Abstract
The neuromuscular junction is known as a strong and reliable synapse. It is strong because it releases an excess of chemical transmitter, beyond what is required to bring the postsynaptic muscle cell to threshold. Because the synapse can sustain suprathreshold muscle activation during short trains of action potentials, it is also reliable. The presynaptic mechanisms that lead to reliability during short trains of activity have only recently been elucidated. It appears that there are relatively few calcium channels in individual active zones, that channels open with a low probability during action potential stimulation and that even if channels open the resulting calcium flux only rarely triggers vesicle fusion. Thus, each synaptic vesicle may only associate with a small number of calcium channels, forming an unreliable single vesicle release site. Strength and reliability of the neuromuscular junction emerge as a result of its assembly from thousands of these unreliable single vesicle release sites. Hence, these synapses are strong while at the same time only releasing a small subset of available docked vesicles during each action potential, thus conserving transmitter release resources. This prevents significant depression during short trains of action potential activity and confers reliability.
 |
Stephen Meriney (left) received his PhD in Physiology/Neuroscience from the University of Connecticut with Guillermo Pilar. He then moved to the Jerry Lewis Neuromuscular Research Centre at UCLA to perform postdoctoral work with Alan Grinnell where he studied synaptic mechanisms at the frog neuromuscular junction. He is currently Professor of Neuroscience and Psychiatry at the University of Pittsburgh. Markus Dittrich (right) received his PhD in Physics with Klaus Schulten from the University of Illinois at Urbana-Champaign. He began working with computer models of neuromuscular synapses as part of his postdoctoral work with Joel Stiles at the Pittsburgh Supercomputing Center. He is currently the Director of the National Resource for Biomedical Supercomputing at the Pittsburgh Supercomputing Center and Carnegie Mellon University. For the past 12 years, the Meriney and Stiles/Dittrich laboratories have collaborated to study presynaptic mechanisms at the neuromuscular junction.
Introduction
As ‘giant’ synapses go, the neuromuscular junction (NMJ) is one that has been extensively studied for over 70 years (Feng, 1941; Katz, 1969). The size and peripheral accessibility of this model synapse have permitted detailed investigation of many conserved synaptic mechanisms (for review, see Grinnell, 1995), and provided the framework for many subsequent studies at CNS synapses (for review, see Bekkers, 1994). Two features of neuromuscular synapses that are critical to their function are strength and reliability. Nervous system-evoked patterned activation of specific skeletal muscles needs to be strong and reliable to allow efficient and consistent movement of body parts in a finely tuned manner. NMJs are strong because they release a large amount of chemical transmitter that is usually more than sufficient to excite postsynaptic muscle cells and lead to their contraction (reviewed by Wood & Slater, 2001). Of course, the NMJ of a given muscle may need to be repeatedly activated during sustained contractions or repetitive movements. The presynaptic mechanisms that contribute to the reliable activation of the NMJ during repeated stimulation have recently been studied at the frog NMJ using a combination of electrophysiology, calcium (Ca2+) imaging and computer modelling. These studies have led to the hypothesis that transmitter release at the NMJ is controlled by the assembly and function of thousands of unreliable single vesicle release sites (Tarr et al. 2013), similar to what has previously been called the ‘synaptosecretosome’ (O’Connor et al. 1993; Bennett, 1996).
The frog NMJ as a model synapse
We have known for many years that the frog NMJ has hundreds of presynaptic transmitter release sites (about 700 ‘active zones’). Each active zone is characterized by two rows of 20–40 docked synaptic vesicles positioned laterally to 200–250 intramembraneous particles (as identified in freeze-fracture replicas and interpreted to represent presynaptic transmembrane proteins) arranged in a precisely organized, long linear array of two parallel double rows (Heuser et al. 1974, 1979; Pawson et al. 1998; Harlow et al. 2001; see Fig. 1B). Therefore, the entire frog NMJ may have a total of 14,000–28,000 docked vesicles ready for release from these 700 active zones. However, following single action potential (AP) stimulation, only about 350 vesicles are released (Katz & Miledi, 1979). In other words, on average each of the 700 active zones has only one synaptic vesicle fusion event following every other AP stimulation. Until recently, it was not clear why the transmitter release probability from active zones in the frog NMJ was so low. Ca2+ imaging at this synapse revealed significant variability in Ca2+ entry during repeated low-frequency stimulation (Wachman et al. 2004). Using an analysis of variability in Ca2+ entry into these active zones during single AP stimuli at low frequency, Luo et al. (2011) provided evidence that there were relatively few Ca2+ channels in these active zones (about 30–50). This represents only a small fraction of the active zone proteins thought to make up the double rows of particles identified in freeze-fracture studies (Pumplin et al. 1981). Interestingly, this number of Ca2+ channels roughly matches the number of synaptic vesicles thought to be docked at active zones. Furthermore, Luo et al. (2011) predicted that the probability of a Ca2+ channel opening during a single presynaptic AP was very low (about 0.2). Thus, this study showed that during a single AP only about 6–10 Ca2+ channels open in each long linear active zone. Considering that on average only a single vesicle is released following every other stimulus, it follows from these recent studies that there must be a very low probability of vesicle fusion following the opening of a Ca2+ channel within these active zones. In the rare instances when Ca2+ entry does trigger vesicle fusion, it appears that the flux through one or only very few open channels is responsible (Yoshikami et al. 1989; Shahrezaei et al. 2006).
Figure 1. Structure and function of the frog neuromuscular junction.
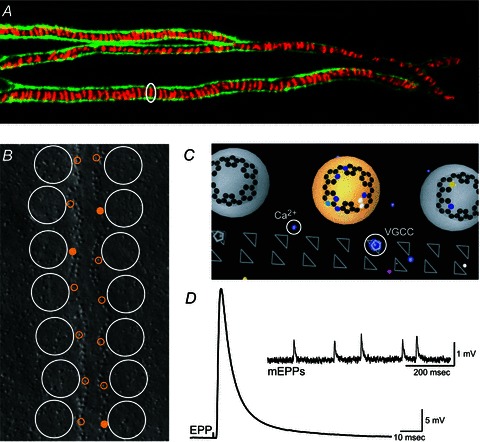
A, portion of a large frog neuromuscular junction stained using FITC-labelled peanut lectin to decorate Schwann cell extracellular matrix (green) and Alexa594-labelled α-bungarotoxin to identify the location of postsynaptic receptor folds (red) immediately opposite presynaptic active zones. A single acetylcholine receptor band that represents the predicted position of one active zone is circled. Image obtained by S.D.M. following the protocols of Ko (1987) and Reddy et al. (2003). B, graphical depiction of the spatial organization of docked synaptic vesicles (large white circles) and presynaptic Ca2+ channels (small yellow circles; filled circles represent the fraction of channels that open on average during an AP stimulus) overlaid onto a freeze-fracture replica of about half of a frog neuromuscular junction active zone (Heuser et al. 1979). Graphic adapted from Luo et al. (2011). C, graphical representation of a small portion of the MCell computer model of the frog neuromuscular junction active zone (bottom view). In this graphic, synaptic vesicles are large grey or yellow spheres, synaptotagmin binding sites are represented as an array of black dots at the base of synaptic vesicles (binding sites with bound Ca2+ are coloured according to the Ca2+ channel contributing the ion), triangles represent the position of presynaptic active zone proteins (some of which are occupied by voltage-gated Ca2+ channels; VGCC), and coloured dots represent Ca2+ ions (Ca2+), colour coded based on the voltage-gated Ca2+ channel of origin. D, endplate potential (EPP) recorded from a single frog neuromuscular junction following exposure to 4 μmμ-conotoxin PIIIA to block selectively postsynaptic sodium channels (average of 10 sweeps). Inset, spontaneous miniature endplate potentials (mEPPs) recorded in the absence of nerve stimulation. Data in D obtained by S.D.M. from the cutaneous pectoris nerve-muscle preparation following the protocol of Shon et al. (1998).
To aid in the interpretation of these data, and to advance new hypotheses that could be tested experimentally, Dittrich and colleagues (Ma et al. 2010, 2011; Dittrich et al. 2013) developed a detailed computer model of a frog NMJ active zone and investigated it using spatially realistic Monte-Carlo diffusion–reaction simulations via the computer program MCell (Monte Carlo Cell; Kerr et al. 2008). Their model included a realistic active zone geometry (Fig. 1C) with synaptic vesicles, voltage-gated Ca2+ channels, Ca2+ buffer and Ca2+ sensor sites on synaptic vesicles representing synaptotagmin molecules (Chapman, 2002). Upon stimulation with an AP waveform, voltage-gated Ca2+ channels opened stochastically and allowed Ca2+ ions to enter the presynaptic space. Ca2+ ions then diffused within the terminal, bound to Ca2+ buffer and/or vesicle-associated Ca2+ sensor sites (synaptotagmin), and eventually triggered vesicle fusion and subsequent release of neurotransmitter.
Using the availability of extensive experimental data (based on years of NMJ study), Dittrich et al. (2013) were able to tightly constrain their model, making it both quantitative and predictive in nature. They could then use their model to test experimental hypotheses and gain detailed insight into biological events inaccessible to experimental study, enabling the design of new experiments. This modelling approach was also important in the validation of a recent single-pixel optical fluctuation analysis study of presynaptic Ca2+ transients (Luo et al. 2011). Recently, this synergistic use of computer modelling and experimental techniques allowed Dittrich et al. (2013) to predict the Ca2+ binding stoichiometry and dynamics that underlie transmitter release from the frog NMJ. They showed that in the presence of an excess of Ca2+ binding sites (modeled after recent data on synaptotagmin copy number), only some of which needed to bind Ca2+ to trigger vesicle fusion, their model accurately predicted normal synaptic physiology. In the future, such a model can be used to provide novel insight not only into the nature of Ca2+-triggered synaptic vesicle release, but also into many other currently open questions regarding synaptic structure and function. For example, as these MCell simulations allow the investigator to follow the diffusion of individual Ca2+ ions within the modelled presynaptic space, it becomes straightforward to predict how many Ca2+ ions from each open voltage-gated Ca2+ channel contribute to the release of a given synaptic vesicle. This, in turn, enables the study of nanodomain versus microdomain coupling of Ca2+ channels to vesicle release. Simulation results for the frog NMJ active zone have already shown the highly localized nature of the interaction of Ca2+ ions from voltage-gated Ca2+ channels with nearby binding sites on vesicles (nanodomain coupling; Ma et al. 2011; Dittrich et al. 2013).
Taken together, the frog NMJ, a classic model system that has been used for over 70 years, continues to serve as a powerful tool with which to elucidate mechanistic details of chemical synaptic transmission. Recent work described above, which combines results from experimental and modelling work, provides significant evidence that active zones at the frog NMJ are assembled from thousands of unreliable single vesicle release sites that each consist of a single vesicle and a small number of closely associated voltage-gated Ca2+ channels.
The mammalian NMJ
Recent evidence from the study of mammalian NMJs suggests that these synapses are also constructed using unreliable single vesicle release sites. In contrast to frog, active zones at the mouse NMJ contain only very short linear arrays (100–200 nm long) of presynaptic transmembrane proteins (Fig. 2B; Nagwaney et al. 2009). These short active zones are tightly associated with about two synaptic vesicles that are positioned between active zone proteins (as opposed to along the lateral edges of active zones in frog NMJs; Nagwaney et al. 2009; see Fig. 2B). The large pretzel-shaped mouse NMJs have been shown to contain 600–800 of these small active zones, evenly spaced about 500 nm from one another (Chen et al. 2012; Fig. 2A). Because each active zone has been shown to have an average of two docked synaptic vesicles, the entire mouse NMJ may have 1200–1600 docked vesicles ready for release during an AP stimulus (Nagwaney et al. 2009). Despite the large number of available vesicle release sites, following a single AP stimulation, only about 60–80 vesicles are released (Wang et al. 2004; Ruiz et al. 2011). Thus, on average these large synapses only release transmitter from about 10% of available active zones with each AP.
Figure 2. Structure and function of the mouse neuromuscular junction.
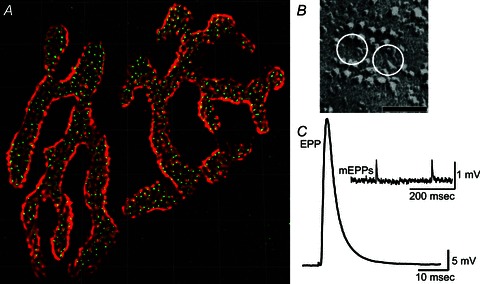
A, an entire single mouse neuromuscular junction stained with Alexa 594 α-bungarotoxin to label postsynaptic acetylcholine receptors (red), and Alexa 488 bassoon antibody to label presynaptic active zones (green spots). Confocal brightest projection image obtained by S.D.M. following the protocol of Nishimune et al. (2004), and processed for deconvolution. Grid lines = 5 μm. B, freeze-fracture replica of a single mouse neuromuscular junction active zone. White circles represent the predicted position of docked synaptic vesicles. Adapted from Nagwaney et al. (2009). Scale bar = 50 nm. C, average endplate potential (EPP) recorded from a single mouse neuromuscular junction following exposure to 1 μmμ-conotoxin GIIIB to block selectively postsynaptic sodium channels (average of 10 sweeps). Inset, spontaneous miniature endplate potentials (mEPPs) recorded in the absence of nerve stimulation. Data in C obtained by S.D.M. from the epitrochleoanconeus nerve-muscle preparation following the protocols of Urbano et al. (2003) and Rogozhin et al. (2008).
Interestingly, when Wang et al. (2010) used a statistical approach, based on variability in postsynaptic currents at the mouse NMJ, to estimate the probability of release and number of release sites, they predicted a probability of release of 0.6–0.9 from a total of 50–70 release sites (when measured at 1–2 mm extracellular Ca2+). Given the large number of total available active zones (600–800) at this synapse, these data imply that only a small subset of active zones contributes to vesicle release during stimulation while the others are inactive. This conclusion was supported by initial studies using pH-sensitive probes for vesicle fusion that revealed a heterogeneous distribution of areas of nerve terminal that participate in releasing transmitter at the mouse NMJ during low-frequency stimulation (Tabares et al. 2007; Wyatt & Balice-Gordon, 2008). Together, these data lead to the intriguing conclusion that only a subset of available vesicle release sites are used. One confound of this conclusion is that the consistent and restricted use of a small number of available release sites would lead to significant short-term depression even during short trains of AP activity, which is not seen experimentally.
Alternatively, each mouse active zone may be constructed using unreliable single vesicle release sites that contain a small number of presynaptic Ca2+ channels, each with a low probability of opening, as was shown at the frog NMJ (Luo et al. 2011). Constructing mammalian NMJs using a large number of spatially distributed release sites, each with a low probability of releasing a synaptic vesicle during single AP activity, is also consistent with previous anatomical and electrophysiological data (Wang et al. 2004; Ruiz et al. 2011). Under this scenario, the specific active zones that participate in vesicle fusion at the mouse NMJ during each AP vary randomly, determined by the stochastic nature of presynaptic Ca2+ channel gating. In preliminary computer modelling using MCell, Ma et al. (2010) found that assembly of mouse active zones using single vesicle release sites designed based on frog data resulted in a mouse active zone model in good agreement with experimental results. If one considers the expected low probability that Ca2+ flux through a single Ca2+ channel would trigger vesicle fusion, one would predict that during any given AP only a small subset of mouse active zones would experience Ca2+-triggered vesicle fusion. Further analysis of Ca2+-triggered vesicle fusion at the mammalian NMJ using tools with increased sensitivity, in combination with more advanced computer models, will be required to determine the sub-active zone mechanisms that control transmitter release at this large synapse.
Summary
Combining experimental and computational approaches, there is significant evidence for the hypothesis that the strength and reliability of frog and mouse NMJs are due to the assembly of large numbers of unreliable single vesicle release sites (Fig. 3; Tarr et al. 2013). Reliability is derived from the low probability of transmitter release from any given site (of which there are thousands), thus ensuring a dependable supply of releasable vesicles under sustained activity. On the other hand, synaptic strength is achieved by assembling sufficiently large numbers of single vesicle release sites into NMJs to bring the postsynaptic muscle cell to threshold in response to a single AP.
Figure 3. Organization of neuromuscular junction active zones based on assembly of unreliable single vesicle release sites.
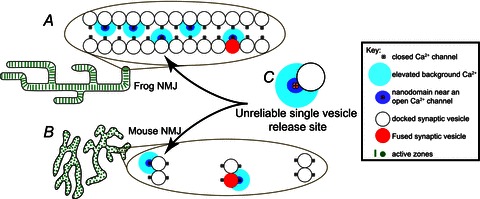
A, each of hundreds of active zones in the frog neuromuscular junction (NMJ) is hypothesized to be constructed using a long linear double array of unreliable single vesicle release sites (C). B, each of hundreds of active zones in the mouse neuromuscular junction are separated from one another by about 500 nm, and are hypothesized to be constructed using a short linear array of only two unreliable single vesicle release sites. C, the basic building block of neuromuscular junctions is hypothesized to be an unreliable single vesicle release site. Graphic adapted from Tarr et al. (2013).
Acknowledgments
This review is dedicated to the memory of Joel R. Stiles, an amazingly insightful and inspiring collaborator, colleague and friend. We thank Tyler Tarr for helpful discussion and comments during the preparation of this manuscript, and Dr. Kenneth Fish for assistance with processing confocal images.
Glossary
- AP
action potential
- MCell
Monte Carlo Cell
Additional information
Competing interests
None.
Funding
Supported by a National Institutes of Health grant (P41RR06009 and P41GM103712) to M.D., and National Science Foundation grants (0844174 to M.D., 0844604 to S.D.M., and 1249546 to M.D. and S.D.M.).
References
- Bekkers JM. Quantal analysis of synaptic transmission in the central nervous system. Curr Opin Neurobiol. 1994;4:360–365. doi: 10.1016/0959-4388(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Bennett MR. Neuromuscular transmission at an active zone: the secretosome hypothesis. J Neurocytol. 1996;25:869–891. doi: 10.1007/BF02284848. [DOI] [PubMed] [Google Scholar]
- Chapman ER. Synaptotagmin: a Ca2+ sensor that triggers exocytosis. Nat Rev Mol Cell Biol. 2002;3:498–508. doi: 10.1038/nrm855. [DOI] [PubMed] [Google Scholar]
- Chen J, Mizushige T, Nishimune H. Active zone density is conserved during synaptic growth but impaired in aged mice. J Comp Neurol. 2012;520:434–452. doi: 10.1002/cne.22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich M, Pattillo JM, King JD, Cho S, Stiles JR, Meriney SD. 2013. An excess calcium binding site model predicts neurotransmitter release at the NMJ Biophysical J. (in press) [DOI] [PMC free article] [PubMed]
- Feng TP. Studies on the neuromuscular junction XXVI. The changes of the end-plate potential during and after prolonged stimulation. Chin J Physiol. 1941;16:341–372. [Google Scholar]
- Grinnell AD. Dynamics of nerve–muscle interaction in developing and mature neuromuscular junctions. Physiol Rev. 1995;75:789–834. doi: 10.1152/physrev.1995.75.4.789. [DOI] [PubMed] [Google Scholar]
- Harlow ML, Ress D, Stoschek A, Marshall RM, McMahan UJ. The architecture of active zone material at the frog's neuromuscular junction. Nature. 2001;409:479–484. doi: 10.1038/35054000. [DOI] [PubMed] [Google Scholar]
- Heuser JE, Reese TS, Landis DM. Functional changes in frog neuromuscular junctions studied with freeze-fracture. J Neurocytol. 1974;3:109–131. doi: 10.1007/BF01111936. [DOI] [PubMed] [Google Scholar]
- Heuser JE, Reese TS, Dennis MJ, Jan Y, Jan L, Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979;81:275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The Release of Neural Transmitter Substances. Liverpool: Liverpool University Press; 1969. [Google Scholar]
- Katz B, Miledi R. Estimates of quantal content during ‘chemical potentiation’ of transmitter release. Proc R Soc Lond B Biol Sci. 1979;205:369–378. doi: 10.1098/rspb.1979.0070. [DOI] [PubMed] [Google Scholar]
- Kerr RA, Bartol TM, Kaminsky B, Dittrich M, Chang JC, Baden SB, Sejnowski TJ, Stiles JR. Fast Monte Carlo simulation methods for biological reaction-diffusion systems in solution and on surfaces. SIAM J Sci Comput. 2008;30:3126. doi: 10.1137/070692017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CP. A lectin, peanut agglutinin, as a probe for the extracellular matrix in living neuromuscular junctions. J Neurocytol. 1987;16:567–576. doi: 10.1007/BF01668509. [DOI] [PubMed] [Google Scholar]
- Luo F, Dittrich M, Stiles JR, Meriney SD. Single-pixel optical fluctuation analysis of calcium channel function in active zones of motor nerve terminals. J Neurosci. 2011;31:11268–11281. doi: 10.1523/JNEUROSCI.1394-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Dittrich M, Tarr T, Meriney SD, Stiles JR. Computational Study of Active Zone Structure and Function at the Mammalian Neuromuscular Junction. Washington, DC: Society for Neuroscience; 2010. Program No. 448.11. 2010 Neuroscience Meeting Planner. Online. http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=5918d739-ab0c-46f4-87ee-39b34e89f097&cKey=5a6369e6-7b2f-42d7-b06d-6a74332b2ab2&mKey=%7bE5D5C83F-CE2D-4D71-9DD6-FC7231E090FB%7d. [Google Scholar]
- Ma J, Kelly L, Price T, Ingram J, Meriney SD, Dittrich M, Stiles J. Computational Study of Persistent Calcium Binding Provides Insight into Short-Term Synaptic Facilitation. Washington, DC: Society for Neuroscience; 2011. Program No. 448.02. 2011 Neuroscience Meeting Planner. Online. http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=bace4c2a-3ebd-4b6c-b9f2-b94276ea96f8&cKey=2793328a-81cd-4500-bdfc-36130fbbdb4a&mKey=%7b8334BE29-8911-4991-8C31-32B32DD5E6C8%7d. [Google Scholar]
- Nagwaney S, Harlow ML, Jung JH, Szule JA, Ress D, Xu J, Marshall RM, McMahan UJ. Macromolecular connections of active zone material to docked synaptic vesicles and presynaptic membrane at neuromuscular junctions of mouse. J Comp Neurol. 2009;513:457–468. doi: 10.1002/cne.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimune H, Sanes JR, Carlson SS. A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature. 2004;432:580–587. doi: 10.1038/nature03112. [DOI] [PubMed] [Google Scholar]
- O’Connor VM, Shamotienko O, Grishin E, Betz H. On the structure of the ‘synaptosecretosome’. Evidence for a neurexin/synaptotagmin/syntaxin, Ca2+ channel complex. FEBS Lett. 1993;326:255–260. doi: 10.1016/0014-5793(93)81802-7. [DOI] [PubMed] [Google Scholar]
- Pawson PA, Grinnell AD, Wolowske B. Quantitative freeze-fracture analysis of the frog neuromuscular junction synapse–I. Naturally occurring variability in active zone structure. J Neurocytol. 1998;27:361–377. doi: 10.1023/a:1006942909544. [DOI] [PubMed] [Google Scholar]
- Pumplin DW, Reese TS, Llinás R. Are the presynaptic membrane particles the calcium channels. Proc Natl Acad Sci U S A. 1981;78:7210–7213. doi: 10.1073/pnas.78.11.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy LV, Koirala S, Sugiura Y, Herrera AA, Ko CP. Glial cells maintain synaptic structure and function and promote development of the neuromuscular junction in vivo. Neuron. 2003;40:563–580. doi: 10.1016/s0896-6273(03)00682-2. [DOI] [PubMed] [Google Scholar]
- Rogozhin AA, Pang KK, Bukharaeva E, Young C, Slater CR. Recovery of mouse neuromuscular junctions from single and repeated injections of botulinum neurotoxin A. J Physiol. 2008;586:3163–3182. doi: 10.1113/jphysiol.2008.153569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz R, Cano R, Casañas JJ, Gaffield MA, Betz WJ, Tabares L. Active zones and the readily releasable pool of synaptic vesicles at the neuromuscular junction of the mouse. J Neurosci. 2011;31:2000–2008. doi: 10.1523/JNEUROSCI.4663-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrezaei V, Cao A, Delaney KR. Ca2+ from one or two channels controls fusion of a single vesicle at the frog neuromuscular junction. J Neurosci. 2006;26:13240–13249. doi: 10.1523/JNEUROSCI.1418-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shon KJ, Olivera BM, Watkins M, Jacobsen RB, Gray WR, Floresca CZ, Cruz LJ, Hillyard DR, Brink A, Terlau H, Yoshikami D. μ-Conotoxin PIIIA, a new peptide for discriminating among tetrodotoxin-sensitive Na channel subtypes. J Neurosci. 1998;18:4473–4481. doi: 10.1523/JNEUROSCI.18-12-04473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabares L, Ruiz R, Linares-Clemente P, Gaffield MA, Alvarez de Toledo G, Fernandez-Chacón R, Betz WJ. Monitoring synaptic function at the neuromuscular junction of a mouse expressing synaptopHluorin. J Neurosci. 2007;27:5422–5430. doi: 10.1523/JNEUROSCI.0670-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr TB, Dittrich M, Meriney SD. Are unreliable release mechanisms conserved from NMJ to CNS. Trends Neurosci. 2013;36:14–22. doi: 10.1016/j.tins.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, Piedras-Rentería ES, Jun K, Shin HS, Uchitel OD, Tsien RW. Altered properties of quantal neurotransmitter release at endplates of mice lacking P/Q-type Ca2+ channels. Proc Natl Acad Sci U S A. 2003;100:3491–3496. doi: 10.1073/pnas.0437991100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachman ES, Poage RE, Stiles JR, Farkas DL, Meriney SD. Spatial distribution of calcium entry evoked by single action potentials within the presynaptic active zone. J Neurosci. 2004;24:2877–2885. doi: 10.1523/JNEUROSCI.1660-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Engisch KL, Li Y, Pinter MJ, Cope TC, Rich MM. Decreased synaptic activity shifts the calcium dependence of release at the mammalian neuromuscular junction in vivo. J Neurosci. 2004;24:10687–10692. doi: 10.1523/JNEUROSCI.2755-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pinter MJ, Rich MM. Ca2+ dependence of the binomial parameters p and n at the mouse neuromuscular junction. J Neurophysiol. 2010;103:659–666. doi: 10.1152/jn.00708.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Slater CR. Safety factor at the neuromuscular junction. Prog Neurobiol. 2001;64:393–429. doi: 10.1016/s0301-0082(00)00055-1. [DOI] [PubMed] [Google Scholar]
- Wyatt RM, Balice-Gordon RJ. Heterogeneity in synaptic vesicle release at neuromuscular synapses of mice expressing synaptopHluorin. J Neurosci. 2008;28:325–335. doi: 10.1523/JNEUROSCI.3544-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikami D, Bagabaldo Z, Olivera BM. The inhibitory effects of omega-conotoxins on Ca channels and synapses. Ann N Y Acad Sci. 1989;560:230–248. doi: 10.1111/j.1749-6632.1989.tb24100.x. [DOI] [PubMed] [Google Scholar]


