Abstract
Functional neural circuit formation during postnatal development involves massive elimination of early-formed redundant synapses and strengthening of necessary synaptic connections. In the cerebellum, one-to-one connection from a climbing fibre (CF) to a Purkinje cell (PC) is established through four distinct phases: (1) strengthening of a single CF among multiple CFs in each PC at postnatal age P3–P7 days, (2) translocation of a single strengthened CF to PC dendrites from around P9, (3) early-phase (P7 to around P11) and (4) late-phase (around P12–P17) elimination of weak CF synapses from PC somata. Mice with PC-selective deletion of the P/Q-type voltage-dependent Ca2+ channel (VDCC) exhibit severe defects in strengthening of single CFs, dendritic translocation of single CFs and CF elimination from P7. In contrast, mice with a mutation of a single allele for the GABA synthesizing enzyme GAD67 show selective impairment of CF elimination from P10. Electrophysiological and Ca2+ imaging data suggest that GABAA receptor-mediated inhibition onto PC somata from putative basket cells influences CF-induced Ca2+ transients and regulates elimination of redundant CF synapses from PC somata at P10–P16. Thus, regulation of Ca2+ influx to PCs through VDCCs is crucial for the four phases of CF synapse elimination during postnatal development.
 |
Masanobu Kano is Professor of Neurophysiology at the University of Tokyo, Graduate School of Medicine. His lab works on refinement of neural circuits in developmental brain, with a special focus on elimination of redundant climbing fiber to Purkinje cell synapses during postnatal cerebellar development. He started to examine climbing fiber synapse elimination in 1990th when he was an assistant professor at Jichi Medical School (Tochigi, Japan). Another main research interest of his lab is endocannabinoid-mediated retrograde modulation of synaptic transmission, which his group found when he was Professor of Physiology at Kanazawa University School of Medicine (Kanazawa, Japan).
Introduction
Precise formation of neural circuits during development is a prerequisite for proper functions of the CNS. Neuronal connections are initially redundant, but they are refined and become functionally mature during postnatal development. This process is known as ‘synapse elimination’ or ‘synapse pruning’, and is widely accepted as an important step to refine initial redundant circuitry into functionally mature versions. (Purves & Lichtman, 1980; Katz & Shatz, 1996; Lichtman & Colman, 2000). The cerebellar climbing fibre (CF)–Purkinje cell (PC) synapse provides an excellent model to study synapse elimination in the CNS. While most PCs in the cerebellum of adult mice receive strong excitatory inputs from single CFs (Ito, 1984), PCs in neonatal cerebellum are innervated by multiple CFs with similar synaptic strengths (Hashimoto & Kano, 2003, 2005; Bosman et al. 2008; Kano & Hashimoto, 2009). From postnatal day 3 (P3) to P7, one CF is selectively strengthened relative to others in each PC (functional differentiation) (Hashimoto & Kano, 2003, 2005; Bosman et al. 2008; Kano & Hashimoto, 2009). Then, only the strengthened CF extends its innervation along growing PC dendrites (CF translocation) (Hashimoto et al. 2009). In parallel, surplus CF synapses on the PC soma are eliminated via two distinct mechanisms from P7 to P11 (early phase of CF elimination) and P12 to P16 (late phase of CF elimination). (Hashimoto & Kano, 2003, 2005; Hashimoto et al. 2009; Kano & Hashimoto, 2009; Watanabe & Kano, 2011).
Previous studies indicate that CF synapse elimination critically depends on neuronal activity. Decreasing PC activity in mice by PC-selective overexpression of chloride channels impairs CF synapse elimination (Lorenzetto et al. 2009). Type 1 metabotropic glutamate receptors (mGluR1) (Kano et al. 1997; Ichise et al. 2000) and NMDA-type glutamate receptors (Rabacchi et al. 1992; Kakizawa et al. 2000) have also been shown to be crucial for CF synapse elimination. In addition to these molecules responsible for mediating neural activity, we have recently reported that the P/Q-type voltage-dependent Ca2+ channel (P/Q-type VDCC) in PCs (Hashimoto et al. 2011) and GABAergic inhibition of the PC soma (Nakayama et al. 2012) play crucial roles in distinct phases of CF synapse elimination. P/Q-type VDCCs provide a major route of Ca2+ entry into PCs, whereas GABAergic inhibition influences the membrane potential of PCs and the opening of VDCCs, and thereby controls Ca2+ influx to PCs. In this Symposium Review, we summarize these two recent papers and discuss how Ca2+ transients in PCs regulate CF synapse elimination.
Analysis of PC-selective P/Q-type VDCC knockout mice
Our previous study demonstrates that global P/Q-type VDCC knockout mice have severe defects in CF synapse development including impaired CF synapse elimination and dendritic translocation of multiple CFs (Miyazaki et al. 2004). However, it remains unclear to what extent the postsynaptic P/Q-type VDCC in PCs is responsible for these developmental defects and to which phase(s) of CF-PC development the P/Q-type VDCC contributes. To address these issues, we generated mice with a PC-selective deletion of CaV2.1 (PC-CaV2.1 KO mice) (Hashimoto et al. 2011). The lack of CaV2.1 mRNA expression was confirmed in PCs at P2. We made whole-cell recordings from PCs in cerebellar slices at P5–P6 and found that voltage-dependent Ca2+ currents from PCs were significantly smaller than those of control PCs and were insensitive to the P/Q-type VDCC blocker ω-agatoxin IVA (Hashimoto et al. 2011). In contrast, the P/Q channel contributed to the same extent to CF to PC synaptic transmission in PC-Cav2.1 KO and control mice (Hashimoto et al. 2011). These results demonstrate that the P/Q channel at the presynaptic terminal is intact and functions normally in PC-Cav2.1 KO mice. Then, we performed simultaneous whole-cell recordings and two-photon Ca2+ imaging from PCs in vivo in control and PC-Cav2.1 KO mice at P7–P11 under isoflurane anaesthesia (Kitamura et al. 2008). We found that PCs exhibited irregular burst firings and occasional trains of burst firing due to CF activity in both PC-Cav2.1 KO and wild-type mice. However, Ca2+ transients induced by spontaneous CF inputs were markedly reduced in PCs of PC-Cav2.1 KO mice (Hashimoto et al. 2011). These results indicate that lack of P/Q-type VDCC in PCs results in reduction of Ca2+ influx into PCs at P7–P11 in PC-Cav2.1 KO mice.
We then examined CF innervations of PCs in PC-Cav2.1 KO mice and their wild-type littermates. We made whole-cell recordings from PCs in cerebellar slices prepared from control and PC-Cav2.1 KO mice at various ages, stimulated CFs in the granule cell layer, and examined CF-EPSCs. At P4–P6, no significant difference was observed in the mean number of CFs innervating each PC between PC-CaV2.1 KO and control mice (Fig. 1A). PCs were innervated by more than four CFs in both mice (Fig. 1A). Thereafter, PC-CaV2.1 KO mice manifested severe defects in CF synapse development and elimination (Hashimoto et al. 2011). First, CF synapse elimination was severely impaired in PC-CaV2.1 KO mice until around P12 (Fig. 1). Second, preferential strengthening of single CF inputs from P5 to P7 was severely impaired in PC-CaV2.1 KO mice (Fig. 2B and C), despite a comparable 4-fold increase in summed amplitudes of multiple CF-EPSCs from P5 to P8 (Fig. 2A). Third, more than one CF translocated to PC dendrites in PC-CaV2.1 KO mice (Hashimoto et al. 2011). These results indicate that Ca2+ influx through P/Q-type VDCCs into PCs is crucial for selective strengthening of single CFs, early-phase elimination and selective translocation of single strengthened CFs to PC dendrites.
Figure 1. Impairment of CF synapse elimination during postnatal development in PC-Cav2.1 KO mice.
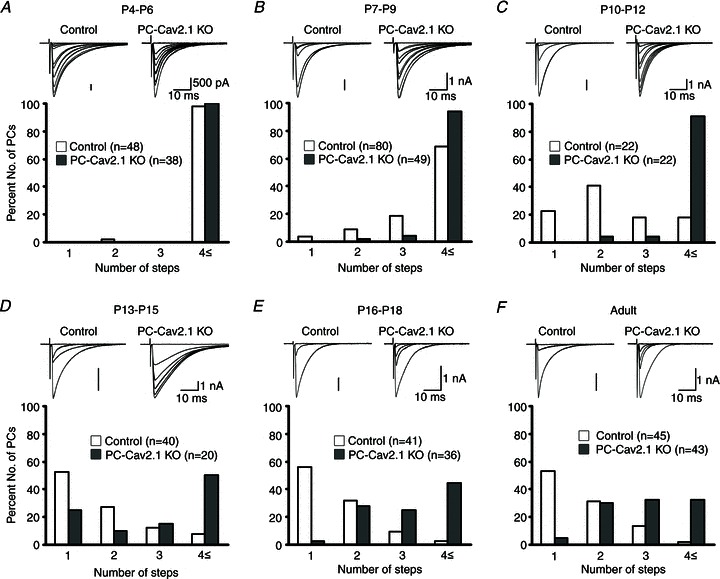
A–F, specimen records of CF-EPSCs (upper panel; two to three traces were superimposed at each threshold intensity; Vh = −10 mV) and frequency distribution histogram for the number of discrete CF-EPSCs (lower panel) for control and PC-Cav2.1 KO mice at indicated ages. There is no significant difference in the frequency distribution in A between control and PC-Cav2.1 KO mice (P = 0.871; Mann–Whitney U test). In contrast, frequency distributions for B–F are significantly different between control and PC-Cav2.1 KO mice (B, P = 0.023; C–F, P < 0.001). Modified with permission from Hashimoto et al. (2011).
Figure 2. Impairment of postnatal development of CF-PC synapses in PC-Cav2.1 KO mice.
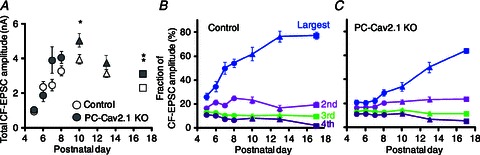
A, postnatal changes in the total amplitudes of CF-EPSCs (at Vh = −20 mV) elicited in each PC. Plots include the data for mono-innervating CFs. The numbers of PCs for each data point are 14–77 for control and 10–61 for PC-Cav2.1 KO PCs. B, C, postnatal changes in the fraction of the largest (blue), second (pink), third (green) and fourth (violet) EPSC amplitude relative to the total CF-EPSC amplitude in control (B) and PC-Cav2.1 KO (C) PCs. Modified with permission from Hashimoto et al. (2011).
In addition to severe defects in the postnatal development of CF–PC synapses, we found that PC-CaV2.1 KO mice exhibit abnormalities in the formation of synapses from parallel fibres (PFs), the other excitatory inputs to PCs. In adult wild-type mice, each PC is innervated by a single CF in proximal dendrites and more than 105 PFs in distal dendrites (Ito, 1984; Altman & Bayer, 1997). We found that the extent of CF territory was restricted to the soma and basal dendrites of PCs and PF territory was expanded reciprocally to the proximal somato-dendritic domain in PC-CaV2.1 KO mice (Miyazaki et al. 2012). Consequently, the soma and proximal dendrites of PCs displayed hyperspiny transformation and are innervated by multiple CFs and numerous PFs (Miyazaki et al. 2012). These results indicate that Ca2+ influx through P/Q-type VDCCs into PCs is crucial not only for homosynaptic competition among multiple CFs but also for heterosynaptic competition between CFs and PFs during postnatal development (Watanabe & Kano, 2011). The schema shown in Fig. 3 illustrates how Ca2+ influx through P/Q-type VDCCs regulates multiple events in CF synapse development during the first 10 postnatal days in rodents. CF inputs elicit large AMPA receptor-mediated EPSPs that are large enough to open P/Q-type VDCCs and induce massive Ca2+ influx into PC somata. Elevated Ca2+ will lead to assignment of ‘resources’ for synaptic strengthening of respective CF synapses in an activity-dependent manner. This will promote ‘functional differentiation’ into a single strong CF and several weak CFs in each PC, and will further facilitate ‘homosynaptic competition’ among multiple CFs. Then, only the strongest CF can start to translocate along growing PC dendrites, which then initiates ‘heterosynaptic competition’ with PF inputs that begin to expand massively during the second and third postnatal weeks (Fig. 3).
Figure 3. Schema showing how Ca2+ influx through P/Q-type VDCCs regulates multiple events of CF synapse development during about the first 10 postnatal days.
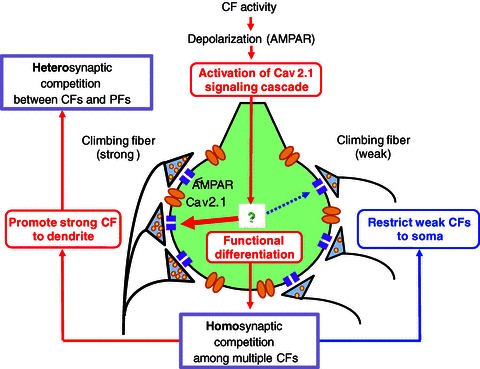
Modified with permission from Watanabe & Kano (2011).
Analysis of GAD67+/GFP mice
To examine whether GABAergic inhibition plays any role in CF synapse development, we used the heterozygous GAD67-GFP (Δneo) knock-in (GAD67+/GFP) mouse, which has a deletion of a single allele of a GABA-synthesizing enzyme, GAD67 (Tamamaki et al. 2003), as a mouse model of diminished GABAergic transmission. We found that GABAergic transmission was attenuated in GAD67+/GFP mice during the second postnatal week, and that GABA caused inhibition of PC activity after P10 in both control and GAD67+/GFP mice (Nakayama et al. 2012). Foliation and the layer structure of the cerebellum, the morphology of PCs, the morphology and density of PF–PC synapses, and basic electrophysiological properties of PF-mediated EPSCs (PF-EPSCs) were normal in GAD67+/GFP mice (Nakayama et al. 2012).
In contrast to the normal morphology and function of PF–PC synapses in adult GAD67+/GFP mice (P21–P52), a higher percentage of PCs were innervated by multiple CFs when compared to control mice (Fig. 4), indicating that developmental CF synapse elimination is impaired in GAD67+/GFP mice. Yet, the basic electrophysiological properties of CF-mediated EPSCs (CF-EPSCs) were normal in GAD67+/GFP mice. During early postnatal development from P5 to P9, there was no significant difference in the mean number of CFs innervating each PC between control and GAD67+/GFP mice (Nakayama et al. 2012). Functional differentiation into a single strong CF and several weak CFs in each PC was also normal in GAD67+/GFP mice (Nakayama et al. 2012). At P10–P12, the difference between the two genotypes became significant such that control PCs were innervated by significantly fewer CFs than GAD67+/GFP PCs (Nakayama et al. 2012). At P13–P15 and P16–P20, the difference became even larger (Nakayama et al. 2012). These results indicate that, in GAD67+/GFP mice, initial CF synapse formation, functional differentiation and maturation of CF synapses, and elimination of surplus CFs until P9 are normal, whereas CF synapse elimination after P10 is specifically impaired.
Figure 4. Persistent multiple CF innervation of PCs in mature GAD67+/GFP mice.
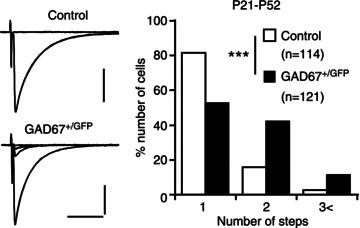
Left panel, specimen records of CF-EPSCs in a control (P26) and a GAD67+/GFP (P22) PC with gradually increasing stimulus intensities. Two to three traces were superimposed at each threshold intensity. Vh = −10 mV. Scale bars, 10 ms and 500 pA. Right panel, summary bar graph showing the number of discrete CF-EPSC steps in PCs from control (□) and GAD67+/GFP (▪) mice. Data from 10 control and 11 GAD67+/GFP mice at P20–P52. ***P < 0.001. Modified with permission from Nakayama et al. (2012).
Similar impairment of CF synapse elimination was observed when the GAD inhibitor 3-mercaptopropionic acid (3-MP) was applied locally and persistently to the cerebellum of control mice (Nakayama et al. 2012) by means of an ethylene-vinyl acetate copolymer (Elvax) from P10 (Kakizawa et al. 2003, 2005). Since 3-MP application from P17 had no effect, the developmental period from P10 to P16 is considered to be the critical period during which CF synapse elimination is sensitive to GAD67 activity in the cerebellar cortex (Nakayama et al. 2012). The impairment of CF synapse elimination in GAD67+/GFP mice was reversed when GABAA receptor sensitivity was enhanced in the cerebellum by local and persistent application of diazepam via Elvax from P10 (Nakayama et al. 2012). Again, diazepam application from P17 was ineffective (Nakayama et al. 2012), confirming that the GABAergic inhibitory tone from P10 to P16 within the cerebellar cortex is an important factor that regulates developmental CF synapse elimination.
It has been reported that GABA elicits depolarization and induces Ca2+ transients in immature rat PCs (Eilers et al. 2001). Therefore, we examined whether GABA excites or inhibits PCs during postnatal development in mice (Nakayama et al. 2012). We found that ionophoretic application of the GABAA receptor agonist muscimol increased the firing rate in about two-thirds of PCs at P4–P6 in both GAD67+/GFP and control mice. By marked contrast, at P10 and thereafter, muscimol decreased the firing rate in most PCs in both strains of mice. This result clearly indicates that GABA inhibits PCs during the developmental period when impairment of CF synapse elimination is manifest in GAD67+/GFP mice (Nakayama et al. 2012).
Several types of GABAergic synapses are present in the neural circuitry in the cerebellar cortex and all of them can be affected in GAD67+/GFP mice. We found that miniature IPSCs in PCs with large amplitude (>100 pA, at holding potential of −70 mV) and fast rise time (<1.5 ms) were much less frequent in GAD67+/GFP mice than in control mice (Nakayama et al. 2012), suggesting that GABAergic inputs to the PC soma are attenuated in GAD67+/GFP mice. Paired recordings from a putative basket cell (BC) and a PC demonstrated that the amplitude of unitary IPSCs in GAD67+/GFP mice was about half of that of control mice (Nakayama et al. 2012), indicating that GABAergic inhibition from putative BCs onto PCs is attenuated in GAD67+/GFP mice.
Reduced GABAergic inhibition is thought to affect depolarization-induced Ca2+ transients by CF inputs. We recorded CF-induced EPSPs and Ca2+ transients simultaneously from the soma of a PC that was multiply innervated by a single “strong” CF (CF-multi-S) and one or two “weak” CFs (CF-multi-W) (Fig. 5A and B). Stimulation of such CF-multi-Ws induced Ca2+ transients in the PC soma but did not elicit measurable Ca2+ elevation in PC dendrites (Fig. 5B). Integration of Ca2+ transients (for 1.5 s from the onset) in the PC soma by stimulating CF-multi-Ws was significantly larger in GAD67+/GFP mice than in control mice (Fig. 5B and C). In contrast to CF-multi-Ws, stimulation of CF-multi-S induced large Ca2+ transients in PC dendrites, but the magnitudes were not different between control and GAD67+/GFP mice (Fig. 5B and D). Importantly, bath application of diazepam (1 μm) significantly reduced the somatic Ca2+ transients induced by CF-multi-W stimulation in GAD67+/GFP mice to the same level as those in control mice without diazepam (Fig. 5E). Thus, diazepam eliminated the difference in the magnitude of somatic Ca2+ transients induced by CF-multi-W stimulation between the two mouse strains, which is considered to be a major cause of diazepam-induced reversal of impaired CF synapse elimination in GAD67+/GFP mice. These results indicate that diminished inhibition of the PC soma permits CF-multi-W to induce much larger somatic Ca2+ transients in GAD67+/GFP mice than in control mice. It should be noted that CF-induced Ca2+ transients are considered to be mediated mainly by P/Q-type VDCCs (Nakayama et al. 2012). Therefore, control of P/Q-type VDCC activity and resultant Ca2+ transients by GABAergic inhibition appears to be crucial for CF synapse elimination from P10 to P16.
Figure 5. Ca2+ transients in the PC soma induced by activation of weak CFs are larger in GAD67+/GFP mice than in control mice.
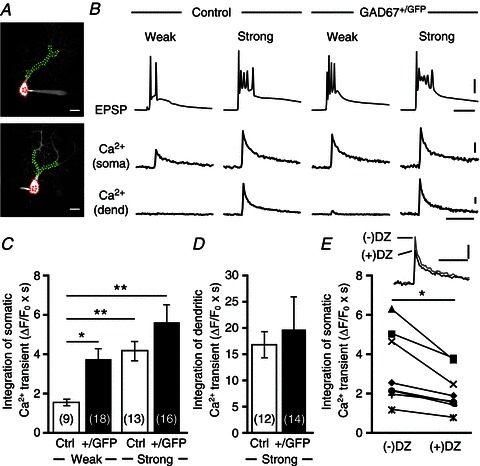
A, representative PC images of a control (upper) and a GAD67+/GFP (lower) mouse. Areas indicated by red and green dotted lines represent ROIs for somatic and dendritic Ca2+ transients, respectively. Scale bars, 20 μm. B, EPSPs and Ca2+ transients recorded in the soma and dendrite of multiply innervated PCs in response to stimulation of a weak or a strong CF in a control and a GAD67+/GFP mouse. Scale bars, 20 mV and 10 ms for EPSPs, 2% (for soma) or 10% (for dendrite) and 1 s for Ca2+ transients. C, D, average magnitudes of Ca2+ transients from the PC soma (C) and dendrites (D) induced by stimulating a weak (CF-multi-W) or a strong (CF-multi-S) CF. Ca2+ transients for 1.5 s from the onset were integrated. For the data in C, statistical analysis was performed using the Kruskal–Wallis test with the Steel-Dwass multiple comparison post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001. E, traces show somatic Ca2+ transients elicited by activation of weak CF in GAD67+/GFP PCs before (–) and after (+) bath application of diazepam (DZ, 1 μm). Data from each cell are plotted in the lower graph. *P < 0.05. Scale bars, 2% and 1 s. Modified with permission from Nakayama et al. (2012).
The schema in Fig. 6 illustrates how GABAergic inhibition to the PC soma regulates the late phase of CF synapse elimination. We hypothesize a mechanism that maintains synapses depending on the Ca2+ level of PCs. In wild-type mice, the somatic Ca2+ transients induced by CF-multi-W will be smaller than the level of synaptic maintenance because of strong somatic inhibition from putative BCs, and therefore CF-multi-Ws will eventually be eliminated (Fig. 6, left panel). In contrast, the somatic Ca2+ transients induced by CF-multi-W in GAD67+/GFP mice will be larger than the level of synaptic maintenance because of the diminished somatic inhibition from putative BCs (Fig. 6, right panel). Consequently, CF-multi-Ws will survive by counteracting developmental synapse elimination, which otherwise prunes CF-multi-Ws during the second postnatal week (Hashimoto et al. 2009).
Figure 6.
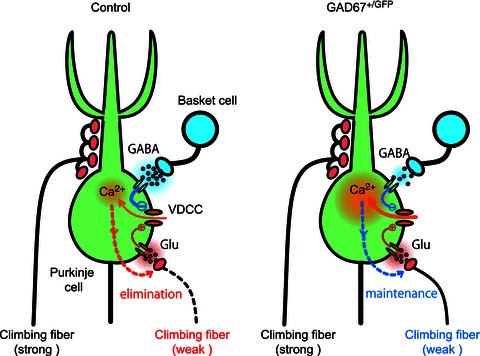
Schema showing a possible mechanism for regulation of the late phase of CF synapse elimination by GABAergic inhibition from BC to PC somata
Conclusions
Our analyses of PC-selective P/Q-type VDCC KO mice demonstrate that Ca2+ influx through P/Q-type VDCCs into PCs is crucial for strengthening of a single CF among multiple CFs in each PC (functional differentiation), translocation of the single strengthened CF to PC dendrites (dendritic translocation), the early phase of CF elimination, and heterosynaptic competition between CF and PF inputs (Hashimoto et al. 2011; Miyazaki et al. 2012). In GAD67+/GFP mice, these four events are normal but elimination of redundant CF synapses from P10 to P16, which largely overlaps the period of the late phase of CF elimination, is selectively impaired (Nakayama et al. 2012). Our analyses demonstrate that diminished inhibition of the PC soma in GAD67+/GFP mice permits weak CFs to induce Ca2+ transients that might be large enough to counteract developmental synapse elimination that otherwise prunes weak CFs during the second postnatal week (Nakayama et al. 2012). Since Ca2+ transients induced by CF activity are considered to be mediated mostly by P/Q-type VDCCs (Nakayama et al. 2012), the results from the analyses of GAD67+/GFP mice indicate that regulation of Ca2+ influx through P/Q-type VDCCs to PCs is crucial for the late phase of CF elimination. Thus, activity of P/Q-type VDCCs and resultant Ca2+ influx to PCs are key factors in the four phases of CF synapse elimination and heterosynaptic competition between CF and PF inputs. A challenge for future work is to identify the signalling molecules downstream of Ca2+ elevation and to elucidate how these molecules contribute to the respective processes of CF synapse elimination and heterosynaptic competition during postnatal cerebellar development.
Acknowledgments
This work was supported by Grants-in Aid for Scientific Research (21700346 to H.N., 17023021, 21220006 and 23650204 to M.K., 17023001 and 19100005 to M.W.), the Strategic Research Program for Brain Sciences (Development of Biomarker Candidates for Social Behaviour), and the Global COE program (Integrative Life Science Based on the Study of Biosignalling Mechanisms) from MEXT, Japan.
References
- Altman J, Bayer SA. Development of the Cerebellar System : In Relation to Its Evolution, Structure, and Functions. Boca Raton, FL, USA: CRC Press; 1997. [Google Scholar]
- Bosman LW, Takechi H, Hartmann J, Eilers J, Konnerth A. Homosynaptic long-term synaptic potentiation of the “winner” climbing fiber synapse in developing Purkinje cells. J Neurosci. 2008;28:798–807. doi: 10.1523/JNEUROSCI.4074-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers J, Plant TD, Marandi N, Konnerth A. GABA-mediated Ca2+ signalling in developing rat cerebellar Purkinje neurones. J Physiol. 2001;536:429–437. doi: 10.1111/j.1469-7793.2001.0429c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Ichikawa R, Kitamura K, Watanabe M, Kano M. Translocation of a “winner” climbing fiber to the Purkinje cell dendrite and subsequent elimination of “losers” from the soma in developing cerebellum. Neuron. 2009;63:106–118. doi: 10.1016/j.neuron.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kano M. Functional differentiation of multiple climbing fiber inputs during synapse elimination in the developing cerebellum. Neuron. 2003;38:785–796. doi: 10.1016/s0896-6273(03)00298-8. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kano M. Postnatal development and synapse elimination of climbing fiber to Purkinje cell projection in the cerebellum. Neurosci Res. 2005;53:221–228. doi: 10.1016/j.neures.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Tsujita M, Miyazaki T, Kitamura K, Yamazaki M, Shin HS, Watanabe M, Sakimura K, Kano M. Postsynaptic P/Q-type Ca2+ channel in Purkinje cell mediates synaptic competition and elimination in developing cerebellum. Proc Natl Acad Sci U S A. 2011;108:9987–9992. doi: 10.1073/pnas.1101488108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, Shigemoto R, Katsuki M, Aiba A. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288:1832–1835. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- Ito M. The Cerebellum and Neural Control. New York: Raven Press; 1984. [Google Scholar]
- Kakizawa S, Miyazaki T, Yanagihara D, Iino M, Watanabe M, Kano M. Maintenance of presynaptic function by AMPA receptor-mediated excitatory postsynaptic activity in adult brain. Proc Natl Acad Sci U S A. 2005;102:19180–19185. doi: 10.1073/pnas.0504359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizawa S, Yamada K, Iino M, Watanabe M, Kano M. Effects of insulin-like growth factor I on climbing fibre synapse elimination during cerebellar development. Eur J Neurosci. 2003;17:545–554. doi: 10.1046/j.1460-9568.2003.02486.x. [DOI] [PubMed] [Google Scholar]
- Kakizawa S, Yamasaki M, Watanabe M, Kano M. Critical period for activity-dependent synapse elimination in developing cerebellum. J Neurosci. 2000;20:4954–4961. doi: 10.1523/JNEUROSCI.20-13-04954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Hashimoto K. Synapse elimination in the central nervous system. Curr Opin Neurobiol. 2009;19:154–161. doi: 10.1016/j.conb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Kurihara H, Watanabe M, Inoue Y, Aiba A, Tonegawa S. Persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking mGluR1. Neuron. 1997;18:71–79. doi: 10.1016/s0896-6273(01)80047-7. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Judkewitz B, Kano M, Denk W, Hausser M. Targeted patch-clamp recordings and single-cell electroporation of unlabeled neurons in vivo. Nat Methods. 2008;5:61–67. doi: 10.1038/nmeth1150. [DOI] [PubMed] [Google Scholar]
- Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25:269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- Lorenzetto E, Caselli L, Feng G, Yuan W, Nerbonne JM, Sanes JR, Buffelli M. Genetic perturbation of postsynaptic activity regulates synapse elimination in developing cerebellum. Proc Natl Acad Sci U S A. 2009;106:16475–16480. doi: 10.1073/pnas.0907298106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Hashimoto K, Shin HS, Kano M, Watanabe M. P/Q-type Ca2+ channel α1A regulates synaptic competition on developing cerebellar Purkinje cells. J Neurosci. 2004;24:1734–1743. doi: 10.1523/JNEUROSCI.4208-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Yamasaki M, Hashimoto K, Yamazaki M, Abe M, Usui H, Kano M, Sakimura K, Watanabe M. Cav2.1 in cerebellar Purkinje cells regulates competitive excitatory synaptic wiring, cell survival, and cerebellar biochemical compartmentalization. J Neurosci. 2012;32:1311–1328. doi: 10.1523/JNEUROSCI.2755-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Miyazaki T, Kitamura K, Hashimoto K, Yanagawa Y, Obata K, Sakimura K, Watanabe M, Kano M. GABAergic inhibition regulates developmental synapse elimination in the cerebellum. Neuron. 2012;74:384–396. doi: 10.1016/j.neuron.2012.02.032. [DOI] [PubMed] [Google Scholar]
- Purves D, Lichtman JW. Elimination of synapses in the developing nervous system. Science. 1980;210:153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- Rabacchi S, Bailly Y, Delhaye-Bouchaud N, Mariani J. Involvement of the N-methyl D-aspartate (NMDA) receptor in synapse elimination during cerebellar development. Science. 1992;256:1823–1825. doi: 10.1126/science.1352066. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kano M. Climbing fiber synapse elimination in cerebellar Purkinje cells. Eur J Neurosci. 2011;34:1781–1794. doi: 10.1111/j.1460-9568.2011.07894.x. [DOI] [PubMed] [Google Scholar]


