Abstract
Objective
To investigate the effectiveness of brief bedside “booster” cardiopulmonary resuscitation (CPR) training to improve CPR guideline compliance of hospital-based pediatric providers.
Design
Prospective, randomized trial.
Setting
General pediatric wards at Children’s Hospital of Philadelphia.
Subjects
Sixty-nine Basic Life Support–certified hospital-based providers.
Intervention
CPR recording/feedback defibrillators were used to evaluate CPR quality during simulated pediatric arrest. After a 60-sec pretraining CPR evaluation, subjects were randomly assigned to one of three instructional/feedback methods to be used during CPR booster training sessions. All sessions (training/CPR manikin practice) were of equal duration (2 mins) and differed only in the method of corrective feedback given to participants during the session. The study arms were as follows: 1) instructor-only training; 2) automated defibrillator feedback only; and 3) instructor training combined with automated feedback.
Measurements and Main Results
Before instruction, 57% of the care providers performed compressions within guideline rate recommendations (rate >90 min−1 and <120 min−1); 71% met minimum depth targets (depth, >38 mm); and 36% met overall CPR compliance (rate and depth within targets). After instruction, guideline compliance improved (instructor-only training: rate 52% to 87% [p .01], and overall CPR compliance, 43% to 78% [p < .02]; automated feedback only: rate, 70% to 96% [p = .02], depth, 61% to 100% [p < .01], and overall CPR compliance, 35% to 96% [p < .01]; and instructor training combined with automated feedback: rate 48% to 100% [p < .01], depth, 78% to 100% [p < .02], and overall CPR compliance, 30% to 100% [p < .01]).
Conclusions
Before booster CPR instruction, most certified Pediatric Basic Life Support providers did not perform guideline-compliant CPR. After a brief bedside training, CPR quality improved irrespective of training content (instructor vs. automated feedback). Future studies should investigate bedside training to improve CPR quality during actual pediatric cardiac arrests.
Keywords: pediatric, cardiopulmonary resuscitation, quality appraisal
Sudden pediatric cardiac arrest is a major public health problem with >8,000 inhospital pediatric arrests occurring each year (1, 2). Survival with good neurologic outcome after these events is not common (3–5). Prompt delivery of high-quality cardiopulmonary resuscitation (CPR) improves outcome substantially (6–8). Unfortunately, the performance of CPR during the actual resuscitation from cardiac arrest is highly variable and often of substandard quality, even when performed by trained healthcare providers (9–13). Difficulties with long interruptions in CPR, overventilation, and chest compressions at inadequate rates and depths have been reported (9–13).
Nurses, resident physicians, and other Basic Life Support (BLS)-certified providers are frequently the first to respond to an acute pediatric event not occurring in an intensive care unit. Although poor baseline CPR performance of adult first-responders has been documented (14), whether these same deficiencies exist in pediatric care providers is unknown. Furthermore, the efficacy of automated feedback devices (15, 16) and focused instructor-facilitated bedside skill training (14) have not been studied extensively in a pediatric population. In this prospective, randomized, interventional trial, we evaluated whether a novel bedside CPR skill-training program could improve CPR performance of hospital-based pediatric BLS providers. We hypothesized that baseline CPR performance would be highly variable. Further, we hypothesized that brief manikin CPR practice sessions (“booster” trainings), with either instructor or defibrillator feedback, would result in >75% of participants delivering high-quality American Heart Association (AHA) guideline-compliant CPR (17) during posttraining evaluations.
MATERIALS AND METHODS
This investigation was a prospective, randomized trial with a primary objective of determining the effectiveness of brief bedside CPR skill training to improve CPR quality as evaluated immediately after training during a simulated pediatric cardiac arrest. This study was conducted as part of a larger trial with an overall objective of determining the effectiveness of these bedside programs to improve CPR skill retention over time and multiple training sessions.
The study protocol, including consent procedures, was approved by the institutional review board at Children’s Hospital of Philadelphia and the University of Pennsylvania. Data-collection procedures were completed in compliance with the guidelines of the Health Insurance Portability and Accountability Act to ensure subject confidentiality. Verbal consent was obtained from all healthcare providers who participated in the simulated resuscitative attempts.
All pediatric inhospital care providers with BLS training (registered nurses, medical resident physicians) working on the general inpatient wards at Children’s Hospital of Philadelphia were eligible for inclusion in this study. To facilitate the study of bedside training effectiveness in a naive population, all intensive care units were excluded because of an existing and ongoing bedside CPR training program in these acute care areas (18). In addition, providers who were unable to perform 120 secs of continuous CPR, and those with previous exposure to the Heartstart MRx defibrillator with Q-CPR system, jointly designed by Philips Healthcare (Andover, MA) and the Laerdal Medical Corporation (Stavanger, Norway), in the last 12 months were also excluded.
The Heartstart MRx defibrillator with Q-CPR system was used in this investigation for training purposes and for recording quantitative CPR data during the evaluation sessions. Each defibrillator has an oval pad that was placed on the lower part of the manikin sternum (pad dimensions, 127 × 62 × 24 mm). The pad contains an accelerometer and a force sensor that detect quantitative chest-compression data by using a method previously validated to an accuracy of 1.6 mm (19). This quantitative information is transmitted to a recording component on the defibrillator and stored internally. The sensing and recording software have also been validated (20). The defibrillator monitor is equipped with a graphical display and loudspeaker that were used to provide real-time automated feedback on CPR technique for participants assigned to study groups in which this function was enabled. The feedback is intended to drive CPR performance toward the following age-appropriate AHA specifications: chest compression depth, ≥ 38 mm (1.5 in); rate, ≥ 90 compressions per min or ≤120 compressions per min; and chest compression pauses, ≤15 secs.
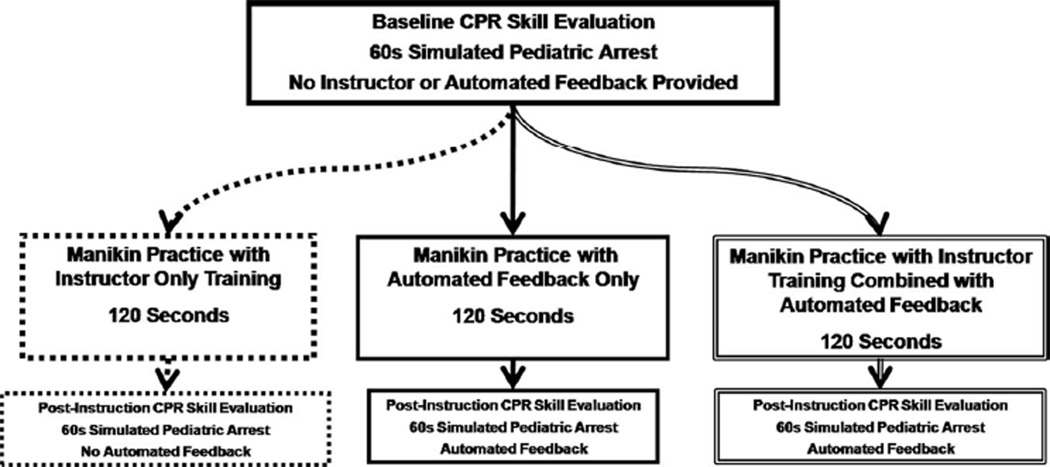
Before they were randomly assigned to the training arms, all subjects completed a 60-sec CPR skill evaluation (without feedback) to evaluate baseline CPR quality performance. To ensure balanced study-group allocation, instructors selected a premarked slip that indicated the allocation assignment from an unmarked envelope (i.e., block-randomization packet containing 25 premarked slips per envelope). Three intervention arms were used to determine the efficacy of brief bedside CPR training to improve CPR quality delivered during simulated pediatric arrest (Fig. 1). All sessions were of equal duration (~120 secs) and differed only in the method of corrective feedback given to participants during the session.
Figure 1.
Study design flow sheet. CPR, cardiopulmonary resuscitation.
Instructor-Only Training
This group received a brief (~120-sec) instructor-led CPR skill training session. This training consisted of a short (~30-sec) scripted verbal instruction on how to perform high-quality CPR immediately followed by manikin practice time for the remainder of the session (~90 secs). During the practice time, participants were given further unscripted verbal feedback on their CPR performance as assessed by the instructor who led the session. After the training session (verbal instruction/subsequent manikin practice), the participants’ performance was reassessed during a 60-sec period of simulated arrest without corrective feedback from the instructor or the defibrillator.
Automated Feedback Only
This group received a brief (~120-sec) CPR skill training session that consisted primarily of defibrillator automated corrective feedback. This training included a short (~30-sec) scripted verbal introduction to the feedback supplied by the MRx defibrillator (i.e., they were familiarized with the auditory prompts and visual feedback) immediately followed by manikin practice for the remainder of the session (~90 secs). During the practice time, participants were given both audio and visual automated feedback assistance. After the training session (verbal introduction to the technology/subsequent manikin practice), a 60-sec epoch of simulated cardiac arrest resuscitation was completed, during which the defibrillator automated feedback was enabled.
Instructor Training Combined with Automated Feedback
A combination of the first two groups, this arm received a brief (120-sec) instructor-led CPR training session that was optimized with defibrillator automated corrective audiovisual feedback. This training included a short (~30-sec) scripted verbal instruction on how to perform high-quality CPR and an introduction to the feedback supplied by the MRx defibrillator. Again, this introduction was immediately followed by manikin practice for the remainder of the session (~90 secs). During the practice time, participants were given not only instructor-led feedback but also audio and visual automated feedback assistance from the MRx. After the training session (verbal introduction/subsequent manikin practice), a 60-sec epoch of simulated cardiac arrest resuscitation was completed, during which the defibrillator automated feedback was enabled.
All CPR (i.e., during both training and evaluation sessions) was performed on a pediatric prototype manikin, the Voice Advisory Manikin Junior (Laerdal Medical Corporation), which is anatomically similar to a 7-yrold child and specifically engineered for pediatric CPR training and evaluation. During the CPR psychomotor skill evaluation sessions, participants performed two-rescuer pediatric BLS CPR according to the current AHA guidelines (15:2 chest compression/ventilation ratio with a target minimal pediatric depth of one-third anterior-posterior chest depth) during 60-sec epochs of simulated pediatric cardiac arrest. During these sessions, the participants were responsible for the provision of chest compressions as an investigator delivered standardized AHA guideline-specified ventilations (1-sec inflation time). By design, there was no change-over of provider role during the evaluations. These booster training sessions were completed during the participant’s normal working hours in the patient care areas (i.e., “at the bedside”). However, all sessions were completed out of view of other participants to avoid contamination of training arms.
Baseline demographic data were collected, including sex, age (yrs), time since last formal BLS education (months), years of formal education (e.g., high school graduate = 12, college graduate = 16), primary training discipline (nurse, physician), years of experience in current training, and current Advanced Cardiac Life Support certification. CPR-quality data for each 60-sec CPR epoch included average chest compression rate (min−1), average compression depth (mm), and CPR no-flow fraction as continuous variables and compression rate compliance (rate ≥90 sec and ≤120 min−1), compression depth compliance (depth, ≥38 mm), and overall CPR compliance (both rate and depth within targets) as dichotomous variables. The primary outcome variable for the analysis plan was overall CPR compliance (both rate and depth within targets); all other variables were secondary. A given instructional session was deemed “successful” if an a priori target of at least 75% of the participants met overall CPR-guideline compliance (both compression rate and depth with targets) during postinstruction testing. In a secondary analysis, we also assessed the efficacy of training content (instructor vs. automated feedback) according to whether there were differences in the variability of CPR performance (chest compression rate and depth) during the posttraining evaluation sessions.
Statistical Analysis
A Microsoft Windows–based software program, Q-CPR Review (Version 2.1.0.0, Laerdal Medical Corporation), was used for initial examination and extraction of the quantitative CPR-quality data. Standard descriptive statistics were calculated as appropriate for the distribution of each variable. Paired analyses were performed to compare measures of CPR quality at the baseline evaluation with those measured during the postinstruction epoch. Continuous parametric variables were analyzed by using a paired t test; nonparametric variables were analyzed by using a Wilcoxon signed-rank test. Categorical variables were compared by using a χ2 test. Levene’s robust test (F test) was used to assess the equality of variance in continuous measures of CPR quality at baseline and after instruction. A p value of <.05 was considered statistically significant. Statistical analysis was completed by using the Stata-IC statistical package (Version 10.0, Stata Corp, College Station, TX).
RESULTS
Subject Characteristics
Sixty-nine hospital-based pediatric care providers were approached for inclusion into the study. All of them (69 of 69 [100%]) met inclusion criteria, consented to study participation, were randomly assigned, and completed all study procedures. Intervention groups were similar in baseline demographics, including sex, age, years of education, time since last CPR instruction, primary training discipline, years of experience, and previous Advanced Cardiac Life Support training (Table 1).
Table 1.
Demographic data of participants
| Instructor Only (n = 23) |
Automated Feedback Only (n = 23) |
Instructor and Feedback (n = 23) |
p | |
|---|---|---|---|---|
| Sex, female, n (%) | 23 (100) | 22 (96) | 20 (87) | .2 |
| Age, mean (SD), yrs | 28.6 (6.6) | 30.2 (7.5) | 28.3 (6.6) | .6 |
| Education, mean (SD), yrs | 17.5 (2.2) | 17.0 (1.6) | 16.8 (1.4) | .4 |
| Last CPR instruction, median (range), mos | 9 (0.25–24) | 6 (0.5–24) | 9 (0.25–24) | .8 |
| Experience primary training, mean (SD), yrs | 3.2 (3.6) | 4.7 (7.0) | 3.4 (5.0) | .8 |
| Current ACLS certification, n (%) | 3 (13) | 4 (17) | 2 (9) | .7 |
| Primary degree, nursing, n (%) | 20 (87) | 20 (87) | 21 (91) | .9 |
CPR, cardiopulmonary resuscitation; ACLS, Advanced Cardiac Life Support.
Primary Outcome Variable: Compliance with CPR Guideline Recommendations
During baseline evaluations, 57% of participants had average compression rates within guideline recommendations (rate, ≥90 mins−1 and ≤120 mins−1); 71% met minimum depth targets (depth, ≥38 mm); and 36% had overall CPR compliance (both rate and depth within targets). There were no statistically significant differences in baseline AHA compliance among the training groups for compression rate, depth, or overall CPR compliance. After instruction, CPR performed in compliance with AHA guidelines was more common in all groups (Table 2). In the instructor-only training group, compression rate compliance improved from 52% to 87% (p = .01), and overall CPR compliance improved from 43% to 78% (p = .02); although depth compliance improved from 74% to 87%, the change was not significant (p = .26). In the automated feedback only group, compression rate compliance improved from 70% to 96% (p = .02), depth compliance improved from 61% to 100% (p = .01), and overall CPR compliance improved from 35% to 96% (p = .01). In the instructor training combined with automated feedback group, compression rate compliance improved from 48% to 100% (p < .01), depth compliance improved from 78% to 100% (p < .02), and overall CPR compliance improved from 30% to 100% (p < .01).
Table 2.
Continuous cardiopulmonary resuscitation quality variables
| Rate, min−1 | Depth, mm | No Flow Fraction, %a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | p | Mean (SD) | p | Mean (SD) | p | |||||||
| Before | After | t Test | F Test | Before | After | t Test | F Test | Before | After | t Test | F Test | |
| Instructor-only training | 103 (20) | 107 (10) | .4 | <.02 | 42 (11) | 45 (6) | .14 | <.04 | 28 (5) | 25 (4) | <.02 | <.04 |
| Automated feedback only | 110 (16) | 102 (6) | .02 | <.01 | 41 (7) | 45 (4) | <.01 | <.01 | 30 (5) | 27 (4) | <.02 | .4 |
| Instructor training combined with automated feedback | 104 (22) | 105 (5) | .9 | <.01 | 42 (7) | 44 (3) | .11 | .02 | 29 (6) | 27 (4) | <.2 | <.04 |
Percentage of arrest time without provision of chest compressions.
Continuous CPR-Quality Variables
Differences between baseline and postinstruction CPR-quality evaluations were seen in the automated feedback only group for the following: mean chest compression rate 110 ± 16 min−1 vs. 102 ± 6 min−1 (p = .02); mean chest compression depth, 41 ± 7 mm vs. 45 ± 4 mm (p < .01); and mean CPR no-flow fraction 30% ± 5% vs. 27% ± 4% (p < .02). In the instructor-only training group, mean CPR no-flow fraction decreased (28% ± 5% vs. 25 ± 4%, p < .02). No significant differences between the means of the continuous CPR quality variables were seen in the instructor training combined with automated feedback group (Table 3).
Table 3.
Compliance with cardiopulmonary resuscitation guideline recommendations
| Guideline Rate (≥90 mins−1 and ≤120 mins−1) |
Guideline Depth (≥38 mm) |
Guideline CPR (Both) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Proportion | Proportion | Proportion | |||||||
| Before | After | p | Before | After | p | Before | After | p | |
| Instructor-only training | 52 | 87 | .01 | 74 | 87 | .26 | 43 | 78 | <.02 |
| Automated feedback only | 70 | 96 | .02 | 61 | 100 | <.01 | 35 | 96 | <.01 |
| Instructor training combined with automated feedback | 48 | 100 | <.01 | 78 | 100 | <.02 | 30 | 100 | <.01 |
CPR, cardiopulmonary resuscitation.
Significant changes were noted in the distribution/spread of these CPR variables in all groups, such that extremes of value (i.e., quality performance outside of guideline recommendations) were less common in the postinstruction testing for all three CPR-quality variables.
Comparison of CPR Skill Variance Among Training Groups After Instruction
Although all interventional groups were able to meet the a priori determination of training success (75% of participants with depth and rate guideline-compliant CPR), there were differences in the variance of the continuous CPR-quality variables noted in the postinstruction CPR epochs between training arms (i.e., content of training seemed to have an effect on the statistical variation of posttraining CPR quality; F test p values for the comparison of variance reported). For compression rate, both automated feedback only and instructor training combined with automated feedback had less statistical variation after training compared with the instructor-only training group (p < .01 for both comparisons). For compression depth, instructor training combined with automated feedback had less statistical variation after training compared to the instructor-only training group (p < .01), and a similar trend was observed in the automated feedback group compared to the instructor-only training group (p < .07). No differences in chest compression variance were noted between the automated feedback only and instructor training combined with automated feedback groups for either compression rate or depth.
DISCUSSION
This study represents the first in-depth evaluation of bedside booster CPR training techniques during simulated pediatric arrest. This investigation establishes that front-line hospital-based pediatric care providers, who have a duty to respond to cardiac arrest, frequently do not perform guideline-compliant CPR. Furthermore, our results show that a focused and brief instructional program, irrespective of training content, was effective and feasible for broad application to BLS providers in an academic medical center. Although all training groups met our a priori target of 75% of participants achieving CPR skill proficiency, the combination of automated feedback technology and human instruction resulted in 100% guideline compliant CPR, a compliance rate that far exceeds previously published interventions (21–24).
During the brief bedside trainings, instructor facilitation alone was successful for improving CPR guideline compliance and chest compression variability. Therefore, our findings demonstrate that a brief booster training program without technologically advanced defibrillators, which may be costly for institutions, can improve CPR skills of providers in the immediate postinstruction period. On the other hand, participants using automated feedback delivered higher-quality CPR, as demonstrated by a further reduction in compression variability after booster training sessions. This pertains especially to chest compression depth, for which instructor training alone did not result in improved guideline compliance. It is important to note that more than 90% of the participants in the training groups who received automated feedback during the evaluation sessions performed guideline compliant CPR for both compression rate and depth. However, more work is needed to determine whether these brief manikin practice sessions are adequate to ensure competency over time.
For CPR training programs to be successful, they must take into account the needs of the busy adult learner (i.e., convenient, relevant, focused, and delivered to the target population) (25–27). Our novel booster training interventions fulfill these requirements. Rather than participants attending formal classroom instruction, we brought the learning to the learners. We concentrated the “curriculum” to focus specifically on previously documented deficiencies and allowed limitation of instruction time to <2 mins. Such brevity increases the convenience of these programs to participants. Furthermore, we have targeted the relevant population: registered nurses and resident physicians, those most likely to respond before the arrival of highly trained intensive care unit providers during those initial critical moments of a pediatric arrest. In short, these facts reinforce the strength of the study design and relevance to pediatric resuscitation education.
Although we have demonstrated improvements in immediate CPR performance after booster training, retention of providers has not been assessed and is the primary objective of our larger ongoing trial. It is likely that repetition of our training programs will be necessary to ensure long-term effects of training. As an analogy, traditional formal CPR training classes may act as an initial “immunization” against disease (CPR skill error). Because CPR skill decay is known to occur over time, “immunity” seems to fade. Our brief training programs can be likened to a booster immunization, whereby an amnestic response is elicited, immunity is restored, and susceptibility to disease (CPR skill decay) is decreased. However, the frequency necessary to maintain immunity from declining CPR skills is unknown at this time.
This simulation manikin training study has several notable limitations. First, although we demonstrated improved CPR skills immediately after training, the durability of improvement (skill retention) is still in question. However, even if repetition is needed on a monthly basis to maintain skills, the limited burden of these brief trainings (~2 mins per subject) would be outweighed by the benefit of improved resuscitative care when concentrated to an area in which arrests are more likely to occur (i.e., in the intensive care units) (18). Second, only modest differences were noted between training groups after instruction. The superiority of instructor feedback vs. automated defibrillator feedback during brief trainings has still not been established. However, because this study is part of a larger ongoing trial to assess skill retention, we hypothesize that instructor presence will improve skill retention by providing a “personalized” aspect to the bedside trainings. In short, although our results show adequate initial skill acquisition for all training groups, the actual content of brief bedside training that is best for improving skill retention over time and multiple trainings is still unknown.
Next, given that most of the study participants were female nurses, there is a theoretical concern that it will be difficult to generalize our findings more broadly to other pediatric care providers. However, the success of this program is most likely attributable to its focus on the needs of the adult learner (brief, convenient, relevant) and should be applicable to not only other pediatric care providers but hospital-based adult responders as well. Because existing BLS training recommendations follow a high-intensity, low-frequency paradigm (recertification every 2 yrs), the success of this low-intensity program is not only interesting but has the potential to change training methods in the future. It is important to note that, although we have demonstrated improvements in CPR quality variables in manikins, we do not know if this improved competence on manikins will translate to higher quality CPR performed during actual resuscitative attempts. We anticipate that a multicentered study will be necessary to answer this question because of the relative rarity of pediatric cardiac arrests. To that end, the information obtained in this investigation, specifically the low rates of CPR compliance in pediatric first-responders (36%), can be used to power future studies aimed at improving CPR quality. In addition, because our sessions were completed during normal working hours, they were required to be as brief as possible so as not to interfere substantially with a given individual’s workday. Due to the brevity of the evaluation sessions, providers were not required to switch roles, which limited our full evaluation of two important aspects of CPR quality: no-flow time and ventilation error. Although the convenience of these programs to participants was preserved in these short evaluation sessions, future studies of bedside training should further evaluate these important CPR quality variables. Finally, although the automated feedback system drives chest-compression depth performance to a target depth of 38 mm (the existing adult minimal depth), recent anthropometric and computed tomography evidence collected from actual children support use of this depth to coach toward the pediatric target of one-third anterior-posterior chest depth (28 –30). Furthermore, although the depth of 38 mm is slightly less than one-third anterior-posterior chest depth (41 mm) of the pediatric manikin used in this study, we believe that the study data demonstrate the effectiveness of multiple, brief, bedside CPR instructional programs to train healthcare personnel to provide CPR to a target rate and depth of compressions determined a priori.
CONCLUSIONS
The results of this randomized interventional study establishes that brief bedside CPR skill training, irrespective of instructional feedback method, improves CPR quality as evaluated immediately after training during simulated pediatric cardiac arrest. Future studies should investigate the durability of these brief trainings (i.e., skill retention) and, more importantly, the effectiveness of bedside training to improve the quality of resuscitation during actual pediatric cardiac arrests.
ACKNOWLEDGMENTS
We thank all of the nurses and pediatric resident physicians on the general inpatient wards at Children’s Hospital of Philadelphia who participated.
This study was supported, in part, by a Laerdal Medical Foundation Center of Excellence Grant, a medical education grant from the University of Pennsylvania School of Medicine, and the Endowed Chair of Pediatric Critical Care Medicine at Children’s Hospital of Philadelphia.
Unrestricted research grant support was provided to Vinay Nadkarni, Dana Niles, and Robert Sutton from the Laerdal Foundation and to Benjamin Abella from Philips Healthcare and Cardiac Science Corporation.
Footnotes
The remaining authors have not disclosed any potential conflicts of interest.
REFERENCES
- 1.Atkins DL, Everson-Stewart S, Sears GK, et al. Epidemiology and outcomes from out-of-hospital cardiac arrest in children: The Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Circulation. 2009;119:1484–1491. doi: 10.1161/CIRCULATIONAHA.108.802678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang PS, Jain R, Nallmothu BK, et al. Rapid response teams: A systematic review and meta-analysis. Arch Intern Med. 2010;170:18–26. doi: 10.1001/archinternmed.2009.424. [DOI] [PubMed] [Google Scholar]
- 3.Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295:50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 4.Donoghue AJ, Nadkarni V, Berg RA, et al. Out-of-hospital pediatric cardiac arrest: An epidemiologic review and assessment of current knowledge. Ann Emerg Med. 2005;46:512–522. doi: 10.1016/j.annemergmed.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 5.Meaney PA, Nadkarni VM, Cook EF, et al. Higher survival rates among younger patients after pediatric intensive care unit cardiac arrests. Pediatrics. 2006;118:2424–2433. doi: 10.1542/peds.2006-1724. [DOI] [PubMed] [Google Scholar]
- 6.Berg RA, Sanders AB, Kern KB, et al. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104:2465–2470. doi: 10.1161/hc4501.098926. [DOI] [PubMed] [Google Scholar]
- 7.Gallagher EJ, Lombardi G, Gennis P. Effectiveness of bystander cardiopulmonary resuscitation and survival following out-of-hospital cardiac arrest. JAMA. 1995;274:1922–1925. [PubMed] [Google Scholar]
- 8.Van Hoeyweghen RJ, Bossaert LL, Mullie A, et al. Quality and efficiency of bystander CPR. Belgian Cerebral Resuscitation Study Group. Resuscitation. 1993;26:47–52. doi: 10.1016/0300-9572(93)90162-j. [DOI] [PubMed] [Google Scholar]
- 9.Abella BS, Sandbo N, Vassilatos P, et al. Chest compression rates during cardiopulmonary resuscitation are suboptimal: A prospective study during in-hospital cardiac arrest. Circulation. 2005;111:428–434. doi: 10.1161/01.CIR.0000153811.84257.59. [DOI] [PubMed] [Google Scholar]
- 10.Abella BS, Alvarado JP, Myklebust H, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005;293:305–310. doi: 10.1001/jama.293.3.305. [DOI] [PubMed] [Google Scholar]
- 11.Aufderheide TP, Lurie KG. Death by hyperventilation: A common and life-threatening problem during cardiopulmonary resuscitation. Crit Care Med. 2004;32(9 Suppl):S345–S351. doi: 10.1097/01.ccm.0000134335.46859.09. [DOI] [PubMed] [Google Scholar]
- 12.Sutton RM, Niles D, Nysaether J, et al. Quantitative analysis of CPR quality during in-hospital resuscitation of older children and adolescents. Pediatrics. 2009;124:494–499. doi: 10.1542/peds.2008-1930. [DOI] [PubMed] [Google Scholar]
- 13.Wik L, Kramer-Johansen J, Myklebust H, et al. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA. 2005;293:299–304. doi: 10.1001/jama.293.3.299. [DOI] [PubMed] [Google Scholar]
- 14.Dine CJ, Gersh RE, Leary M, et al. Improving cardiopulmonary resuscitation quality and resuscitation training by combining audiovisual feedback and debriefing. Crit Care Med. 2008;36:2817–2822. doi: 10.1097/CCM.0b013e318186fe37. [DOI] [PubMed] [Google Scholar]
- 15.Abella BS, Edelson DP, Kim S, et al. CPR quality improvement during in-hospital cardiac arrest using a real-time audiovisual feedback system. Resuscitation. 2007;73:54–61. doi: 10.1016/j.resuscitation.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Kramer-Johansen J, Myklebust H, Wik L, et al. Quality of out-of-hospital cardiopulmonary resuscitation with real time automated feedback: A prospective interventional study. Resuscitation. 2006;71:283–292. doi: 10.1016/j.resuscitation.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 17.American Heart Association: 2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: Pediatric Basic Life Support. Pediatrics. 2006;117:e989–e1004. doi: 10.1542/peds.2006-0219. [DOI] [PubMed] [Google Scholar]
- 18.Niles D, Sutton RM, Donoghue A, et al. “Rolling refreshers”: A novel approach to maintain CPR psychomotor skill competence. Resuscitation. 2009;80:909–912. doi: 10.1016/j.resuscitation.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Aase SO, Myklebust H. Compression depth estimation for CPR quality assessment using DSP on accelerometer signals. IEEE Trans Biomed Eng. 2002;49:263–268. doi: 10.1109/10.983461. [DOI] [PubMed] [Google Scholar]
- 20.Handley AJ, Handley SA. Improving CPR performance using an audible feedback system suitable for incorporation into an automated external defibrillator. Resuscitation. 2003;57:57–62. doi: 10.1016/s0300-9572(02)00400-8. [DOI] [PubMed] [Google Scholar]
- 21.Brennan RT, Braslow A. Skill mastery in cardiopulmonary resuscitation training classes. Am J Emerg Med. 1995;13:505–508. doi: 10.1016/0735-6757(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 22.Brennan RT, Braslow A. Skill mastery in public CPR classes. Am J Emerg Med. 1998;16:653–657. doi: 10.1016/s0735-6757(98)90167-x. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton R. Nurses’ knowledge and skill retention following cardiopulmonary resuscitation training: A review of the literature. J Adv Nurs. 2005;51:288–297. doi: 10.1111/j.1365-2648.2005.03491.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaye W, Mancini ME. Retention of cardiopulmonary resuscitation skills by physicians, registered nurses, and the general public. Crit Care Med. 1986;14:620–622. doi: 10.1097/00003246-198607000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Brennan RT. Student, instructor, and course factors predicting achievement in CPR training classes. Am J Emerg Med. 1991;9:220–224. doi: 10.1016/0735-6757(91)90080-4. [DOI] [PubMed] [Google Scholar]
- 26.Braslow A, Brennan RT, Newman MM, et al. CPR training without an instructor: Development and evaluation of a video self-instructional system for effective performance of cardiopulmonary resuscitation. Resuscitation. 1997;34:207–220. doi: 10.1016/s0300-9572(97)01096-4. [DOI] [PubMed] [Google Scholar]
- 27.Eisenburger P, Safar P. Life supporting first aid training of the public: review and recommendations. Resuscitation. 1999;41:3–18. doi: 10.1016/s0300-9572(99)00034-9. [DOI] [PubMed] [Google Scholar]
- 28.Braga MS, Dominguez TE, Pollock AN, et al. Estimation of optimal CPR chest compression depth in children by using computer tomography. Pediatrics. 2009;124:e69–e74. doi: 10.1542/peds.2009-0153. [DOI] [PubMed] [Google Scholar]
- 29.Kao PC, Chiang WC, Yang CW, et al. What is the correct depth of chest compression for infants and children? A radiological study. Pediatrics. 2009;124:49–55. doi: 10.1542/peds.2008-2536. [DOI] [PubMed] [Google Scholar]
- 30.Sutton RM, Niles D, Nysaether J, et al. Pediatric CPR quality monitoring: Analysis of thoracic anthropometric data. Resuscitation. 2009;80:1137–1141. doi: 10.1016/j.resuscitation.2009.06.031. [DOI] [PubMed] [Google Scholar]