Abstract
The main aim of the study was to examine the effects of transcranial polarization on neurons in two descending motor systems, rubro- and reticulospinal. Anodal DC current was applied through an electrode in contact with the skull over the contralateral sensori-motor cortex, against an electrode placed between the skull and the ipsilateral temporal muscles in deeply anaesthetized cats. Its effects were estimated from changes in descending volleys evoked by electrical stimuli applied in the red nucleus (RN), medial longitudinal fascicle (MLF; to reticulospinal fibres) and the pyramidal tract (PT; to corticospinal or corticoreticular fibres). The descending volleys were recorded from the surface of the spinal cord at a cervical level. Rubrospinal neurones were activated either directly or indirectly, via interpositorubral fibres. Reticulospinal neurons were likewise activated directly and indirectly, via other reticulospinal or corticospinal fibres. Transcranial polarization facilitated transsynaptic activation of both rubrospinal and reticulospinal neurons, shortening the latency of the indirect descending volleys and/or increasing them, Direct activation of descending axons was much less affected. The facilitation of all subcortical neurons examined was potentiated by repeated applications of transcranial direct current stimulation (tDCS) and outlasted the polarization by at least 1–2 h, replicating tDCS effects on indirect activation of cortical neurons. The results indicate that the beneficial effects of tDCS on motor performance in humans may be due to more efficient activation of not only cortical but also subcortical neuronal systems. Combined actions of tDCS on cortical and subcortical neurones might thus further improve recovery of motor functions during rehabilitation after central injuries. 249/250
Key points
Transcranial constant current polarization of the human brain is on the increase in neurological practice because it improves several motor and cognitive functions of the human nervous system and because it is non-invasive and technically simple.
Here we show that transcranial brain polarization in anaesthetized animals not only affects cortical neurons, as is often assumed, but also facilitates activation of neurons in all investigated subcortical motor systems.
In addition, the subcortical facilitation greatly outlasts (by at least hours) the period of transcranial polarization. These findings provide new evidence of plasticity at subcortical levels, the mechanisms for which remain to be investigated.
In clinical practice, the subcortical effects of transcranial polarization may thus make an essential contribution to the beneficial effects of the treatment of motor impairments.
Introduction
Brain polarization modulates neuronal activity in a very potent way. In acute experiments on animals, or on brain tissue in vitro, weak polarization was found to induce changes in activity of nerve cells and the probability of their activation (see e.g. Morrell, 1961; Bindman et al. 1964; Purpura & McMurtry, 1965; Bikson et al. 2004). In awake humans, transcranial direct current stimulation (tDCS) was found to affect a great variety of brain functions and their range is continuously increasing. In addition these effects have been found to occur not only during, but also following tDCS, and are enhanced by repeated tDCS applications, which should be highly beneficial for rehabilitation after CNS injuries (for latest reviews and references, see Nitsche et al. 2008; Brunoni et al. 2011b, 2012). Studies in humans have linked the effects of tDCS primarily to synaptic modifications within the cortex and plasticity in the operations of cortical neuronal networks (see e.g. Nitsche & Paulus, 2000; Lang et al. 2004a, 2005, 2011; Di Lazzaro et al. 2008; Stagg & Nitsche, 2011) and the issue of whether tDCS-induced effects are primarily intracortical, or also occur subcortically has been left unresolved. Nevertheless it has been already demonstrated that sustained changes in cortical actions elicited by subcortical neurons can be evoked by tDCS over primary motor cortex (Polania et al. 2012). tDCS may also induce changes in regional cerebral blood flow in the brain, including such subcortical structures as the thalamus, globus pallidus and nucleus accumbens (Lang et al. 2005). These findings thus set the stage for investigating the effects of tDCS at a subcortical level and their possible contribution to the beneficial effects of tDCS in humans.
Taking into account both widespread changes in the blood flow during and after tDCS and the known extent of spread of current in the nervous tissue, the main aims of the present study were twofold: firstly, to investigate whether any effects of anodal tDCS, similar to the effects on corticospinal neurons, occur on subcortical descending tract neurons and, secondly, to find out whether such subcortical effects outlast tDCS.
In order to investigate the subcortical effects of tDCS we selected two descending motor systems, rubro- and reticulospinal. They were selected because they are sufficiently well known to allow qualitative comparison of their activation by controlled electrical stimuli before, during and after application of tDCS. However additional observations were also made on the vestibulospinal and cerebello-rubrospinal, cerebello-vestibulospinal or cerebello-reticulospinal neuronal systems. The tests that we used are described in the first section of the Results. The effects of the tDCS were analysed in acute experiments on deeply anaesthetized animals which allowed much easier access to the subcortical neurons and stimulation and recording possibilities exceeding those in humans. Nevertheless, despite differences in both species and experimental conditions, transcranial polarization was found to facilitate responses of subcortical neurons to a similar extent as responses evoked by stimulation of motor cortex in awake humans.
Methods
Ethical approval
All experimental procedures were approved by a regional Ethics Committee for Animal Research (Göteborgs Djurförsöksetiska Nämnd) and comply with NIH and EU guidelines for animal care and with the ethical policies and regulations of The Journal of Physiology (Drummond et al. (2010)). The cats were bred and housed under veterinary supervision at the Laboratory of Experimental Biomedicine at Sahlgrenska Academy.
Preparation
The experiments were performed on 12 deeply anaesthetized cats weighing 2.2–3.4 kg. Anaesthesia was induced with sodium pentobarbital (Apoteksbolaget, Sweden; 40–44 mg kg−1, i.p.) and maintained with intermittent doses of α-chloralose (Rhône-Poulenc Santé, France; 5 mg kg−1 administered every 1–3 h, up to 65 mg kg−1, i.v.). Additional doses of α-chloralose were given when motor reactions were evoked during dissection and when increases in the continuously monitored blood pressure or heart rate were evoked by any experimental procedures. During recordings, neuromuscular transmission was blocked by pancuronium bromide (Pavulon, Organon, Sweden; 0.3 mg kg−1 i.v. supplemented with about 0.2 mg kg−1 h−1) and the animals were artificially ventilated. Mean blood pressure was kept at 100–130 mmHg and end-tidal CO2 at 4–4.5% by adjusting the parameters of artificial ventilation and the rate of a continuous infusion of a bicarbonate buffer solution with 5% glucose (1–2 ml h−1 kg−1). The body temperature was kept at about 37.5°C by servo-controlled infrared lamps. The experiments were terminated by a lethal dose of pentobarbital i.v. followed by formalin perfusion.
Following the initial vein, artery and tracheal canulation, the head was fixed in a stereotactic frame and several parts of the brain were exposed by craniotomy to allow insertion of stimulating electrodes to the right red nucleus (RN), the left medullary longitudinal fascicle (MLF), the right pyramidal tract (PT) or the right motor cortex, the left lateral vestibular nucleus (LVN) and two regions in the cerebellum. The area in RN was selected by recording antidromic field potentials evoked by stimulation of the contralateral lateral funiculus, aiming at an area at Horsley-Clarke coordinates A3.5, R1.5, H –3.5, as described by Hongo et al. (1969). MLF and PT were reached via cerebellum aiming at Horsley-Clarke co-ordinates P9, L0.6, H-5 and P5–7, R1, H-10, at an angle of 20 or 25 deg from vertical (tip directed rostrally). The area in LVN was selected by recording antidromic field potentials following stimuli applied to the ipsilateral lateral funiculus, aiming at Horsley-Clarke coordinates P7.7, L4, H –4, at an angle of 25 deg. The cerebellar electrodes were placed rostral or caudal to the fastigial nucleus. The electrode to be used for stimulation of the motor cortex (for comparison with effects of subcortical stimulation) was inserted under visual control into the postcruciate gyrus a few millimetres from the midline, aiming at the bottom of the cruciate sulcus exposed by craniotomy. The electrodes were left at locations from which distinct descending volleys were evoked from the surface of the lateral funiculus at the C1/C2 (in four experiments) or the C3/C4 (in eight experiments) at stimulus intensities of 20 μA or less.
At the end of the experiments the stimulation sites were marked with electrolytic lesions and verified histologically (see Fig. 1).
Figure 1. Location of stimulation sites.
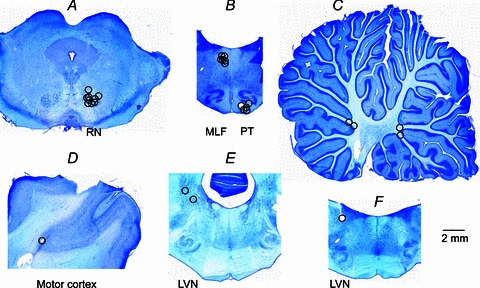
A–F, location of electrolytic lesions made at the stimulation sites by passing 0.2 mA constant current for 15 s. Locations of these stimulation sites were subsequently verified on 100 μm thick sections of the brain cut, using a vibratome, in the frontal or parasagittal plane, mounted on slides, counterstained with Cresyl Violet, scanned and indicated on representative sections of the brain. A, contralateral RN; B, medulla in a frontal plane corresponding to P9 with MLF stimulation sites dorsally and PT stimulation sites ventrally; C, cerebellum in a sagittal plane about 1.5 mm laterally; D, pericruciate area of the motor cortex in a sagittal plane; E, medulla in a frontal plane corresponding to Horsley-Clarke coordinate P7 with stimulation sites in the region of the vestibular nuclei F; F, medulla in a frontal plane corresponding to P8 with LVN stimulation site.
Stimulation and recording
Single 0.2 ms constant current stimuli and trains of two to five stimuli at intensities 20–100 μA were applied monopolarly via tungsten electrodes (impedance 30–150 kΩ). The parameters of the stimuli at each location were chosen to ensure that they induced not only directly but also indirectly evoked volleys, as outlined in the first part of the Results and schematically indicated in Fig. 2.
Figure 2. Diagrams of stimulation–recording arrangements for analysis of the directly and indirectly evoked descending volleys in the rubrospinal, corticospinal and reticulospinal tract fibres.
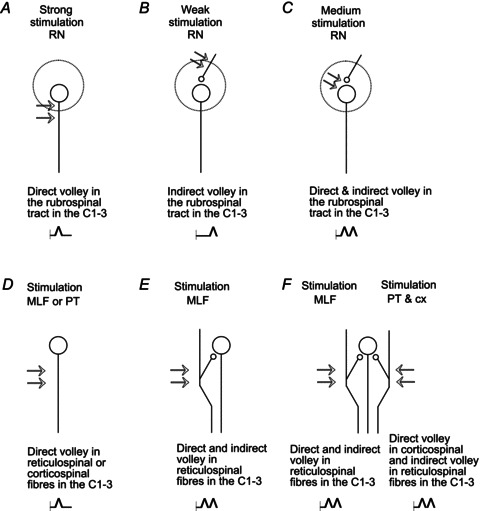
The sites of the stimulation are indicated by double arrows. The results of the stimulation are indicated at the bottom in A–F. When the stimuli activated neurons, or their axons, they initiated direct descending volleys (A, D) which are schematically indicated by single notches in the horizontal trace line just after the shock artifacts (vertical lines). In contrast, when only the presynaptic fibres were stimulated (B), action potentials in these fibres induced activation of postsynaptic neurons with one synaptic delay and an indirectly initiated descending volley at a longer latency. When both presynaptic fibres and postsynaptic neurons were stimulated (C, E, F), descending volleys displayed two components, direct at a latency of about 0.5 ms and indirect further delayed by up to 1 ms. In the illustrated records in Figs 3 and 5–9 the direct volleys will be indicated by ‘d’ and the indirect volleys by ‘i’. For further explanations see text.
The descending volleys were recorded monopolarly with one electrode in contact with the intact dura mater and the reference electrode in contact with one of the neck or back muscles. Both single records and averages of 10–50 records were stored on-line (with the time resolution of 30 μs per address) and were analysed off-line using software for sampling and analysis developed by E. Eide, T. Holmström and N. Pihlgren (University of Gothenburg).
Parameters of tDCS
One of the preliminary issues of this study was to select parameters of transcranial stimulation that would replicate effects of such stimulation in humans. The stimulation was applied to the area over the major part of the contralateral (right) pericruciate area corresponding to the human sensorimotor cortex. The polarizing anodal current was applied via 3% agar-agar in saline contained in a chamber attached to the skull with a contact area of about 200 mm2 about 3–10 mm from the midline. The reference electrode was in contact with about twice that area, between the ipsilateral (left) lateral aspect of the skull and the temporal muscles, about 20 mm caudal and 20 mm lateral from that over the sensorimotor cortex. Current intensities used were 0.2 or 0.5 mA, corresponding to about 1 or 2.5 μA mm−2. They exceeded the level of 0.3 μA mm−2 used in humans (1 mA over 35 cm2) but were within the range of intensities that were used in the original acute experiments on anaesthetized rats (0.25 or 10 μA mm−2) (Bindman et al. 1964), awake rabbits or cats (10 μA mm−2) (Morrell, 1961), awake rats (30 μA mm−2) (Liebetanz et al. 2006) or unanaesthetized decerebrate cats (30–80 μA mm−2) (Purpura & McMurtry, 1965) and the levels in more recent studies (1–57 μA mm−2; see Table 1 in Brunoni et al. 2011a).The reason for using higher current intensities than in most studies in humans was that the density of current within the target area at depth drops significantly when the size of the electrode is decreased (Miranda et al. 2009). In two experiments the polarizing current was applied first via titanium screws inserted into the skull and then via the usual agar-agar filled chamber. The screws were placed above the contralateral posterior sigmoid gyrus lateral to the ipsilateral coronal sulcus. They were used to test the effects of this kind of application of tDCS in future chronic experiments. The current was applied most often during several periods of 5 min separated by 5 min intervals.
Analysis
Effects of tDCS were estimated from changes in latencies and/or sizes of descending volleys evoked during control periods and during, or after application of tDCS using averages of 20 single records for comparison. These changes were estimated by overlaying subsequent records (Fig. 3A–C; Fig. 4C and D) and by off-line subtracting control records from records obtained during later periods. Changes in size were then quantified by comparing areas of differences between these records as indicated in Fig. 3D–F. The areas were related to the areas of the control volleys within the same time window and expressed as a percentage of control volleys. Comparing computer generated differences had also the advantage of eliminating stimulus artifacts on which the earliest direct volleys (especially from MLF and PT) were superimposed (see e.g. Fig. 3A).
Figure 3. Examples of descending volleys on which effects of tDCS were tested.
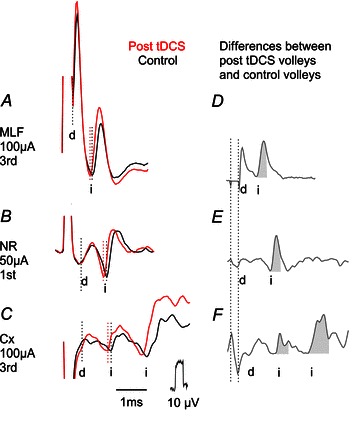
Averaged records of descending volleys (n= 20) evoked by stimuli applied in the ipsilateral MLF, contralateral NR and the motor cortex. A, an example of large direct volleys (d) followed by smaller indirect volleys (i). B, an example of small direct volleys followed by larger indirect volleys. C, an example of primarily indirect volleys following very small direct volleys. The illustrated volleys were evoked by the 3rd stimulus in A and C and by the 1st stimulus in B, with the stimulation–recording arrangements corresponding to those in Fig. 2E, C and F. Black traces, control responses. Red traces, responses evoked during the last period of tDCS. Dotted lines indicate the latency of the volleys. D–F, differences between black and red traces in A–C; with the time windows used to measure the areas shaded. Note two distinct indirect volleys evoked from the motor cortex, one at latency as similar the indirect volley evoked from the RN (compatible with one synaptic delay) and another one at about 1 ms longer latency indicating an additional synaptic delay. Note also that latencies of all illustrated indirect volleys were shortened after tDCS while the latency of the direct volley (in A) was not changed. Some of these volleys were also increased, including the direct volley in A although its area could not be reliably measured. In this and the following figures the negativity is upward and the largest shock artifacts are truncated.
Figure 4. Development of facilitation of indirect volleys evoked by stimulation of RN by tDCS.
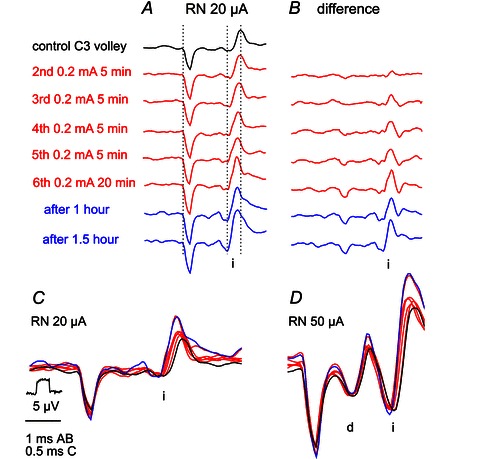
A, records of indirect volleys (i) evoked by weak rubral stimuli before, during and after indicated periods of application of 0.2 mA tDCS stimulation. The illustrated volleys were evoked by the 3rd stimulus in a train applied at 400 Hz. Note decrease in the latency (by up to 0.15 ms) and increase in the amplitude of the subsequently evoked volleys. B, computer generated differences between the black control records and the subsequent records. Note increases in amplitude of these differences and their timing. Note also their slow development and persistence after the end of the tDCS. Three dotted lines in A indicate the beginning of the stimulus artifact, the onset of the volleys evoked at the shortest latency and the peak of the control volley to ease the comparison of their timing. C, superimposed twice expanded records in A. D, superimposed records of similarly twice expanded volleys recorded in the same experiment as those in A but when stronger stimuli also evoked a direct volley (d). The stimulus–recording arrangement was as in Figs 2B and C and 3B. Note weaker facilitation of indirect volleys evoked by 50 μA than by 20 μA stimuli and much weaker facilitation of direct volleys evoked by these stimuli. With respect to some changes in shock artifacts in A it may be pointed out that the changes in indirect volleys in A and B are highly unlikely to be related to them because the indirect volleys increased during the first two illustrated periods of tDCS and the two periods after tDCS when the artifacts were identical. In addition the shortening of the latencies of the indirect volleys is unlikely to be related to the recording conditions which are possibly reflected in the changes in the shock artifacts.
Differences between data sets were assessed for statistical significance by using Student's t test (for unpaired or paired samples assuming equal variances and the two tail distribution; using Statistica 5.1, StatSoft.
Results
Tests used to estimate effects of tDCS
The effects of tDCS were investigated on responses evoked by electrical stimuli applied at different sites in the brain. Direct activation of the corticospinal and reticulospinal fibres was evoked by low intensity stimuli from within the pyramidal tract (PT) or the reticulospinal tract at the level of the medial longitudinal fascicle (MLF). The excitability of these fibres, and the effectiveness of the stimuli, was monitored at spinal level by comparing volleys of action potentials induced in these fibres, as indicated in Fig. 2D. Similarly selective direct activation of rubrospinal tract fibres was not possible because these fibres run too close to other fibres or cells along their trajectory between the red nucleus (RN) and the spinal cord. Furthermore, stimuli applied within the RN may activate neurons in this nucleus both directly and indirectly. Predominantly direct activation is evoked by strong single stimuli applied close to the axons of RN neurons, as indicated in Fig. 2A. Predominantly indirect activation is evoked via action potentials induced in presynaptic terminals of neurons providing input to RN neurons, in the first instance the interposito-rubral neurons, as indicated in Fig. 2B. As described by Baldissera et al. (1972), the excitability of these terminals is higher than that of the neurons and therefore the lowest intensity stimuli applied in the RN may excite RN neurons only transsynaptically, especially when a train of a few stimuli is used. It is therefore possible to choose a stimulus intensity at which RN neurons are activated only transsynaptically, or some neurons are activated directly and others indirectly; hence either only indirect or both direct and indirect components of the descending volleys in rubrospinal tract fibres are evoked, as indicated in Fig. 2C and illustrated in Fig. 3B. This manner of activating RN neurons replicates the way corticospinal neurons are activated by stimulation of the motor cortex: indirectly at a lower intensity and directly at a higher intensity (Jankowska et al. 1975; Asanuma et al. 1976; Phillips & Porter, 1977), thus giving rise to the I and D waves of the corticospinal volleys (for more recent references see Lemon et al. 2004; Di Lazzaro et al. 2008; Lemon, 2008). Corticospinal volleys recorded in the present series of experiments are illustrated in Fig. 3C. By using descending volleys that are directly and indirectly evoked in rubrospinal and corticospinal neurons one may thus compare the effects of tDCS on excitability of these neurons (or their axons) and on their transsynaptic activation in relation to changes in the excitability of presynaptic terminals.
Indirect activation of reticulospinal neurons is also possible since according to recent studies axon collaterals of both reticulospinal and corticospinal fibres provide input to some reticulospinal neurons and this provides the means for their transsynaptic activation (Edgley et al. 2004; Jankowska & Stecina, 2007; Stecina & Jankowska, 2007). As indicated in Fig. 2E–G and illustrated in Figs 3A and 8A, when trains of stimuli are applied in the MLF and PT, they induce not only directly but also indirectly evoked descending volleys and the effects of tDCS may be examined on both of these.
Figure 8. Facilitation of descending volleys evoked from the pyramids and the motor cortex by tDCS.
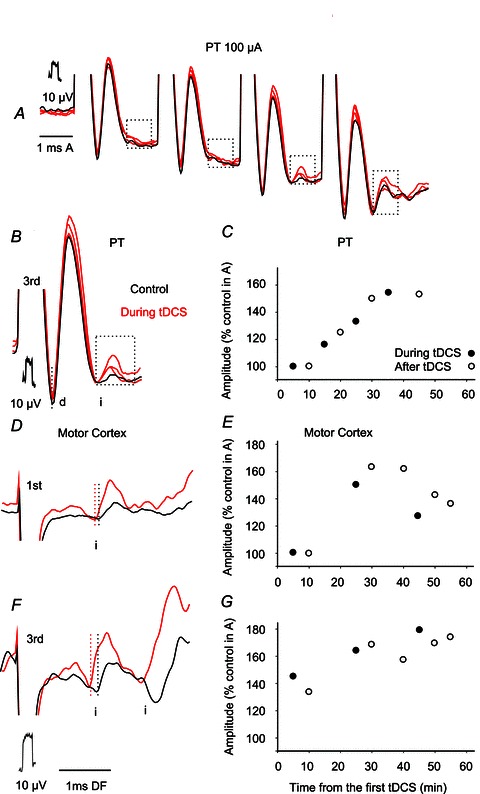
A, superimposed averaged records (n= 20) of volleys evoked by 100 μA stimuli applied in PT before (black; control) and during the 3rd, 4th and 5th 5 min periods of 0.5 mA tDCS. Parts of the records during which indirect volleys (i) appeared are boxed. B, expanded part of records in A including boxed direct and indirect volleys evoked by the 3rd stimuli. C, time course of changes in the areas of indirect volleys in B. D and F, examples of indirect volleys evoked by 1st and 3rd stimuli applied within the motor cortex (left stimulation site in Fig. 1) before (black) and during (red) the 4th 5 min period of 0.2 mA tDCS. Averaged records (n= 20). E and G, time course of changes in the areas of the earlier and later indirect volleys in F.
In humans little or no facilitation by tDCS of responses evoked by direct stimulation of corticospinal neurons in the motor cortex was seen, while indirectly evoked responses were facilitated (Lang et al. 2011). In view of this, direct volleys following subcortical stimulation in the present experiments were expected to be only marginally affected by tDCS. However, it turned out that not only indirect but also some direct volleys were affected, albeit to a lesser extent, consistent with results of more recent studies in humans (Di Lazzaro et al. 2012).
The direct volleys were evoked at latencies of 0.4–0.9 ms, depending on their origin and the length of the axons between their cell origin and the cervical segments. The earliest direct volleys were evoked from the MLF (0.4–0.9 ms) and the later ones from the RN (0.7–0.9 ms) and PT (0.7–0.8 ms). However, the indirect volleys attributable to monosynaptically evoked transsynaptic actions followed all these direct volleys with similar additional delays of about 0.7–0.9 ms, at total latencies of 1.2–1.9 ms from MLF, 1.3–1.7 ms from RN and 1.6–1.7 ms from PT.
Changes in the latency and/or size of descending volleys evoked from the red nucleus
Indirect volleys evoked from RN were facilitated by tDCS in all eight experiments in which they were tested. The facilitation resulted in a shortening of the latency of the control volleys by 0.1–0.2 ms (in two experiments) and/or in an increase in the area of the volleys to 143 ± 13.39% (mean ± SEM). Both these effects were evoked by tDCS at an intensity of 0.2 mA (in five experiments, including those illustrated in Figs 3 and 4) as well as an intensity of 0.5 mA. Direct volleys evoked by the same or stronger stimuli were either unchanged (n= 5; e.g. in Fig. 3B), or increased, but the increase was smaller than that of the indirect volleys (n= 1; Fig. 4D).
Development of facilitatory effects of tDCS
The facilitation developed gradually. In none of the experiments could it be detected at the beginning of the first 5 min period of tDCS, whether at 0.2 or 0.5 mA, indicating that it was not due to mere depolarization of RN neurons. In one experiment the first signs of facilitation were detected after only 4 min of polarization and the facilitation usually appeared during the 2nd or subsequent periods of tDCS. It increased during the succeeding periods, as illustrated in Fig. 4B and summarized in Fig. 5A, and reached maximum after 4th or 5th periods.
Figure 5. Time course of facilitation of indirect volleys evoked from RN and MLF by tDCS.
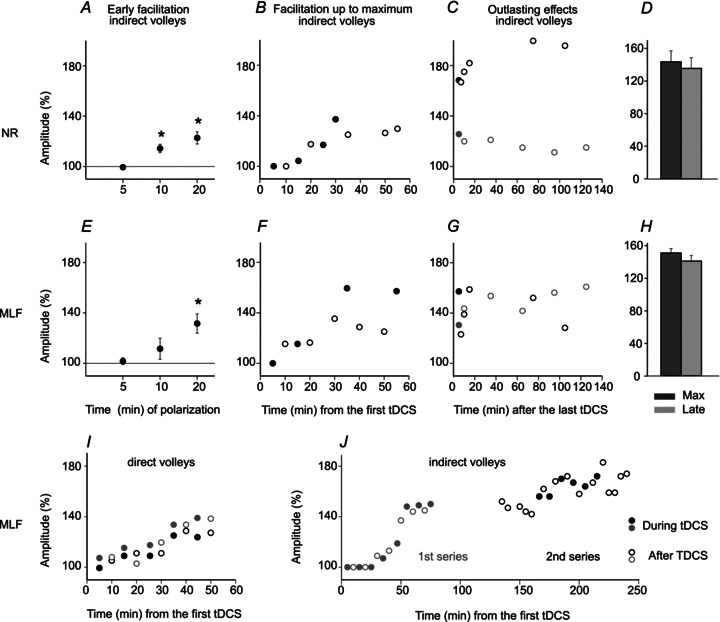
Areas of indirect descending volleys evoked from RN (A–D) and from MLF (E–I). A, mean increases (± SD) during the first three 5 min periods of tDCS (0.2 or 0.5 mA; n= 7) expressed as a percentage of the control values (100%). B, changes in area of one of the indirect volleys as a percentage of control volleys during successive 5 min periods of tDCS of 0.2 mA (filled symbols) and the following 5 min periods (open symbols), illustrated in Fig. 3A–C. C, changes in areas of two other indirect volleys, indicated by black and grey symbols, respectively, following the last tDCS of 0.2 mA in two separate experiments. D, mean areas of five indirect volleys during the last tDCS period and areas of these volleys after the following 90 min ± SD. No statistically significant differences were revealed by Student's t test. E–H, as in A–F for indirect volleys from the MLF (for 6 volleys in E and H). Note in B and F that the facilitation outlasted each of the 5 min periods of polarization and in C and G that the degree of facilitation continued to increase after the last period of polarization, remained constant, or started to decline after 1.5–2 h. I, as in F, but for two direct volleys from the MLF. J, as in F except for the use of two series of 5 min tDCS; the second series started about 1 h after the first series. In the first series 0.5 mA DC current was applied via screws traversing the bone and in contact with the dura mater (see Methods). In the second series similar intensity current was applied transcranially. Note an additional facilitation during and after the second series. Similar enhancement of facilitation was seen in another experiment when transcranial stimulation was repeated (on MLF-evoked volleys and on volleys from RN). Statistically significant differences in A and E were found using Student's t test for paired samples of 20 averaged records (*P < 0.01).
In Fig. 4C and D the progression of changes in indirect volleys evoked by near-threshold and somewhat stronger stimuli is illustrated by superimposing records obtained during successive periods of tDCS. The time course of these changes is plotted in Fig. 5B and C.
Duration of facilitatory effects of tDCS
Analysis of the after-effects of tDCS, by comparing areas of volleys evoked at the end of each successive 5 min polarization period and after the immediately following 5 min period, showed no systematically occurring differences. Volleys evoked after tDCS sometimes remained similar, sometimes decreased and sometimes increased and no statistically significant differences were revealed by applying Student's t test when the two sets of data were compared.
The long term effects following the last tDCS were monitored for 1.5–2 h in seven experiments. In some (3/7) cases the areas of the volleys continued to increase during these periods by at least 10–20%, so that the volleys reached the maximal size long after the polarization had been terminated (Fig. 5C). In three cases there was a decline in their size by 10–20% after about 1 h, (bottom plot in Fig. 5C), and in one case the size remained unchanged during nearly 2 h. However, for the whole sample (n= 6) the comparison of areas of maximal volleys recorded just after and 1–1.5 h after tDCS did not reveal statistically significant differences (Fig. 5D).
Changes in the latency and/or the size of descending volleys in axons of reticulospinal neurons
Indirect activation of reticulospinal neurons was facilitated by tDCS in all seven experiments, closely resembling the effects on rubrospinal neurons. In three of the seven experiments the latencies of the indirect volleys from the MLF were shortened by tDCS by 0.1–0.2 ms but the effects of tDCS manifested themselves primarily in an increase of the areas of the volleys. As in the case of rubrospinal neurons the facilitation developed gradually so that significant increases in the indirect reticulospinal volleys started during the second or later periods of tDCS (Fig. 5E, F and I) and reached a maximum during the 5–6th tDCS periods. Subsequent to the last tDCS the facilitation increased, declined or remained unchanged during the next 1–1.5 h (Fig. 5G) and no statistically significant difference in mean values (n= 6) was found (Fig. 5H). The facilitation was further enhanced when tDCS was reapplied 30 min – 1 h after the last series of tDCS (Fig. 5I). This is consistent with the effects of tDCS in humans, showing that when the second stimulation was performed during the after-effects of the first, a prolongation and enhancement of tDCS-induced effects after stimulation was observed (Monte-Silva et al. 2010).
The only more essential difference between effects of tDCS on rubrospinal and reticulospinal neurons appeared to be related to changes in direct volleys. In contrast to rubrospinal volleys, only three of the six measurable direct volleys from the MLF were not found to be affected by tDCS (while the concurrently evoked indirect volleys were increased to 145–186%). Three other direct volleys increased (examples shown in Fig. 3A and Fig. 7), though less (122–140%) than the indirect volleys (140–180%). The facilitation of indirect volleys in reticulospinal neurons might thus be either secondary to the increased input from MLF to reticulospinal neurons or be independent of the number of excited MLF fibres and due primarily to facilitation of synaptic transmission and/or excitability of reticulospinal neurons.
Figure 7. Time course of facilitation of indirect volleys evoked from MLF by tDCS.
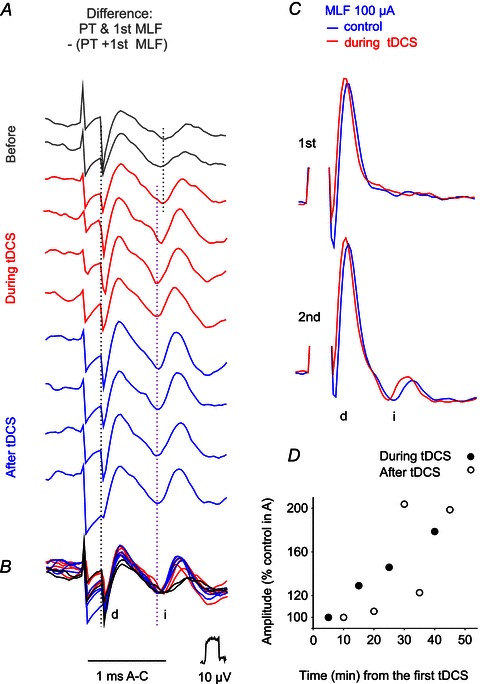
A, a series of differences between indirect volleys (i) evoked by single MLF stimuli applied separately or preceded by a train of four PT stimuli, obtained as illustrated in Fig. 5A–D in another experiment. The difference traces are for volleys evoked before, during successive periods of 5 min 0.5 mA tDCS, and after the last tDCS, all of which are superimposed in B. Note shortening in the latency as well as increase in the areas of the indirect volleys. Changes in the areas of these volleys are plotted in D. C, records of descending volleys evoked by MLF stimuli used in the series in A, before and during tDCS and in the same scale as records in A. They show that indirect volleys absent after single MLF stimuli appeared when they were preceded by another MLF stimulus. The early parts of the differences illustrated in A most likely reflect changes in the shock artifacts and not in the indirect volleys because records in C show only minute increases in peak amplitudes of these volleys. Dotted lines in A and B indicate the end of the stimulus artifacts and the onsets of indirect volleys evoked before and during the tDCS. With respect to decreases or increases in shock artifacts at the beginning of traces in A, it may be pointed out that they are not consistently related to the increases in indirect volleys. In addition, they represent differences between three records, as indicated above A, and not the original shock artifacts.
In order to estimate the mode of actions of tDCS on reticulospinal neurons, some of its effects were tested under conditions when reticulospinal neurons were co-activated by joint actions of PT and MLF fibres, due to convergence depicted in Fig. 3H. PT stimuli applied before tDCS increased small MLF-evoked indirect volleys (Fig. 6A and B purple), or caused the previously absent indirect volley to appear (Fig. 6E and F purple); tDCS was found to result in a further increase of these indirect volleys (compare red traces in Fig. 6C and D and in Fig. 6E and F). Facilitation of joint actions of PT and MLF was found on four out of six volleys evoked by MLF stimuli following PT stimuli.
Figure 6. Examples of facilitation of indirect volleys evoked from MLF and PT by tDCS.
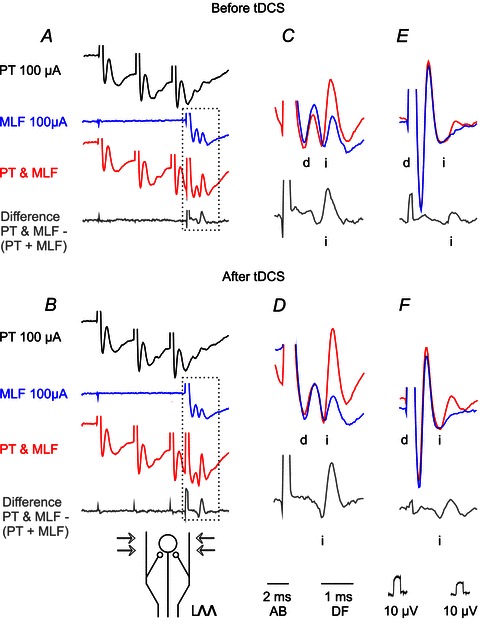
A–D and E–F, average records of descending volleys (n= 20) evoked by single MLF stimuli in two experiments, recorded from the C1 and C3 segment respectively. The stimulation–recording arrangement below B corresponds to that in Fig. 2F. A, a series of records before application of tDCS with direct volleys evoked by 3 PT stimuli, by single stimuli applied just lateral to MLF (the most lateral stimulation site in Fig. 1) and by joint application of the same PT and MLF stimuli. Difference traces show the indirect volleys (i) evoked by joint application of PT and MLF stimuli from which volleys evoked by separate PT or MLF stimuli were subtracted. B, as in A but after 25 min of 0.5 mA tDCS. Note similar direct MLF volley, slightly smaller indirect MLF volley and larger indirect volley after PT & MLF stimuli. C and D, superimposed three times expanded boxed parts of records in A and B respectively. E and F, similarly superimposed records of volleys evoked by single MLF stimuli applied separately or preceded by a train of PT stimuli in another experiment and the differences between them before and after 10 min of 0.2 mA tDCS. Note that joint actions of PT and MLF stimuli were considerably stronger after 0.5 mA tDCS (B, D, F) than under control conditions (A, C, E).
Figure 7 illustrates some additional features of the facilitation of joint actions of PT and MLF stimuli. Records in A show that the facilitation developed slowly, reached maximum after 20–30 min of tDCS and involved both shortening of the latencies of indirect volleys and increase in their area (plotted in panel D). The comparison of volleys evoked by the first and second MLF stimuli which were not preceded by PT stimuli before and during tDCS (black and green in Fig. 7C) shows furthermore that tDCS alone did not bring about the indirect volley after the first stimulus.
Changes in the latency and/or amplitude of descending volleys evoked from the pyramids and from the motor cortex
The effects of tDCS on indirect volleys following PT stimulation were more difficult to see than those on indirect volleys evoked by other subcortically applied stimuli. Nevertheless indirect volleys from PT were found to be facilitated in four of the five experiments in which very small or hardly any indirect volleys followed direct volleys evoked by the 3rd, 4th or 5th stimulus in a train. In the case of volleys illustrated in Fig. 8A and B, facilitation of indirect volleys manifested itself primarily after the 3rd direct PT volley and in parallel with an increase in this direct volley. On the basis of previous evidence (Stecina & Jankowska, 2007) it was assumed that indirect volleys following PT stimuli within about 1 ms delay are relayed by reticulospinal neurons. The selective enhancement of the indirect volleys evoked by the 3rd but not by other stimuli could be related to the differences in the effects of these stimuli on reticulospinal neurons. If the input after the first two PT stimuli was too weak to discharge reticulospinal neurons, while the 4th stimulus was near-maximal, the facilitation would only appear on the effects of the 3rd stimuli. The time course of facilitation of the indirect PT volleys illustrated in Fig. 8C was generally similar to that of the facilitation of volleys from RN and MLF. In particular, it appeared during the 2nd period of 0.5 mA tDCS and outlasted tDCS. When no indirect components followed direct volleys evoked by PT stimulation before tDCS (in three other experiments), we could only note that indirect volleys appeared in an all-or-none fashion during or after tDCS, but were unable to relate them to the original indirect volleys.
In order to verify that subcortical tDCS actions are comparable to the effects of tDCS at the level of the motor cortex under the same conditions, in one experiment tDCS was tested in parallel on descending volleys evoked from the pericruciate cortex, RN (illustrated in Fig. 4) and MLF. Figure 8D–G shows that at moderate stimulus intensities (100 μA) cortical stimuli evoked hardly any direct volleys but an early indirect volley was evoked by the 1st stimulus (Fig. 8D) and both early and later indirect volleys by the 2nd and 3rd stimuli (Fig. 8F). Both were facilitated by tDCS as indicated by shortening of their latencies and increases in their size. The two indirect volleys might be attributed to indirect activation of PT neurons and to a subsequent activation of reticulospinal neurons (see above) respectively. The time course of facilitation of these indirect volleys was therefore plotted separately. Figure 7E and F shows that the development of effects of tDCS on these volleys was similar.
Effects of tDCS on indirect descending volleys evoked from other subcortical regions
The effects of tDCS were tested on descending volleys evoked by stimuli applied in or close to the lateral vestibular nucleus (LVN), and within the cerebellum at the sites indicated in Fig. 1C, E and F. Application of tDCS facilitated indirect volleys evoked from the lateral vestibular nucleus (Fig. 1F) and from one of the two areas rostral to it (Fig. 1E). The time course (Fig. 9A) and the degree of this facilitation (Fig. 9B,C) were the same as those evoked from the RN and the MLF.
Figure 9. Examples of facilitation of descending volleys evoked from the lateral vestibular nucleus and the cerebellum.
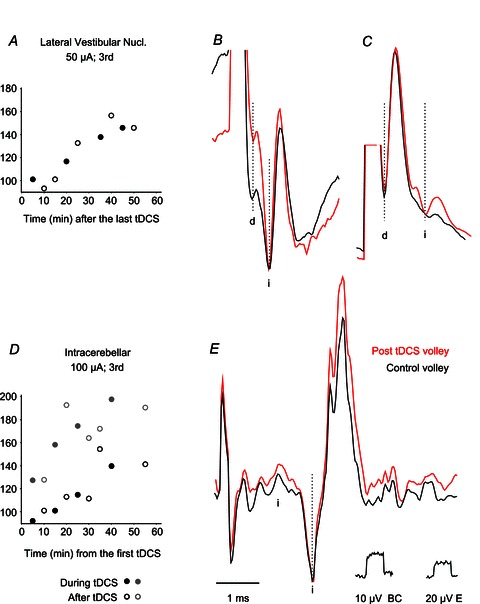
A–C, facilitation of indirect volleys (i) from two regions in and rostral to the lateral vestibular nucleus. A, changes in the area of averaged records (n= 20) of indirect volleys illustrated in B. D–E, changes in the areas of averaged records (n= 20) of indirect volleys evoked from two regions in the anterior lobe of the cerebellum, with the time course of both (with black and grey symbols respectively, one of these illustrated. Note the generally weaker and slower developing effects. Note also the very long latency (about 2 ms) of indirect volleys compatible with trisynaptic rather than disynaptic coupling. If these volleys were relayed by rubrospinal neurons, the latency would be compatible with up to 1.4 ms latency of activation of neurons in the RN by stimuli applied in the nucleus interpositus (Eccles et al. 1975) and conduction time from RN of about 0.7 ms. Dotted lines indicate the onset of direct (d) and indirect (i) volleys.
Indirect volleys from two stimulation sites within the anterior lobe of the cerebellum rostral to the nucl. fastigius and/or nucl. interpositus (Fig. 1B) were facilitated (Fig. 9D and E), in contrast to those from a region caudal to nucl. fastigius which were not changed by tDCS.
Discussion
The reported results show that tDCS applied in the anaesthetized cat facilitates transsynaptic activation of subcortical descending tract neurons and, even more importantly, that the facilitation outlasts the tDCS. They show also that the facilitation is enhanced by repeated applications of tDCS. Thereby they give positive answers to the main questions of this study.
Methodological problems
Effects of tDCS or of other factors?
In order to relate changes in descending volleys to tDCS, we had to ensure that tDCS, and no other factors, such as the general state of the animal, or changes in the recording conditions, was responsible for them. One of the strongest indications for specific effects of tDCS that would differentiate them from effects of changes in the general state are parallel changes in the direct and indirect descending volleys, because any changes in the latency, or the areas of the direct volleys evoked by electrical stimuli should depend on the excitability of the stimulated fibres and a shorter utilization time, but not on the animal state. In addition, a decrease but not an increase in the size of direct volleys would be attributable to changes in the recording conditions. For example, collection of fluid around the recording electrode, or a higher resistance, are generally associated with a decrease rather than increase in amplitude of the potentials recorded from the surface of the spinal cord and they would be unlikely to cause a shortening of the latencies of either direct or indirect volleys. The same argument applies to any changes in the amplitude of indirect volleys which might be related to changes in the recording conditions reflected in e.g. changes in the shock artifacts because shortening of the latencies of indirect volleys should be independent of the variations in the recording conditions (for further comments on the relationships between the volleys and changes in shock artifacts see legend of Fig. 4). In addition, other records, e.g. in Fig. 3D and E, show distinct changes in the indirect volleys without associated changes in the shock artifacts.
Changes due to the state of the animal were also obviated by comparing volleys evoked during several 5 min periods before, during and after tDCS. As this was done after the animals had reached a deep level of anaesthesia and when the blood pressure, heart rate, level of CO2 in the respired air and temperature remained stable, changes occurring within 10–20 min of application of tDCS, especially stepwise increases in the area of the indirect volleys like those illustrated in Figs 4 and 6, would be unlikely to be secondary to changes in the state of the animals.
Changes occurring within longer periods could be more related to factors other than tDCS but at least some of these could be minimized. Thus in some experiments application of tDCS was delayed by 1, 2 or 3 h during which only the stability of the recording was verified. Volleys recorded during both short and long control periods then remained generally unchanged and sometimes showed smaller rather than larger amplitudes. Furthermore, once the degree of facilitation of indirect volleys reached a plateau, it often remained stable, despite changes in the depth of anaesthesia (e.g. when it was supplemented after 2–3 h) or in the blood pressure (when it dropped at the end of some experiments). Moreover, although it could be expected that changes in the general state of the animal would affect indirect activation of more than one type of neuron, even when indirect volleys evoked from MLF or RN were strongly facilitated, indirect volleys from PT, or from the cerebellum sometimes remained unchanged. There are therefore no reasons to postulate that more effective activation of neurons resulting in larger or earlier indirect volleys within a couple of hours after application of tDCS was secondary to changes in the state of the animal rather than due to tDCS, even though some specific effects of tDCS could have been evoked on the background of a general increase in neuronal excitability.
Comparison of subcortical effects of tDCS in anaesthetized cats and in humans
To what extend tDCS applied in humans would have subcortical facilitatory effects similar to the effects in anaesthetized cats investigated in the present study is currently a matter of conjecture. However, positron emission tomography (PET) of regional cerebral blood flow (rCBF) in humans revealed widespread increases in rCBF during and following tDCS in posterior brain regions (Lang et al. 2005) including cerebellar vermis and the thalamus, as mentioned by the authors. However, effects projected on the medial surfaces of the brain show that changes occurred in a number of other subcortical structures as well, in particular at the location of the RN and the mesencephalic or pontine reticular nuclei (see legend to Fig. 10). Modifications of functional coupling between thalamus or caudate nucleus and cortical areas when tDCS was applied over the human primary motor cortex (M1) was also demonstrated by fMRI analysis, but with no indication of facilitation at cortical or subcortical levels (Polania et al. 2012).
Figure 10. Indications for subcortical effects of tDCS applied in humans.
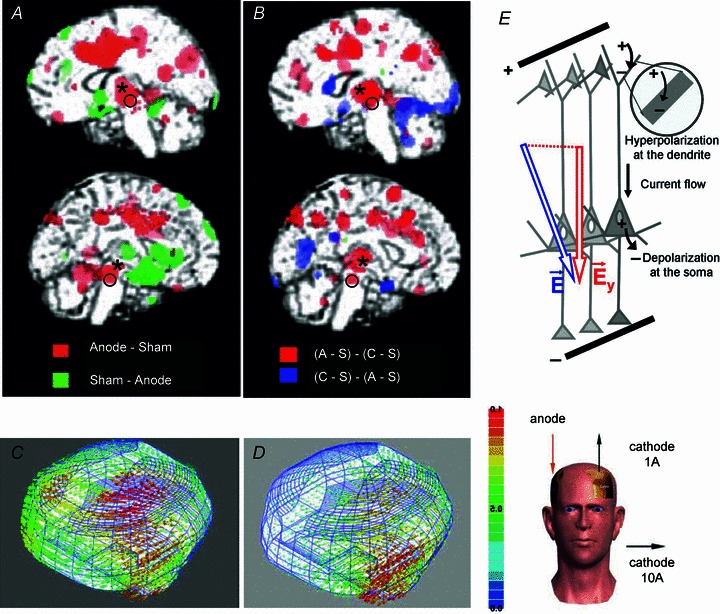
A and B, changes in the regional cerebral blood flow (rCBF; increases indicated in red) reported by Lang et al. (2005), modified from their Figs 3 and 4 with permission. The areas indicated by * and o appear to correspond to those of the RN and the mesencephalic reticular formation (when compared to structures outlined in the Harvard atlas of the human brain (parasaggital slides 24 and 19 in Jackson and Becker, http://www.med.harvard.edu). C and D, models of the distribution of current densities during tDCS, with anode over the M1 region and cathode either over the supraorbital region (C) or over neck muscles (D) as indicated to the right. Diagrams modified from Figs 1, 2 (montage 1A) and 5 (montage 10A) in Wagner et al. (2007) with permission. The illustrated current densities were projected on the surface of the cerebral cortex, but the spread of current barely escaped the subcortical tissue which would indicate involvement of several deep structures. E, the diagram of differential effects of DC current on dendrites and on the initial segment of the axon of PT cells when anodal current is applied close to their apical dendrites (modified from Fig. 2 of Molaee-Ardekani et al. (2012) with permission.
In keeping with these observations, modelling of spread of current during tDCS indicated that tDCS should be able to affect all of these subcortical structures, as well as more distant nuclei down to the most ventral regions. This would however depend on the location of the focal and reference electrodes, as indicated in Fig. 10C and D. The spread of current would be relatively more effective dorsally with the reference electrode at the supraorbital site (Wagner et al. 2007) but might be more effective ventrally with the reference electrode placed in an extracephalic position, e.g. in contact with neck muscles (Wagner et al. 2007; see also Faria et al. (2011)).
Whether, the smaller size of the cat brain would favour more effective subcortical effects at the level of the RN and the reticular formation than in the larger human skull would again be a matter of conjecture. However, considering the smaller size of the cat skull as well as the smaller area of application of the tDCS (see Methods) and the smaller distances between the site of application of the tDCS and the subcortical structures, we reduced the intensity of the tDCS. It was reduced to 0.2 mA compared to 2 mA used in humans, i.e. 10 times. Considering the smaller size of the tDCS electrodes this resulted in the surface current density (about 1 μA mm−2) that was not much different from that used in human (0.6 μA mm−2).
The resulting differences, or similarities, in the current density at various levels in humans and in cats could not be estimated, but the degree of facilitation at the level of cat subcortical and cortical neurons was comparable. At both levels the facilitation was evoked by anodal tDCS and it replicated the effects of anodal tDCS in humans within the cortex as well as on thalamo- and striato-cortical networks (Polania et al. 2012). When the subcortical effects of cathodal tDCS in the cat were compared to those of anodal tDCS in a pilot experiment, any effects of cathodal tDCS on direct and indirect volleys evoked from the RN, PT and MLF were only marginal and with the tendency to be depressive. Much stronger effects of anodal tDCS were therefore considered as of much greater interest and only these were examined.
From the theoretical point of view, the facilitatory effects of transcranial anodal polarization on corticospinal neurons are fully in keeping with the original observations of Phillips and colleagues (Phillips, 1956; Hern et al. 1962) of a generally lower threshold for excitation and depolarizing effects of anodal surface stimulation on axons of deeper located cortical neurons. The results of both these and earlier studies (for references see Hern et al. 1962) can be explained by the morphology of these neurons with their long apical dendrites entering the superficial cortical layers and their axons crossing the most ventral layers before they enter the white matter. Anodal current applied at the surface would thus hyperpolarize the dendrites and depolarize the initial segments of the axons where the action potentials are generated, while surface cathodal stimulation would have an opposite effect (see the latest diagram of this situation in Fig. 10E).
Whether anodal tDCS would polarize rubral and reticular output neurons in the same way as corticospinal neurons cannot be predicted. However the morphology of rubrospinal and at least some reticulospinal neurons, and in particular the trajectory of medio-ventro-caudally directed axons of these neurons (see e.g. Cajal (Cajal, 1953; vol II, Fig. 166)) would make it plausible. Depolarization of the initial segment of the axons of these neurons associated with the facilitatory effects of tDCS on them would be favoured in this situation.
Could facilitation by tDCS be related to pre-, post- or transsynaptic actions of tDCS?
The depolarization of the initial segment of the investigated projection neurons might be one of the mechanisms of the facilitatory effects of the anodal tDCS. However, the reported effects of tDCS involved transsynaptic activation of neurons in two subcortical nuclei (RN and LVN) where a relatively high proportion of presynaptic fibres synapsing with these neurons should be activated by electrical stimuli in parallel with the neurons themselves. tDCS could thus increase the excitability of presynaptic and postsynaptic neurons to electrical stimuli as well as facilitate synaptic transmission in synapses between them. Under our experimental conditions the effects of tDCS on presynaptic fibres in RN and LVN could not be estimated but effects on the first components of descending volleys reflecting activation of neurons in these nuclei by electrical stimuli could be used as a measure of changes in the excitability of these neurons. As direct volleys from RN were found to be increased in only two of seven experiments and only small changes, if any, were detected in direct volleys from LVN (see Fig. 9B and C), tDCS might have only weakly depolarized neurons in these nuclei. If so, the main effects of tDCS might involve either presynaptic fibres or synaptic transmission between these fibres and RN or LVN neurons.
The effects of tDCS on transsynaptic activation of reticulospinal neurons were found under different experimental conditions, because these neurons were activated by presynaptic fibres stimulated at quite long distances from them. The presynaptic fibres included collaterals of axons of other reticulospinal neurons stimulated within the MLF (Ito & McCarley, 1987; Edgley et al. 2004), at least a few millimetres away, and collaterals of the PT fibres (Jankowska & Stecina, 2007; Stecina & Jankowska, 2007) stimulated at even greater distances. Direct volleys following MLF stimuli would thus not give a measure of changes in excitability of reticulospinal neurons themselves. Facilitation of some direct MLF and PT volleys indicates that the facilitatory effects of tDCS could to some extent be due to an increase in input from MLF and PT to reticulospinal neurons. However, as facilitation of indirect volleys following MLF and PT stimulation was not always associated with facilitation of direct volleys, i.e. an increased number of MLF or PT fibres, facilitation in at least one-half of our tests might have been primarily due to facilitation of synaptic transmission between presynaptic fibres and reticulospinal neurons. Furthermore the increased number of MLF and PT fibres after tDCS does not give the measure of fibres actually acting on reticulospinal neurons; it is only compatible with this possibility.
The facilitatory effects of tDCS were expressed in two ways: firstly, in an increase in the area of the descending volleys and, secondly, in shortening of the latency and/or of the time to peak of these volleys, and both might result from an increase in excitability of the postsynaptic neurons as well as the effectiveness of synaptic transmission. When the same number of presynaptic fibres were involved and the same number of postsynaptic neurons activated before and after the tDCS, as judged by the same amplitudes of direct and indirect volleys, shortening of the latencies of the indirect volleys (see e.g. Fig. 3B) might be preferentially associated with the amount of transmitter released, the dynamics of transmitter release, or the temporal characteristics of the resulting EPSPs. Generation of action potentials in postsynaptic neurons generally occurs within the rising phase or at the peak of the EPSPs and both depolarization of the neurons and an increase in the slope of the EPSPs would assist in reaching the threshold for the action potentials earlier. The maximal degree of shortening of latencies of indirect volleys evoked from RN and MLF may thus be predicted from the timing of synaptic activation of rubral and reticular neurons. Published records show action potentials delayed by 0.1–0.5 ms with respect to the onset of EPSPs evoked in the RN and in reticular neurons by stimuli applied in the nucleus interpositus (Eccles et al. 1975) or other reticular nuclei (McCarley et al. 1987). They also show about 0.5 ms jitter in timing of activation of extracellularly recorded reticular neurons (Iwakiri et al. 1995). Even if these delays were halved, one cannot thus expect the indirect activation of RN, RS and LVN neurons to be advanced by more than about 0.2 ms, which corresponds to the maximal decreases of latencies of indirect volleys following tDCS found in this study.
Among the reported effects of tDCS on humans we noted two possible cases of shortening of latencies of epidurally recorded descending volleys and of EMG responses evoked by magnetic brain stimulation or during voluntary movements, but in both of these only increases in amplitude but not changes in the latency were indicated. These were the effects of tDCS illustrated in Fig. 1 of Lang et al. (2011) and in Fig. 2 in McCambridge et al. (2011).
In contrast to indirect volleys, shortening of latencies of direct volleys was at best 0.1 ms. Some changes in the threshold as well as in conduction velocity of electrically stimulated fibres were found to be associated with previous stimuli and/or with previously induced action potentials (Swadlow & Waxman, 1975, 1976; Waxman & Swadlow, 1977; Kocsis et al. 1979; Kocsis & Waxman, 1982; Malenka et al. 1983) and similar changes might be induced by tDCS. Changes in excitability of electrically stimulated MLF and PT fibres may be irrelevant to the effects of tDCS under other conditions, e.g. on EMG responses evoked by transcranial magnetic stimulation. Nevertheless they demonstrate that tDCS may change the excitability of fibres providing input to subcortical neurons and that tDCS may thereby affect any motor reactions depending on the excitability of either presynaptic fibres or postsynaptic neurons.
Induction and after-effects of tDCS
In humans, tDCS of sufficiently high intensity sometimes had practically immediate effects (e.g. within 1 min) but usually they manifested themselves only after a few minutes (see e.g. Fig. 3 in Nitsche & Paulus, 2000; Nitsche et al. 2005).
Under the experimental conditions of the present study the earliest effects of positive tDCS on descending volleys from the motor cortex started to appear a few minutes after the onset of polarization. The earliest facilitation of responses of RN neurons in the same experiment was found during the same period of polarization, but in other experiments only during the 2nd, 3rd or 4th periods of polarization and/or one or two intervening periods. The induction of subcortical facilitation might thus require longer periods of time. However, the question of whether this could depend on the distance between the source of the polarizing current and the involved descending tract neurons, anaesthesia, or other factors has not yet been addressed.
Brain polarization has been reported to have after-effects of varying duration, depending on the intensity and duration of the tDCS and most likely on the size of the polarized areas, their location and a number of other experimental conditions. After a single 5 min period of polarization the residual facilitation sometimes started to decline within 2 min, with the return to control values during the next few minutes (Fig. 2 in Nitsche & Paulus, 2000) but in other cases the after-effects of polarization remained stable for an hour or more (Nitsche & Paulus, 2001; Nitsche et al. 2003; Lang et al. 2004a,b) and after repeated periods of polarization the after-effects of polarization of motor cortex were considerably longer lasting, up to several weeks (Boggio et al. 2007; Reis et al. 2009; Reis & Fritsch, 2011).
In the present study, once the facilitation of indirect activation of subcortical neurons developed, it always outlasted the tDCS, even though it appeared to develop more slowly than in humans. Subsequent to two to three effective periods of polarization a stable degree of facilitation was observed for up to 2–3 h and both shortening of the latencies and increases in amplitude of the descending volleys became even more pronounced after the tDCS had been terminated.
Functional consequences of subcortical effects of tDCS
The effects of tDCS on subcortical neurons, including both their expressions and timing, closely replicate effects of tDCS on cortical neurons, but they also resemble the effects of the polarization of the human spinal cord (Cogiamanian et al. 2008, 2011, 2012; Winkler et al. 2010; Lamy et al. 2012). It may thus be justifiable to postulate that similar mechanisms underlie the effects of the polarizing current on cortical and subcortical neurons as well as spinal neurons. However, such a conclusion would have two main implications.
The first of these would be that the previous conclusions on mechanisms of effects of tDCS on cortical neurons should apply to both subcortical and spinal neurons. Therefore, if polarity-driven alterations of resting membrane potentials represent the crucial mechanisms of the tDCS-induced after-effects, leading to a change in NMDA receptor activation of cortical neurons, as proposed by Liebetanz et al. (2002), changes in NMDA receptor dependent activation should also be expected in the case of rubrospinal, reticulospinal and vestibulospinal neurons and any neurons affected by spinal cord polarization. Similarly, if the after-effects of tDCS are due to shifts in intracortical inhibition and facilitation, as proposed by Nitsche et al. (2005), similar long-lasting changes in the operation of neuronal networks in the red nucleus, in the reticular formation and in the spinal cord might be expected. Such expectations would need to be verified in future studies.
The second implication of the similarities of effects of tDCS on cortical, subcortical and spinal neurons might be that they depend on much more general cellular mechanisms, involved in the operation of any neurons and not only those considered to have as high degree of plasticity as cortical neurons. Again, such mechanisms should be defined in future studies, considering that they underlie the effects of tDCS on a variety of neurons and may be involved in long-lasting increases of excitability of both neurons and fibres.
In view of the firmly established beneficial effects of tDCS it would be redundant to dwell on the value of its clinical applications. Nevertheless, it is worth pointing out that rehabilitation of motor ímpairments after spinal cord injury or stroke may benefit from taking into account that tDCS affects subcortical as much as cortical neurons. To this end it will be of relevance to address the effects on rubrospinal, reticulospinal and various spinal neuronal systems more systematically. Successful physiotherapy undoubtedly involves the activation of a number of motor systems. Parallel activation of several non-injured pathways that have become more efficient through tDCS therapy may increase the probability of activation of motoneurons or result in stronger output from them, both of which should be of benefit for motor recovery.
Translational perspective
Studies on the effects of transcranial direct current stimulation (tDCS) have so far focused on the range of these effects and on defining optimal parameters of the tDCS. Much less attention has been paid to the mechanisms of these effects. In addition, on the tacit assumption that they are intricately related to the operation of cortical neurons, very little has been done to analyse any effects on other neurons. We provide evidence that tDCS in deeply anaesthetized animals facilitates activation of neurons in several subcortical motor systems, including the rubrospinal and reticulospinal systems, thus replicating the effects of tDCS on cortical neurons in both animals and humans. We show also a considerable degree of plasticity of the subcortical effects of tDCS as they outlast the duration of polarization by at least some hours. The mechanisms of subcortical effects remain to be investigated, but there already exist several indications of how they might be enhanced, for instance by adjusting parameters of transcranial polarization and/or the placement of the focal and reference electrodes. It is also possible that beneficial effects on rehabilitation of motor impairments may result from the combined effects of tDCS on cortical and subcortical neuronal systems. It might be also relevant that the subcortical actions of tDCS could to some extent be monitored by positron emission tomography of regional cerebral blood flow, or other techniques.
Acknowledgments
We wish to thank Drs Gerta Vrbova and Paolo Cavallari for comments on preliminary versions of this paper, Jytte Grännsjö for excellent technical assistance during experiments and with histological control and Drs E. Nilsson and P. Geborek for participation in some experiments. The work was supported by the National Institutes of Health (grant number R01 NS040863 to E.J.).
Glossary
- EMG
electromyographic
- EPSP
excitatory postsynaptic potentials
- LVN
lateral vestibular nucleus
- MEP
motor evoked potential
- MLF
medial longitudinal fascicle
- PT
pyramidal tract
- RN
red nucleus
- RS
reticulospinal
- tDCS
transcranial direct current stimulation
- TMS
transcranial magnetic stimulation
Author contributions
The experiments were performed at the Department of Physiology, University of Gothenburg. All authors contributed to the design of the experiments as well as to the collection, analysis and interpretation of the data and to the drafting of the article, and all approved the final version of the manuscript.
References
- Asanuma H, Arnold A, Zarzecki P. Further study on the excitation of pyramidal tract cells by intracortical microstimulation. Exp Brain Res. 1976;26:443–461. doi: 10.1007/BF00238820. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Lundberg A, Udo M. Stimulation of pre- and postsynaptic elements in the red nucleus. Exp Brain Res. 1972;15:151–167. doi: 10.1007/BF00235579. [DOI] [PubMed] [Google Scholar]
- Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, Jefferys JG. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiol. 2004;557:175–190. doi: 10.1113/jphysiol.2003.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OC, Redfearn JW. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol. 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25:123–129. [PubMed] [Google Scholar]
- Brunoni AR, Ferrucci R, Bortolomasi M, Vergari M, Tadini L, Boggio PS, Giacopuzzi M, Barbieri S, Priori A. Transcranial direct current stimulation (tDCS) in unipolar vs. bipolar depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011a;35:96–101. doi: 10.1016/j.pnpbp.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Fregni F, Pagano RL. Translational research in transcranial direct current stimulation (tDCS): a systematic review of studies in animals. Rev Neurosci. 2011b;22:471–481. doi: 10.1515/RNS.2011.042. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, Edwards DJ, Valero-Cabre A, Rotenberg A, Pascual-Leone A, Ferrucci R, Priori A, Boggio PS, Fregni F. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012;5:175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal SR. Histologie du Systeme Nerveux de l’Homme & des Vertebres. Madrid: Instituto Ramon y Cajal; 1953. [Google Scholar]
- Cogiamanian F, Ardolino G, Vergari M, Ferrucci R, Ciocca M, Scelzo E, Barbieri S, Priori A. Transcutaneous spinal direct current stimulation. Front Psychiatry. 2012;3:63. doi: 10.3389/fpsyt.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogiamanian F, Vergari M, Pulecchi F, Marceglia S, Priori A. Effect of spinal transcutaneous direct current stimulation on somatosensory evoked potentials in humans. Clin Neurophysiol. 2008;119:2636–2640. doi: 10.1016/j.clinph.2008.07.249. [DOI] [PubMed] [Google Scholar]
- Cogiamanian F, Vergari M, Schiaffi E, Marceglia S, Ardolino G, Barbieri S, Priori A. Transcutaneous spinal cord direct current stimulation inhibits the lower limb nociceptive flexion reflex in human beings. Pain. 2011;152:370–375. doi: 10.1016/j.pain.2010.10.041. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Ziemann U, Lemon RN. State of the art: Physiology of transcranial motor cortex stimulation. Brain Stim. 2008;1:345–362. doi: 10.1016/j.brs.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Ranieri P, Profice P, Pilato F, Mazzone P, Capone F, Insola A, Oliviero A. Transcranial direct current stimulation effects on the excitability of corticospinal axons of the human cerebral cortex. Brain Stim. 2012 doi: 10.1016/j.brs.2012.09.006. doi j.brs.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Drummond GB, Paterson DJ, McGrath JC ARRIVE: new guidelines for reporting animal research. J Physiol. 2010;588:2517. doi: 10.1113/jphysiol.2010.192260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Scheid P, Taborikova H. Responses of red nucleus neurons to antidromic and synaptic activation. J Neurophysiol. 1975;38:947–964. doi: 10.1152/jn.1975.38.4.947. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. J Neurosci. 2004;24:7804–7813. doi: 10.1523/JNEUROSCI.1941-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria P, Hallett M, Miranda PC. A finite element analysis of the effect of electrode area and inter-electrode distance on the spatial distribution of the current density in tDCS. J Neural Eng. 2011;8:066017. doi: 10.1088/1741-2560/8/6/066017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hern JE, Landgren S, Phillips CG, Porter R. Selective excitation of corticofugal neurones by surface-anodal stimulation of the baboon's motor cortex. J Physiol. 1962;161:73–90. doi: 10.1113/jphysiol.1962.sp006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Lundberg A. The rubrospinal tract. I. Effects on alpha-motoneurones innervating hindlimb muscles in cats. Exp Brain Res. 1969;7:344–364. doi: 10.1007/BF00237320. [DOI] [PubMed] [Google Scholar]
- Ito K, McCarley RW. Physiological studies of brainstem reticular connectivity. I. Responses of mPRF neurons to stimulation of bulbar reticular formation. Brain Res. 1987;409:97–110. doi: 10.1016/0006-8993(87)90745-1. [DOI] [PubMed] [Google Scholar]
- Iwakiri H, Oka T, Takakusaki K, Mori S. Stimulus effects of the medial pontine reticular formation and the mesencephalic locomotor region upon medullary reticulospinal neurons in acute decerebrate cats. Neurosci Res. 1995;23:47–53. [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Tanaka R. The mode of activation of pyramidal tract cells by intracortical stimuli. J Physiol. 1975;249:617–636. doi: 10.1113/jphysiol.1975.sp011034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Stecina K. Uncrossed actions of feline corticospinal tract neurones on lumbar interneurones evoked via ipsilaterally descending pathways. J Physiol. 2007;580:133–147. doi: 10.1113/jphysiol.2006.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis JD, Swadlow HA, Waxman SG, Brill MH. Variation in conduction velocity during the relative refractory and supernormal periods: a mechanism for impulse entrainment in central axons. Exp Neurol. 1979;65:230–236. doi: 10.1016/0014-4886(79)90263-2. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Waxman SG. Intra-axonal recordings in rat dorsal column axons: membrane hyperpolarization and decreased excitability precede the primary afferent depolarization. Brain Res. 1982;238:222–227. doi: 10.1016/0006-8993(82)90787-9. [DOI] [PubMed] [Google Scholar]
- Lamy JC, Ho C, Badel A, Arrigo RT, Boakye M. Modulation of soleus H reflex by spinal DC stimulation in humans. J Neurophysiol. 2012;108:906–914. doi: 10.1152/jn.10898.2011. [DOI] [PubMed] [Google Scholar]
- Lang N, Nitsche MA, Dileone M, Mazzone P, De Andres-Ares J, Diaz-Jara L, Paulus W, Di Lazzaro V, Oliviero A. Transcranial direct current stimulation effects on I-wave activity in humans. J Neurophysiol. 2011;105:2802–2810. doi: 10.1152/jn.00617.2010. [DOI] [PubMed] [Google Scholar]
- Lang N, Nitsche MA, Paulus W, Rothwell JC, Lemon RN. Effects of transcranial direct current stimulation over the human motor cortex on corticospinal and transcallosal excitability. Exp Brain Res. 2004a;156:439–443. doi: 10.1007/s00221-003-1800-2. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ernst D, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects. Biol Psychiatry. 2004b;56:634–639. doi: 10.1016/j.biopsych.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, Rothwell JC, Lemon RN, Frackowiak RS. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain. Eur J Neurosci. 2005;22:495–504. doi: 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN, Kirkwood PA, Maier MA, Nakajima K, Nathan P. Direct and indirect pathways for corticospinal control of upper limb motoneurons in the primate. Prog Brain Res. 2004;143:263–279. doi: 10.1016/S0079-6123(03)43026-4. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. An Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Klinker F, Hering D, Koch R, Nitsche MA, Potschka H, Loscher W, Paulus W, Tergau F. Anticonvulsant effects of transcranial direct-current stimulation (tDCS) in the rat cortical ramp model of focal epilepsy. Epilepsia. 2006;47:1216–1224. doi: 10.1111/j.1528-1167.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Ito K, Rodrigo-Angulo ML. Physiological studies of brainstem reticular connectivity. II. Responses of mPRF neurons to stimulation of mesencephalic and contralateral pontine reticular formation. Brain Res. 1987;409:111–127. doi: 10.1016/0006-8993(87)90746-3. [DOI] [PubMed] [Google Scholar]
- McCambridge AB, Bradnam LV, Stinear CM, Byblow WD. Cathodal transcranial direct current stimulation of the primary motor cortex improves selective muscle activation in the ipsilateral arm. J Neurophysiol. 2011;105:2937–2942. doi: 10.1152/jn.00171.2011. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Kocsis JD, Waxman SG. The supernormal period of the cerebellar parallel fibers effects of [Ca2+]o and [K+]o. Pflugers Arch. 1983;397:176–183. doi: 10.1007/BF00584354. [DOI] [PubMed] [Google Scholar]
- Miranda PC, Faria P, Hallett M. What does the ratio of injected current to electrode area tell us about current density in the brain during tDCS. Clin Neurophysiol. 2009;120:1183–1187. doi: 10.1016/j.clinph.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molaee-Ardekani B, Marquez-Ruiz J, Merlet I, Leal-Campanario R, Gruart A, Sanchez-Campusano R, Birot G, Ruffini G, Delgado-Garcia JM, Wendling F. Effects of transcranial Direct Current Stimulation (tDCS) on cortical activity: A computational modeling study. Brain Stimul. 2012 doi: 10.1016/j.brs.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Monte-Silva K, Kuo MF, Liebetanz D, Paulus W, Nitsche MA. Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS) J Neurophysiol. 2010;103:1735–1740. doi: 10.1152/jn.00924.2009. [DOI] [PubMed] [Google Scholar]
- Morrell F. Effect of anodal polarization on the firing pattern of single cortical cells. Ann N Y Acad Sci. 1961;92:860–876. doi: 10.1111/j.1749-6632.1961.tb40962.x. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A. Transcranial direct current stimulation: State of the art 2008. Brain stimulation. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation–technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255–276. doi: 10.1016/s1567-424x(09)70230-2. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W, Tergau F. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568:291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C. Cortical motor threshold and the thresholds and distribution of excited Betz cells in the cat. Q J Exp Physiol. 1956;41:71–83. doi: 10.1113/expphysiol.1956.sp001164. [DOI] [PubMed] [Google Scholar]
- Phillips CG, Porter R. London: Academic Press; 1977. Corticospinal neurones. Their role in movement; p. 450. [PubMed] [Google Scholar]
- Polania R, Paulus W, Nitsche MA. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum Brain Mapp. 2012;33:2499–2508. doi: 10.1002/hbm.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Reis J, Fritsch B. Modulation of motor performance and motor learning by transcranial direct current stimulation. Curr Opin Neurol. 2011;24:590–596. doi: 10.1097/WCO.0b013e32834c3db0. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Stecina K, Jankowska E. Uncrossed actions of feline corticospinal tract neurones on hindlimb motoneurones evoked via ipsilaterally descending pathways. J Physiol. 2007;580:119–132. doi: 10.1113/jphysiol.2006.122721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA, Waxman SG. Observations on impulse conduction along central axons. Proc Natl Acad Sci U S A. 1975;72:5156–5159. doi: 10.1073/pnas.72.12.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA, Waxman SG. Variations in conduction velocity and excitability following single and multiple impulses of visual callosal axons in the rabbit. Exp Neurol. 1976;53:128–150. doi: 10.1016/0014-4886(76)90288-0. [DOI] [PubMed] [Google Scholar]
- Wagner T, Fregni F, Fecteau S, Grodzinsky A, Zahn M, Pascual-Leone A. Transcranial direct current stimulation: A computer-based human model study. Neuro-Image. 2007;35:1113–1124. doi: 10.1016/j.neuroimage.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Swadlow HA. The conduction properties of axons in central white matter. Prog Neurobiol. 1977;8:297–324. doi: 10.1016/0301-0082(77)90009-0. [DOI] [PubMed] [Google Scholar]
- Winkler T, Hering P, Straube A. Spinal DC stimulation in humans modulates post-activation depression of the H-reflex depending on current polarity. Clin Neurophysiol. 2010;121:957–961. doi: 10.1016/j.clinph.2010.01.014. [DOI] [PubMed] [Google Scholar]


