Abstract
Horizontal cells send inhibitory feedback to photoreceptors, helping form antagonistic receptive fields in the retina, but the neurotransmitter and the mechanisms underlying this signalling are not known. Since the proteins responsible for conventional Ca2+-dependent release of GABAergic synaptic vesicles are present in mammalian horizontal cells, we investigated this conventional mechanism as the means by which horizontal cells inhibit photoreceptors. Using Ca2+ imaging in rat retinal slices, we confirm that horizontal cell depolarization with kainate inhibits and horizontal cell hyperpolarization with NBQX disinhibits the Ca2+ signals produced by pH-sensitive activation of voltage-gated calcium channels (Ca channels) in photoreceptors. We show that while 100 μm Co2+ reduces photoreceptor Ca2+ signals, it disinhibits them at 10 μm, an effect reminiscent of earlier studies where low [Co2+] eliminated feedback. The low [Co2+] disinhibition is pH sensitive. We localized L-, N- and P/Q-type Ca channels in rat horizontal cells, and showed that both the N-type Ca channel blocker ω-conotoxin GVIA and the P/Q-type Ca channel blocker ω-agatoxin IVA increased Ca2+ signals in photoreceptors in a pH-sensitive manner. Pronounced actions of GABAergic agents on feedback signals to photoreceptors were observed, and are pH sensitive, but are inconsistent with direct inhibition by GABA of photoreceptor [Ca2+]. Patch-clamp studies revealed that GABA activates a conductance having high bicarbonate permeability in isolated horizontal cells, suggesting that the commonality of pH sensitivity throughout the results could arise from a GABA autofeedback action in horizontal cells. This could change cleft pH with concomitant inhibitory influences on photoreceptor Ca channels.
Key points
Inhibitory feedback from horizontal cells to photoreceptors regulates synaptic gain and contributes to centre–surround receptive field formation via mechanisms that are not fully understood.
We show that horizontal cell calcium channels and ionotropic GABA receptors mediate the inhibitory feedback, and that the results of their actions are blocked by strong pH buffering with Hepes.
GABA appears to act not upon the photoreceptor but instead upon the horizontal cell itself. The horizontal cell GABA receptors are permeable to chloride and bicarbonate, meaning their activation can produce changes in synaptic cleft pH.
These results suggest that activation of calcium channels in a depolarized horizontal cell releases GABA, which acts in an autaptic manner to increase bicarbonate permeability. The resulting influx of bicarbonate contributes to acidification of the synaptic cleft, inhibiting photoreceptor calcium channels, the hallmark of inhibitory feedback at this synapse.
Introduction
Depolarization of the horizontal cell membrane leads to inhibition of Ca2+ signals in rod and cone photoreceptors while hyperpolarization does the opposite, reflecting the voltage dependence of inhibitory signalling from horizontal cells to photoreceptors (Hirasawa & Kaneko, 2003; Vessey et al. 2005; Cadetti & Thoreson, 2006; Thoreson et al. 2008; Babai & Thoreson, 2009; Fahrenfort et al. 2009; reviewed in Thoreson & Mangel, 2012). This inhibitory feedback from horizontal cells to photoreceptors is known to underlie synaptic gain control and the formation of centre–surround receptive fields in the retina, the underlying mechanisms for which remain incompletely described (Thoreson & Mangel, 2012). Mammalian horizontal cells express an array of voltage-gated calcium channels (Ca channels), including L-, N- and P/Q-types (Ueda et al. 1992; Löhrke & Hofmann, 1994; Schubert et al. 2006; Witkovsky et al. 2006), as well as the proteins that typically mediate Ca2+-dependent transmitter release, including those involved in vesicle fusion and exocytosis (synaptosome-associated protein SNAP-25, syntaxin-1a and -4, and vesicle-associated membrane protein VAMP-1) and transmitter synthesis and packaging (glutamic acid decarboxylase (GAD) and vesicular GABA transporter (VGAT)), with the latter pointing toward GABA as a transmitter (Hirano et al. 2005, 2007; Guo et al. 2010; Lee & Brecha, 2010). While most electrophysiological evidence refutes a role for GABA at these synapses (Thoreson & Burkhardt, 1990; Verweij et al. 1996; Thoreson et al. 2008; reviewed in Thoreson & Mangel, 2012), some from lower vertebrates lends support for such a role (Yang & Wu, 1989, 1993; Tatsukawa et al. 2005; Endeman et al. 2012). With recent reports supporting a proton- and/or hemichannel-mediated mechanism as the means by which horizontal cells communicate with photoreceptors (Fahrenfort et al. 2009), we sought to determine whether horizontal cell feedback to photoreceptors was mediated via Ca channel-regulated release of GABA and how this action could be related to pH-sensitive and ephaptic effects at this synapse.
The robust modulation of photoreceptor Ca2+ signalling caused by horizontal cell membrane potential manipulation (Hirasawa & Kaneko, 2003; Vessey et al. 2005; Cadetti & Thoreson, 2006; Thoreson et al. 2008; Babai & Thoreson, 2009) was used to test the role of voltage-gated Ca channels and GABAergic agents in feedback. Photoreceptors only express L-type Ca channels (Wilkinson & Barnes, 1996; Taylor & Morgans, 1998; Kourennyi & Barnes, 2000; Morgans et al. 2005; Witkovsky et al. 2006) and there is broad agreement from studies in many species that the feedback from horizontal cells is expressed as a modulation of those L-type Ca channels (Verweij et al. 1996; Kamermans & Spekreijse, 1999). The involvement of amacrine cells in releasing dopamine in a Ca2+-dependent manner onto cells of the outer retina was investigated, as well as other retinal cell-derived neurotransmitters accounting for the inhibitory signalling.
The findings presented here support a role for voltage-gated Ca channels and a GABAergic mechanism in the feedback, but do not support GABA as the inhibitory transmitter acting at photoreceptors. Instead, this work reveals that all of the signalling mechanisms leading to photoreceptor [Ca2+] modulation that we investigated are neutralized by Hepes and that the pronounced actions of GABA are upon the horizontal cell itself and lead to pH modulation. These results offer a new, potentially unifying mechanism for inhibitory action at this synapse.
Methods
Electrophysiological, imaging and immunohistochemical experiments were performed in accordance with the guidelines for the welfare of experimental animals issued by the U.S. Public Health Service Policy on Human Care and Use of Laboratory Animals (2002) and the University of California-Los Angeles Animal Research Committee.
Immunohistochemical labelling
Adult Sprague-Dawley rats (Charles River Lab, Wilmington, MA, USA) of either sex were used for these studies. Following deep anaesthesia with isofluorane (IsoFlo, Abbott Laboratories, Abbott Park, IL, USA), the animal was decapitated, the eyes enucleated, and the anterior chamber and lens were removed. The eyecups were immersion-fixed in 4% (w/v) paraformaldehyde (PFA) or 2% PLP (2% PFA, 75 mm l-lysine, and 10 mm sodium periodate) in 0.1 m phosphate buffer (PB), pH 7.4, for 15–30 min, cryoprotected in 30% sucrose, and sectioned vertically at 12–14 μm on a cryostat onto gelatin-coated slides. Immunostaining was performed using the indirect fluorescence method (Hirano et al. 2005). In brief, retinal sections were incubated in a blocking solution containing 10% normal goat serum (NGS), 1% bovine serum albumin (BSA), 0.5% Triton X-100, 0.05% sodium azide (NaN3) in 0.1 m PB, pH 7.4 for 1 h. The primary antibody was diluted in 3% NGS, 1% BSA, 0.5% Triton X-100, 0.05% NaN3, in 0.1 m PB, for 12–16 h at room temperature. The specific immunolabelling was visualized using Alexa Fluor 488-, 568- or 594-conjugated anti-rabbit or mouse secondary antibodies (Invitrogen, Grand Island, NY, USA) at 1:500 dilutions for 40–60 min at room temperature. The immunostaining was examined on a Zeiss Laser Scanning Microscope 510 Meta (Carl Zeiss, Inc., Thornwood, NY, USA) with Zeiss C-Apochromat 40× (1.2 NA) or C-Apochromat 63× (1.2 NA) corrected water objectives. Confocal images were analysed using the Zeiss LSM 510 proprietary software (version 3.2). Intensity levels and brightness/contrast were adjusted in Adobe Photoshop CS5 v.12.1 (Adobe Systems, San Jose, CA, USA). Antibodies used included rabbit polyclonal antibodies against voltage-gated calcium channel α-subunits (Cav2.2, Alomone ACC-002, lot AN-21, 1:1000–1500; Cav1.2, Alomone ACC-003, 1:250–1:500, Jerusalem, Israel; Cav2.1, Synaptic Systems 152 103, Göttingen, Germany, 1:7500–1:15,000, Sigma C1353, St Louis, MO, USA, 1:200). A mouse monoclonal antibody against calbindin (Sigma C9848, 1:3000) was used to label horizontal cells (Hirano et al. 2005, 2011).
Ca2+ imaging in retinal slices
Adult Sprague-Dawley rats (Charles River Lab, Wilmington, MA, USA) were deeply anaesthetized with isofluorane (IsoFlo, Abbott Laboratories, Abbott Park, IL, USA) and decapitated. The eyes were enucleated, and the anterior portion of an eye including the lens removed. The resulting eyecup was trimmed and a section of retina with scleral backing was placed vitreal side down on a piece of filter (Millipore; 2 × 5 mm, type GS, 0.2 μm pores). After the retina had adhered to the filter, the sclera was peeled away and the retina and filter paper were cut into 150–200 μm slices using a tissue chopper (Stoelting Tissue Slicer, Stoelting Co., Wood Dale, IL, USA) mounted with a razor blade (No. 121–6; Ted Pella Inc., Redding, CA, USA) and the slices rotated 90 deg to permit viewing of the retinal layers. Intracellular Ca2+ changes were assessed with the Ca2+-sensitive dye fluo-4, which was prepared as a 1 mm stock solution in DMSO and diluted in Hibernate® A (Brain Bits, Springfield, IL, USA) to a final concentration of 10 μm. Retinal slices were incubated in fluo-4 for 1 h at room temperature in darkness. Images were collected with a Zeiss LSM 5 Pascal microscope mounted on an upright microscope (Zeiss Axioplan 2) equipped with a Axoplan 40× (NA 0.8) water-immersion objective. The 488 nm laser line of an argon laser provided excitation and the emission was collected through a 505 nm LP filter on a photomultiplier tube. Slices were superfused via a gravity driven fast flow system (ALA, Farmingdale, NY, USA) with a solution containing (in mm) 125 NaCl, 3 KCl, 2 CaCl2, 1.25 NaH2PO4, 1 MgCl2, 25 NaHCO3 and 10 glucose, bubbled continuously with 95% O2–5% CO2. To activate voltage-dependent Ca2+ channels in photoreceptors and test the effects of drugs on the activity of these channels, retinal cells were depolarized by increasing [K+]o from 3 to 30 mm for 30 s twice. NaCl was reduced by 27 mm to maintain osmolarity. We found a sigmoid-shaped dose–response curve for [K+]out versus[Ca2+]in, with an EC50 of 17.5 mm K+. To prevent movement of the slices, we optimized the flow rate of solutions and tested the rate of exchange, finding that the bath changed exponentially with a time constant of ∼15 s. Neurons and glia in the retina express a variety of ion exchangers whose activity could be altered by these changes in the K+ gradient, including KCC2 (potassium–chloride cotransporter 2) and NKCC (sodium–potassium–chloride cotransporter) which are present in photoreceptors, bipolar cells and horizontal cells (Vardi et al. 2000; Vu et al. 2000; Zhang et al. 2006; Li & Shen, 2007; Li et al. 2008; Shen et al. 2008, 2013). In consideration of potential confounding effects of elevated K+ upon ion exchangers and pumps, we limited stimulation with K+ to 30 mm and the time of exposure to 30 s. Images were acquired at 5 s intervals. For analysis, regions of interest (ROIs) were drawn over rod photoreceptor somata near the outer plexiform layer (OPL) and changes in fluorescence recorded. In the figures, fluorescence traces are shown normalized to the first of the paired responses to elevated K+ application. The change produced in the presence of the test drug during the second of the paired high K+ pulses was compared with the change produced during the first high K+ pulse. Many ROIs corresponding to individual rods were averaged in each slice and all experiments were performed on three to nine preparations, each considered an independent observation for statistical testing using one-way ANOVA, with P < 0.05 considered significant. Data in bar graphs show mean values from multiple retinal slices and the value n equals the number of slices, from which each provided a value averaged from 5 to 20 individual rods.
Patch clamp recording from isolated horizontal cells and rods in slices
Slices were prepared as described above for Ca2+ imaging experiments, but isolated horizontal cells were prepared following incubation of retinas in Hanks’ Balanced Salt Solution (HBSS; Hyclone, Logan, UT, USA) containing 18 U ml−1 papain and 100 U ml−1 DNase I (Worthington Biochemical, Freehold, NJ, USA) at 37°C for 40 min. Isolated cells were obtained by gentle trituration after digestion. The cells were kept in Dulbecco's Modified Eagle Medium (DMEM; Life Technologies, Grand Island, NY, USA) with 10% fetal bovine serum (Life Technologies) in a CO2 incubator at 37°C. To record membrane potential and current, photoreceptors and isolated horizontal cells were bathed in the same extracellular solution as the slices in the Ca2+ imaging experiments. For horizontal cell recordings, the patch pipette contained (in mm): 140 KCl, 0.1 CaCl2, 1 EGTA, 10 Hepes, 3 Mg-ATP, 0.2 Li-GTP, and 8 phosphocreatine, at pH 7.2. For recording from rods, the pipette contained (in mm): 140 KCl, 0.1 CaCl2, 1 EGTA, 10 Hepes, and 240 μg ml−1 amphotericin B, at pH 7.2. Room-temperature (21–24°C) solutions were superfused via a gravity driven fast flow system. Patch electrodes with 5–15 MΩ tip resistances were pulled from borosilicate glass capillary tubes (A-M Systems, Sequim, WA, USA) using a micropipette puller (Sutter Instrument, Novato, CA, USA). The bath reference electrode consisted of an AgCl wire in a side chamber. Cell voltage was clamped with an Axopatch-200B amplifier (Axon Instruments, Foster City, CA, USA) using whole cell capacitance and series resistance compensation. The current signal was filtered at 2 kHz and digitized at 10 kHz with an Axon Digidata 1320A for storage on the hard disk of a computer running pCLAMP 8.2 acquisition software.
To determine the bicarbonate:chloride permeability ratio of the GABAA receptor in horizontal cells, we applied muscimol to patch clamped isolated cells in a bathing solution containing (in mm): 138 NaCl, 3 KCl, 2 CaCl2, 1.25 NaH2PO4, 1 MgCl2, 10 glucose and 10 Hepes, at pH 7.4. We switched the pipette solution between a high concentration Cl− solution composed of (in mm): 140 CsCl, 1 CaCl2, 11 EGTA, 2 MgCl2, and 10 Hepes, at pH 7.2, and a high concentration bicarbonate solution composed of (in mm): 140 CsHCO3, 1 CaCl2, 11 EGTA, 2 MgCl2, and 10 Hepes, at pH 7.2. We obtained reversal potential measurements for the currents elicited by muscimol in both conditions using ramp voltage clamp protocols, and calculated the difference between the averaged reversal potentials after correcting for liquid junction potentials estimated with JPCalc (Axon Instruments, Foster City, CA, USA). We then solved the Goldman–Hodgkin–Katz voltage equation:
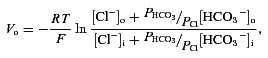 |
for PHCO3/PCl, where Vo was the measured change in reversal potential, R and F have the usual values, and T is 298 K.
Drugs and chemicals
All chemicals and reagents, unless otherwise noted, were obtained from Sigma-Aldrich (St Louis, MO, USA). NBQX, ω-conotoxin GVIA (CTX), muscimol, spiperone, SKF38393, CYN-154806 and AM-251 were obtained from Tocris (Ellisville, MO, USA). ω-Conotoxin MVIIC, ω-agatoxin IVA (ATX), and l-NMMA were obtained from Ascent Scientific (Princeton, NJ, USA). Fluo-4 AM was purchased from Invitrogen (Grand Island, NY, USA). Nifedipine was prepared as stock solutions in DMSO, frozen at −20°C and thawed immediately before experiments.
Data analysis
All data are reported as the mean ± standard error of the mean (SEM). Graphing and statistical analyses were performed using Matlab 7.6 (MathWorks, Inc., Natick, MA, USA).
Results
Recently, Babai & Thoreson (2009) showed that direct depolarization of horizontal cells with injected current in mouse retinal slices inhibited photoreceptor L-type Ca channel gating and reduced photoreceptor Ca2+ signalling. In that study, and in others performed in other non-mammalian vertebrate species, this inhibition of photoreceptor L-type Ca channels was reproduced with drugs that depolarize the horizontal cells (e.g. kainate), while disinhibition of photoreceptor Ca channels was seen with drugs that hyperpolarize the horizontal cell membrane (e.g. NBQX or CNQX), or direct hyperpolarization of the horizontal cell with injected current and even with light (Hirasawa & Kaneko, 2003; Vessey et al. 2005; Cadetti & Thoreson, 2006; Thoreson et al. 2008; Babai & Thoreson, 2009; Fahrenfort et al. 2009). Thus the results presented in this work are considered initially within the framework of a hypothesis for feedback in which horizontal cells signal inhibition to photoreceptors. Tests of other inhibitory modulation models are considered as well, and these should be borne in mind throughout the presentation of results.
Photoreceptor [Ca2+]i is sensitive to horizontal cell membrane potential in a pH-sensitive manner
Figure 1A shows fluo-4 labelling of photoreceptor cell bodies on the scleral side of the outer plexiform layer which allowed assessment of increased intracellular photoreceptor [Ca2+] levels during depolarization with 30 mm K+ pulses. Patch clamp recordings in rat retinal slice showed that superfusion with the 30 mm K+ solution depolarized rods from −53.2 ± 6.7 mV to −40.9 ± 4.9 mV (n= 9; not shown), values close to the values of −54.4 mV ± 2.8 mV (control) and −35.7 mV ± 1.4 mV (30 mm K+ stimulated) previously reported in mouse rods by Babai & Thoreson (2009). In accord with reports where imaging of Ca2+ in retinal slices was undertaken in several different vertebrate species, modulation of the horizontal cell membrane potential with direct current injection or glutamatergic agents altered the feedback inhibition of photoreceptor [Ca2+]i (Hirasawa & Kaneko, 2003; Vessey et al. 2005; Cadetti & Thoreson, 2006; Thoreson et al. 2008; Babai & Thoreson, 2009; Fahrenfort et al. 2009). When we applied the ionotropic glutamate receptor (iGluR) agonist kainate at 50 μm during the second K+ pulse, photoreceptor [Ca2+]i was reduced relative to control (Fig. 1B). Application of the iGluR antagonist NBQX (50 μm) during the second pulse enhanced photoreceptor [Ca2+]i (Fig. 1C). In separate patch clamp recordings of isolated rat horizontal cells, we found that, under current clamp, kainate depolarized the cells from −35.4 ± 7.11 mV to −13.1 ± 2.9 mV (n= 5; not shown). This is a somewhat smaller value than that obtained in salamander retinal slices, where it depolarizes horizontal cells by 31 ± 5 mV (Babai & Thoreson, 2009), and where NBQX hyperpolarizes horizontal cells by 12 ± 4 mV and where both glutamatergic agents have no direct effect on the photoreceptors themselves. In our sample of cells, control paired K+ pulses (bar labelled 30K in Fig. 1D) produce a small pair-wise decrement of 2 ± 4%, while kainate inhibits by 36 ± 6%, and NBQX disinhibits photoreceptor [Ca2+]i by 22 ± 5%. These results are the same as those obtained using similar protocols in zebra fish, mouse, salamander and goldfish (Vessey et al. 2005; Cadetti & Thoreson, 2006; Thoreson et al. 2008; Babai & Thoreson, 2009; Fahrenfort et al. 2009). To extend further the comparison between these different species tested, we showed that Hepes (10 mm) also blocked completely the kainate- and NBQX-induced changes in rat photoreceptor Ca2+ signalling (Fig. 1D).
Figure 1. Depolarization horizontal cells inhibits photoreceptor [Ca2+]i in a pH-sensitive manner in rat retina.
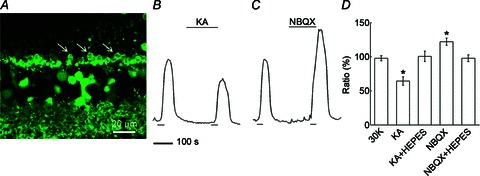
A, fluo-4 labelling of rod cell bodies (3 examples marked with arrows) in the outer nuclear layer of a rat retinal slice. Image was sampled during a control 30 s pulse of elevated K+ solution, which depolarizes photoreceptors, activates their L-type Ca channels and increases [Ca2+]i. B, depolarization of the horizontal cells via application of the iGluR agonist kainate (KA; 50 μm) during the second K+ pulse reduced photoreceptor [Ca2+]i. The fluorescence traces are shown normalized to the first elevated [K+] application in this and all subsequent figures. C, hyperpolarization of the horizontal cells with application of the iGluR antagonist nitro-benzo-quinoxaline (NBQX; 50 μm) during the second K+ pulse enhanced photoreceptor [Ca2+]i. D, summary of feedback modulation of photoreceptor [Ca2+]i. Control paired K+ pulses (bar labelled 30 K) show an insignificant reduction of 2 ± 4% (n= 9), while kainate inhibits by 36 ± 6% (n= 5, P < 0.05), and NBQX disinhibits photoreceptor [Ca2+]i by 22 ± 5% (n= 5, P < 0.05). In the presence of 10 mm Hepes, kainate failed to inhibit (0 ± 8% (n= 6, P > 0.05) and NBQX failed to increase photoreceptor [Ca2+]i (2 ± 5%; n= 3, P > 0.05). Data in this bar graph (and those in subsequent figures) show normalized mean values from multiple retinal slices (1 data point per slice), each averaged from 5–20 individual rods in preparations from 3–5 different eyes.
Ca channel block with cobalt shows concentration-dependent, pH-sensitive effects on photoreceptor [Ca2+]i
At relatively high concentrations, Co2+ blocks Ca2+ spikes in photoreceptors (Fain et al. 1977), Ca channels in photoreceptors (Corey et al. 1984), and transmission from photoreceptors to second order neurons (Thoreson & Burkhardt, 1990). It has also been noted that at lower concentrations, depending on the preparation used, Co2+ selectively eliminates feedback to cones and the surround antagonistic receptive field of ganglion cells without impairing vertical signalling (Thoreson & Burkhardt, 1990; Vigh & Witkovsky, 1999; Fahrenfort et al. 2004). Figure 2A shows that while application of 100 μm Co2+ strongly suppressed photoreceptor [Ca2+]i, when 10 μm Co2+ was added, photoreceptor [Ca2+]i was greatly increased (Fig. 2B). This implies that low Co2+ reduces inhibition and essentially reproduces the concentration-dependent actions of Co2+ on feedback reported previously. Similar to the Hepes-sensitive signalling shown in Fig. 1, the low-Co2+ enhancement of photoreceptor [Ca2+]i was eliminated in 20 mm Hepes (Fig. 2C). A summary of these effects is shown in Fig. 2E. We also tested the sensitivity of isolated horizontal cell Ca channels to 10 μm Co2+ under voltage clamp and found this to be low (inhibition of 14.4 ± 5%n= 6; data not shown).
Figure 2. Cobalt has concentration-dependent and pH-sensitive effects on photoreceptor [Ca2+]i but inhibition of L-type Ca channels only decreases photoreceptor [Ca2+]i.
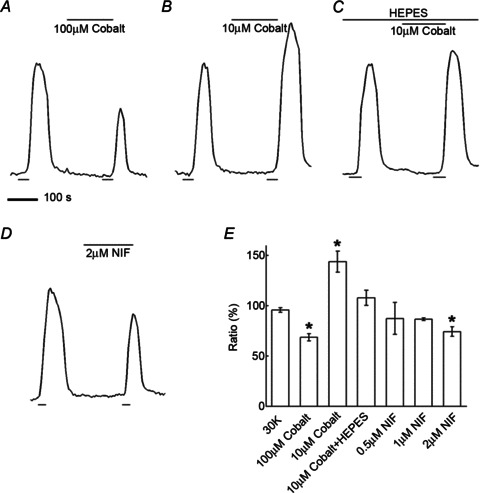
A, application of Co2+ (100 μm) during the second K+ pulse strongly suppressed photoreceptor [Ca2+]i. B, 10 μm Co2+ applied during the second K+ pulse enhanced photoreceptor [Ca2+]i. C, in the presence of 20 mm Hepes, 10 μm Co2+ increased photoreceptor [Ca2+]i significantly less than Co2+ alone. D, the L-type Ca channel blocker nifedipine (NIF; 2 μm) added during the second K+ pulse reduced photoreceptor [Ca2+]i. E, summary of concentration and pH-sensitive effects of Co2+ and nifedipine showing 100 μm Co2+ decreasing the signal by 32 ± 4% (n= 3, P < 0.05), 10 μm Co2+ increasing photoreceptor [Ca2+]i by 44 ± 10% (n= 4, P < 0.05), 20 mm Hepes reducing the low Co2+-enhanced photoreceptor [Ca2+]i to 8 ± 8% (n= 6, P < 0.05 compared to Co2+ alone), and applications of 0.5, 1, and 2 μm nifedipine decreasing photoreceptor [Ca2+]i by 13 ± 15% (n= 3, P > 0.05), 13 ± 1% (n= 3, P > 0.05), and 26 ± 5% (n= 3, P < 0.05), respectively, for the three concentrations.
Inhibition of L-type Ca channels decreases photoreceptor [Ca2+]i
Since photoreceptors express L-type Ca channels (Wilkinson & Barnes, 1996; Taylor & Morgans, 1998; Kourennyi & Barnes, 2000), it was expected that the L-type Ca channel blocker nifedipine would block the photoreceptor Ca channels, reducing photoreceptor [Ca2+]i. Indeed, application of nifedipine (2 μm) during the second of paired high K pulses, shown in Fig. 2D, caused a ∼26% reduction of photoreceptor [Ca2+]i. However, since horizontal cells also express L-type Ca channels (Sullivan & Lasater, 1992; Ueda et al. 1992; Schubert et al. 2006), we tested lower concentrations of nifedipine seeking to find a point where, as seen with low Co2+, enhanced Ca2+ signalling might occur. Summarized data in Fig. 2E shows that this approach, using 0.5 and 1 μm nifedipine, was not successful, with reductions of photoreceptor [Ca2+]i by 13 ± 15% and 13 ± 1% persisting, respectively.
Immunohistochemical localization of N-, L-, and P/Q-type Ca channels in horizontal cells
Schubert et al. (2006) reported three types of voltage-gated Ca channels present in mouse horizontal cells based on pharmacological separation with ω-conotoxin GVIA, nifedipine/verapamil, and ω-agatoxin IVA, indicating the presence of N-type, L-type, and P/Q-type Ca channels, respectively. We performed immunohistochemical analysis to determine the localization of N-type, L-type and P/Q-type Ca channel primary subunits in rat horizontal cells. Figure 3 shows fluorescence micrographs of immunoreactivity for Ca channel α1 subunits Cav2.2 (N-type; α1B), Cav1.2 (L-type; α1C), and Cav2.1 (P/Q-type; α1A), and calbindin, a cell marker for horizontal cells in the outer retina. The merged images show co-localization of the Ca channel subunit immunolabelling in horizontal cell processes and tips. The immunolabelling images for N- and L-type Ca channels stand out as they indicate distinctive localization to the invaginating tips of horizontal cells (arrows in A–C, D–F), while the immunolabelling image for P/Q-type Ca channels indicates smaller puncta more diffusely present on horizontal cell processes and tips (G–I).
Figure 3. Immunohistochemical localization of N-, L-, and P/Q-type Ca channels in horizontal cells.
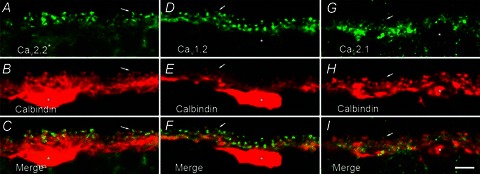
A, fluorescence micrograph of labelling by antibodies to N-type Ca channel α1B subunits (Cav2.2, in green). B, fluorescence micrograph of labelling of calbindin, a cell marker for horizontal cells in the outer plexiform layer, on the same section (in red). C, the merged image of these two images. D. labelling of L-type Ca channel α1C subunits (Cav1.2, in green). E, labelling of calbindin (in red). F, the merge of these two images. G, labelling of P/Q-type Ca channel α1A subunits (Cav2.1, in green). H, the same section labelled for calbindin. I, the merge of these two images. The merged images indicate co-localization of calbindin and Ca channel immunolabelling. Each image is a projection of three optical sections. Asterisks denote horizontal cell bodies, and the arrows point to the invaginating tips of horizontal cell processes. Scale bar, 5 μm.
Photoreceptor [Ca2+]i increases in a pH-sensitive manner when horizontal cell N- and P/Q-type Ca channels are blocked
Just as hyperpolarization of the horizontal cell membrane with NBQX reduced inhibition of photoreceptors and produced increased Ca2+ signalling during stimulation with 30 mm K+, application of the N-type Ca channel blocker ω-conotoxin GVIA (CTX; 1 μm) during the second pulse enhanced photoreceptor [Ca2+]i (Fig. 4A). Likewise, when the P/Q-type Ca channel antagonist ω-agatoxin IVA (ATX; 400 nm) was applied during the second pulse, photoreceptor [Ca2+]i was enhanced (Fig. 4C) and application of ω-conotoxin GVIA (1 μm) together with ω-agatoxin IVA (400 nm) during the second pulse enhanced photoreceptor [Ca2+]i by approximately the same amount (Fig. 4E). In the presence of 20 mm Hepes (Fig. 4B), application of ω-conotoxin GVIA enhanced photoreceptor [Ca2+]i, but much more modestly. Similarly, Fig. 4D shows that 20 mm Hepes mostly eliminated the enhancement seen with ω-agatoxin IVA alone. The enhancement was significantly less than that without Hepes. Figure 4F gives a summary of these N- and P/Q-type channel blocking effects on feedback, as well as the similar effects of the non-selective N- and P/Q-type channel blocker ω-conotoxin MVIIC (with details in the figure legend).
Figure 4. Block of N- and P/Q-type Ca channels increases photoreceptor [Ca2+]i.
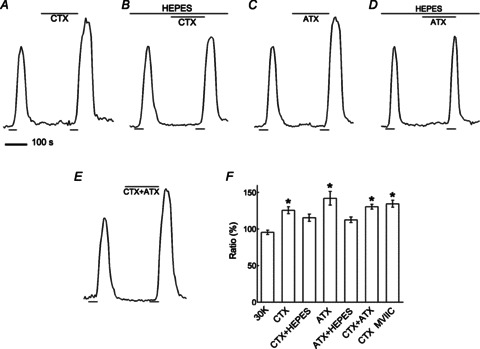
A, application of the N-type Ca channel blocker ω-conotoxin GVIA (CTX; 1 μm) during the second K+ pulse enhanced photoreceptor [Ca2+]i. B, when Hepes (10 mm) was added, ω-conotoxin GVIA enhanced photoreceptor [Ca2+]i less. C, application of the P/Q-type Ca channel blocker ω-agatoxin IVA (ATX; 400 nm) during the second K+ pulse enhanced photoreceptor [Ca2+]i. D, in the presence of Hepes, the enhancement of photoreceptor [Ca2+]i by ω-agatoxin IVA was reduced. E, application of ω-conotoxin GVIA and ω-agatoxin IVA together (CTX + ATX; 1 μm and 400 nm, respectively) during the second K+ pulse enhanced photoreceptor [Ca2+]i. F, summary of N- and P/Q-type channel blocking effects on feedback showing ω-conotoxin GVIA increasing photoreceptor [Ca2+]i by 26 ± 5% (n= 9, P < 0.05), an increase of 12 ± 3% (n= 6, P < 0.05 compared to CTX alone) induced by ω-conotoxin GVIA in the presence of Hepes (CTX + Hepes), ω-agatoxin IVA increasing the signal by 42 ± 9% (n= 5, P < 0.05), an increase of 13 ± 4% (n= 6, P < 0.05 compared to ATX alone) induced by ω-agatoxin IVA in the presence of Hepes (ATX + Hepes), ω-conotoxin GVIA together with ω-agatoxin IVA increasing the signal by 31 ± 3% (n= 4, P < 0.05) and ω-conotoxin MVIIC (non-selective N- and P/Q-type channel blocker) increasing the signal by 34 ± 5% (n= 4, P < 0.05).
To see if the actions of these Ca channel blockers was mediated by hyperpolarization of the horizontal cell, as the actions of NBQX appear to be in Fig. 1C, we current clamped isolated horizontal cells and found that ω-conotoxin GVIA and ω-agatoxin IVA altered the membrane potential only very modestly, depolarizing the cells from −38.3 ± 12.2 mV to −37.9 ± 11.4 mV (n= 7), and from −41.9 ± 9.9 mV to −40.8 ± 9.2 mV (n= 10), respectively (data not shown). We also tested the sensitivity of isolated horizontal cell Ca channels to ω-agatoxin IVA under voltage clamp and found a mean inhibition of 18% (n= 5; not shown).
The actions of N-and P/Q-type Ca channel blockers cannot be attributed to direct effects upon photoreceptors since there appear to be only L-type Ca channels in these cells (Taylor & Morgans, 1998; Morgans et al. 2005; Witkovsky et al. 2006). If what we recorded were non-selective actions occurring at the L-type Ca channels of photoreceptors, they would be classified as enhancement, not block, because the photoreceptor Ca2+ signals increased. We note that no change of resting [Ca2+]i levels in photoreceptors during application of Ca channel blockers was seen and that effects were observed only during high K+ depolarization. Likewise, no change of resting [Ca2+]i was seen during superfusion with kainate or NBQX. This implies that in photoreceptors, under these light-adapted conditions in slices, Ca2+ signals are only stimulated appreciably during the ∼12 mV depolarization induced by 30 mm K+.
Actions of GABA are not mediated directly at the photoreceptor membrane and are pH sensitive
Since all of the components for the Ca2+-dependent release of an inhibitory neurotransmitter are present in horizontal cells (Lee & Brecha, 2010; Guo et al. 2010; Hirano et al. 2005, 2007, 2011; Sherry et al. 2006), we investigated whether GABA might fulfil a classic role at this site. We first tested the effects of the GABAR antagonist picrotoxin on the kainate-induced inhibition of photoreceptors. Fig. 5A and B shows that picrotoxin blocks the inhibition of photoreceptor [Ca2+]i caused by kainate, an action seemingly consistent with a GABAergic inhibition at this synapse. However, the picrotoxin sensitivity seen here implies a GABA-mediated, ionotropic mechanism in photoreceptors, presumably a Cl− conductance change, which is inconsistent with the Ca channel activation curve shifts shown to underlie feedback (Verweij et al. 1996; Hirasawa & Kaneko, 2003; Babai & Thoreson, 2009).
Figure 5. GABAergic agents that alter photoreceptor [Ca2+]i are pH sensitive and act on horizontal cells.
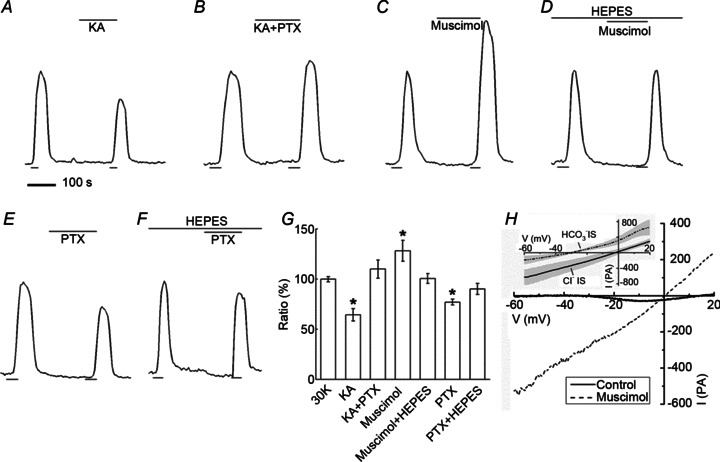
Ca2+ signal changes in rods during application of kainate (KA), picrotoxin (PTX), and muscimol. A, application of kainate (50 μm) caused a decrease of the response. B, the GABA receptor antagonist PTX (200 μm) blocked the kainite-induced decrease. C, application of GABAR agonist muscimol (400 μm) caused an increase of the Ca2+ signal in photoreceptors. D, this response was blocked by 10 mm Hepes. E, application of PTX (200 μm), now by itself, caused a decrease of the Ca2+ response. F, this decrease was blocked by Hepes. G, summary of GABAergic effects on photoreceptor [Ca2+]i: KA control reduced [Ca2+]i by 36 ± 6% (n= 5, P < 0.05) while KA+PTX together increased it by 10 ± 9% (n= 7, P > 0.05). Muscimol alone increased [Ca2+]i by 28 ± 10% (n= 4, P < 0.05) and 10 mm Hepes reduced this increase to 1 ± 5% (n= 5, P > 0.05). PTX alone decreased the photoreceptor [Ca2+]i. by 23 ± 3% (n= 8, P < 0.05) while in 10 mm Hepes the reduction was only 10 ± 6% (n= 8, P > 0.05). H, whole cell patch clamp of a horizontal cell responding to muscimol (100 μm), showing an increase in current reversing near 0 mV. Inset shows average of difference currents from 6 horizontal cells recorded with Cl− intracellular solution-filled patch pipettes and 6 cells measured with HCO3− intracellular solution. Shaded regions indicate standard deviations.
We next tested the actions of the GABA receptor agonist muscimol on the photoreceptor Ca2+ signal. Muscimol caused strong enhancement of the Ca2+ signal (Fig. 5C), not an inhibition. And picrotoxin, applied now in the absence of kainate, inhibited photoreceptor Ca2+ signals (Fig. 5E), the opposite action expected for a GABA-mediated inhibition. These actions, summarized in Fig. 5G, are inconsistent with a direct, ionotropic inhibition of photoreceptors by GABA. Furthermore, the effects of muscimol and picrotoxin on photoreceptor Ca2+ responses were blocked in the presence of 10 Hepes, suggesting a non-conventional action of GABA.
It is improbable that picrotoxin and muscimol are acting at the photoreceptor membrane. To explain these results, we considered an alternative site of action for GABAergic drugs, the horizontal cell membrane itself, since GABA has been shown to activate GABARs in these cells (Gilbertson et al. 1991; Kamermans & Werblin, 1992; Yang et al. 1999; Feigenspan & Weiler, 2004). In Fig. 5G we show that in rat retina the GABAA receptor agonist muscimol activates a large conductance that reverses near 0 mV in conditions of symmetrical [Cl−]. Due to the pH sensitivity of feedback inhibition, we sought to determine the ionic permeability of this conductance by replacing intracellular Cl− with HCO3−. Such replacement caused the reversal potential of the muscimol-activated conductance to become more negative by 29.4 mV, giving a permeability ratio for HCO3− to Cl−, calculated from the Goldman–Hodgkin–Katz (GHK) equation, of 0.29, a value somewhat larger than previous estimates in other GABARs (Bormann et al. 1987). This indicates that bicarbonate ions are highly permeant in horizontal cell GABAR channels, raising the possibility that activation of these channels could modulate cleft pH. Inward flux of HCO3−, as would occur were the membrane potential positive to the equilibrium potential for HCO3− (Ebicarb) would tend to contribute to acidification of the extracellular space, while outward HCO3− flux, should the membrane potential be negative to Ebicarb, would tend to alkalinize the extracellular space.
Actions by other retinal neuromodulatory systems on photoreceptor [Ca2+]i
We considered other synaptic pathways by which N- or P/Q-type Ca channel block might affect photoreceptor [Ca2+]i, including the Ca2+-dependent release of the inhibitory transmitter dopamine from interplexiform amacrine cells onto photoreceptors. Amacrine cell-released dopamine has broad actions throughout the retina (Witkovsky, 2004), one of which is modulation of L-type Ca channels via dopamine D2 receptors (D2Rs) in rods and cones (Stella & Thoreson, 2000). Basal release of dopamine, which may occur in the slice experiments, might be further increased by the high K stimulus used in the present study, modulating the photoreceptor Ca2+ signal via this dopaminergic mechanism. Blocking N- or P/Q-type Ca channels at amacrine cell synaptic endings in the OPL could potentially modulate Ca2+ signals in photoreceptors. Thus as an alternative mechanism, not even involving horizontal cells, high K+-evoked dopamine release could modulate Ca channels in photoreceptors directly via D2Rs on photoreceptor terminals, an effect that Ca channel block in amacrine cells would reduce. Figure 6A shows that application of the D2R antagonist spiperone had no modulatory effect on photoreceptor Ca2+ signals, suggesting that the observed modulation of [Ca2+]i did not involve dopamine receptors on photoreceptors themselves.
Figure 6. Inhibition of retinal neurotransmitter systems does not modulate photoreceptor [Ca2+]i.
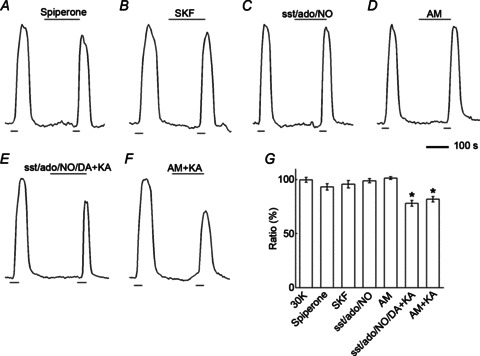
A, application of the D2R antagonist spiperone (20 μm) did not change photoreceptor [Ca2+]i. B, application of the D1R agonist SKF38393 (SKF; 20 μm) did not change photoreceptor [Ca2+]i. C, application of a cocktail, labelled ‘sst/ado/NO’, containing antagonists of somatostatin sst2A receptors (CYN-154806; 1 μm), adenosine A2 receptors (DMPX; 50 μm) and nitric oxide production (l-NMMA; 100 μm) had no effect on photoreceptor [Ca2+]i. D, application of the cannabinoid CB1 receptor antagonist AM-251 (AM; 10 μm) did not change photoreceptor [Ca2+]i. E, application of kainate (KA) in the presence of a new cocktail labelled ‘sst/ado/NO/DA’, containing the same antagonists as used in C but now with the dopamine D2 antagonist, spiperone (10 μm) added, reduced photoreceptor [Ca2+]i. F, when kainate was applied in the presence of AM-251, photoreceptor [Ca2+]i was reduced. G, summary of antagonist effects on Ca2+ signals in photoreceptors: Spiperone decreased the signal by 7 ± 3% (n= 6, P > 0.05); SKF decreased photoreceptor [Ca2+]i by 4 ± 3% (n= 6, P > 0.05); The sst/ado/NO cocktail decreased the signal by 1 ± 2% (n= 6, P > 0.05); the sst/ado/NO/DA plus kainate decreased the signal by 22 ± 3% (n= 6, P < 0.05); AM-251 increased the signal by 1 ± 1% (n= 5, P > 0.05); AM-251 plus kainate decreased the signal by 18 ± 2% (n= 4, P < 0.05).
An indirect action of Ca channel block in amacrine cells could operate via D1Rs on horizontal cells (Witkovsky, 2004). Dopaminergic amacrine cells also release dopamine onto D1Rs in horizontal cells, potentially increasing, in turn release of inhibitory messengers from the horizontal cells (Herrmann et al. 2011). Thus, block of N- and P/Q-type Ca channels in amacrine cells and the subsequent reduction of a dopamine-mediated stimulation of horizontal cell transmitter release could have the effect of reducing inhibition of photoreceptors and increasing photoreceptor Ca signals. Figure 6B shows that when the D1R agonist SKF38393 was applied, it also had no modulatory effect on photoreceptor Ca2+ signals. Fig. 6G summarizes the actions of these dopaminergic agents.
Other reported modulators of photoreceptor Ca signalling include somatostatin (via sst2A receptors; Akopian et al. 2000), adenosine (via A2 receptors; Stella et al. 2009), nitric oxide (Kurenny et al. 1994; Straiker & Sullivan, 2003) and cannabinoids (via CB1 receptors; Fan & Yazulla, 2003; Jackman et al. 2011). Application of a cocktail containing antagonists of somatostatin sst2A receptors, adenosine A2 receptors and nitric oxide production had no effect on photoreceptor [Ca2+]i (Fig. 6C). Application of the cannabinoid CB1 receptor antagonist AM-251 during the second K+ pulse also did not change photoreceptor [Ca2+]i (Fig. 6D). When antagonists of somatostatin sst2A receptors, adenosine A2 receptors, nitric oxide production and dopamine D2 receptors were applied, photoreceptor [Ca2+]i was significantly reduced by kainate (Fig. 6E), ruling out an action of these receptors on the kainate stimulated effect observed in Fig. 1. Similarly, co-application of kainate with the cannabinoid CB1 receptor antagonist AM-251 significantly reduced photoreceptor [Ca2+]i (Fig. 6F), as did kainate by itself.
Discussion
This work reveals a commonality between the intercellular signalling events that lead to modulation of photoreceptor [Ca2+]i: They are all neutralized by elevated pH buffering by Hepes. Modulation of photoreceptor [Ca2+]i evoked by (1) depolarization and hyperpolarization of the horizontal cell, (2) application of low [Co2+], (3) block of N- and P/Q-type voltage-gated Ca channels in rat horizontal cells, and (4) actions of GABAR agonists and antagonists, is reversed with strengthened pH buffering. On this basis, we propose a potentially unifying mechanism for inhibitory action at the horizontal cell–photoreceptor synapse in rat retina, in which the pH sensitivity arises from recurrent GABA actions in horizontal cells mediated by the large bicarbonate permeability of the GABAR channels. Direct inhibitory actions mediated by GABARs in photoreceptors are not supported by these results. We discuss additional possible ramifications of Ca channel-mediated release and GABAR function in horizontal cells in the regulation of photoreceptor inhibition.
Effects of Ca channel block in horizontal cells on photoreceptor [Ca2+]i
Consistent with the presence of vesicular fusion proteins in horizontal cells, an interpretation of our results is that N- and P/Q-type Ca channels regulate the release of the inhibitory signal sent to photoreceptors. We saw that during selective block of N- and P/Q-type Ca channels, disinhibition in photoreceptors was recorded as an increase in photoreceptor [Ca2+]i. By blocking the Ca channels in horizontal cells, inhibitory signal transmission is reduced and the photoreceptors become disinhibited. This view rests on previously published results, in which increases or decreases in photoreceptor [Ca2+]i are interpreted to be mediated by reductions or enhancements, respectively, of inhibitory feedback from horizontal cells (Hirasawa & Kaneko, 2003; Vessey et al. 2005; Thoreson et al. 2008; Babai & Thoreson, 2009). Both Ca2+ imaging and patch clamp electrophysiological assays contribute to this interpretation, and it has been directly shown with dual patch recordings that depolarization of horizontal cells leads, in photoreceptors, to Ca channel inhibition, while hyperpolarization leads to greater Ca channel activation (Cadetti & Thoreson, 2006; Thoreson et al. 2008, Babai & Thoreson, 2009). In many of these assays and in many species, Hepes and other pH buffers block inhibitory feedback to photoreceptors (Hirasawa & Kaneko, 2003; Vessey et al. 2005; Babai & Thoreson, 2009; Fahrenfort et al. 2009; Crook et al. 2011; Kamiji et al. 2012). When Hepes was co-applied with N- and P/Q-type Ca channel blockers in this work, disinhibition of the photoreceptor Ca2+ signal was reduced, a finding consistent with the pH sensitivity of feedback.
Horizontal cell L-type Ca channels may also contribute to release of the inhibitory signal. The failure to observe disinhibition of photoreceptors when nifedipine was applied is most easily explained by the fact that nifedipine directly blocks photoreceptor L-type Ca channels, but it remains possible that L-type Ca channels do not take part in release of the inhibitory signal.
A similar argument may explain how high and low [Co2+] differentially affect photoreceptor [Ca2+]i. Reduction of the Ca2+ signal in photoreceptors by 100 μm Co2+ is most likely due to direct block of photoreceptor Ca channels. Several previous reports show that low[Co2+] selectively blocks feedback to and not synaptic output from photoreceptors (Thoreson & Burkhardt, 1990; Vigh & Witkovsky, 1999; Fahrenfort et al. 2004). We found an increase in Ca2+ signal at 10 μm Co2+, as would occur were inhibitory feedback selectively blocked. This concentration-dependent action could mean that horizontal cell Ca channels are particularly sensitive to Co2+. We found that 10 μm Co2+ inhibited horizontal cell Ca channels by about 14%, and other reports show Co2+ block of high voltage activated (HVA) channels with IC50 values between 65 μm and 280 μm (Narahashi et al. 1987; Wakamori et al. 1998; Castelli et al. 2003). This degree of block appears small, but we note that agatoxin, which potently blocked inhibitory feedback, reduced our rat horizontal cell Ca channel current by 18%, similar to the 12% block reported for mouse by Feigenspan & Weiler (2004).
Enhancement by Co2+ of photoreceptor Ca2+ signal could also be due to block of some other ion conducting channels or transporters. Fahrenfort et al. (2004) showed that hemichannels are Co2+ sensitive and proposed this as the mechanism by which Co2+ blocked feedback. The IC50 of ∼125 μm found in that report may be too high to account for the feedback block seen here at 10 μm Co2+.
Direct modulation by retinal transmitters does not explain the photoreceptor Ca2+ increase
We sought to show whether reported modulators of photoreceptor Ca signalling were involved in the disinhibition observed in the present study. The GABAR antagonist picrotoxin blocked the inhibition of photoreceptor Ca2+ signal caused by kainate, but when applied by itself, it had the opposite effect, inhibiting photoreceptor [Ca2+]i, in contradiction of a direct action on photoreceptors. The application of the GABAR agonist, muscimol, produced strong enhancement, a result also inconsistent with activation of GABAAR in photoreceptors. Direct GABAergic inhibition of photoreceptors is a possibility previously investigated over several decades without definitive conclusion (Thoreson & Burkhardt, 1990; Kamermans & Spekreijse, 1999; Tatsukawa et al. 2005). Most accounts consider the feedback inhibition of photoreceptors to be a Ca channel modulation rather than an ionotropic action (Verweij et al. 1996), but our contradictory findings of [Ca2+]i enhancement caused by muscimol and inhibition of [Ca2+]i by picrotoxin, when applied alone, indicate a markedly different action by GABA at this synapse. We investigated additional neurotransmitter systems which might lead to disinhibition of photoreceptors, including dopamine, somatostatin, adenosine, nitric oxide and cannabinoids. None blocked the inhibitory actions of kainate on photoreceptor [Ca2+]i. We conclude that these transmitter systems are not responsible for the photoreceptor signal modulation observed.
Other models of Ca2+ dependent inhibitory signalling by horizontal cells
Although evidence for hemichannels in the tips of mammalian horizontal cell processes is not fully developed (Kranz et al. 2013) we consider the possible role of these channels in mediating photoreceptor disinhibition via an ephaptic mechanism during block of N- and P/Q-type Ca channels. Acidification of the horizontal cell cytoplasm is believed to reduce hemichannel conductance (Fahrenfort et al. 2009). Ca2+ influx leads to intracellular acidification (Dixon et al. 1993) via activation of Ca2+-ATPases which bring protons into horizontal cells (Kreitzer et al. 2007; Jacoby et al. 2012). Block of Ca channels would reduce Ca2+-ATPase activity, result in less acidification, increase hemichannel conductance and produce more inhibition. In contrast, we observed that Ca channel block produced less inhibition.
Ca channels mediate the fusion of vesicles, whose contents are acidic. Cleft acidification during vesicle fusion inhibits photoreceptor Ca channels (DeVries, 2001), and cleft acidification is postulated in the pH-sensitive mechanism that could play a role in feedback to photoreceptors (Hirasawa & Kaneko, 2003; Vessey et al. 2005; Davenport et al. 2008; Babai & Thoreson, 2009). Block of Ca channels and reduced vesicle fusion would weaken cleft acidification and lead to larger Ca2+ signals in photoreceptors, as observed.
Ca channel-mediated vesicle fusion would also bring the vesicular proton pump V-ATPase to the plasma membrane. The depolarization-activated V-ATPase has been proposed to be a mechanism by which horizontal cells acidify the cleft to modulate photoreceptor Ca channels (Jouhou et al. 2007; but see Jacoby et al. 2012). Membrane delivery of vesicle V-ATPases has been shown to contribute to synaptic dynamics via pH effects (Zhang et al. 2010). Blocking Ca channels would reduce this action, leading to photoreceptor disinhibition, as seen.
Unconventional GABA action at the feedback synapse
Since GABARs appear unlikely to directly inhibit the photoreceptor membrane, we now consider two alternative roles for GABA which may account for these unconventional results. Both consider the effects of horizontal cell-released GABA to be mediated via GABARs on the horizontal cell membrane rather than at the photoreceptors. First, an increase in Cl− conductance caused by GABA in fish cones shunts the interstitial current flow responsible the ephaptic effect (Endeman et al. 2012). Within this framework, our results with muscimol and picrotoxin, acting at the horizontal cell membrane to increase or decrease Cl− conductance, might shunt or strengthen the interstitial current, reducing or increasing feedback inhibition. The block of kainate-induced inhibition by picrotoxin is not explained by this interpretation, since block of the shunt by picrotoxin when the horizontal cell is maximally depolarized and ephaptic current flow is already at a minimum could produce only a small change in cleft potential. This would be in contrast to the results shown in Fig. 5B where picrotoxin application reverses the kainate inhibition.
The second alternative, a novel hypothesis for GABAergic inhibition, considers the role that the bicarbonate permeability of horizontal cell ionotropic GABARs might play in contributing to the changes of the synaptic cleft pH and is summarized in Fig. 7. We show in Fig. 5H that rat horizontal cells have GABARs permeable to bicarbonate as well as chloride. We propose that inhibition of photoreceptor Ca2+ signalling induced by kainate is the result of the horizontal cell membrane potential being driven positive to the equilibrium potential for HCO3− (Ebicarb). Together with depolarization-induced GABA release and subsequent activation of GABARs, this would produce an inward HCO3− flux that would augment acidification of the cleft. In this model, when picrotoxin blocks GABAR channels, entry of HCO3− and cleft acidification does not occur, accounting for the block of the kainate-induced inhibition by picrotoxin. The actions of muscimol and picrotoxin alone are also explained by HCO3− flux, but work in different directions due to different membrane potentials. In the absence of kainate, which in the previous example had depolarized the horizontal cell membrane positive to Ebicarb, the large muscimol-induced GABAR conductance would produce an outward HCO3− flux from the hyperpolarized horizontal cell, as seen at many GABAergic synapses (Farrant & Kaila, 2007). HCO3− efflux would contribute to alkalinization of the cleft and disinhibition of the photoreceptor Ca2+ signal, as seen here. Picrotoxin, applied by itself without the depolarizing influence of kainate, may block basal GABAR conductance in horizontal cells, interrupting HCO3− efflux and halting cleft alkalinization. These models depend critically on the values of Ebicarb and horizontal cell membrane potential. In addition, the opening of the inhibitory feedback loop by kainate might have a destabilizing effect such that any bias, such as that resulting from a remnant of positive feedback (Jackman et al. 2011), might push the system to an extreme value. There are many pH regulating membrane transport mechanisms in horizontal cells to be considered in addition to the GABARs. Full examination of these influences, along with analysis of the bicarbonate and Cl− equilibrium potentials, will benefit from further investigation.
Figure 7. Model of calcium-dependent, GABA-mediated changes in synaptic cleft pH.
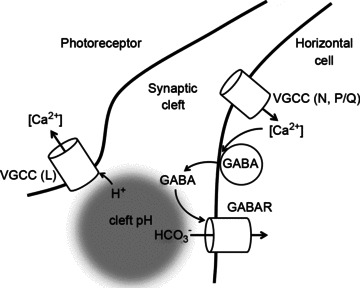
Activation of horizontal cell N- and P/Q-type Ca channels (VGCCs; L-type not ruled out) increases GABA release from the horizontal cell. GABA then acts upon horizontal cell membrane GABA receptors (GABAR) to increase chloride and bicarbonate permeability. Depolarization of the membrane positive to the equilibrium potential for HCO3− (Ebicarb) produces an influx of bicarbonate that would promote acidification in the synaptic cleft, conditions under which inhibition of photoreceptor L-type Ca channels (VGCC – L) has been shown. Activation of GABARs in horizontal cells could also shunt interstitial current flow to glutamate receptors or hemichannels, weakening the ephaptic effect.
In lower vertebrates, horizontal cell membrane GABA transporters are reported to have auto-regulatory and stabilizing roles in horizontal cell membrane activity (Gilbertson et al. 1991; Kamermans & Werblin, 1992; Yang et al. 1999). GABAR channels in mammalian horizontal cells, permeable to Cl− and HCO3−, could stabilize membrane potential and produce changes in the strength of inhibitory feedback to photoreceptors.
The results reported here invoke voltage-gated Ca channel-mediated release of GABA in establishing inhibitory feedback from horizontal cells to photoreceptors. Of the three principal models under consideration (vesicular release, pH-sensitive, and ephaptic), these new results complement many features of a classical vesicle-mediated transmitter release model well, except that the neurotransmitter action is directly upon the cell releasing it. The results explain aspects of the pH-sensitive mechanism which we propose includes cleft pH regulation mediated in part via the HCO3− conductance of the GABARs present in the horizontal cell membrane. The results are also congruent with GABA shunting in the ephaptic hypothesis, albeit at the horizontal cell and not the cone membrane (Endeman et al. 2012). Thus, these results potentially link Ca2+-dependent vesicular GABA release by horizontal cells with the pH-sensitive and ephaptic mechanisms for inhibitory feedback from horizontal cells to photoreceptors.
Glossary
- ATX
ω-agatoxin IVA
- CTX
ω-conotoxin GVIA
- D1R
dopamine type 1 receptor
- D2R
dopamine type 2 receptor
- DMSO
dimethyl sulfoxide
- GABA
γ-aminobutyric acid
- Hepes
hydroxyethyl piperazine ethanesulfonic acid
- GABAR
GABA receptor
- KA
kainic acid
- l-NMMA
NG-monomethyl-l-arginine acetate
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline-2,3,dione
- PTX
picrotoxin
Additional information
Competing interests
None.
Author contributions
Conception and design of the experiments: X.L., A.A.H., N.C.B., S.B. Collection, analysis and interpretation of data: X.L., A.A.H., X.S., S.B. Drafting the article or revising it critically for important intellectual content: X.L., A.A.H., N.C.B., S.B. Experiments were carried out at the University of California, Los Angeles. All authors approved submission of this manuscript.
Funding
Support for this work was provided by grants from the National Eye Institute (EY15573 to N.C.B.), a UCLA Oppenheimer Seed Grant (A.A.H.), the Plum Foundation (S.B. and N.C.B.), the Canadian Institutes of Health Research (MOP-10968 to S.B.), the National Science and Engineering Research Council (Discovery Award to S.B.), and a Veterans Administration Senior Career Scientist Award (N.C.B.).
References
- Akopian A, Johnson J, Gabriel R, Brecha N, Witkovsky P. Somatostatin modulates voltage-gated K+ and Ca2+ currents in rod and cone photoreceptors of the salamander retina. J Neurosci. 2000;20:929–936. doi: 10.1523/JNEUROSCI.20-03-00929.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babai N, Thoreson WB. Horizontal cell feedback regulates calcium currents and intracellular calcium levels in rod photoreceptors of salamander and mouse retina. J Physiol. 2009;587:2353–2364. doi: 10.1113/jphysiol.2009.169656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and γ-aminobutyric acid in mouse cultured spinal neurons. J Physiol. 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byzov AL, Shura-Bura TM. Electrical feedback mechanism in the processing of signals in the outer plexiform layer of the retina. Vision Res. 1986;26:33–44. doi: 10.1016/0042-6989(86)90069-6. [DOI] [PubMed] [Google Scholar]
- Cadetti L, Thoreson WB. Feedback effects of horizontal cell membrane potential on cone calcium currents studied with simultaneous recordings. J Neurophysiol. 2006;95:1992–1995. doi: 10.1152/jn.01042.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli L, Tanzi F, Taglietti V, Magistretti J. Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J Membrane Biol. 2003;195:121–136. doi: 10.1007/s00232-003-0614-2. [DOI] [PubMed] [Google Scholar]
- Corey DP, Dubinsky JM, Schwartz EA. The calcium current in inner segments of rods from the salamander (Ambystoma tigrinum) retina. J Physiol. 1984;354:557–575. doi: 10.1113/jphysiol.1984.sp015393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook JD, Manookin MB, Packer OS, Dacey DM. Horizontal cell feedback without cone type-selective inhibition mediates “red-green” colour opponency in midget ganglion cells of the primate retina. J Neurosci. 2011;31:1762–1772. doi: 10.1523/JNEUROSCI.4385-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport CM, Detwiler PB, Dacey DM. Effects of pH buffering on horizontal and ganglion cell light responses in primate retina: evidence for the proton hypothesis of surround formation. J Neurosci. 2008;28:456–464. doi: 10.1523/JNEUROSCI.2735-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH. Exocytosed protons feedback to suppress the Ca2+current in mammalian cone photoreceptors. Neuron. 2001;32:1107–1117. doi: 10.1016/s0896-6273(01)00535-9. [DOI] [PubMed] [Google Scholar]
- Dixon DB, Takahashi KI, Copenhagen DR. l-Glutamate suppresses HVA calcium current in catfish horizontal cells by raising intracellular proton concentration. Neuron. 1993;11:267–277. doi: 10.1016/0896-6273(93)90183-r. [DOI] [PubMed] [Google Scholar]
- Endeman D, Fahrenfort I, Sjoerdsma T, Steijaret M, Eikelder HT, Kamermans M. Chloride currents in cones modify feedback from horizontal cells to cones in goldfish retina. J Physiol. 2012;590:5581–5595. doi: 10.1113/jphysiol.2012.240325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenfort I, Sjoerdsma T, Ripps H, Kamermans M. Cobalt ions inhibit negative feedback in the outer retina by blocking hemichannels on horizontal cells. Vis Neurosci. 2004;21:501–511. doi: 10.1017/S095252380421402X. [DOI] [PubMed] [Google Scholar]
- Fahrenfort I, Steijaert M, Sjoerdsma T, Vickers E, Ripps H, van Asselt J, Endeman D, Klooster J, Numan R, ten Eikelder H, von Gersdorff H, Kamermans M. Hemichannel-mediated and pH-based feedback from horizontal cells to cones in the vertebrate retina. PLoS One. 2009;4:e6090. doi: 10.1371/journal.pone.0006090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, Quandt FN, Gerschenfeld HM. Calcium-dependent regenerative responses in rods. Nature. 1977;269:707–710. doi: 10.1038/269707a0. [DOI] [PubMed] [Google Scholar]
- Fan SF, Yazulla S. Biphasic modulation of voltage-dependent currents of retinal cones by cannabinoid CB1 receptor agonist WIN 55212–2. Vis Neurosci. 2003;20:177–188. doi: 10.1017/s095252380320208x. [DOI] [PubMed] [Google Scholar]
- Farrant M, Kaila K. The cellular, molecular and ionic basis of GABAA receptor signalling. Prog Brain Res. 2007;160:59–87. doi: 10.1016/S0079-6123(06)60005-8. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Weiler R. Electrophysiological properties of mouse horizontal cell GABAA receptors. J Neurophysiol. 2004;92:2789–801. doi: 10.1152/jn.00284.2004. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Borges S, Wilson M. The effects of glycine and GABA on isolated horizontal cells from the salamander retina. J Neurophysiol. 1991;66:2002–2013. doi: 10.1152/jn.1991.66.6.2002. [DOI] [PubMed] [Google Scholar]
- Guo C, Hirano AA, Stella SL, Jr, Bitzer M, Brecha NC. Guinea pig horizontal cells express GABA, the GABA-synthesizing enzyme GAD 65, and the GABA vesicular transporter. J Comp Neurol. 2010;518:1647–1669. doi: 10.1002/cne.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann R, Heflin SJ, Hammond T, Lee B, Wang J, Gainetdinov RR, Caron MG, Eggers ED, Frishman LJ, McCall MA, Arshavsky VY. Rod vision is controlled by dopamine-dependent sensitization of rod bipolar cells by GABA. Neuron. 2011;72:101–110. doi: 10.1016/j.neuron.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano AA, Brandstätter JH, Brecha NC. Cellular distribution and subcellular localization of molecular components of vesicular transmitter release in horizontal cells of rabbit retina. J Comp Neurol. 2005;488:70–81. doi: 10.1002/cne.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano AA, Brandstätter JH, Vila A, Brecha NC. Robust syntaxin-4 immunoreactivity in mammalian horizontal cell processes. Vis Neurosci. 2007;24:489–502. doi: 10.1017/S0952523807070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano AA, Brandstätter JH, Morgans CW, Brecha NC. SNAP25 expression in mammalian retinal horizontal cells. J Comp Neurol. 2011;519:972–988. doi: 10.1002/cne.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa H, Kaneko A. pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J Gen Physiol. 2003;122:657–671. doi: 10.1085/jgp.200308863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, Babai N, Chambers JJ, Thoreson WB, Kramer RH. A positive feedback synapse from retinal horizontal cells to cone photoreceptors. PLoS Biol. 2011;9:e1001057. doi: 10.1371/journal.pbio.1001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby J, Kreitzer MA, Alford S, Qian H, Tchernookova BK, Naylor ER, Malchow RP. Extracellular pH dynamics of retinal horizontal cells examined using electrochemical and fluorometric methods. J Neurophysiol. 2012;107:868–879. doi: 10.1152/jn.00878.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouhou H, Yamamoto K, Homma A, Hara M, Kaneko A, Yamada M. Depolarization of isolated horizontal cells of fish acidifies their immediate surrounding by activating V-ATPase. J Physiol. 2007;585:401–412. doi: 10.1113/jphysiol.2007.142646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science. 2001;292:1178–1180. doi: 10.1126/science.1060101. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Spekreijse H. The feedback pathway from horizontal cells to cones. A mini review with a look ahead. Vision Res. 1999;39:2449–2468. doi: 10.1016/s0042-6989(99)00043-7. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Werblin F. GABA-mediated positive autofeedback loop controls horizontal cell kinetics in tiger salamander retina. J Neurosci. 1992;12:2451–2463. doi: 10.1523/JNEUROSCI.12-07-02451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiji NL, Yamamoto K, Hirasawa H, Yamada M, Usui S, Kurokawa M. Proton feedback mediates the cascade of colour-opponent signals onto H3 horizontal cells in goldfish retina. Neurosci Res. 2012;72:306–315. doi: 10.1016/j.neures.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Kourennyi DE, Barnes S. Depolarization-induced calcium channel facilitation in rod photoreceptors is independent of G proteins and phosphorylation. J Neurophysiol. 2000;84:133–138. doi: 10.1152/jn.2000.84.1.133. [DOI] [PubMed] [Google Scholar]
- Kranz K, Dorgau B, Pottek M, Herrling R, Schultz K, Bolte P, Monyer H, Penuela S, Laird DW, Dedek K, Weiler R, Janssen-Bienhold U. Expression of Pannexin1 in the outer plexiform layer of the mouse retina and physiological impact of its knock-out. J Comp Neurol. 2013;521:1119–1135. doi: 10.1002/cne.23223. [DOI] [PubMed] [Google Scholar]
- Kreitzer MA, Collis LP, Molina AJ, Smith PJ, Malchow RP. Modulation of extracellular proton fluxes from retinal horizontal cells of the catfish by depolarization and glutamate. J Gen Physiol. 2007;130:169–182. doi: 10.1085/jgp.200709737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurenny DE, Moroz LL, Turner RW, Sharkey KA, Barnes S. Modulation of ion channels in rod photoreceptors by nitric oxide. Neuron. 1994;13:315–324. doi: 10.1016/0896-6273(94)90349-2. [DOI] [PubMed] [Google Scholar]
- Lee H, Brecha NC. Immunocytochemical evidence for SNARE protein-dependent transmitter release from guinea pig horizontal cells. Eur J Neurosci. 2010;31:1388–1401. doi: 10.1111/j.1460-9568.2010.07181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, McKernan K, Shen W. Spatial and temporal distribution patterns of Na-K-2Cl cotransporter in adult and developing mouse retinas. Vis Neurosci. 2008;25:109–123. doi: 10.1017/S0952523808080164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Shen W. Cation Cl− cotransporters in the dendrites of goldfish bipolar cells. Neuroreport. 2007;18:625–629. doi: 10.1097/WNR.0b013e3280ba49b0. [DOI] [PubMed] [Google Scholar]
- Löhrke S, Hofmann HD. Voltage-gated currents of rabbit A- and B-type horizontal cells in retinal monolayer cultures. Vis Neurosci. 1994;2:369–378. doi: 10.1017/s0952523800001711. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Bayley PR, Oesch NW, Ren G, Akileswaran L, Taylor WR. Photoreceptor calcium channels: Insight from night blindness. Vis Neurosci. 2005;22:561–568. doi: 10.1017/S0952523805225038. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Tsunoo A, Yoshii M. Characterization of two types of calcium channels in mouse neuroblastoma cells. J Physiol. 1987;383:231–249. doi: 10.1113/jphysiol.1987.sp016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert T, Weiler R, Feigenspan A. Intracellular calcium is regulated by different pathways in horizontal cells of the mouse retina. J Neurophysiol. 2006;96:1278–1292. doi: 10.1152/jn.00191.2006. [DOI] [PubMed] [Google Scholar]
- Shen W, Jiang Z, Li B. Glycine input induces the synaptic facilitation in salamander rod photoreceptors. J Biomed Sci. 2008;15:743–754. doi: 10.1007/s11373-008-9263-x. [DOI] [PubMed] [Google Scholar]
- Shen W, Purpura LA, Li B, Nan C, Chang IJ, Ripps H. Regulation of synaptic transmission at the photoreceptor terminal: A novel role for the cation–chloride co-transporter NKCC1. J Physiol. 2013;591:133–147. doi: 10.1113/jphysiol.2012.241042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry DM, Mitchell R, Standifer KM, Du Plessis B. Distribution of plasma membrane-associated syntaxins 1 through 4 indicates distinct trafficking functions in the synaptic layers of the mouse retina. BMC Neurosci. 2006;7:54. doi: 10.1186/1471-2202-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella SL, Jr, Hu WD, Brecha NC. Adenosine suppresses exocytosis from cone terminals of the salamander retina. Neuroreport. 2009;20:923–929. doi: 10.1097/WNR.0b013e32832ca4b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella SL, Jr, Thoreson WB. Differential modulation of rod and cone calcium currents in tiger salamander retina by D2 dopamine receptors and cAMP. Eur J Neurosci. 2000;12:3537–3548. doi: 10.1046/j.1460-9568.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- Straiker A, Sullivan JM. Cannabinoid receptor activation differentially modulates ion channels in photoreceptors of the tiger salamander. J Neurophysiol. 2003;89:2647–2654. doi: 10.1152/jn.00268.2002. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Lasater EM. Sustained and transient calcium currents in horizontal cells of the white bass retina. J Gen Physiol. 1992;99:85–107. doi: 10.1085/jgp.99.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsukawa T, Hirasawa H, Kaneko A, Kaneda M. GABA-mediated component in the feedback response of turtle retinal cones. Vis Neurosci. 2005;22:317–324. doi: 10.1017/S0952523805223076. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Morgans C. Localization and properties of voltage-gated calcium channels in cone photoreceptors of Tupaia belangeri. Vis Neurosci. 1998;15:541–552. doi: 10.1017/s0952523898153142. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Babai N, Bartoletti TM. Feedback from horizontal cells to rod photoreceptors in vertebrate retina. J Neurosci. 2008;28:5691–5695. doi: 10.1523/JNEUROSCI.0403-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Burkhardt DA. Effects of synaptic blocking agents on the depolarizing responses of turtle cones evoked by surround illumination. Vis Neurosci. 1990;5:571–583. doi: 10.1017/s0952523800000730. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Mangel SC. Lateral interactions in the outer retina. Prog Retinal and Eye Research. 2012;31:407–441. doi: 10.1016/j.preteyeres.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Kaneko A, Kaneda M. Voltage-dependent ionic currents in solitary horizontal cells isolated from cat retina. J Neurophysiol. 1992;4:1143–1150. doi: 10.1152/jn.1992.68.4.1143. [DOI] [PubMed] [Google Scholar]
- Vardi N, Zhang LL, Payne JA, Sterling P. Evidence that different cation chloride cotransporters in retinal neurons allow opposite responses to GABA. J Neurosci. 2000;20:7657–7663. doi: 10.1523/JNEUROSCI.20-20-07657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij J, Kamermans M, Spekreijse H. Horizontal cells feedback to cones by shifting the cone calcium-current activation range. Vision Res. 1996;36:3943–3953. doi: 10.1016/s0042-6989(96)00142-3. [DOI] [PubMed] [Google Scholar]
- Vessey JP, Stratis AK, Daniels BA, Da Silva N, Jonz MG, Lalonde MR, Baldridge WH, Barnes S. Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J Neurosci. 2005;25:4108–4117. doi: 10.1523/JNEUROSCI.5253-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigh J, Witkovsky P. Sub-millimolar cobalt selectivity inhibits the receptive field surround of retinal neurons. Vis Neurosci. 1999;16:159–168. doi: 10.1017/s095252389916111x. [DOI] [PubMed] [Google Scholar]
- Vu TQ, Payne JA, Copenhagen DR. Localization and developmental expression patterns of the neuronal K-Cl cotransporter (KCC2) in the rat retina. J Neurosci. 2000;20:1414–1423. doi: 10.1523/JNEUROSCI.20-04-01414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamori M, Strobeck M, Niidome T, Teramoto T, Imoto K, Mori Y. Functional characterization of ion permeation pathway in the N-type Ca2+ channel. J Neurophysiol. 1998;79:622–634. doi: 10.1152/jn.1998.79.2.622. [DOI] [PubMed] [Google Scholar]
- Wilkinson MF, Barnes S. The dihydropyridinesensitive calcium channel subtype in cone photoreceptors. J Gen Physiol. 1996;107:621–630. doi: 10.1085/jgp.107.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Shen C, McRory J. Differential distribution of voltage-gated calcium channels in dopaminergic neurons of the rat retina. J Comp Neurol. 2006;497:384–396. doi: 10.1002/cne.20995. [DOI] [PubMed] [Google Scholar]
- Yang XL, Gao F, Wu SM. Modulation of horizontal cell function by GABAA and GABAC receptors in dark- and light-adapted tiger salamander retina. Vis Neurosci. 1999;16:967–979. doi: 10.1017/s0952523899165167. [DOI] [PubMed] [Google Scholar]
- Yang XL, Wu SM. Effects of prolonged light exposure, GABA, and glycine on horizontal cell responses in tiger salamander retina. J Neurophysiol. 1989;61:1025–1035. doi: 10.1152/jn.1989.61.5.1025. [DOI] [PubMed] [Google Scholar]
- Yang XL, Wu SM. Effects of GABA on horizontal cells in the tiger salamander retina. Vision Res. 1993;33:1339–1344. doi: 10.1016/0042-6989(93)90041-t. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Fina ME, Vardi N. Regulation of KCC2 and NKCC during development: membrane insertion and differences between cell types. J Comp Neurol. 2006;499:132–143. doi: 10.1002/cne.21100. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Nguyen KT, Barrett EF, David G. Vesicular ATPase inserted into the plasma membrane of motor terminals by exocytosis alkalinizes cytosolic pH and facilitates endocytosis. Neuron. 2010;68:1097–1108. doi: 10.1016/j.neuron.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]


