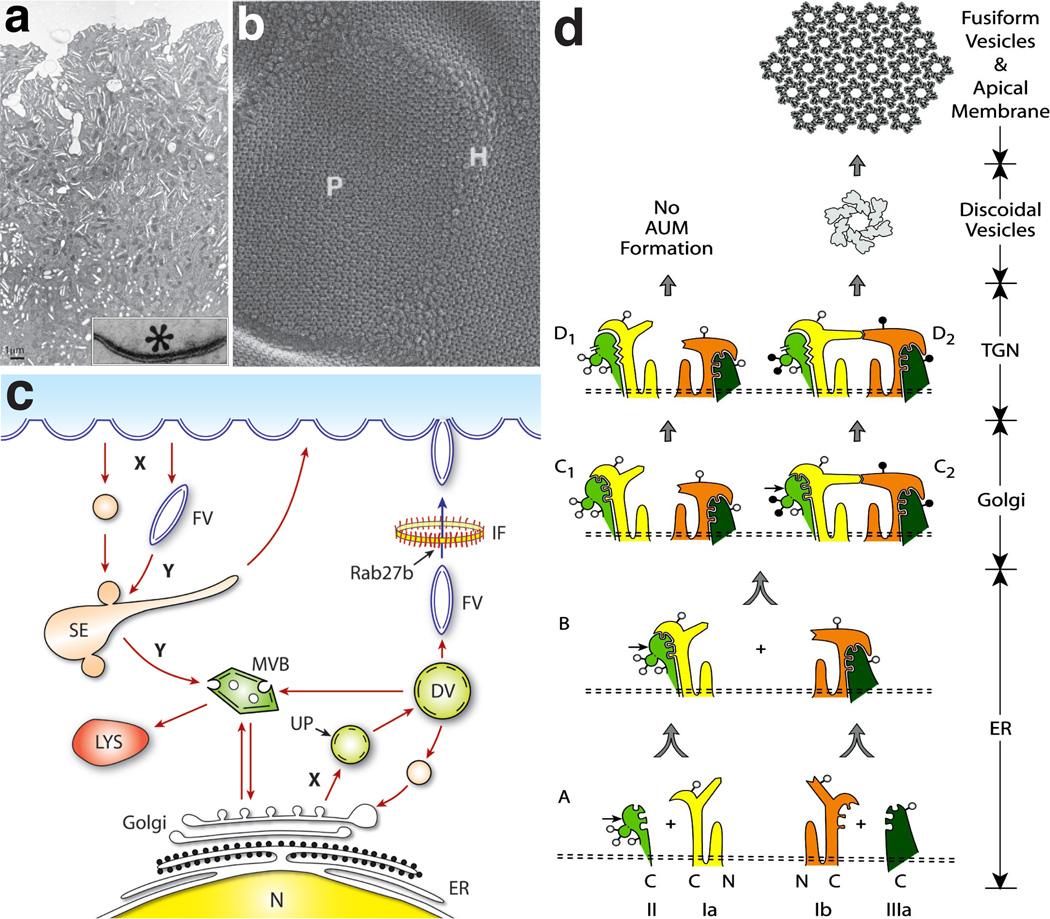
Urothelial plaques are rigid-looking, concave-shaped biomembrane structures that are visible only by electron microscopy. On cross-sections, the plaques (0.3–1 µm in diameter) exhibit outer membrane leaflets twice as thick as the inner ones, hence the name asymmetric unit membrane (or AUM) (Refs. 9–11; Fig. 1a, inset). These plaques occupy ~90% of the urothelial apical surface, interrupted by narrow “hinge” regions (Ref. 12; Fig. 1a and b). Negative staining and quick-freeze/deep-etch of the urothelial plaques revealed hexagonally arranged, 16-nm protein particles 13–18. Not only are the plaques found on the luminal surface, they are also present in great abundance in fusiform vesicles in the cytoplasm of the superficial umbrella cells (Fig. 1a and 1c). These cytoplasmic vesicles were thought to travel reversibly between the luminal surface and cytoplasm to adjust the apical surface area during the urothelial contraction/extension cycle 7,8,10,12, although whether the movement is uni- or bi-directional remains an open question 19,20. Despite the abundance and the striking structural features of these urothelial plaques, relatively little was known about their protein composition and their exact role(s) in urothelial physiology and diseases. In the following paragraphs, we will review the identification and characterization of the protein components of the urothelial plaques and their biological functions and disease implications. The recently discovered secretory 21 and neuronal properties of the urothelium and their possible roles in bladder functions have been detailed in excellent reviews 22,23 and will not be discussed here.
Figure 1.
Structure, assembly and trafficking of uroplakins. (a) Transmission electron micrograph of a mouse urothelial superficial umbrella cell. Note dense clusters of fusiform vesicles (flattened disks), which most likely mature from the spherical discoidal vesicles deep in the cytoplasm. Inset shows a higher magnification view of a rigid-looking plaque showing asymmetric unit membrane. (b) Quick-freeze deep-etch image of the apical surface of a mouse umbrella cell showing urothelial plaques (P) containing hexagonal arrays of 16-nm particles interconnected by particle-free hinge (H) areas 16. (c) Schematic diagram illustrating the vesicular traffic in umbrella cells. Uroplakin hetreodimers assembled in the ER and modified in the Golgi apparatus accumulate in small vesicles budding off the trans Golgi network (TGN). They form discoidal vesicles (DVs) where assembly of crystalline arrays continues. The mature fusiform vesicles (FVs) traverse a meshwork of intermediate filaments (IF; Ref. 150) and fuse with the apical plasma membrane in a regulated fashion mediated by Rab27b. Degradation of UPs requires the formation of endocytic vesicles and/or of modified FVs that form sorting endosomes (SE) and multivesicular bodies (MVB), which fuse with mature lysosomes (LYS). (d) A model depicting the assembly of the four major uroplakins (UPIa, Ib, II, and IIIa) into 2D crystals of urothelial particles. Stages A and B: The four uroplakins acquire high-mannose glycans in the ER and form two heterodimers (UPIa/II and UPIb/IIIa), which undergo major conformational changes. Symbols: the small, horizontal arrows on UPII mark the furin cleavage site at the end of the prosequence; the open and closed circles represent high-mannose and complex glycans, respectively. In normal urothelium (pathway on the right), the glycans on two of the three N-glycosylation sites on the prosequence of UPII become complex glycans in the Golgi apparatus (stage C2), and the furin-mediated cleavage/removal of the prosequence in the TGN (stage D2) then triggers oligomerization to form a 16-nm particle. In cultured urothelial cells (pathway on the left), the differentiation-dependent glycosylation of pro-UPII does not occur, thus hampering the formation of the uroplakin heterotetramer and the 16-nm particle, thus no AUM assembly (Adapted from Ref. 43 with permission).
Uroplakins are the protein building blocks of urothelial plaques
Although urothelial plaques are a hallmark of urothelial differentiation, earlier attempts to define their protein composition were hampered by difficulties in their purification and inability to generate antibodies to any plaque-associated proteins 24–26. Using sucrose gradient centrifugation coupled with detergent wash, we succeeded in isolating milligram quantities of highly purified bovine urothelial plaques 27. These plaques exhibited typical 2D crystals of hexagonally packed 16-nm particles and contained four major proteins of 15, 27, 28 and 47 kDa. By generating monospecific antibodies and EM-localizing each of these proteins to the urothelial plaques in situ, we named these novel integral membrane proteins uroplakins Ia (27 kDa), Ib (28 kDa), II (15 kDa) and IIIa (47 kDa) 28–30. Subsequent studies demonstrated that the urothelial plaques isolated from a wide range of species including mouse and human were ultrastructurally identical, and all contained the four uroplakins, demonstrating their high degree of conservation during mammalian evolution 31,32. The onset of uroplakin expression during urothelial differentiation varies, however, from species to species. In higher mammals such as cattle and human that possess a thick urothelium, uroplakins are detected primarily in the superficial umbrella cells, whereas in rodents that possess a thin 3–4 cell layered urothelium they are found in all urothelial layers including the basal cells (Refs. 27, 33–35; also see below). Such a species and/or urothelial thickness-dependent expression of uroplakins may be related to the expression patterns of certain transcription factors 36.
Among the uroplakins (UP), UPIa and UPIb share ~40% of their amino acid sequence, and both have four transmembrane domains (TMD) with a minor (1st) and a major (2nd) hydrophilic domain extending extracellularly 30. These two UPs belong to the tetraspanin family comprised of many leukocyte differentiation antigens such as CD9, CD27, CD63, CD81 and CD83 37,38. UPII possesses an N-terminal signal peptide followed by a heavily glycosylated pro-peptide of 59 amino acids ending in an RGRR consensus substrate for furin, a trans-Golgi network-associated processing enzyme. The mature UPII is not glycosylated and has only a single TMD located close to its C-terminus, with very little cytoplasmic domain 29. Similar to UPII, UPIIIa has a single TMD, which divides the protein into a long (189 residue) N-terminal luminal domain and a C-terminal (52 residue) cytoplasmic domain 28. The N-terminal luminal domain is heavily glycosylated with 20-kDa equivalents of complex-type carbohydrates, while the cytoplasmic domain harbors several potential serine/threonine and tyrosine phosphorylation sites. Interestingly, UPIIIa shares a stretch of about 12 amino acid residues, located on the N-terminal side of the single TMD, with a similarly located domain in UPII suggesting that these two UPs are related 28,29. Since UPIIIa is the only UP that possesses a significant cytoplasmic domain, this raises the interesting possibility that UPIIIa may be involved in anchoring the urothelial plaques to the underlying cytoskeleton 39 and that its C-terminal phosphorylation may be involved in regulating such an association 28. It is notable that, overall, the mass of UPs’ extracellular domains greatly exceeds that of their cytoplasmic domains (Fig. 1d). Such an asymmetric mass distribution of UPs across the lipid bilayer explains the TEM observation that the outer leaflets of urothelial plaques are almost twice as thick as the inner ones 28–30.
Three lines of evidence suggest that the four major UPs form two specific pairs. First, treatment of purified bovine urothelial plaques with bifunctional crosslinking reagents led to the crosslinking of UPIa and UPIb, the two tetraspanin-related uroplakins, to UPII and UPIIIa, respectively, suggesting the existence of UPIa/II and UPIb/IIIa heterodimers 40. Second, ion exchange chromatography co-purified UPIa with II and UPIb with IIIa 41. Third, transfection of 293T cells with single uroplakin cDNAs resulted in UPs being trapped in the ER (except UPIb which can exit by itself). In contrast, double transfection of UPIa/II or UPIb/IIIa enabled the heterodimers to reach the cell surface 42. These results clearly indicate that the formation of UPIa/II or UPIb/IIIa heterodimers is the first step of UP assembly that is required for ER-exit (Refs. 42, 43; Fig. 1d).
Formation of 2D crystals by UPs provides structural insights into these proteins
The existence of UPs in naturally occurring 2D crystals of the urothelial plaques has offered unique opportunities for in-depth structural studies. Quick-freeze deep-etch 16, negative staining coupled with image processing 14,15,17,18, cryo-EM 44–46 and atomic force microscopy 47 resolved each 16-nm particle into six inner and six outer subdomains forming two concentric rings. One inner and one outer subdomain interconnect to form a subunit - one sixth of the hexagonal particle. Cryo-EM 3D reconstructions revealed that each subdomain contains a total of 5 transmembrane helices, corresponding to one tetraspanin and a single-pass uroplakin (Refs. 45, 46; Fig. 2). EM localization using the E. coli FimH adhesin, which specifically binds to UPIa, indicates that the UPIa/II pair occupies the inner subdomain, whereas the UP Ib/III pair occupies the outer subdomain Ref. 47; Figs. 2 and 3a and b). The four transmembrane helices of UPIa and UPIb are tightly packed into bundles aligned with the extracellular domains, giving these two UPs an overall cylindrical shape. The single-pass UPII and UPIIIa adopt an inverted “L”-shape, anchored by its transmembrane helix packed against the four transmembrane helices of their partner tetraspanin UPs, thus forming a five-helix bundle within each subdomain. The long arm of the inverted “L” continues up against the cylindrical UP tetraspanins. The short arm of the “L” extends to join the short arm of the other inverted “L” from the paired subdomain within the same subunit, thus forming a “joint” 45. Interestingly, the joint provides the only contact between the two subdomains within a subunit, while the two tetraspanins, UPIa and UPIb, do not appear to have any direct contact 45. This type of loose connection between the two subdomains within a subunit suggests a flexible interaction between the inner and outer subdomains, thus providing a basis for possible structural changes of UPs upon binding to the E. coli FimH adhesin.
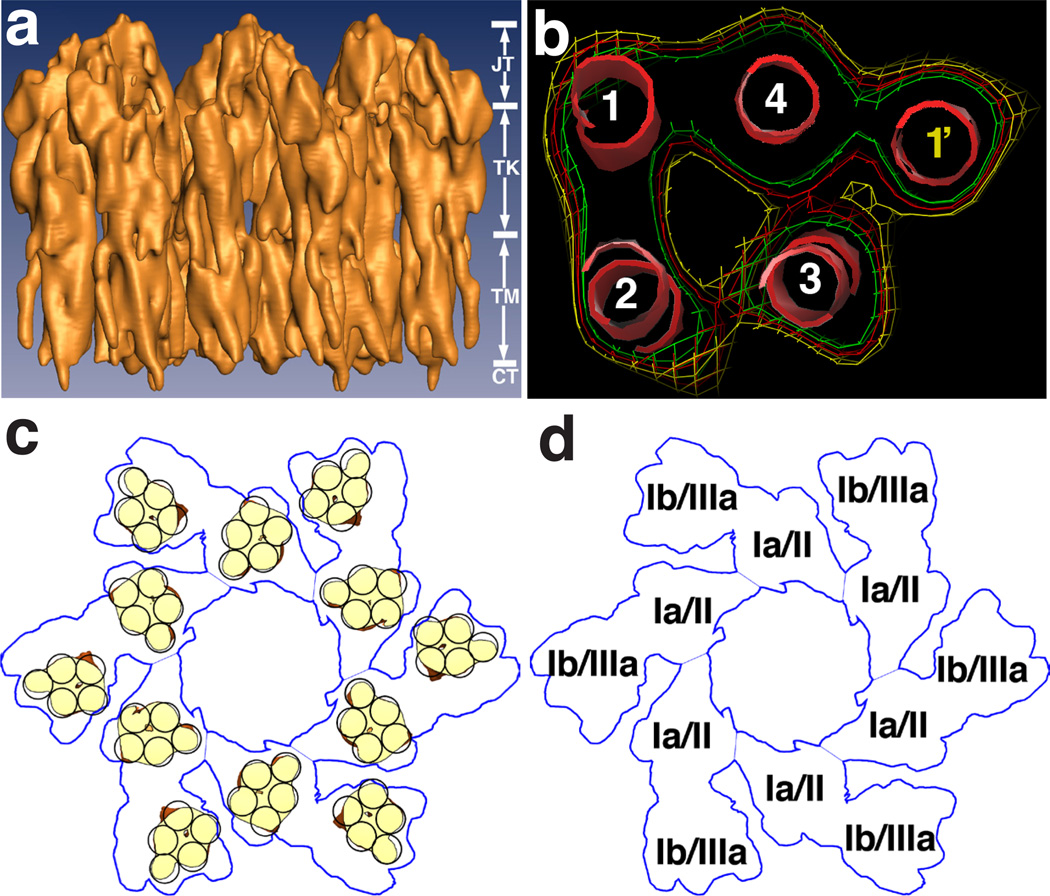
Figure 2.
Structure of the 16-nm uroplakin particle. (a) The side view of the cryo-EM structure of the 16-nm mouse uroplakin particle at 6Å resolution. The vertical dimension of the particle can be divided into four regions: the joint (JT), the trunk (TK), the transmembrane region (TM), and the cytoplasmic region (CT). (b) Electron densities of the transmembrane region of an inner subdomain of the particle, showing a five-helix bundle formed by the transmembrane helices from the tetranspanin UPIa (helices 1–4) and the single transmembrane domain from UPII (helix 1’). (c) Positions of the transmembrane helices in the 16-nm particle (yellow). (d) A model showing that the inner and outer subdomains of the particle are formed by the UPIa/II and UPIb/IIIa pair, respectively (see text). Panels a and b are reprinted with permission from Ref. 45.
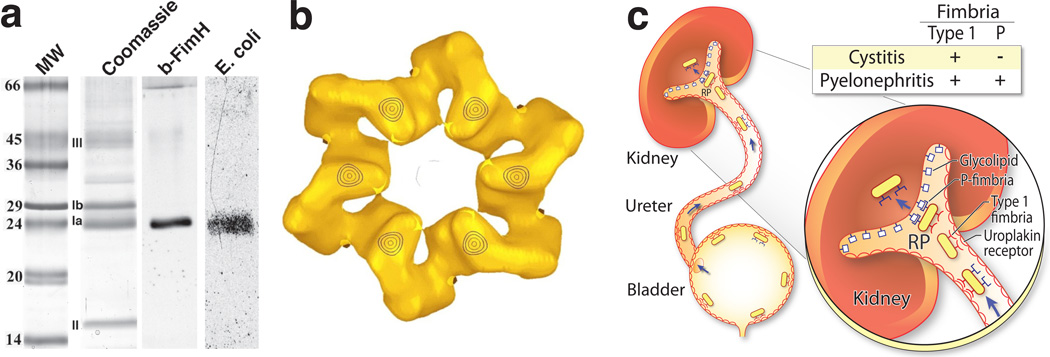
Figure 3.
Identification of uroplakin Ia as bacterial receptor and its role in urinary tract infection. (a) The four major mouse uroplakins (Ia, Ib, II and IIIa) were revolved by SDS-PAGE, electroblotted (lane 2) and incubated with biotinylated, bacterial FimH/C complex (lane 3) or with type 1-fimbriated E. coli that were metabolically labeled with 35S-methionine (lane 4). Note the specific binding of FimH and the uropathogenic E. coli to UPIa. MW: molecular weight standards. (b) Localization of the FimH binding site to the six inner subdomains of the mouse 16-nm uroplakin particle, based on the difference map of the mouse urothelial plaques that have been negatively stained with and without a saturating amount of FimH. (c) Schematic model of ascending UTI in mice by uropathogenic E. coli. E. coli expressing the type 1 fimbriae can attach to the uroplakin receptor and cause bladder infection (cystitis). While these type-1 positive bacteria can bind to the uroplakin receptor on the ureteral urothelial surface and ascend to the kidneys, they usually do not cause kidney infection (pyelonephritis) because they lack P fimbria, which is required for binding to the kidney’s glycolipids. In contrast, E. coli expressing both type 1 and P fimbriae can not only ascend to the renal pelvis (RP), but also bind to and invade into the renal parenchyma causing pyelonephritis. Once there, the expression of type 1 fimbria can sometimes be turned off via phase variation. Panels a and b are reprinted with permission from Refs. 95 and 47, respectively.
Assembly of UPs into 2D crystals is a dynamic and highly regulated process
A crucial step in elucidating the biological functions of urothelial plaques is to understand how UPs are assembled into higher order protein complexes and how the resultant urothelial plaques are delivered to the urothelial apical surface. Transfection studies showed that, immediately after their synthesis in the ER, the four uroplakins acquire high-mannose glycans and form two heterodimers (UPIa/II and UPIb/IIIa) 42. Recent data suggest a model in which major conformational changes occur during UP dimerization leading to a more stable protein complex that can exit from the ER 43. The glycans on two of the three N-glycosylation sites of the pro-peptide of UPII become complex glycans in the Golgi apparatus, causing conformational changes in pro-UPII and its partner UPIa and thereby allowing heterotetramer formation (Refs. 43, 48; Fig. 1d). Furin-mediated removal of the pro-peptide in the TGN then triggers oligomerization to form a 16-nm particle in which UPIa/II and UPIb/IIIa are associated with the inner and outer subdomains, respectively 47, and to later form 2D crystals (Fig. 1d). Interestingly, in cultured bovine urothelial cells, the differentiation-dependent glycosylation of pro-UPII does not occur, thus hampering the formation of the UP heterotetramer and the 16-nm particle 42,49.
While UPII and UPIIIa are transported to the plasma membrane only after heterodimerization with UPIa and UPIb, respectively, UPIb when expressed alone can exit the ER and move to the plasma membrane. In contrast, the structurally closely related UPIa by itself remains trapped in this compartment 42,50. This may explain why UPIb is the only UP that is expressed by itself in tissues other than the urothelium, namely the cornea, conjunctiva, and possibly the lung 51,52. The finding that UPIb can exit from the ER without forming a heterodimeric complex with UPIIIa is also consistent with the results from UPIIIa-knockout mice where UPIb can be detected at the urothelial cell surface 53.
The identification of specific UPIb domains that are required for this tetraspanin to exit the ER was greatly facilitated by swapping UPIb domains with its closely related tetraspanin UPIa. As it turns out, the four TMDs of UPIb do not simply provide hydrophobic anchors; they contain sequence and structural information that affects intra-TMD interactions which are critical for the transport of UPIb from the ER to the Golgi apparatus 50. Mutations in such TMDs cause UPIb to aggregate, perhaps due to interactions with chaperone-like proteins that recognize improperly assembled TMDs resulting in their retention in the ER 54,55. However, deletion of the N-linked oligosaccharide from UPIb or elimination of the disulfide bridges does not result in the ER-retention of UPIb. Therefore, these two posttranslational modifications are apparently not essential for the proper folding of the large loop of UPIb 50. This is in striking contrast to studies on other tetraspanins such as CD81 and CD82, which after reduction of their disulfide bridges, can no longer exit from the ER 56.
How are pre-assembled crystalline arrays of UPs delivered to the urothelial apical surface? We have attempted to address this question by investigating the possible role of Rab proteins. Using RT-PCR, we identified in urothelial cells 12 Rab or Rab-related proteins: Rab4, Rab5, Rab8, Rab11, Rab13, Rab15, Rab27b, Rab28, Rab32, RhoA, RhoC and Ras1 57. While most Rabs were broadly distributed, Rab27b was exceptionally enriched in urothelium, representing as much as 0.1% of the total urothelial proteins. Furthermore, on Western blots, only Rab27b, but not its isoform Rb27a, was detected in urothelium 57. The abundance of Rab27b in urothelium and its differentiation-dependent co-expression with the UPs raise the interesting possibility that the expression of Rab27b and the formation of UP-containing fusiform vesicles are highly coordinated, and that Rab27b may play a role in the delivery of urothelial plaques to the plasma membrane (see e.g., Ref. 58). Consistent with this idea, we demonstrated by immuno-EM that Rab27b was associated with the UP-containing fusiform vesicles 57. These results indicate that Rab27b is involved in targeting UP-containing fusiform vesicles to the plasma membrane of urothelial umbrella cells (Fig. 1c). Recent results indicate the involvement of an additional Rab protein, Rab11a, in the exocytosis of urothelial plaques 59.
Urothelia of different anatomic locations represent different cell lineages
Although the term “urothelium” is ordinarily used to describe the epithelial lining of both upper and lower urinary tracts, recent data indicate that urothelia at different anatomical sites are ultrastructurally and biochemically different due to intrinsic divergence acquired during development 60. For instance, ureteral urothelium contains fewer cytoplasmic fusiform vesicles and less uroplakin proteins per total cellular proteins than does the bladder urothelium 60–62. Importantly, this difference in uroplakin content persists even after the bovine bladder and ureteral urothelial cells have been serially subcultured under identical cell culture conditions using lethally irradiated 3T3 cells as a feeder layer. Moreover, such cultured bovine bladder urothelial cells exhibits a higher growth rate than the ureteral urothelial cells. These results indicate that the observed phenotypic differences cannot be due to extrinsic regulation 63–66. Feeding vitamin A-deficient diet to mice induces bladder urothelial keratinization (bladder metaplasia) that originates not from the bladder dome but from the bladder neck/proximal urethra (Ref. 60; Fig. 4a). Combined with the fact that renal pelvis/ureteral urothelium is mesoderm-derived, whereas the bladder/urethral urothelium is endoderm-derived 67,68, these observations suggest that urothelium consists of at least three cell lineages, i.e., (i) renal pelvis/ureter, (ii) bladder and (iii) bladder neck/proximal urethra 60. Therefore, to use the general term “urothelium” without specifying its anatomic location can be confusing and misleading. The heterogeneity of urothelium has implications about the cellular basis of urothelial metaplasia (Fig. 4b–f), and raises questions about the common practice of using human ureteral urothelium, which is readily available from surgical specimens, as a surrogate for adult human bladder urothelium, which is more difficult to obtain and to grow in culture. Specifically, autologous bladder urothelial cells provide an ideal source for bladder reconstruction 69. Ureteral urothelial cells may be used as a substitute for bladder urothelial cells, although these two cell types may have different functional and biomechanical properties 70,71. Finally, urothelia at different locations appear to respond differently to various carcinogens. While experimental carcinogens like N-butyl-N-(4-hydroxybutyl)nitrosamine or N-nitrosomethylurea cause primarily urothelial carcinomas of the bladder 72, aristolochic acid, a mycotoxin contaminant in wheat and a component in certain Chinese herbal products, induces urothelial carcinomas of only the renal pelvis and the upper ureter 73,74. It will be very interesting to determine whether intrinsic properties underlying the urothelial heterogeneity are responsible for the varied susceptibilities of the urothelia of different anatomic sites to different carcinogens.
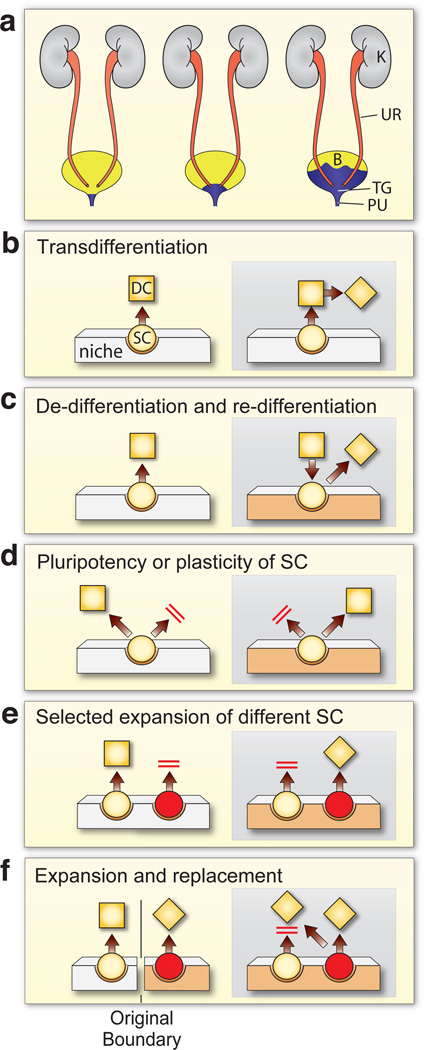
Figure 4.
Models of squamous metaplasia of the mouse urothelium induced by vitamin A deficiency. (a) Schematic diagram showing gradual expansion of the vitamin A deficiency-induced squamous metaplasia from the proximal urethra (PU) and the trigone (TG) region to the rest of the bladder (B). UR, ureter; K, kidney. (b–f) Models for various cellular mechanisms of bladder metaplasia. (b) The transdifferentiation model: A terminally differentiated cell (DC; square) can directly transform into a different kind of terminally differentiated cell (diamond). (c) The dedifferentiation-and-redifferentiation model: A terminally differentiated cell can revert back to an undifferentiated or stem cell, which can then differentiate along a different pathway yielding a distinct phenotype in response to environmental and/or mesenchymal changes (beige). (d) The pluripotent stem cell model: Under normal conditions, the pluripotent stem cells give rise to terminal differentiated cells of a particular phenotype; mesenchymal changes may induce such a stem cell to undergo an alternative pathway of differentiation. (e) The selective expansion model. The tissue contains two separate populations of stem cells: one of them (yellow) normally gives rise to the main phenotype, whereas the other lies dormant. Mesenchymal and/or environmental changes including alterations of the stem cell niche suppress the growth and differentiation of the originally dominant stem cell while activating the originally dormant stem cell (red) that now gives rise to a different phenotype. (f) The expansion and replacement model. The tissue contains two separate cell lineages that occupy different domains separated by well-defined boundaries. Mesenchymal and/or environmental changes such as vitamin A deficiency favor the expansion of one cell lineage over another, thus allowing one cell type to expand and invade into another cell lineage’s domain. This last model can best explain existing data on urothelial keratinizing squamous metaplasia that is induced by vitamin A deficiency. (d–f) The parallel red bars denote that the process/pathway is blocked. SC, stem cell. (adapted from Ref. 60).
Gene inactivation reveals important physiological functions of UPs
A strategy was taken to ablate mouse genes encoding UPII or UPIIIa because inactivating these two UPs should abolish the formation of UPIa/II and UPIb/III pairs, respectively 53,75. Knockout of the UPIIIa gene led to the loss of about 70–80% of the urothelial plaques of the urothelial apical surface 53. The retention of some plaques in the UPIIIa knockouts may be due to the presence of a minor UPIII isoform, UPIIIb, which was identified in a bovine urothelial subtraction cDNA library 76. However, ablation of the UPII gene, which does not appear to have an isoform, led to the complete loss of urothelial plaques 75. These results establish UPs as the integral protein subunits of the urothelial plaques.
Physiological measurements of bladder mucosa using modified Ussing chambers showed that the permeability of the UPII- or UPIIIa-deficient mouse urothelium to 14C-urea and 3H-water was significantly higher than that of the wild-type mice, suggesting that uroplakin plaques contribute to the urothelial permeability barrier function 53,75,77. It is likely that under normal conditions the crystalline network of UPs imposes structural constraints on the lipid molecules, limiting their ability to undergo lateral diffusion and thus enhancing the barrier function 6,46,77. Together, these results indicate that the UP-containing urothelial plaques, in conjunction with the lipids and the tight junctions, play an important part in the urothelial permeability barrier function 5,49,78. It is worth noting that increased urothelial permeability is a common manifestation in interstitial cystitis, an excruciatingly painful bladder syndrome 79–81. Whether defects of UPs and their associated proteins, possibly including the “glycocalyx” components 80,82, are involved in the pathogenesis of this important disease remains to be seen.
Other prominent features in mice lacking UPII, UPIIIa or both are severe vesicoureteral reflux (VUR), hydronephrosis and impaired renal function 53,75. The retrograde overflow of urine into the upper tract appeared to result from enlarged ureteral orifices in the knockout mice 53. In an attempt to determine whether UP defects might be involved in human VUR, we examined a panel of 76 well-documented VUR patients and 90 race-matched controls for single nucleotide polymorphisms (SNPs) of all four major UPs. Most SNPs found were not significantly associated with VUR, although several SNPs were marginally associated 83. The fact that no truncation or frame shift mutations were found in any of the VUR patients, coupled with our recent finding that some breeding pairs of UPIIIa and UPII knockout mice yielded litters that die neonatally 53,75, raises the intriguing possibility that major UP mutations are not tolerated in humans. By analyzing a few SNPs of only the UPIIIa gene, others have come to a similar conclusion 84,85. More recent data indicate that certain UPIIIa mutations can be correlated with human renal hypodysplasia and adysplasia, which can lead to renal failure 86,87.
UPs play a role in E. coli adhesion, invasion and upper tract dissemination
The first and essential step for uropathogenic E. coli (UPEC), which causes ~85% of all urinary tract infections, to colonize the bladder is for the bacteria to adhere to the urothelial surface via specific interactions between UPEC’s FimH adhesin and the urothelial surface’s mannosylated glycoprotein(s) 88–93. However, despite much conjecture, the identity of such urothelial receptors had long been elusive. We tested whether some of the glycosylated UPs, as the principal protein components that account for over 90% of the urothelial surface, can serve as the urothelial receptor(s) for type 1-fimbriated E. coli. We discovered that UPEC strains expressing the type 1-fimbriae, but not those expressing P-fimbriae or no fimbriae, bound to purified urothelial plaques in large numbers, in a saturable and species-conserved manner 94. The binding was completely abolished by pre-incubating type 1-fimbriated E. coli with D-mannose. Geloverlay assay revealed that the type 1-fimbriated E. coli bound specifically to protein bands in the UPIa/Ib region, without binding to UPII and III. The fact that the highly glycosylated UPIIIa did not interact with type 1-fimbriae suggested that only UPIa/Ib contained unmodified, terminal mannose moieties that represent the structural fit for the FimH binding pocket. Indeed, endo-H treatment, which removes the high mannose residues, or the addition of D-mannose, completely abolished the E. coli-UPIa/Ib binding. These results provided the first experimental evidence indicating that the urothelial surface contains a principal mannosylated glycoprotein (UPIa/Ib) that is capable of interacting with type 1-fimbriated E. coli 94.
This conclusion was further refined using mouse UPs and recombinant FimH/FimC as a probe. Unlike bovine UPIa and UPIb, which were not well resolved on SDS-PAGE because of their close molecular masses, the mouse counterparts were well separated, allowing the conclusive identification of mouse UPIa as the sole binder for FimH (Ref. 95; Fig. 3a). Additional studies of the glycomoieties of UPIa and UPIb established that only UPIa contains high mannose glycans that are capable of interacting with FimH, making it the sole urothelial receptor for FimH in vitro 95,96. Glycopeptide analysis also established Asn169 of UPIa as the site that bears the Man(6)GlcNAc(2). Importantly, this glyco-moiety is conserved in UPIa isolated from mouse, bovine and human bladder urothelia 96.
Mounting evidence suggests that the interaction of UPECs with the urothelial surface is critical not only for adhesion, but also for triggering a cascade of events that lead to bacterial invasion into the urothelial cells 97,98. UPECs appear to exploit the membrane trafficking machinery that normally exists in the urothelial cells. Abraham and colleagues recently showed that UPECs initially reside in the Rab27b-positive, UP-containing, fusiform vesicles 99. Once inside the urothelial cells, UPECs are insulated from the attack of host immunity and are resistant to antibiotic treatment. Hultgren and colleagues showed that the intracellular UPECs can not only survive, but also proliferate, forming so-called intracellular bacterial communities (IBCs) 100,101. Some of these IBCs can either stay dormant forming “quiescent intracellular reservoirs” or break out of the urothelial cells to seed a new round of infection. Recent evidence indicates that IBC formation can occur in many mouse strains and is reproducible with different clinical E. coli isolates from human UTIs 102. More importantly, desquamated urothelial cells from UTI patients harbor IBCs 103. The discovery of UPECs as intracellular pathogens is highly significant because it reveals a potentially important mechanism for recurrent UTIs. Thus, instead of re-inoculation of the urinary system with E. coli from the peri-urethral region, UPECs causing recurrent UTIs could be derived from those inhabiting the urothelial cells between active infections. Clearly, this concept, if further explored, can have major impact on devising novel treatment strategies for recurrent UTIs.
In addition to its role in mediating urothelial adhesion and invasion within the confines of the bladder, the binding of UPECs to UPIa may also facilitate the ascent of the bacteria into the kidney to cause upper tract infections (Fig. 3c). This scenario is based on the fact that UPIa is also present on the surfaces of the ureteral and renal pelvic urothelia 60,62,83. The interactions of type 1-fimbriae with UPIa in these anatomic locations may help the bacteria resist the urine flow and enable them to migrate upwards (Ref. 94; Fig. 3c). Most UPECs that cause pyelonephritis express both type 1 and P fimbriae 104. While type 1 fimbriae are crucial for cystitis, P fimbriae seem dispensable in the lower urinary tract, perhaps due to the lack of specific receptors for P fimbriae on the urothelial cells 105–107. Once attaching to the renal papillary epithelium and invading into the kidney parenchyma where nutrients are more abundant, the expression of type 1 fimbriae ceases due to a transcriptionally controlled process called “phase variation” 108. There, P fimbriae can bind to the globo-series of the glycolipids expressed by the renal tubular cells 109–111. Epigenetic control of P fimbrial expression can also suppress type 1 fimbrial expression via cross-talk that is not yet completely understood 112. Taken together, the discrete uroplakin/glycolipid receptor expression at different anatomic locations within the urinary tract, as depicted in Figure 3c, can explain the ability of UPECs to spread from lower to upper tract as well as the highly characteristic fimbria expression patterns of the UPEC strain isolates from cystitis and pyelonephritis patients.
UPs are useful markers for diagnosis, detection and prognostic prediction of urothelial carcinomas
As discussed in the preceding sections, UPs are expressed abundantly by the normal urothelium, but are undetectable in non-urothelial tissues in humans. This urothelium-specificity is by and large maintained after urothelial transformation, as UPs are readily detected by immunohistochemistry in urothelial carcinomas, but not in non-urothelial tumors 113–115. The detection rate in urothelial carcinomas has been fairly consistent among different cohorts, ranging from 50 to 60%. Somewhat surprisingly, UP expression is not strictly correlative with low pathological grade, despite their being the terminal differentiation products of normal urothelium 116,117. About half of the muscle-invasive urothelial carcinomas and lymph node metastases retain UPs. The clinico-pathological implications of UP detection are manifold. First, although a lack of UP expression does not rule out a urothelial carcinoma, positive identification of UPs is strongly indicative of a carcinoma of urothelial origin. UPs can therefore be of significant value, particularly when combined with other urothelium-restricted markers such as CK20, in the differential diagnosis of poorly differentiated pelvic carcinomas whose sites of origin are in question 113,116,118–122. Second, UP detection by RT-PCR is more sensitive than histopathology for detecting local tissue and lymph node spread of urothelial carcinoma cells. Copp and colleagues observed that, among the pathologically node-negative patients, 33% turned out to be node-positive upon UPII-based RT-PCR and in 91% of these PCR-positive cases the urothelial carcinomas recurred 123. Thus, UP identification by RT-PCR appears to be an excellent tool for aiding pathologists to detect lymph node metastasis. Third, detection of UP mRNAs in the circulation by RT-PCR could be an indication of micro-metastastic spread of urothelial carcinomas into the bloodstream 124–128. In extending this concept, the UP status in the peripheral blood could be used to monitor the efficacy of chemotherapeutic regimens, although prospective studies establishing this utility have yet to be carried out. Overall, challenges remain with RT-PCR regarding its sensitivity and specificity. Nested PCR and increased cycles both improved the sensitivity, while inevitably compromising the specificity. Genomic DNA contamination of the RNA samples, a universal problem associated with the use of commercial kits for RNA isolation, can lead to false positive results, although digestion of DNA in the RNA samples and using PCR primers on separate exons can alleviate some of these problems. Like other tissue-specific markers, it requires refinement and standardization before the RT-PCR detection of UPs can be employed reliably in a clinical setting. Finally, we have recently shown by using immunohistochemistry of arrayed human urothelial carcinomas that the absence of UPs expression was significantly associated with advanced pathologic stage, lymph node metastases, disease recurrence and bladder cancer-specific mortality in univariate analyses 117. The UP status is independent of cell cycle regulators p53, pRb, p27 and cyclin D1, and can therefore be a useful adjunct marker to predict the prognosis of patients with advanced urothelial carcinomas.
Mouse UPII promoter provides an in vivo platform for studying bladder tumorigenesis
A major byproduct from characterizing UPs was the successful isolation of the mouse UPII promoter 129. Although this promoter functions both in bladder and ureteral urothelia, it apparently is much more active in the former so that it has become an excellent tool for driving bladder-specific gene expression and interrogating the divergent molecular pathways of bladder tumorigenesis. This transgenic approach would not have been feasible, had the UPII promoter been active only in terminally differentiated urothelial umbrella cells, which would be highly refractory to oncogenic transformation. Fortunately, unlike the human urothelium, UP expression in mouse urothelium has a much earlier onset, beginning in the basal layer 34. The ability to drive gene expression even in the urothelial basal layer is an important requirement for tumorigenesis because this layer is where the stem cells most likely reside and oncogenic transformation originates.
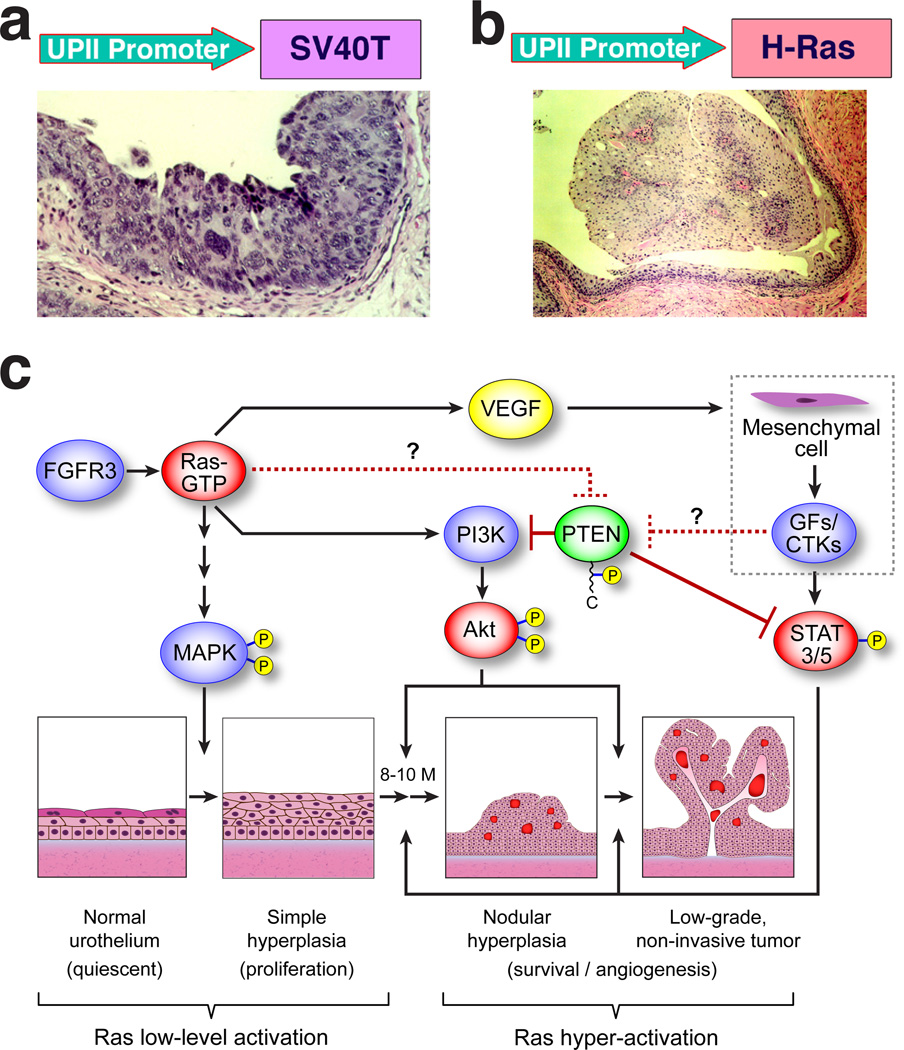
Using the mouse UPII promoter to express activated oncogenes and/or inactivate specific tumor suppressor genes in the urothelium, we have systematically evaluated whether specific molecular defects are capable of driving bladder tumorigenesis along divergent phenotypic pathways (Fig. 5a and b). We discovered that activation of the ras pathway, given sufficient gene dosage, induces exclusively low-grade, non-invasive superficial papillary bladder tumors (Ref. 130; Fig. 5b). Molecularly, the over-activation of Akt and Stat3/5 and the functional inactivation of PTEN by C-terminal hyper-phosphorylation seem crucial for triggering the nodular urothelial hyperplasia and low-grade papillary bladder tumors (Ref. 130; Fig. 5c). The onset and time course of these lesions are, however, not affected by the concurrent deficiency of p16Ink4a and p19Arf – an event found to synergize with ras activation in a wide range of tissues 131–133. These results provide compelling experimental evidence indicating that deficiency of the INK4a gene, long-thought to be a critical event in urothelial tumor initiation 134, is unnecessary for urothelial tumor initiation; and that hyper-activation of ras per se is sufficient to trigger urothelial tumors. The results obtained in mice are highly relevant to humans because ras activation via point mutations and over-expression occurs in 30% and 50%, respectively, of the human low-grade, superficial papillary bladder tumors 135,136. Interestingly, mutations of fibroblast growth factor receptor 3 (FGFR3), which can activate the ras signaling pathways, are found in up to 70% of these papillary tumors 137–139. Given the fact that ras and FGFR3 mutations are almost always mutually exclusive 140, perhaps reflecting the fact that they function in the same signaling pathway, there is strong reason to believe that ras pathway activation, via ras mutation/overexpression or FGFR3 mutation/overexpression, occurs in an overwhelming majority of the low-grade, superficial papillary bladder tumors in humans (Refs. 130, 136; Fig. 5c). Finally, patients with Costello syndrome, a genetic disease caused by germline mutations in the Ha-ras gene, frequently develop low-grade papillary bladder tumors 141, further supporting the relevance of ras pathway activation in this type of bladder tumors.
Figure 5.
Divergent molecular pathways of urothleial tumorigenesis. Transgenic mouse models recapitulate the two main phenotypes of urothelial carcinomas. Whereas urothelial expression of SV40 large T antigen elicits exclusively high-grade, flat urothelial lesions (a) bearing strong resemblance to carcinoma in situ of the human counterparts, urothelial expression of activated Ha-ras induces exclusively low-grade, non-invasive superficial papillary urothelial tumors (b). (c) Dosage-dependence of ras activation in triggering urothelial tumorigenesis. While low-level ras activation activates the MAPK pathway resulting in urothelial hyperplasia, it is insufficient to induce tumorigenesis. Hyperactivation of ras can, however, activate the PI3K/AKT and STAT3/5 pathways, leading to nodular urothelial hyperplasia and low-grade, superficial papillary tumors. The activation of these two pathways may also be facilitated by the functional inactivation of PTEN via its C-terminal hyper-phosphorylation. FGFR3, whose mutations occur in up to 80% of the low-grade, superficial papillary tumors in humans, may trigger this urothelial tumor variant through similar signal mechanisms (adapted from Ref. 130).
Contrary to the Ha-ras mutation, which consistently induces low-grade papillary bladder tumors, urothelial expression of a Simian virus 40 large T antigen (SV40T) elicited exclusively high-grade, flat urothelial carcinomas that strongly resembled carcinoma in situ (CIS) in humans (Ref. 35; Fig. 5a). Over time, these CIS-like lesions evolve to high-grade papillary tumors that are occasionally muscle-invasive and metastatic to lymph node, liver and lungs 142. In support of our findings, Sandgren and colleagues showed by using a keratin 5 promoter to drive the SV40T expression in urothelium and other epithelial tissues, that bladder-invasive tumors were a consistent finding 143. Together, these results suggest that SV40T-mediated functional inactivation of the p53 and Rb tumor suppressors plays a critical role in bladder tumorigenesis along the high-grade, invasive pathway. Interestingly, in humans, defects affecting both p53 and Rb are rare in low-grade, superficial papillary bladder tumors but occur in over half of the high-grade, invasive bladder carcinomas 144–146. While the SV40T data from the transgenic mice are strongly supportive of the human data on p53 and Rb, one needs to be cautious because SV40T exerts wider oncogenic effects than simply inactivating p53 and Rb 147,148. For this reason, specific inactivation of both p53 and Rb genes in urothelium should provide more insightful information regarding the role of these two genes in triggering muscle-invasive bladder tumors. Recently, we have developed a Cre-recombinase based system that allows urothelium-specific ablation of genes flanked by the loxP sequences 34. With this system, we have been able to inactivate p53 or Rb or both in the urothelium, thus avoiding the embryonic lethality and premature death associated with the global knockout of these two genes. Experiments are underway to determine whether p53 and/or Rb deficiency is sufficient to trigger invasive bladder tumors. Overall, the UPII promoter-based transgenic and knockout systems have been instrumental in defining the in vivo roles of genetic alterations in bladder tumor initiation and progression. Given their resemblance to the human counterparts and their highly predictable time course and biological behavior, the transgenic models of bladder tumors will also be useful for evaluating novel preventive and therapeutic strategies 149.
Summary and Perspectives
Over the last two decades, the field of urothelial biology has seen considerable advances in understanding how the urothelial cells differentiate to perform their unique biological functions. We now know that uroplakins are the essential structural components of the urothelial apical surface whose deficiency compromises the urothelial permeability barrier and leads to global urinary tract anomalies. The assembly of UPs and subsequent translocation of the urothelial plaques from the cytoplasm to the apical surface are a highly coordinated and regulated process. As one of the few known urothelium-specific markers that are retained by most of its malignancies, UPs can serve as excellent adjunct biomarkers for differential diagnosis and early identification of urothelial cancer cell spread to local tissues, lymph nodes and blood stream. UPs are major mediators in the adhesion, host cell invasion and upward urinary tract spread of type 1-fimbriated E. coli. Finally, the uroplakin promoter-based transgenic and knockout models have yielded new insights into the molecular pathways of urothelial tumorigenesis and progression and will provide a platform for evaluating novel preventive and therapeutic approaches. Notwithstanding the progress, many important questions remain. For instance, how is uroplakin-trafficking between the cytoplasm and the apical surface of the urothelial umbrella cells regulated in the non-stretched vis-à-vis the stretched state of the urothelium? What are the relative contributions of different Rab family proteins in tethering the uroplakin-containing fusiform vesicles to the urothelial apical surface, and precisely what cellular machineries control the degradative pathway of uroplakins? Are these membrane trafficking processes involved in bacterial invasion into and exit from the urothelial cells? Through what specific mechanisms do urothelial plaques interact with a meshwork of underlying cytoskeletons allowing dynamic membrane movement during the normal micturition cycle as well as the pathophysiological processes such as bacterial invasion? Exactly how are the signals from FimH/UPIa interaction at the extracellular surface propagated across the membrane to the cytoplasm? Is it possible to resolve the uroplakin complex at atomic resolution so that the structure/function relationship of these membrane proteins can be eventually elucidated? Finally, will gene expression and/or knockout in urothelium in a temporally controlled manner yield more realistic models of urothelial diseases including carcinomas over the current, constitutive approaches? Answers to some of these questions will undoubtedly further advance the field of urothelial biology and, more importantly, they should shed light on the molecular mechanisms whereby urothelium-related diseases arise and evolve.
Acknowledgement
The work summarized in this review has been supported by grants from the United States National Institutes of Health (DK52206, DK39753 and DK69688); a Merit Review Award from Veterans Affairs Administration; and a grant-in-aid from the Weinstein Foundation for Urological Research at New York University School of Medicine.
Footnotes
Note: The authors regret that due to space constraints not all important publications in the urothelial field could be cited.
REFERENCES
- 1.Melicow MM. The urothelium: a battleground for oncogenesis. J Urol. 1978;120:43–47. doi: 10.1016/s0022-5347(17)57034-2. [DOI] [PubMed] [Google Scholar]
- 2.Koss LG. Tumors of the Bladder. In: Koss LG, editor. Diagnostic Cytology of the Urinary Tract: with Histopathologic and Clinical Correlations. Philadelphia: Lippincott-Raven; 1996. pp. 71–139. [Google Scholar]
- 3.Walker RE. Renewal of cell populations in the female mouse. Am. J. Anat. 1960;102:95–100. doi: 10.1002/aja.1001070202. [DOI] [PubMed] [Google Scholar]
- 4.Hicks RM. The mammalian urinary bladder: an accommodating organ. Biol Rev Camb Philos Soc. 1975;50:215–246. doi: 10.1111/j.1469-185x.1975.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 5.Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol. 2000;278:F867–F874. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- 6.Negrete HO, Lavelle JP, Berg J, et al. Permeability properties of the intact mammalian bladder epithelium. Am J Physiol. 1996;271:F886–894. doi: 10.1152/ajprenal.1996.271.4.F886. [DOI] [PubMed] [Google Scholar]
- 7.Minsky BD, Chlapowski FJ. Morphometric analysis of the translocation of lumenal membrane between cytoplasm and cell surface of transitional epithelial cells during the expansion-contraction cycles of mammalian urinary bladder. J Cell Biol. 1978;77:685–697. doi: 10.1083/jcb.77.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis SA, de Moura JL. Incorporation of cytoplasmic vesicles into apical membrane of mammalian urinary bladder epithelium. Nature. 1982;297:685–688. doi: 10.1038/297685a0. [DOI] [PubMed] [Google Scholar]
- 9.Porter KR, Bonneville MA. An Introduction to the Fine Structure of Cells and Tissues. Philadelphia: Lea & Febiger; 1963. [Google Scholar]
- 10.Hicks RM. The fine structure of the transitional epithelium of rat ureter. J Cell Biol. 1965;26:25–48. doi: 10.1083/jcb.26.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koss LG. The asymmetric unit membranes of the epithelium of the urinary bladder of the rat. An electron microscopic study of a mechanism of epithelial maturation and function. Lab Invest. 1969;21:154–168. [PubMed] [Google Scholar]
- 12.Porter KR, Kenyon K, Badenhausen S. Specializations of the unit membrane. Protoplasma. 1967;63:262–274. [PubMed] [Google Scholar]
- 13.Vergara J, Longley W, Robertson JD. A hexagonal arrangement of subunits in membrane of mouse urinary bladder. J Mol Biol. 1969;46:593–596. doi: 10.1016/0022-2836(69)90200-9. [DOI] [PubMed] [Google Scholar]
- 14.Hicks RM, Ketterer B. Hexagonal lattice of subunits in the thick luminal membrane of the rat urinary bladder. Nature. 1969;224:1304–1305. doi: 10.1038/2241304a0. [DOI] [PubMed] [Google Scholar]
- 15.Taylor KA, Robertson JD. Analysis of the three-dimensional structure of the urinary bladder epithelial cell membranes. J Ultrastruct Res. 1984;87:23–30. doi: 10.1016/s0022-5320(84)90113-8. [DOI] [PubMed] [Google Scholar]
- 16.Kachar B, Liang F, Lins U, et al. Three-dimensional analysis of the 16 nm urothelial plaque particle: luminal surface exposure, preferential head-to-head interaction, and hinge formation. J Mol Biol. 1999;285:595–608. doi: 10.1006/jmbi.1998.2304. [DOI] [PubMed] [Google Scholar]
- 17.Brisson A, Wade RH. Three-dimensional structure of luminal plasma membrane protein from urinary bladder. J Mol Biol. 1983;166:21–36. doi: 10.1016/s0022-2836(83)80048-5. [DOI] [PubMed] [Google Scholar]
- 18.Walz T, Haner M, Wu XR, et al. Towards the molecular architecture of the asymmetric unit membrane of the mammalian urinary bladder epithelium: a closed "twisted ribbon" structure. J Mol Biol. 1995;248:887–900. doi: 10.1006/jmbi.1995.0269. [DOI] [PubMed] [Google Scholar]
- 19.Truschel ST, Ruiz WG, Shulman T, et al. Primary uroepithelial cultures. A model system to analyze umbrella cell barrier function. J Biol Chem. 1999;274:15020–15029. doi: 10.1074/jbc.274.21.15020. [DOI] [PubMed] [Google Scholar]
- 20.Truschel ST, Wang E, Ruiz WG, et al. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell. 2002;13:830–846. doi: 10.1091/mbc.01-09-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng FM, Ding M, Lavker RM, et al. Urothelial function reconsidered: a role in urinary protein secretion. Proc Natl Acad Sci U S A. 2001;98:154–159. doi: 10.1073/pnas.98.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apodaca G, Balestreire E, Birder LA. The uroepithelial-associated sensory web. Kidney Int. 2007;72:1057–1064. doi: 10.1038/sj.ki.5002439. [DOI] [PubMed] [Google Scholar]
- 23.Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol. 2007;4:46–54. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ketterer B, Hicks RM. Proteins of the plasma membrane lining the lumen of the rat bladder. Biochem J. 1971;122:66P. doi: 10.1042/bj1220066pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ketterer B, Hicks RM, Christodoulides L, et al. Studies of the chemistry of the luminal plasma membrane of rat bladder epithelial cells. Biochim Biophys Acta. 1973;311:180–190. doi: 10.1016/0005-2736(73)90265-4. [DOI] [PubMed] [Google Scholar]
- 26.Vergara J, Zambrano F, Robertson JD, et al. Isolation and characterization of luminal membranes from urinary bladder. J Cell Biol. 1974;61:83–94. doi: 10.1083/jcb.61.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu XR, Manabe M, Yu J, et al. Large scale purification and immunolocalization of bovine uroplakins I, II, and III. Molecular markers of urothelial differentiation. J Biol Chem. 1990;265:19170–19179. [PubMed] [Google Scholar]
- 28.Wu XR, Sun TT. Molecular cloning of a 47 kDa tissue-specific and differentiation-dependent urothelial cell surface glycoprotein. J Cell Sci. 1993;106:31–43. doi: 10.1242/jcs.106.1.31. [DOI] [PubMed] [Google Scholar]
- 29.Lin JH, Wu XR, Kreibich G, et al. Precursor sequence, processing, and urothelium-specific expression of a major 15-kDa protein subunit of asymmetric unit membrane. J Biol Chem. 1994;269:1775–1784. [PubMed] [Google Scholar]
- 30.Yu J, Lin JH, Wu XR, et al. Uroplakins Ia and Ib, two major differentiation products of bladder epithelium, belong to a family of four transmembrane domain (4TM) proteins. J Cell Biol. 1994;125:171–182. doi: 10.1083/jcb.125.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu XR, Lin JH, Walz T, et al. Mammalian uroplakins. A group of highly conserved urothelial differentiation-related membrane proteins. J Biol Chem. 1994;269:13716–13724. [PubMed] [Google Scholar]
- 32.Garcia-Espana A, Chung PJ, Zhao X, et al. Origin of the tetraspanin uroplakins and their co-evolution with associated proteins: implications for uroplakin structure and function. Mol Phylogenet Evol. 2006;41:355–367. doi: 10.1016/j.ympev.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 33.Yu J, Manabe M, Wu XR, et al. Uroplakin I: a 27-kD protein associated with the asymmetric unit membrane of mammalian urothelium. J Cell Biol. 1990;111:1207–1216. doi: 10.1083/jcb.111.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mo L, Cheng J, Lee EY, et al. Gene deletion in urothelium by specific expression of Cre recombinase. Am J Physiol Renal Physiol. 2005;289:F562–F568. doi: 10.1152/ajprenal.00368.2004. [DOI] [PubMed] [Google Scholar]
- 35.Zhang ZT, Pak J, Shapiro E, et al. Urothelium-specific expression of an oncogene in transgenic mice induced the formation of carcinoma in situ and invasive transitional cell carcinoma. Cancer Res. 1999;59:3512–3517. [PubMed] [Google Scholar]
- 36.Chopra B, Hinley J, Oleksiewicz MB, et al. Trans-species comparison of PPAR and RXR expression by rat and human urothelial tissues. Toxicol Pathol. 2008;36:485–495. doi: 10.1177/0192623308315672. [DOI] [PubMed] [Google Scholar]
- 37.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 38.Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005;5:136–148. doi: 10.1038/nri1548. [DOI] [PubMed] [Google Scholar]
- 39.Staehelin LA, Chlapowski FJ, Bonneville MA. Lumenal plasma membrane of the urinary bladder. I. Three-dimensional reconstruction from freeze-etch images. J Cell Biol. 1972;53:73–91. doi: 10.1083/jcb.53.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu XR, Medina JJ, Sun TT. Selective interactions of UPIa and UPIb, two members of the transmembrane 4 superfamily, with distinct single transmembrane-domained proteins in differentiated urothelial cells. J Biol Chem. 1995;270:29752–29759. doi: 10.1074/jbc.270.50.29752. [DOI] [PubMed] [Google Scholar]
- 41.Liang FX, Riedel I, Deng FM, et al. Organization of uroplakin subunits: transmembrane topology, pair formation and plaque composition. Biochem J. 2001;355:13–18. doi: 10.1042/0264-6021:3550013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tu L, Sun TT, Kreibich G. Specific heterodimer formation is a prerequisite for uroplakins to exit from the endoplasmic reticulum. Mol Biol Cell. 2002;13:4221–4230. doi: 10.1091/mbc.E02-04-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu CC, Liang FX, Zhou G, et al. Assembly of urothelial plaques: tetraspanin function in membrane protein trafficking. Mol Biol Cell. 2005;16:3937–3950. doi: 10.1091/mbc.E05-02-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oostergetel GT, Keegstra W, Brisson A. Structure of the major membrane protein complex from urinary bladder epithelial cells by cryo-electron crystallography. J Mol Biol. 2001;314:245–252. doi: 10.1006/jmbi.2001.5128. [DOI] [PubMed] [Google Scholar]
- 45.Min G, Wang H, Sun TT, et al. Structural basis for tetraspanin functions as revealed by the cryo-EM structure of uroplakin complexes at 6-A resolution. J Cell Biol. 2006;173:975–983. doi: 10.1083/jcb.200602086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Min G, Zhou G, Schapira M, et al. Structural basis of urothelial permeability barrier function as revealed by Cryo-EM studies of the 16 nm uroplakin particle. J Cell Sci. 2003;116:4087–4094. doi: 10.1242/jcs.00811. [DOI] [PubMed] [Google Scholar]
- 47.Min G, Stolz M, Zhou G, et al. Localization of uroplakin Ia, the urothelial receptor for bacterial adhesin FimH, on the six inner domains of the 16 nm urothelial plaque particle. J Mol Biol. 2002;317:697–706. doi: 10.1006/jmbi.2002.5442. [DOI] [PubMed] [Google Scholar]
- 48.Hu CC, Bachmann T, Zhou G, et al. Assembly of a membrane receptor complex: roles of the uroplakin II prosequence in regulating uroplakin bacterial receptor oligomerization. Biochem J. 2008;414:195–203. doi: 10.1042/BJ20080550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun TT. Altered phenotype of cultured urothelial and other stratified epithelial cells: implications for wound healing. Am J Physiol Renal Physiol. 2006;291:F9–F21. doi: 10.1152/ajprenal.00035.2006. [DOI] [PubMed] [Google Scholar]
- 50.Tu L, Kong XP, Sun TT, et al. Integrity of all four transmembrane domains of the tetraspanin uroplakin Ib is required for its exit from the ER. J Cell Sci. 2006;119:5077–5086. doi: 10.1242/jcs.03285. [DOI] [PubMed] [Google Scholar]
- 51.Kallin B, de Martin R, Etzold T, et al. Cloning of a growth arrest-specific and transforming growth factor beta-regulated gene, TI 1, from an epithelial cell line. Mol Cell Biol. 1991;11:5338–5345. doi: 10.1128/mcb.11.10.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adachi W, Okubo K, Kinoshita S. Human uroplakin Ib in ocular surface epithelium. Invest Ophthalmol Vis Sci. 2000;41:2900–2905. [PubMed] [Google Scholar]
- 53.Hu P, Deng FM, Liang FX, et al. Ablation of uroplakin III gene results in small urothelial plaques, urothelial leakage, and vesicoureteral reflux. J Cell Biol. 2000;151:961–972. doi: 10.1083/jcb.151.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schamel WW, Kuppig S, Becker B, et al. A high-molecular-weight complex of membrane proteins BAP29/BAP31 is involved in the retention of membrane-bound IgD in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2003;100:9861–9866. doi: 10.1073/pnas.1633363100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kota J, Ljungdahl PO. Specialized membrane-localized chaperones prevent aggregation of polytopic proteins in the ER. J Cell Biol. 2005;168:79–88. doi: 10.1083/jcb.200408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cannon KS, Cresswell P. Quality control of transmembrane domain assembly in the tetraspanin CD82. Embo J. 2001;20:2443–2453. doi: 10.1093/emboj/20.10.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y, Guo X, Deng FM, et al. Rab27b is associated with fusiform vesicles and may be involved in targeting uroplakins to urothelial apical membranes. Proc Natl Acad Sci U S A. 2003;100:14012–14017. doi: 10.1073/pnas.2436350100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tiwari S, Italiano JE, Jr, Barral DC, et al. A role for Rab27b in NF-E2-dependent pathways of platelet formation. Blood. 2003;102:3970–3979. doi: 10.1182/blood-2003-03-0977. [DOI] [PubMed] [Google Scholar]
- 59.Khandelwal P, Ruiz WG, Balestreire-Hawryluk E, et al. Rab11a-dependent exocytosis of discoidal/fusiform vesicles in bladder umbrella cells. Proc Natl Acad Sci U S A. 2008;105:15773–15778. doi: 10.1073/pnas.0805636105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang FX, Bosland MC, Huang H, et al. Cellular basis of urothelial squamous metaplasia: roles of lineage heterogeneity and cell replacement. J Cell Biol. 2005;171:835–844. doi: 10.1083/jcb.200505035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riedel I, Liang FX, Deng FM, et al. Urothelial umbrella cells of human ureter are heterogeneous with respect to their uroplakin composition: different degrees of urothelial maturity in ureter and bladder? Eur J Cell Biol. 2005;84:393–405. doi: 10.1016/j.ejcb.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 62.Romih R, Korosec P, de Mello W, Jr, et al. Differentiation of epithelial cells in the urinary tract. Cell Tissue Res. 2005;320:259–268. doi: 10.1007/s00441-004-1005-4. [DOI] [PubMed] [Google Scholar]
- 63.Li Y, Liu W, Hayward SW, et al. Plasticity of the urothelial phenotype: effects of gastro-intestinal mesenchyme/stroma and implications for urinary tract reconstruction. Differentiation. 2000;66:126–135. doi: 10.1046/j.1432-0436.2000.660207.x. [DOI] [PubMed] [Google Scholar]
- 64.Cunha GR, Fujii H, Neubauer BL, et al. Epithelial-mesenchymal interactions in prostatic development. I. morphological observations of prostatic induction by urogenital sinus mesenchyme in epithelium of the adult rodent urinary bladder. J Cell Biol. 1983;96:1662–1670. doi: 10.1083/jcb.96.6.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doran TI, Vidrich A, Sun TT. Intrinsic and extrinsic regulation of the differentiation of skin, corneal and esophageal epithelial cells. Cell. 1980;22:17–25. doi: 10.1016/0092-8674(80)90150-6. [DOI] [PubMed] [Google Scholar]
- 66.Oottamasathien S, Williams K, Franco OE, et al. Bladder tissue formation from cultured bladder urothelium. Dev Dyn. 2006;235:2795–2801. doi: 10.1002/dvdy.20886. [DOI] [PubMed] [Google Scholar]
- 67.Batourina E, Tsai S, Lambert S, et al. Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet. 2005;37:1082–1089. doi: 10.1038/ng1645. [DOI] [PubMed] [Google Scholar]
- 68.Viana R, Batourina E, Huang H, et al. The development of the bladder trigone, the center of the anti-reflux mechanism. Development. 2007;134:3763–3769. doi: 10.1242/dev.011270. [DOI] [PubMed] [Google Scholar]
- 69.Atala A, Bauer SB, Soker S, et al. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 70.Korossis S, Bolland F, Ingham E, et al. Review: tissue engineering of the urinary bladder: considering structure-function relationships and the role of mechanotransduction. Tissue Eng. 2006;12:635–644. doi: 10.1089/ten.2006.12.635. [DOI] [PubMed] [Google Scholar]
- 71.Staack A, Hayward SW, Baskin LS, et al. Molecular, cellular and developmental biology of urothelium as a basis of bladder regeneration. Differentiation. 2005;73:121–133. doi: 10.1111/j.1432-0436.2005.00014.x. [DOI] [PubMed] [Google Scholar]
- 72.Masui T, Mann AM, Macatee TL, et al. Direct DNA sequencing of the rat neu oncogene transmembrane domain reveals no mutation in urinary bladder carcinomas induced by N-butyl-N-(4-hydroxybutyl)nitrosamine, N-[4-(5-nitro-2-furyl)-2-thiazolyl]formamide or N-methyl-N-nitrosourea. Carcinogenesis. 1991;12:1975–1978. doi: 10.1093/carcin/12.10.1975. [DOI] [PubMed] [Google Scholar]
- 73.Grollman AP, Jelakovic B. Role of environmental toxins in endemic (Balkan) nephropathy. October 2006, Zagreb, Croatia. J Am Soc Nephrol. 2007;18:2817–2823. doi: 10.1681/ASN.2007050537. [DOI] [PubMed] [Google Scholar]
- 74.Grollman AP, Shibutani S, Moriya M, et al. Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc Natl Acad Sci U S A. 2007;104:12129–12134. doi: 10.1073/pnas.0701248104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kong XT, Deng FM, Hu P, et al. Roles of uroplakins in plaque formation, umbrella cell enlargement, and urinary tract diseases. J Cell Biol. 2004;167:1195–1204. doi: 10.1083/jcb.200406025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deng FM, Liang FX, Tu L, et al. Uroplakin IIIb, a urothelial differentiation marker, dimerizes with uroplakin Ib as an early step of urothelial plaque assembly. J Cell Biol. 2002;159:685–694. doi: 10.1083/jcb.200204102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu P, Meyers S, Liang FX, et al. Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. Am J Physiol Renal Physiol. 2002;283:F1200–F1207. doi: 10.1152/ajprenal.00043.2002. [DOI] [PubMed] [Google Scholar]
- 78.Apodaca G. The uroepithelium: not just a passive barrier. Traffic. 2004;5:117–128. doi: 10.1046/j.1600-0854.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 79.Graham E, Chai TC. Dysfunction of bladder urothelium and bladder urothelial cells in interstitial cystitis. Curr Urol Rep. 2006;7:440–446. doi: 10.1007/s11934-006-0051-8. [DOI] [PubMed] [Google Scholar]
- 80.Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology. 2007;69:9–16. doi: 10.1016/j.urology.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 81.Lavelle JP, Meyers SA, Ruiz WG, et al. Urothelial pathophysiological changes in feline interstitial cystitis: a human model. Am J Physiol Renal Physiol. 2000;278:F540–F553. doi: 10.1152/ajprenal.2000.278.4.F540. [DOI] [PubMed] [Google Scholar]
- 82.Hurst RE, Moldwin RM, Mulholland SG. Bladder defense molecules, urothelial differentiation, urinary biomarkers, and interstitial cystitis. Urology. 2007;69:17–23. doi: 10.1016/j.urology.2006.03.083. [DOI] [PubMed] [Google Scholar]
- 83.Jiang S, Gitlin J, Deng FM, et al. Lack of major involvement of human uroplakin genes in vesicoureteral reflux: implications for disease heterogeneity. Kidney Int. 2004;66:10–19. doi: 10.1111/j.1523-1755.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 84.Giltay JC, van de Meerakker J, van Amstel HK, et al. No pathogenic mutations in the uroplakin III gene of 25 patients with primary vesicoureteral reflux. J Urol. 2004;171:931–932. doi: 10.1097/01.ju.0000094802.50650.3d. [DOI] [PubMed] [Google Scholar]
- 85.Kelly H, Ennis S, Yoneda A, et al. Uroplakin III is not a major candidate gene for primary vesicoureteral reflux. Eur J Hum Genet. 2005;13:500–502. doi: 10.1038/sj.ejhg.5201322. [DOI] [PubMed] [Google Scholar]
- 86.Jenkins D, Bitner-Glindzicz M, Malcolm S, et al. De novo Uroplakin IIIa heterozygous mutations cause human renal adysplasia leading to severe kidney failure. J Am Soc Nephrol. 2005;16:2141–2149. doi: 10.1681/ASN.2004090776. [DOI] [PubMed] [Google Scholar]
- 87.Schonfelder EM, Knuppel T, Tasic V, et al. Mutations in uroplakin IIIA are a rare cause of renal hypodysplasia in humans. Am J Kidney Dis. 2006;47:1004–1012. doi: 10.1053/j.ajkd.2006.02.177. [DOI] [PubMed] [Google Scholar]
- 88.Hasty DL, Wu X-R, Dykuizen DE, et al. Allelic Variation of the FimH Lectin of Escherichia coli Type 1 Fimbriae and Uropathogenesis. In: Nataro JP, Cohen PS, Mobley HLT, Weiser JN, editors. Colonization of Mucosal Surfaces. American Society for Microbiology Press; 2005. [Google Scholar]
- 89.Mulvey MA, Schilling JD, Martinez JJ, et al. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc Natl Acad Sci U S A. 2000;97:8829–8835. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Langermann S, Palaszynski S, Barnhart M, et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 91.Sokurenko EV, Chesnokova V, Dykhuizen DE, et al. Pathogenic adaptation of Escherichia coli by natural variation of the FimH adhesin. Proc Natl Acad Sci U S A. 1998;95:8922–8926. doi: 10.1073/pnas.95.15.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ofek I, Hasty DL, Abraham SN, et al. Role of bacterial lectins in urinary tract infections. Molecular mechanisms for diversification of bacterial surface lectins. Adv Exp Med Biol. 2000;485:183–192. doi: 10.1007/0-306-46840-9_25. [DOI] [PubMed] [Google Scholar]
- 93.Stamm WE. Theodore e. Woodward award: host-pathogen interactions in community-acquired urinary tract infections. Trans Am Clin Climatol Assoc. 2006;117:75–84. [PMC free article] [PubMed] [Google Scholar]
- 94.Wu XR, Sun TT, Medina JJ. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: relation to urinary tract infections. Proc Natl Acad Sci U S A. 1996;93:9630–9635. doi: 10.1073/pnas.93.18.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou G, Mo WJ, Sebbel P, et al. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J Cell Sci. 2001;114:4095–4103. doi: 10.1242/jcs.114.22.4095. [DOI] [PubMed] [Google Scholar]
- 96.Xie B, Zhou G, Chan SY, et al. Distinct glycan structures of uroplakins Ia and Ib: structural basis for the selective binding of FimH adhesin to uroplakin Ia. J Biol Chem. 2006;281:14644–14653. doi: 10.1074/jbc.M600877200. [DOI] [PubMed] [Google Scholar]
- 97.Mulvey MA, Lopez-Boado YS, Wilson CL, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 98.Martinez JJ, Mulvey MA, Schilling JD, et al. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. Embo J. 2000;19:2803–2812. doi: 10.1093/emboj/19.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bishop BL, Duncan MJ, Song J, et al. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13:625–630. doi: 10.1038/nm1572. [DOI] [PubMed] [Google Scholar]
- 100.Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anderson GG, Palermo JJ, Schilling JD, et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 102.Garofalo CK, Hooton TM, Martin SM, et al. Escherichia coli from urine of female patients with urinary tract infections is competent for intracellular bacterial community formation. Infect Immun. 2007;75:52–60. doi: 10.1128/IAI.01123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rosen DA, Hooton TM, Stamm WE, et al. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lane MC, Mobley HL. Role of P-fimbrial-mediated adherence in pyelonephritis and persistence of uropathogenic Escherichia coli (UPEC) in the mammalian kidney. Kidney Int. 2007;72:19–25. doi: 10.1038/sj.ki.5002230. [DOI] [PubMed] [Google Scholar]
- 106.Mo L, Zhu XH, Huang HY, et al. Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Renal Physiol. 2004;286:F795–F802. doi: 10.1152/ajprenal.00357.2003. [DOI] [PubMed] [Google Scholar]
- 107.Connell I, Agace W, Klemm P, et al. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci U S A. 1996;93:9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gunther NWt, Lockatell V, Johnson DE, et al. In vivo dynamics of type 1 fimbria regulation in uropathogenic Escherichia coli during experimental urinary tract infection. Infect Immun. 2001;69:2838–2846. doi: 10.1128/IAI.69.5.2838-2846.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stapleton AE, Stroud MR, Hakomori SI, et al. The globoseries glycosphingolipid sialosyl galactosyl globoside is found in urinary tract tissues and is a preferred binding receptor In vitro for uropathogenic Escherichia coli expressing pap-encoded adhesins. Infect Immun. 1998;66:3856–3861. doi: 10.1128/iai.66.8.3856-3861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dodson KW, Pinkner JS, Rose T, et al. Structural basis of the interaction of the pyelonephritic E. coli adhesin to its human kidney receptor. Cell. 2001;105:733–743. doi: 10.1016/s0092-8674(01)00388-9. [DOI] [PubMed] [Google Scholar]
- 111.Striker R, Nilsson U, Stonecipher A, et al. Structural requirements for the glycolipid receptor of human uropathogenic Escherichia coli. Mol Microbiol. 1995;16:1021–1029. doi: 10.1111/j.1365-2958.1995.tb02327.x. [DOI] [PubMed] [Google Scholar]
- 112.Holden NJ, Totsika M, Mahler E, et al. Demonstration of regulatory cross-talk between P fimbriae and type 1 fimbriae in uropathogenic Escherichia coli. Microbiology. 2006;152:1143–1153. doi: 10.1099/mic.0.28677-0. [DOI] [PubMed] [Google Scholar]
- 113.Moll R, Wu XR, Lin JH, et al. Uroplakins, specific membrane proteins of urothelial umbrella cells, as histological markers of metastatic transitional cell carcinomas. Am J Pathol. 1995;147:1383–1397. [PMC free article] [PubMed] [Google Scholar]
- 114.Lobban ED, Smith BA, Hall GD, et al. Uroplakin gene expression by normal and neoplastic human urothelium. Am J Pathol. 1998;153:1957–1967. doi: 10.1016/S0002-9440(10)65709-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Olsburgh J, Harnden P, Weeks R, et al. Uroplakin gene expression in normal human tissues and locally advanced bladder cancer. J Pathol. 2003;199:41–49. doi: 10.1002/path.1252. [DOI] [PubMed] [Google Scholar]
- 116.Wu RL, Osman I, Wu XR, et al. Uroplakin II gene is expressed in transitional cell carcinoma but not in bilharzial bladder squamous cell carcinoma: alternative pathways of bladder epithelial differentiation and tumor formation. Cancer Res. 1998;58:1291–1297. [PubMed] [Google Scholar]
- 117.Huang HY, Shariat SF, Sun TT, et al. Persistent uroplakin expression in advanced urothelial carcinomas: implications in urothelial tumor progression and clinical outcome. Hum Pathol. 2007;38:1703–1713. doi: 10.1016/j.humpath.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu X, Kakehi Y, Zeng Y, et al. Uroplakin II as a promising marker for molecular diagnosis of nodal metastases from bladder cancer: comparison with cytokeratin 20. J Urol. 2005;174:2138–2142. doi: 10.1097/01.ju.0000181214.32390.75. discussion 2142-2133. [DOI] [PubMed] [Google Scholar]
- 119.Riedel I, Czernobilsky B, Lifschitz-Mercer B, et al. Brenner tumors but not transitional cell carcinomas of the ovary show urothelial differentiation: immunohistochemical staining of urothelial markers, including cytokeratins and uroplakins. Virchows Arch. 2001;438:181–191. doi: 10.1007/s004280000315. [DOI] [PubMed] [Google Scholar]
- 120.Parker DC, Folpe AL, Bell J, et al. Potential utility of uroplakin III, thrombomodulin, high molecular weight cytokeratin, and cytokeratin 20 in noninvasive, invasive, and metastatic urothelial (transitional cell) carcinomas. Am J Surg Pathol. 2003;27:1–10. doi: 10.1097/00000478-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 121.Harnden P, Allam A, Joyce AD, et al. Cytokeratin 20 expression by non-invasive transitional cell carcinomas: potential for distinguishing recurrent from non-recurrent disease. Histopathology. 1995;27:169–174. doi: 10.1111/j.1365-2559.1995.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 122.Mhawech P, Uchida T, Pelte MF. Immunohistochemical profile of high-grade urothelial bladder carcinoma and prostate adenocarcinoma. Hum Pathol. 2002;33:1136–1140. doi: 10.1053/hupa.2002.129416. [DOI] [PubMed] [Google Scholar]
- 123.Copp HL, Chin JL, Conaway M, et al. Prospective evaluation of the prognostic relevance of molecular staging for urothelial carcinoma. Cancer. 2006;107:60–66. doi: 10.1002/cncr.21953. [DOI] [PubMed] [Google Scholar]
- 124.Okegawa T, Kinjo M, Nutahara K, et al. Value of reverse transcription polymerase chain assay in peripheral blood of patients with urothelial cancer. J Urol. 2004;171:1461–1466. doi: 10.1097/01.ju.0000118648.29024.b7. [DOI] [PubMed] [Google Scholar]
- 125.Kurahashi T, Hara I, Oka N, et al. Detection of micrometastases in pelvic lymph nodes in patients undergoing radical cystectomy for locally invasive bladder cancer by real-time reverse transcriptase-PCR for cytokeratin 19 and uroplakin II. Clin Cancer Res. 2005;11:3773–3777. doi: 10.1158/1078-0432.CCR-04-2297. [DOI] [PubMed] [Google Scholar]
- 126.Yuasa T, Yoshiki T, Isono T, et al. Expression of transitional cell-specific genes, uroplakin Ia and II, in bladder cancer: detection of circulating cancer cells in the peripheral blood of metastatic patients. Int J Urol. 1999;6:286–292. doi: 10.1046/j.1442-2042.1999.00064.x. [DOI] [PubMed] [Google Scholar]
- 127.Osman I, Kang M, Lee A, et al. Detection of circulating cancer cells expressing uroplakins and epidermal growth factor receptor in bladder cancer patients. Int J Cancer. 2004;111:934–939. doi: 10.1002/ijc.20366. [DOI] [PubMed] [Google Scholar]
- 128.Li SM, Zhang ZT, Chan S, et al. Detection of circulating uroplakin-positive cells in patients with transitional cell carcinoma of the bladder. J Urol. 1999;162:931–935. doi: 10.1097/00005392-199909010-00093. [DOI] [PubMed] [Google Scholar]
- 129.Lin JH, Zhao H, Sun TT. A tissue-specific promoter that can drive a foreign gene to express in the suprabasal urothelial cells of transgenic mice. Proc Natl Acad Sci U S A. 1995;92:679–683. doi: 10.1073/pnas.92.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mo L, Zheng X, Huang HY, et al. Hyperactivation of Ha-ras oncogene, but not Ink4a/Arf deficiency, triggers bladder tumorigenesis. J Clin Invest. 2007;117:314–325. doi: 10.1172/JCI30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aguirre AJ, Bardeesy N, Sinha M, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Uhrbom L, Kastemar M, Johansson FK, et al. Cell type-specific tumor suppression by Ink4a and Arf in Kras-induced mouse gliomagenesis. Cancer Res. 2005;65:2065–2069. doi: 10.1158/0008-5472.CAN-04-3588. [DOI] [PubMed] [Google Scholar]
- 133.Chin L, Pomerantz J, Polsky D, et al. Cooperative effects of INK4a and ras in melanoma susceptibility in vivo. Genes Dev. 1997;11:2822–2834. doi: 10.1101/gad.11.21.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cordon-Cardo C, Cote RJ, Sauter G. Genetic and molecular markers of urothelial premalignancy and malignancy. Scand J Urol Nephrol Suppl. 2000:82–93. doi: 10.1080/003655900750169338. [DOI] [PubMed] [Google Scholar]
- 135.Dinney CP, McConkey DJ, Millikan RE, et al. Focus on bladder cancer. Cancer Cell. 2004;6:111–116. doi: 10.1016/j.ccr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 136.Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer. 2005;5:713–725. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 137.Bakkar AA, Wallerand H, Radvanyi F, et al. FGFR3 and TP53 gene mutations define two distinct pathways in urothelial cell carcinoma of the bladder. Cancer Res. 2003;63:8108–8112. [PubMed] [Google Scholar]
- 138.Rieger-Christ KM, Mourtzinos A, Lee PJ, et al. Identification of fibroblast growth factor receptor 3 mutations in urine sediment DNA samples complements cytology in bladder tumor detection. Cancer. 2003;98:737–744. doi: 10.1002/cncr.11536. [DOI] [PubMed] [Google Scholar]
- 139.van Rhijn BW, van der Kwast TH, Vis AN, et al. FGFR3 and P53 characterize alternative genetic pathways in the pathogenesis of urothelial cell carcinoma. Cancer Res. 2004;64:1911–1914. doi: 10.1158/0008-5472.can-03-2421. [DOI] [PubMed] [Google Scholar]
- 140.Jebar AH, Hurst CD, Tomlinson DC, et al. FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene. 2005;24:5218–5225. doi: 10.1038/sj.onc.1208705. [DOI] [PubMed] [Google Scholar]
- 141.Gripp KW. Tumor predisposition in Costello syndrome. Am J Med Genet C Semin Med Genet. 2005;137:72–77. doi: 10.1002/ajmg.c.30065. [DOI] [PubMed] [Google Scholar]
- 142.Cheng J, Huang H, Pak J, et al. Allelic loss of p53 gene is associated with genesis and maintenance, but not invasion, of mouse carcinoma in situ of the bladder. Cancer Res. 2003;63:179–185. [PubMed] [Google Scholar]
- 143.Grippo PJ, Sandgren EP. Highly invasive transitional cell carcinoma of the bladder in a simian virus 40 T-antigen transgenic mouse model. Am J Pathol. 2000;157:805–813. doi: 10.1016/S0002-9440(10)64594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cordon-Cardo C, Zhang ZF, Dalbagni G, et al. Cooperative effects of p53 and pRB alterations in primary superficial bladder tumors. Cancer Res. 1997;57:1217–1221. [PubMed] [Google Scholar]
- 145.Grossman HB, Liebert M, Antelo M, et al. p53 and Rb expression predict progression in T1 bladder cancer. Clin Cancer Res. 1998;4:829–834. [PubMed] [Google Scholar]
- 146.Cote RJ, Dunn MD, Chatterjee SJ, et al. Elevated and absent pRb expression is associated with bladder cancer progression and has cooperative effects with p53. Cancer Res. 1998;58:1090–1094. [PubMed] [Google Scholar]
- 147.Ahuja D, Saenz-Robles MT, Pipas JM. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24:7729–7745. doi: 10.1038/sj.onc.1209046. [DOI] [PubMed] [Google Scholar]
- 148.Zalvide J, DeCaprio JA. Role of pRb-related proteins in simian virus 40 large-T-antigen-mediated transformation. Mol Cell Biol. 1995;15:5800–5810. doi: 10.1128/mcb.15.10.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Buckley MT, Yoon J, Yee H, et al. The histone deacetylase inhibitor belinostat (PXD101) suppresses bladder cancer cell growth in vitro and in vivo. J Transl Med. 2007;5:49. doi: 10.1186/1479-5876-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Veranic P, Jezernik K. Trajectorial organisation of cytokeratins within the subapical region of umbrella cells. Cell Motil Cytoskeleton. 2002;53:317–325. doi: 10.1002/cm.10077. [DOI] [PubMed] [Google Scholar]