Abstract
Regioselective ring expansion of alkynyl cyclopropanes to highly substituted cyclobutenes was developed. The reaction involves a copper-catalyzed cycloaddition of an alkyne with an arylsulfonyl azide and a silver-catalyzed carbene formation followed by ring expansion of a cyclopropyl carbene intermediate.
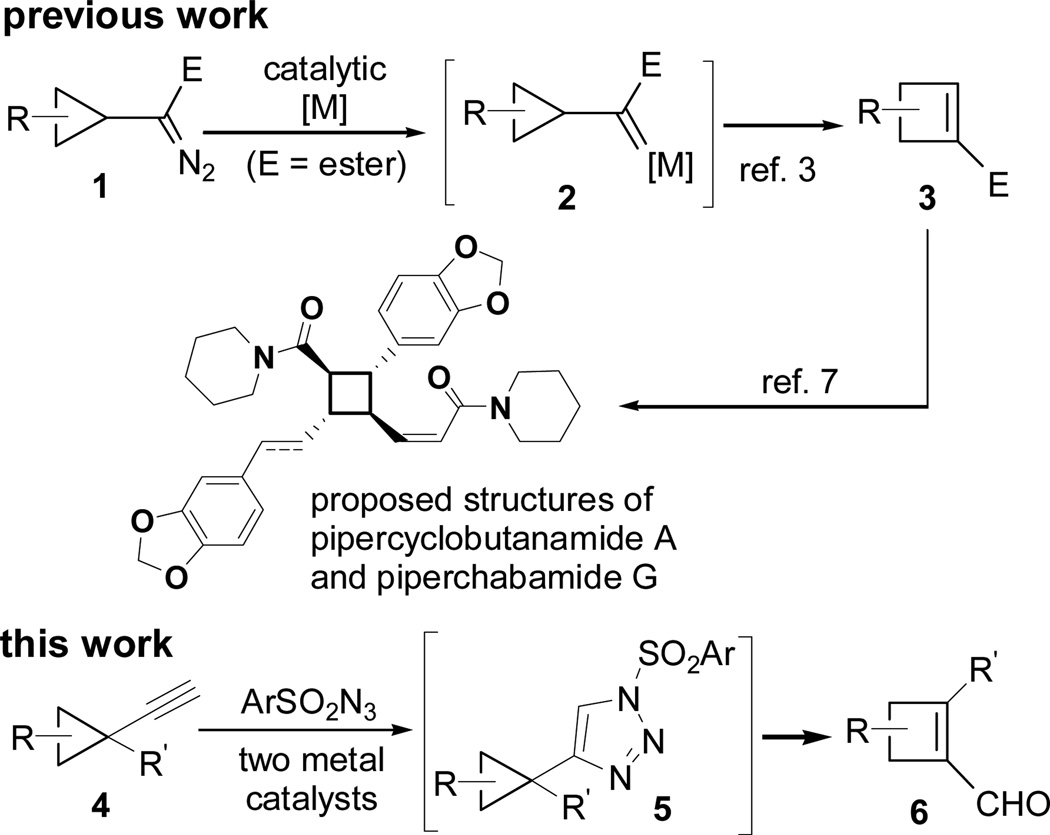
Four-membered rings are frequently presented in bioactive natural products and employed as key intermediates for the preparation of complex targets.1 Efficient synthesis of functionalized four-membered rings still stimulate the development of new selective methods that complements existing technologies.2 We previously developed a method for the synthesis of highly substituted cyclobutenes from cyclopropyl metal carbenes derived from Rh(II), Ag(I), or Cu(I)-catalyzed decomposition of diazo compounds (Scheme 1).3, 4 We took advantage of the well-documented stereoselective cyclopropanation methods5 and transferred the substituents and stereochemistry of cyclopropanes to cyclobutenes.6 This method was recently applied to the diastereo- and enantioselective synthesis of the proposed structures of natural products pipercyclobutanamide A and piperchabamide G.7 However, cyclopropyl diazo compound 1 is not very stable and its preparation is often lengthy.
Scheme 1.
Ring Expansion of Cyclopropyl Metal Carbene
To search for a more convenient and stable precursor for cyclopropyl carbenes, we turned our attention to N-sulfonyl 1,2,3-triazoles,8 a diazo compound equivalent developed by Fokin and Gevorgyan for annulation, cyclopropanation and C-H insertion reactions.9, 10, 11 We found that cyclobutene carboxylaldehyde 6 could be prepared directly from alkynyl cyclopropane 4 through triazole intermediate 5 (Scheme 1), whose isolation was not necessary when two metal catalysts were employed.
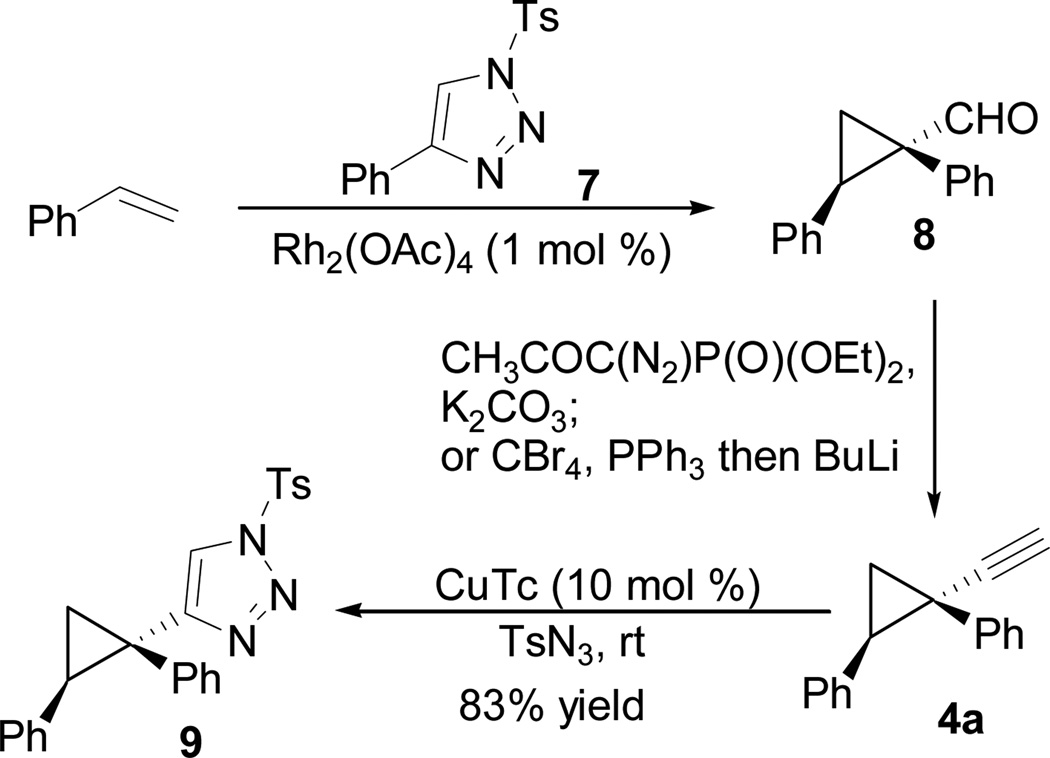
Known aldehyde 8 was prepared by cyclopropanation of styrene with tosyl triazole 7.10, 11 Homologation of aldehyde 8 then afforded cyclopropyl acetylene 4a (Scheme 2).12 The acetylene in 4a could be converted to tosyl triazole 9 following literature procedures.13
Scheme 2.
Preparation of Substituted Cyclopropanes from Alkenes
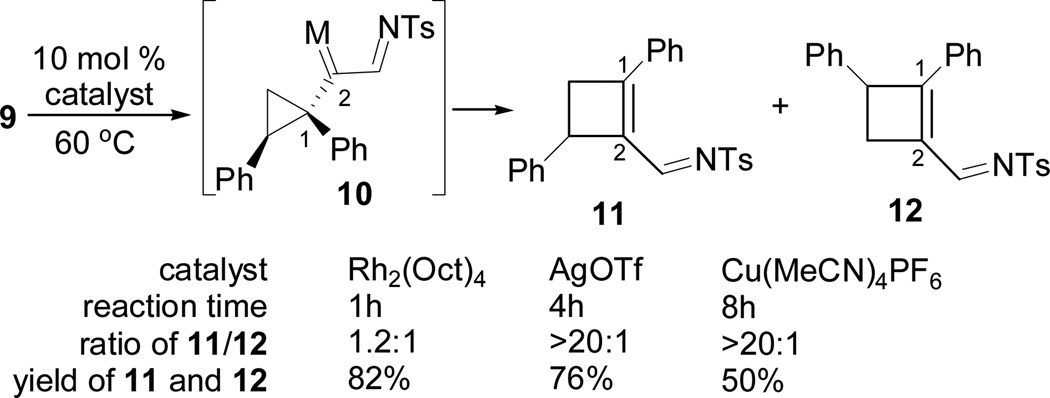
Triazole 9 was then treated with three catalysts that we previously used for the decomposition of cyclopropyl diazo compounds (Scheme 3).3 Although Rh2(Oct)4 was a more reactive catalyst than AgOTf and Cu(MeCN)4PF6, the latter two provided much higher regioselectivity for the formation of cyclobutene 11 over isomer 12. Through the formation of tosyl triazoles 7 and 9, carbons 1 and 2 in intermediate 10 were converted to metal carbenes from alkynes conveniently.
Scheme 3.
Effect of Catalysts on the Regioselectivity of Ring Expansion
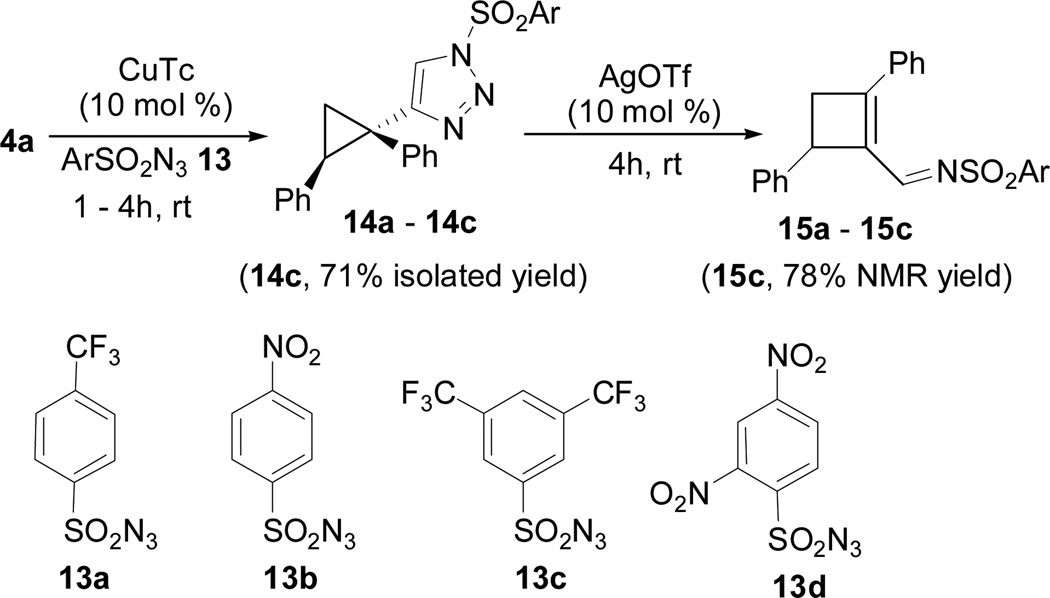
We then examined the effect of arylsulfonyl azide on triazole formation and ring expansion (Scheme 4). It has been reported that the reactivity of N-sulfonyl 1,2,3-triazole increases and its stability decreases when the tosyl group was replaced by a triflate.11 We prepared triazoles 14a-14c from alkyne 4a and azides 13a-13c, respectively. We found that the ring expansion of triazole 14c could be completed in 4h at room temperature in the presence of a Ag(I) catalyst, while low conversions were observed for triazoles 14a and 14b under the same condition. In the absence of any catalyst, no reaction was observed at room temperature after 24h. Triazole 14d derived from alkyne 4a and azide 13d was not stable enough to be isolated. Triazole 14c has the balanced reactivity and stability.
Scheme 4.
Effect of Azides on Ring Expansion
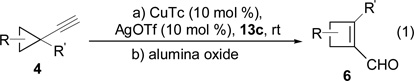
During the preparation of triazole 14c, we also observed small amount of cyclobutene product 15c, suggesting that CuTc was capable of catalyzing the decomposition of triazole. However, even after we extended the reaction time from 4h to 12h, the ratio of 15c/14c was only about 1:2. Base on results in Scheme 3, AgOTf catalyst has higher reactivity than Cu(MeCN)4PF6 and higher selectivity than Rh2(Oct)4. We then decided to treat cyclopropyl acetylene 4a with azide 13c in the presence of both CuTc and AgOTf catalysts. We were pleased to find that these two catalysts did not interfere with each other and cyclobutene carboxyaldehyde 6a was isolated in good yield and selectivity (entry 1, Table 1).
Table 1.
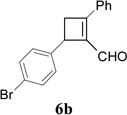
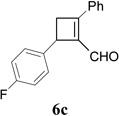
Synthesis of Cyclobutene Aldehydes 6 from Alkynyl cyclopropanesa
 | |||
|---|---|---|---|
| Entry | Substrate | Productb | Yield |
| 1 | 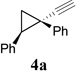 |
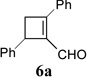 |
85% |
| 2 | 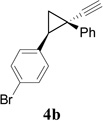 |
 |
80% |
| 3 | 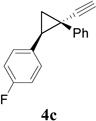 |
 |
80% |
| 4 | 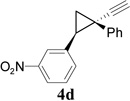 |
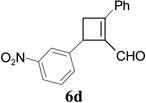 |
73% |
| 5 | 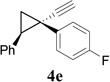 |
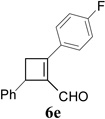 |
82% |
| 6 | 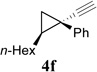 |
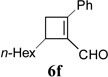 |
86% |
Conditions: 1) CuTc (10 mol % ), AgOTf (10 mol %), 13c (1 equivalent), rt, 4-8h; 2) alumina oxide;
Regioselectivity (>20:1) was determined by 1H NMR of the crude product.
We then studied the scope of the ring expansion of different cyclopropyl acetylenes facilitated by the dual catalyst in the presence of azide 13c (Table 1). We first examined different aryl groups on the 2- and 1-position of the cyclopropane ring (entries 2–5). High regioselectivity (>20:1) was observed in all cases. The selective formation of cyclobutenes with a 1,3-diaryl over 1,2-diaryl relationship may be due to steric interactions between the two aryl groups during the ring expansion. Alkyl groups could also be tolerated on the 2-position of cyclopropanes (entry 6).
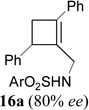
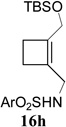
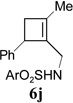
In addition to hydrolysis, imine intermediate 15c could also be reduced in-situ to form sulfonamide 16 (Table 2). The chirality in cyclopropane 4a was also successfully transferred to the product (entry 1). This paved the way for enantioselective synthesis of four-membered rings from chiral alkynyl cyclopropanes. Commercially available cyclopropyl acetylene 4g could be converted to sulfonamide 16g in 85% yield (entry 2). Alkyl substituent on the 1-position of cyclopropane could also be tolerated (entry 3). We found that the cyclobutenyl aldehyde derived from acetylene 4i was not very stable at room temperature. Direct reduction of the imine intermediate afforded stable sulfonamide 16i in good yield and high regioselectivity (entry 4). Substrate 4j with an alkyl group on the 1-position and aryl group on the 2-position of the cyclopropane also worked well (entry 5).
Table 2.
Synthesis of Cyclobutene Sulfonamides 16 from Alkynyl Cyclopropanes (Ar = 3,5-(CF3)2C6H3)a
 | |||
|---|---|---|---|
| Entry | Substrate | Productb | Yield |
| 1 | 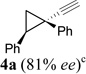 |
 |
81% |
| 2 | 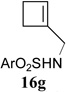 |
85% | |
| 3 | 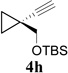 |
 |
80% |
| 4 | 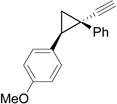 |
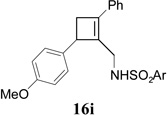 |
78% |
| 5 | 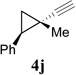 |
 |
76% |
Conditions: CuTc (10 mol %), AgOTf (10 mol %), 13c (1 equivalent), rt, 1-8h; 2) LiAlH4;
Regioselectivity (>20:1) was determined by 1H NMR of the crude product.
This is based on the ee of the aldehyde precursor.
In summary, we have developed an efficient method for the preparation of highly substituted cyclobutenes from alkynyl cyclopropanes selectively. The tandem process was facilitated by a dual catalyst system (CuTc and AgOTf). This new protocol eliminated the need of isolating diazo or triazole intermediates. Various cyclobutenes with aldehyde or sulfonamide functionality could be prepared. The synthesis of cyclobutenes is greatly simplified by using N-sulfonyl-1,2,3-triazoles as the carbene precursor for cyclopropanation of alkene and ring expansion of cyclopropanes.
Supplementary Material
Acknowledgments
We thank NIH (R01 GM088285) and the University of Wisconsin for financial support and a Young Investigator Award (to W.T.) from Amgen.
Footnotes
Electronic Supplementary Information (ESI) available: [1H NMR, 13C NMR, HRMS, and IR data and copies of NMR specta for all starting materials and products.]. See DOI: 10.1039/b000000x/
Notes and references
- 1.a) Hansen TV, Stenstrom Y. Organic Synthesis: Theory and Applications. vol. 5. Elsevier Science Ltd.; 2001. p. 1. [Google Scholar]; b) Dembitsky VM. J. Nat. Med. 2008;62:1. doi: 10.1007/s11418-007-0166-3. [DOI] [PubMed] [Google Scholar]; c) Lee-Ruff E, Mladenova G. Chem. Rev. 2003;103:1449. doi: 10.1021/cr010013a. [DOI] [PubMed] [Google Scholar]; d) Gauvry N, Lescop C, Huet F. Eur. J. Org. Chem. 2006:5207. [Google Scholar]
- 2.For selected examples of cyclobutene synthesis, see: Inanaga K, Takasu K, Ihara M. J. Am. Chem. Soc. 2005;127:3668. doi: 10.1021/ja042661s. Liu YH, Liu MN, Song ZQ. J. Am. Chem. Soc. 2005;127:3662. doi: 10.1021/ja042636m. Canales E, Corey EJ. J. Am. Chem. Soc. 2007;129:12686. doi: 10.1021/ja0765262. Lopez-Carrillo V, Echavarren AM. J. Am. Chem. Soc. 2010;132:9292. doi: 10.1021/ja104177w.
- 3.Xu H, Zhang W, Shu D, Werness JB, Tang W. Angew. Chem. Int. Ed. 2008;47:8933. doi: 10.1002/anie.200803910. [DOI] [PubMed] [Google Scholar]
- 4. Um JM, Xu H, Houk KN, Tang W. J. Am. Chem. Soc. 2009;131:6664. doi: 10.1021/ja9016446.; For related studies, see: Barluenga J, Riesgo L, Lopez LA, Rubio E, Tomas M. Angew. Chem. Int. Ed. 2009;48:7569. doi: 10.1002/anie.200903902. Li C-W, Pati K, Lin G-Y, Abu Sohel SM, Hung H-H, Liu R-S. Angew. Chem. Int. Ed. 2010;49:9891. doi: 10.1002/anie.201004647.
- 5.For selected reviews on cyclopropanations, see: Doyle MP. Chem. Rev. 1986;86:919. Ye T, McKervey MA. Chem. Rev. 1994;94:1091. Doyle MP, Forbes DC. Chem. Rev. 1998;98:911. doi: 10.1021/cr940066a. Doyle MP, McKervey MA, Ye T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds. New York: John Wiley & Sons; 1998. Lebel H, Marcoux J-F, Molinaro C, Charette AB. Chem. Rev. 2003;103:977. doi: 10.1021/cr010007e. Zhang Z, Wang J. Tetrahedron. 2008;64:6577. Pellissier H. Tetrahedron. 2008;64:7041. Davies HML, Denton JR. Chem. Soc. Rev. 2009;38:3061. doi: 10.1039/b901170f. Concellon JM, Rodriguez-Solla H, Concellon C, del Amo V. Chem. Soc. Rev. 2010;39:4103. doi: 10.1039/b915662c.
- 6.For a comprehensive review on transition metal mediated reactions involving cyclopropanes, see: Rubin M, Rubina M, Gevorgyan V. Chem. Rev. 2007;107:3117. doi: 10.1021/cr050988l.
- 7. Liu R, Zhang M, Wyche TP, Winston-McPherson GN, Bugni TS, Tang W. Angew. Chem. Int. Ed. 2012;51:7503. doi: 10.1002/anie.201203379.; b) For a contemporary synthesis of the proposed structure of pipercyclobutanamide A, see: Gutekunst WR, Gianatassio R, Baran PS. Angew. Chem. Int. Ed. 2012;51:7507. doi: 10.1002/anie.201203897.
- 8.For a recent review on transition metal-catalyzed reactions of 1,2,3-trizoles, see: Chattopadhyay B, Gevorgyan V. Angew. Chem. Int. Ed. 2012;51:862. doi: 10.1002/anie.201104807.
- 9.a) Horneff T, Chuprakov S, Chernyak N, Gevorgyan V, Fokin VV. J. Am. Chem. Soc. 2008;130:14972. doi: 10.1021/ja805079v. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chuprakov S, Malik JA, Zibinsky M, Fokin VV. J. Am. Chem. Soc. 2011;133:10352. doi: 10.1021/ja202969z. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chattopadhyay B, Gevorgyan V. Org. Lett. 2011;13:3746. doi: 10.1021/ol2014347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuprakov S, Kwok SW, Zhang L, Lercher L, Fokin VV. J. Am. Chem. Soc. 2009;131:18034. doi: 10.1021/ja908075u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimster N, Zhang L, Fokin VV. J. Am. Chem. Soc. 2010;132:2510. doi: 10.1021/ja910187s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a) Muller S, Liepold B, Roth GJ, Bestmann HJ. Synlett. 1996:521. [Google Scholar]; b) Corey EJ, Fuchs PL. Tetrahedron Lett. 1972;13:3769. [Google Scholar]
- 13.Raushel J, Fokin VV. Org. Lett. 2010;12:4952. doi: 10.1021/ol102087r. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.