Abstract
Dr. Robert K. Yu's research showed for the first time that the composition of glycosphingolipids is tightly regulated during embryo development. Studies in our group showed that the glycosphingolipid precursor ceramide is also critical for stem cell differentiation and apoptosis. Our new studies suggest that ceramide and its derivative, sphingosine-1-phosphate (S1P), act synergistically on embryonic stem (ES) cell differentiation. When using neural precursor cells (NPCs) derived from ES cells for transplantation, residual pluripotent stem (rPS) cells pose a significant risk of tumor formation after stem cell transplantation. We show here that rPS cells did not express the S1P receptor S1P1, which left them vulnerable to ceramide or ceramide analog (N-oleoyl serinol or S18)-induced apoptosis. In contrast, ES cell-derived NPCs expressed S1P1 and were protected in the presence of S1P or its pro-drug analog FTY720. Consistent with previous studies, FTY720-treated NPCs differentiated predominantly toward oligodendroglial lineage as tested by the expression of the oligodendrocyte precursor cell (OPC) markers Olig2 and O4. As the consequence, a combined administration of S18 and FTY720 to differentiating ES cells eliminated rPS cells and promoted oligodendroglial differentiation. In addition, we show that this combination promoted differentiation of ES cell-derived NPCs toward oligodendroglial lineage in vivo after transplantation into mouse brain.
Keywords: ceramide, sphingosine-1-phosphate, embryonic stem cells, apoptosis, differentiation, oligodendrocyte precursors
Introduction
In the last ten years, the methodology to replace damaged tissue by grafting of progenitor cells in vitro differentiated from embryonic stem (ES) cells has made tremendous progress. Highlights of recent research were the successful integration of mouse and human ES cells into mouse brain, giving rise to functional neurons and oligodendrocytes that generate new myelin [1-9]. Studies in Dr. Robert K. Yu's laboratory were among the first to clearly show the integration of embryonic development and stem cell differentiation with glycosphingolipid metabolism [10-21]. Based on these studies, our group developed new approaches using the cell signaling function of sphingolipids to guide ES cell differentiation [3, 22-34].
The reliability and safety of current differentiation protocols is still a matter of controversy. Roughly half of the studies showed that stem cell transplantation leads to the formation of teratomas, although similar protocols were used for the in vitro differentiation of ES cells [3, 35-52]. Teratomas are stem cell-derived tumors that contain cells of all three germ layers in a disordered and displacing fashion, fatal for the patient if it occurs in the brain or heart. Since teratoma formation is the gold standard for pluripotency, teratomas can arise from any type of pluripotent cells, including induced pluripotent stem (iPS) cells. Therefore, they are a major safety concern, in particular when using larger numbers of ES or iPS cell-derived cells as deemed necessary for human stem cell therapy [49]. While lengthy and extensive differentiation of ES or iPS cells is a safe way to enrich neural precursor cells (NPCs), it may not be feasible for all clinical applications of human ES or iPS cells, in particular if new grafts have to be quickly generated from undifferentiated cells. In our laboratory, we have introduced for the first time an alternative way to get rid of residual pluripotent stem (rPS) cells forming tumors from stem cells grafts [3, 30]. We have found that rPS cells that still express the transcription factor Oct-4 cause teratomas with high frequency and are not suitable for stem cell therapy. These cells co-express prostate apoptosis response 4 (PAR-4), a protein that makes rPS cells sensitive toward ceramide and its analogs [3].
Ceramide and sphingosine-1-phosphate (S1P) are two sphingolipids that antagonize each other in their cell signaling function for cell survival and apoptosis (Fig.1 for structures) [30, 31, 53-59]. Previous studies in our laboratory have shown that ceramide and the novel ceramide analog N-oleoyl serinol (S18) induces apoptosis in differentiating ES cells [3, 22, 25, 26]. S18 has been, for the first time, designed and synthesized in our laboratory as a water-soluble analog of ceramide (Fig. 1). We have shown that S18 promotes binding of atypical PKC (aPKC) to PAR-4, which inhibits the aPKC-activated NF-κB cell survival pathway and induces apoptosis in differentiating ES cells [3, 22, 24, 60]. Others have shown that S1P promotes cell survival and differentiation of primary cultures of oligodendroglial precursor cells (OPCs) [61-63]. Both, ceramide and S1P are essential for embryonic development as documented by the embryonic lethal phenotypes of knockout mice deficient in ceramide or S1P biosynthesis [64, 65]. The ceramide/S1P antagonism can be twofold, metabolic and functional. The concentration of ceramide and S1P is counter-balanced by enzymes that convert the two lipids into each other. Ceramide can be hydrolyzed by ceramidase to sphingosine, which is then phosphorylated to S1P by sphingosine kinase. S1P can be hydrolyzed to sphingosine that is then acylated to ceramide by ceramide synthase. These metabolic reactions render the regulation of the ceramide/S1P balance complicated and dependent on distinct lipid pools and enzyme activities within a cell.
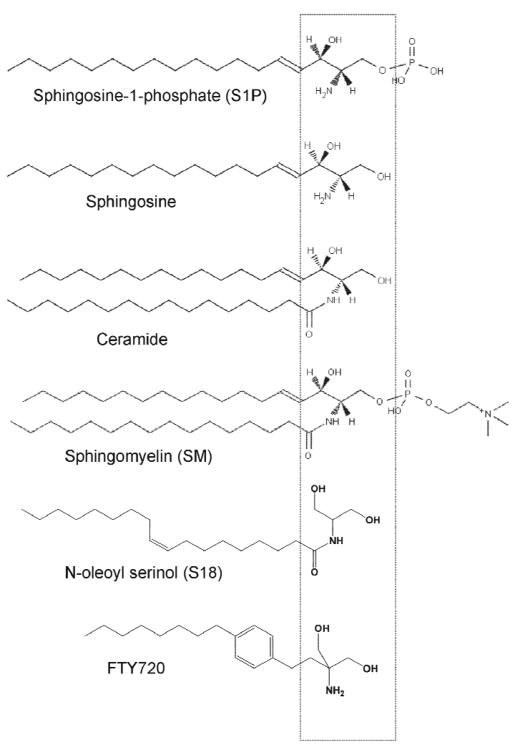
Figure 1. Ceramide and ceramide analogs.
Structural comparison of ceramide and ceramide precursors/derivatives (sphingosine/S1P) and analogs of ceramide (S18) or S1P (FTY720, pro-drug phosphorylated by sphingosine kinase to yield the S1P analog). A β-hydroxy alkylamine (or amide) motif in ceramide was used for the design of the novel ceramide analog S18 (N-oleoyl serinol) (Box shows relative position of this or a similar structural motif in various compounds). For details of S18 synthesis see [24, 60].
However, even in the absence of metabolic conversion, S1P can functionally antagonize ceramide by activating pro-survival cell signaling pathways. In particular, S1P receptor-mediated activation of PI3K/Akt may counteract its ceramide/PAR-4-induced inactivation [25, 30, 31, 62, 66-79]. Therefore, ceramide and S1P are two compounds that if combined can sustain cell survival and induce apoptosis of distinct cell types depending on whether S1P receptors are expressed and pro-apoptotic pathways are activated. In this study, we show that rPS cells do not express the S1P receptor S1P1, which leaves them vulnerable to ceramide or S18-induced apoptosis. In contrast, ES cell-derived NPCs express S1P1 and are protected in the presence of S1P or its pro-drug analog FTY720. As the consequence, a combined administration of S18 and FTY720 to differentiating ES cells eliminates rPS cells and promotes neural differentiation. We also show that this combination of drugs promotes differentiation of ES cell-derived NPCs toward oligodendroglial lineage in vitro and in vivo.
Material and Methods
Materials
ES-J1 and feeder fibroblasts were purchased from the ES core facility (A. Eroglu, Medical College of Georgia, Augusta, GA). Olig2-GFP expressing ES cells were obtained from the American Type Culture Collection (ATCC) (Manassas, VA). Myelin basic protein (MBP), O1 and O4 antibodies were a generous gift from Dr. Somsankar Dasgupta (Medical College of Georgia, Augusta, GA) and Dr. Narayan Bhat, Medical University of South Carolina, Charleston, SC). Knockout DMEM, Knockout serum replacement, ES qualified FBS, N2 supplement, FGF-2, and Vybrant CM-diI (Molecular Probes) were obtained from Invitrogen (Carlsbad, CA). DMEM/F-12 50/50 mix was purchased from Cellgro (Manassas, VA). The sphingosine kinase inhibitor SKI was purchased from EMD Chemicals/Calbiochem (Gibbstown, NJ). Non-enzymatic cell dissociation solution, Hoechst 33258, and goat anti–rabbit IgG HRP conjugate were obtained from Sigma-Aldrich. Monoclonal anti–Oct-4 mouse IgG and anti-β tubulin III (Tuj-1) mouse monoclonal IgG (clone TU-20), and ESGRO (LIF) were from Millipore (Chemicon) (Temecula, CA). Polyclonal anti-Edg-1 (S1P1) rabbit IgG were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). . Donkey anti–mouse, –rabbit, and –goat IgG Cy2, Cy3, and Cy5 conjugates, goat anti–mouse IgG HRP conjugate, and normal donkey serum were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). All reagents were of analytical grade or higher.
In vitro differentiation of ES cells
In vitro differentiation of mouse ES cells (ES-J1) followed a serum deprivation protocol [80, 81]. In brief, ES cells were grown on γ-irradiated feeder fibroblasts for 4 days in knockout DMEM/15% knockout serum replacement, supplemented with ESGRO (LIF). ES cells were then passaged onto gelatin-coated tissue culture dishes without feeder fibroblasts and incubated for 4 days in knockout DMEM/15% heat-inactivated ES-qualified FBS, supplemented with 1,000 U/ml ESGRO (LIF). On trypsinization, ES cells were transferred to bacterial culture dishes without gelatin in order to allow for embryoid body (EB) formation. EBs were incubated for 4 days in knockout DMEM/10% heat-inactivated ES-qualified FB. On the fifth day, floating and loosely attached EBs were rinsed off, transferred to tissue culture dishes, and incubated overnight in knockout DMEM, 10% heat-inactivated ES-qualified FBS to the allow the EBs to attach to the dish. Neural differentiation due to serum deprivation was induced by cultivation for 3 days in DMEM/Ham's F12 (50/50), N2 supplement (1:100 of commercially available formulation). During this time, attached EBs were treated with S18 and S1P or FTY720. Serum-deprived EBs were then trypsinized or treated with a non-enzymatic cell dissociation solution, plated on poly-L-ornithine/laminin–coated tissue culture dishes and grown for 4 days in DMEM/Ham's F12 (50/50), supplemented with N2 and 10 ng/ml FGF-2 (neural progenitor or NP medium) and then for another 3-4 days in neurobasal medium supplemented with 5% heat-inactivated FBS (differentiation medium).
Statistical evaluation
Antigen-specific immunostaining was quantified by counting cells that showed signals twofold or more above background fluorescence. Cell counts were performed in five areas of approximately 200 cells that were obtained from three independent immunostaining reactions. A Chi-square test with one degree of freedom was applied for the statistical analysis of the distribution of two immunostained antigens. The expected frequency for double staining if two antigens were independently distributed (first null hypothesis to be refuted) was the frequency product for immunostaining of A or B in the total population, f(A and B) = f(A) × f(B). The second null hypothesis to be refuted was that the frequency of antigen B in the subpopulation A was identical to its frequency in the total population, f(B in A) = f(B in A × B).
Transplantation of mouse EBCs
EB-derived cells (EBCs) were incubated for 48 h with 100 μM S18 and 300 nM FTY720 in EB medium, further cultivated for 48 h in 50 μM S18 and 300 nM FTY720 in NP medium, and then used for transplantation into mouse brain. EBCs were non-enzymatically dissociated and labeled with Vybrant CM-diI according to the manufacturer's protocol (Molecular Probes). Vybrant CM-diI has been used in our laboratory and by others for persistent fluorescent labeling of transplanted stem cells [3, 82, 83]. The cells were transplanted into the striatum of 10-days-old C57CLB6 mice by intracranial injection (bregma - 1 mm, right hemisphere 2 mm off suture, 2 mm deep) of 105 EBCs in 5 μl of 0.9% sterile saline solution [3, 35]. We transplanted equal numbers of viable untreated and S18/FTY720-treated cells as determined by trypan blue staining. The pups were grown for one week or until myelination was accomplished (about 4 weeks) and then sacrificed. Brains were fixed and cryosectioned (coronal).
Immunocytochemistry
Differentiating ES cells (EBs and EBCs) on poly-L-ornithine/laminin-coated coverslips or frozen brain sections were fixed with 4% p-formaldehyde in PBS and then permeabilized by incubation with 0.2% Triton X-100 in PBS for 5 min at RT. The immunostaining of fixed cells or brain sections followed procedures described previously using a blocking solution of 3% ovalbumin/2% donkey serum in PBS and concentrations of 5 μg/ml primary or secondary antibody in 0.1% ovalbumin/PBS [3]. Cell nuclei were stained with 2 μg/ml of Hoechst 33258 in PBS for 30 min at RT. Antigen specific immunostaining was quantified by counting cells that showed signals twofold or more above background fluorescence. Confocal fluorescence microscopy was performed with a confocal scanning microscope (model LSM 510; Carl Zeiss MicroImaging, Inc.; equipped with Argon-488 and He-Neon 543, 633 lasers) using 40 (NA 1.3, oil, plan-neofluor) and 63 (NA 1.4, oil, apochromat) objectives. Spot Software (Scientific Diagnostics) and LSM 510 Meta 3.2 software (Carl Zeiss MicroImaging, Inc.) was used for image acquisition from confocal microscopy. Adobe Photoshop 7.0 software was used for background reduction, pseudo-colorizing, and overlaying of pseudo-colorized grayscale images.
Results
S1P and FTY720 induce OPCs from EBCs
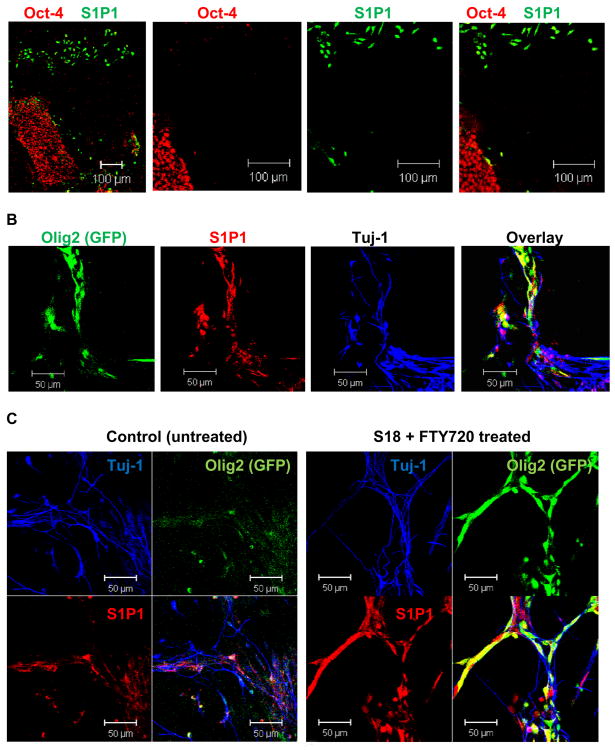
The in vitro differentiation protocol used in our group followed Dr. Ron McKay's original procedure of inducing neural differentiation by serum deprivation of mouse ES cells in the presence of basic fibroblast growth factor (FGF-2) [4, 26]. This protocol consistently yields a homogenous population of neural precursor cells (NPCs) that are self-renewing and express well-characterized NPC markers such as Sox1, Nestin, and FGF-2 receptor. Feeder fibroblast-free ES cells were cultivated for four days as suspension EBs and then for another four days as attached EBs. At this stage, the cells showed up-regulation of the S1P receptor S1P1 in NPCs that migrated out of the attached EB (Fig. 2A). Residual pluripotent stem (rPS) cells in the attached EB expressed the pluripotency marker Oct-4, but they did not express S1P1 (Fig. 2A).
Figure 2. Non-pluripotent EBCs express S1P1 and are induced to OPC lineage specification by incubation with S18 and FTY720.
A. Immunocytochemistry using attached EBs and antibodies against Oct-4 (green) and S1P1 (red). Note that EBCs migrating out of the center are Oct-4 (-) and express S1P1. Pluripotent (Oct-4(+)) cells in the central EB do not express S1P1. B. Attached EBs were incubated for 48 h with 100 μM S18 and 300 nM FTY720, dissociated, and EBCs further cultivated for 4 days in the presence of 50 μM S18 and 300 nM FTY720, followed by 2 days in differentiation medium without S18 or FTY720. Differentiating EBCs express Olig2 as indicated by the expression of GFP (green) under control of the Olig2 promoter. These cells also express S1P1 (red). Additional cell types undergo neuronal differentiation as indicated by the expression of β-tubulin III (Tuj-1, blue). C. The number of Olig2 expressing OPCs is 4-5-fold higher when EBCs were incubated with S18 and FTY720.
Based on the observation that NPCs, but not rPS cells express S1P1, we treated attached EBs with S1P or S1P analogs to test the effect on ES cell differentiation. We used mouse ES cells that express GFP under control of the Olig2 promoter [84, 85]. Olig2 is a transcription factor that is expressed in EBCs that differentiate toward oligodendroglial lineage. These cells are considered to be equivalent to oligodendrocyte precursor cells (OPCs). Table 1 shows that S1P, FTY720, and the S1P1 agonists VPC24191 and SEW2871 increased the number of OPCs by 5-to-15-fold. Because of this significant enhancement we termed these cells induced OPCs or iOPCs. The sphingosine kinase inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl) thiazole (SKI) or the S1P1 receptor antagonist VPC23019 obliterated the effect of FTY720, indicating that phosphorylation of FTY720 by sphingosine kinase and FTY720-P-mediated activation of S1P1 was required to promote oligodendroglial differentiation from EBCs. However, this effect was likely to involve FTY720-independent generation of S1P as well because SKI has been described to be specific for sphingosine kinase 1, while FTY720 has been reported to be predominantly phosphorylated by sphingosine kinase 2 [86, 87]. Additional effects of FTY720 such as inhibition of sphingosine kinase 1 or ceramide synthase were unlikely because these effects have only been reported when FTY720 was used in the micromolar concentration range [88-90].
Table 1. S1P, FTY720, and other S1P1 agonists induce OPCs from EBCs.
EBCs were incubated for 48 h with S1P (1 μM), FTY720 (300 nM), the S1P1 agonists VPC24191 or SEW2871 (300 nM) in NP medium. S1P and S1P1 agonists elevate the number of Olig2 (GFP) expressing cells by more than 4-fold. The S1P1 antagonist VPC23019 (2 μM) inhibits the effect of S1P and S1P1 agonists, indicating that the pro-survival effect of S1P required activation of S1P1. The sphingosine kinase inhibitor SKI (5 μM) compromises the OPC- inducing effect of FTY720 (see Results for detailed discussion). (n=4 independent experiments for each effector).
To test the combinatorial effect of S18 and FTY720 we treated attached EBs with a combination of S18 and FTY720. Consistent with our previous studies, rPS cells in the center of the EBs underwent apoptosis and came off the plate [3]. The remaining NPCs started to express Olig2 in similar proportions as described for cells only incubated with S1P or its analogs (Table 1). We concluded from these results that a combination of S18 and S1P or FTY720 can be used to eliminate rPS cells and at the same time, promote oligodendroglial differentiation.
iOPCs associate with early neurons and differentiate toward oligodendrocytes in vitro and in vivo
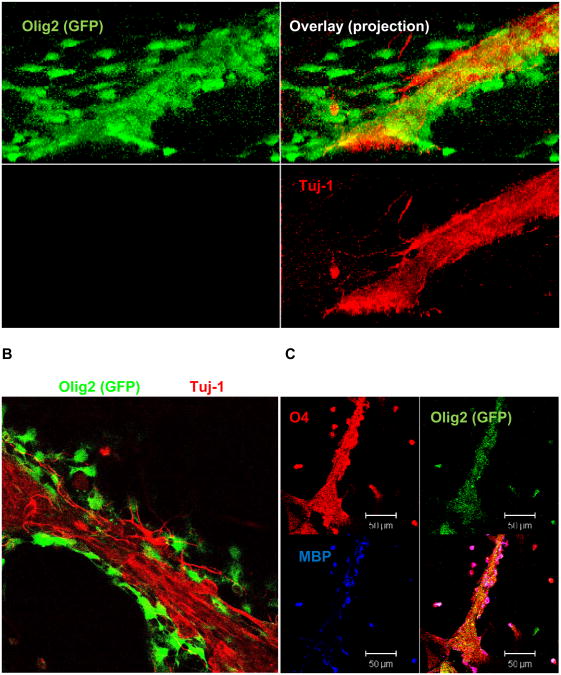
Because iOPCs can potentially myelinate neurons we have focused on the further characterization of differentiating iOPCs. Using immunocytochemistry we tested for the expression of the OPC marker O4 and the mature oligodendrocyte marker myelin basic protein (MBP). We also tested for the expression of Tuj-1 (β-tubulin III), a marker for early neuron differentiation. Figs. 2 (A and B) and 3 (A and B) show that at early stages of differentiation, Olig2 (+) iOPCs associate with Tuj-1 (+) early neurons. Interestingly, iOPCs maintain the expression of S1P1, which suggests that S1P or S1P analogs are beneficial for further differentiation even after induction of oligodendroglial lineage. Olig2 (+) iOPCs also express O4 and O1 (Fig. 3C, O1 is not shown), which clearly demonstrates that these cells undergo oligodendroglial differentiation. The expression of Olig2 is significantly higher in S18 and FTY720 treated cells than in untreated controls (Fig. 2C), which is consistent with the results shown in Table 1. After 72 h of further incubation in differentiation medium, expression of Olig2 fades away while that of O4 increases. Also, a portion of the O4 (+) cells start to express MBP, indicating that these cells undergo further differentiation to mature oligodendrocytes. Interestingly, iOPCs associated closely with Tuj1 (+) early neurons suggesting that they could form a tissue complex required for myelination (Figs. 2B, C and 3).
Figure 3. iOPCs associate with early neurons and undergo further oligodendrocyte differentiation.
EBCs were treated as described in the legend for Fig. 2B. A. A projection reconstruction of a Z-stack image shows that the Olig2 expressing OPCs (green) associate with early neurons (Tuj-1(+), red). B. Experiment as in A, but view on one confocal plane. C. Further incubation in differentiation medium for 72 h shows that Olig2 expressing cells undergo oligodendrocyte differentiation as indicated by the appearance of O4 and MBP.
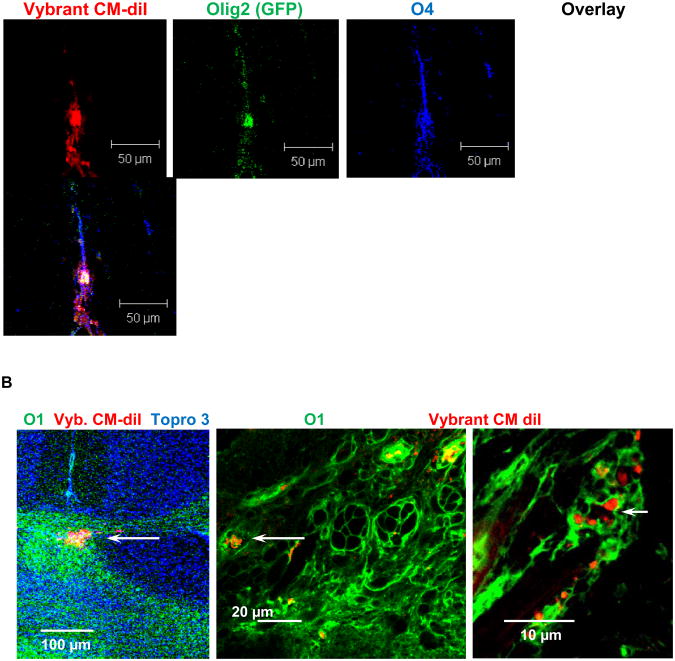
To test the differentiation potential of iOPCs in vivo, EB-derived cells were treated with 100 μM S18 and 300 nM FTY720 for 48 h. The cells were re-plated and then further treated with 50 μM S18 and 300 nM FTY720 for 48 h in NP medium prior to the injection into the brain (striatum) of 10 days old mouse pups. Two sets of 14 mice per set were sacrificed, one set prior (Fig. 4A) and the other set after completion of myelination (Fig. 4B). Fig. 4A shows that the injected cells expressed Olig2 and O4 indicating that the graft underwent oligodendroglial differentiation as described for the in vitro differentiated iOPCs. Fig. 4B shows that the majority of the injected cells settled in myelin-rich areas of the brain as indicated by immunocytochemistry for O1 (Fig. 4B). The co-distribution of the fluorescent Vybrant CM-diI signal with immunostaining for O1 suggested that the grafted cells took part in or supported myelination. EBCs that were not treated showed teratoma formation in about 20% of the transplanted animals as described before [3]. Therefore, the combined treatment of EBCs with the sphingolipid analogs S18 and FTY720 is a novel approach to prevent teratoma formation and to engineer OPCs from ES cells.
Figure 4. Transplanted iOPCs undergo oligodendrocyte differentiation in vivo.
EBCs were treated as described in the legend for Fig. 2, but only incubated for two days, labeled with Vybrant CM-diI (red) and then injected into the striatum of 10 days old mouse pups. A. Mice sacrificed 7 days post-injection, coronal cryosection, and immunocytochemistry for O4. Olig2 expressing cells spread at the injection site and undergo oliogdendrocyte differentiation as indicated by staining for O4. C. Mice sacrifice 4 weeks post-injection, coronal cryosection, and immunocytochemistry for O1. Injected cells migrate to myelinated areas (corpus callosum) in the brain as indicated by staining for Vybrant CM-diI in O1 expressing tissue.
Discussion
Studies in Dr. Robert K. Yu's laboratory about 30 years ago have pioneered the extensive use of glycosphingolipid surface markers for the identification and isolation of distinct cell types and differentiation stages during embryonic brain development [10, 13, 91, 92]. Highlight of this work is the characterization of c-series gangliosides (in particular GT3 and GQ1c) as antigenic epitope for the A2B5 antibody [91]. This antibody is now widely used for the purification and identification of glial-restricted precursor cells and OPCs [92-103]. Other examples for surface glycosphingolipids used for the characterization of consecutive oligodendroglial differentiation stages are O4 (anti-galactosulfatide) and O1 (anti-galactocerebroside), two markers specific for pre- (O4 (+)/O1 (−)) or immature (O4 (+)/O1 (+)) oligodendrocytes arising from A2B5(+) OPCs [104-108]. The most recent achievement of Dr. Yu's group is the identification of the ganglioside GD3 for specific labeling (and sorting) of subventricular zone NP cells [10, 19, 20]. In light of these examples of glycosphingolipids as specific surface markers of distinct differentiation stages, one will inquire into their function in development. Most recently, this research has focused on the role of glycosphingolipids, and in particular gangliosides as raft-forming lipids for growth or differentiation factor receptor activation.
Our initial work attempted to determine the function of glycosphingolipids by blocking their biosynthesis with enzyme inhibitors such as the glucosyltransferase inhibitor PDMP [23]. As a side observation of these early studies, we noticed that a) ceramide is elevated when blocking glucosylceramide biosynthesis, and b) particular cell types are sensitive to ceramide elevation and undergo apoptosis, while others do not. Using the novel ceramide analog S18 synthesized for the first time in our laboratory we found that the expression of the protein PAR-4 renders pluripotent stem cells sensitive to S18 or ceramide-induced apoptosis [24, 26]. PAR-4 is an endogenous inhibitor of aPKC first described in prostate cancer cells. It is critical for tumor suppression by inducing apoptosis [109-112]. In subsequent studies, we found that ceramide promotes binding of aPKC to PAR-4, which suppresses activation of cell survival pathways such as NF-kB by aPKC in pluripotent stem cells [22, 26, 113]. When stem cells further differentiate to NPCs, PAR-4 expression is downregulated, which renders these cells insensitive to ceramide-induced apoptosis. We have used this regulation to selectively eliminate rPS cells from EBC-derived grafts by incubation with S18, which significantly enhanced the safety of stem cell transplantation by preventing teratoma formation from rPS cells [3, 30].
Once NPCs are formed, the role of ceramide and other sphingolipids appears to change dramatically. We have found that ceramide is now beneficial in two ways: a) it promotes cell polarity and migration of NPCs, which is likely to involve the ceramide/aPKC complex in association with cell polarity-related proteins, and b) it will serve as a metabolic precursor for S1P [30-32]. Ceramide generated at the cell membrane by the (growth factor-dependent) activation of sphingomyelinase(s) will be internalized by the endo-lysosomal vesicle trafficking pathway and then hydrolyzed by acid ceramidase to fatty acid and sphingosine. Sphingosine will be converted to S1P by sphingosine kinase 1 or 2, which will lead to S1P-dependent regulation of intracellular targets (e.g., histone deacetylase) or binding to cell surface receptors such as S1P1 [54, 65, 114-117]. In particular, activation of S1P1 has been demonstrated to sustain cell survival of OPCs and to promote oligodendroglial differentiation. FTY720, a pro-drug that is phosphorylated by sphingosine kinase to yield a pharmacological analog of S1P is now used for treatment of multiple sclerosis [61, 63, 118-120]. While it has been described to suppress the S1P-dependent autoimmune reaction against myelin, several studies suggest that it has a second activity by direct neurological effects and promoting OPC survival [62, 120-122]. We have used this activity to generate iOPCs for transplantation experiments.
We have shown that the combination of two analogs of ceramide (S18) and S1P (FTY720) will have two effects on differentiating ES cells: a) teratoma-forming rPS cells are eliminated because they are sensitive to S18 (PAR-4 is expressed) and at the same time, they are not protected by FTY720 or S1P (S1P1 is not expressed), and b) NPCs will survive and undergo oligodendroglial differentiation because they are insensitive to S18 (PAR-4 is not expressed) and at the same time, OPC differentiation is promoted by FTY720 or S1P (S1P1 is expressed). It should be noted that at later oligodendrocyte differentiation stages, the activity of S1P and FTY720 has been reported to involve a more complex regulation by various S1P receptors including S1P1, S1P3, and S1P5 [120].
Our results show that the cell population of iOPCs resulting from the incubation of attached EBs with S18 and FTY720 engrafts into various brain areas and expresses markers for oligendrocyte maturation and myelin formation. Future studies in our laboratory will now determine if iOPCs will functionally restore brain tissue in mouse models for dys- or demyelination diseases. In summary, we have developed are novel protocol for the generation of OPCs from ES cells based on the specific function of the sphingolipids ceramide and S1P. The results of these studies will contribute to the evanescent field of research on the function of sphingolipids in stem cell differentiation and embryo development. From studies pioneered in Dr. Robert K. Yu's laboratory and now further developed in our and many other groups it is clear that there is more to a lipid than just being a fat.
Acknowledgments
This work was supported in part by the NIH grants R01AG034389 and R01NS046835 to EB. The author also acknowledges institutional support (under directorship of Dr. Lin Mei) at the Medical College of Georgia/Georgia Health Sciences University, Augusta, GA. We are thankful to the Imaging Core Facility (under directorship of Dr. Paul McNeil) for assistance with confocal microscopy.
Abbreviations
- aPKC
atypical PKC
- EB
embryoid body
- EBC
EB-derived cell
- ES
embryonic stem
- iOPC
induced oligodendrocyte precursor cell
- MBP
myelin basic protein
- NP
neural progenitor
- NPC
neural precursor cell
- OPC
oligodendrocyte precursor cell
- PAR-4
prostate apoptosis response 4
- rPS
residual pluripotent stem
- S1P
sphingosine-1-phoshate
- S18
N-oleoyl serinol
Footnotes
Special Issue in Honor of Dr. Robert K. Yu
References
- 1.Strathmann FG, Wang X, Mayer-Proschel M. Identification of two novel glial-restricted cell populations in the embryonic telencephalon arising from unique origins. BMC Dev Biol. 2007;7:33. doi: 10.1186/1471-213X-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjorklund LM, Sanchez-Pernaute R, Chung S, Andersson T, Chen IY, McNaught KS, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, Isacson O. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci U S A. 2002;99:2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bieberich E, Silva J, Wang G, Krishnamurthy K, Condie BG. Selective apoptosis of pluripotent mouse and human stem cells by novel ceramide analogues prevents teratoma formation and enriches for neural precursors in ES cell-derived neural transplants. J Cell Biol. 2004;167:723–734. doi: 10.1083/jcb.200405144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 5.Duncan ID. Oligodendrocytes and stem cell transplantation: their potential in the treatment of leukoencephalopathies. J Inherit Metab Dis. 2005;28:357–368. doi: 10.1007/s10545-005-7058-z. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Bouza A, Glaser T, Brustle O. ES cell-derived glial precursors contribute to remyelination in acutely demyelinated spinal cord lesions. Brain Pathol. 2005;15:208–216. doi: 10.1111/j.1750-3639.2005.tb00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu BY, Du ZW, Zhang SC. Differentiation of human oligodendrocytes from pluripotent stem cells. Nat Protoc. 2009;4:1614–1622. doi: 10.1038/nprot.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiel ME, Chen CP, Sadowski D, McKinnon RD. Stem cell-derived therapeutic myelin repair requires 7% cell replacement. Stem Cells. 2008;26:2229–2236. doi: 10.1634/stemcells.2008-0218. [DOI] [PubMed] [Google Scholar]
- 9.Maire CL, Buchet D, Kerninon C, Deboux C, Baron-Van Evercooren A, Nait-Oumesmar B. Directing human neural stem/precursor cells into oligodendrocytes by overexpression of Olig2 transcription factor. J Neurosci Res. 2009;87:3438–3446. doi: 10.1002/jnr.22194. [DOI] [PubMed] [Google Scholar]
- 10.Goldman JE, Hirano M, Yu RK, Seyfried TN. GD3 ganglioside is a glycolipid characteristic of immature neuroectodermal cells. J Neuroimmunol. 1984;7:179–192. doi: 10.1016/s0165-5728(84)80017-x. [DOI] [PubMed] [Google Scholar]
- 11.Yu RK. Development regulation of ganglioside metabolism. Prog Brain Res. 1994;101:31–44. doi: 10.1016/s0079-6123(08)61938-x. [DOI] [PubMed] [Google Scholar]
- 12.Suetake K, Liour SS, Tencomnao T, Yu RK. Expression of gangliosides in an immortalized neural progenitor/stem cell line. J Neurosci Res. 2003;74:769–776. doi: 10.1002/jnr.10802. [DOI] [PubMed] [Google Scholar]
- 13.Cochran FB, Ledeen RW, Yu RK. Gangliosides and proteins in developing chicken brain myelin. Brain Res. 1982;282:27–32. doi: 10.1016/0165-3806(82)90171-7. [DOI] [PubMed] [Google Scholar]
- 14.Yu RK, Macala LJ, Taki T, Weinfield HM, Yu FS. Developmental changes in ganglioside composition and synthesis in embryonic rat brain. J Neurochem. 1988;50:1825–1829. doi: 10.1111/j.1471-4159.1988.tb02484.x. [DOI] [PubMed] [Google Scholar]
- 15.Yu RK, Macala LJ, Farooq M, Sbaschnig-Agler M, Norton WT, Ledeen RW. Ganglioside and lipid composition of bulk-isolated rat and bovine oligodendroglia. J Neurosci Res. 1989;23:136–141. doi: 10.1002/jnr.490230203. [DOI] [PubMed] [Google Scholar]
- 16.Zeng G, Gao L, Freischutz B, Tokuda A, Yu RK. Developmental expression of rat brain GD3-and GT3-synthases. Ann N Y Acad Sci. 1998;845:430. doi: 10.1111/j.1749-6632.1998.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 17.Ngamukote S, Yanagisawa M, Ariga T, Ando S, Yu RK. Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J Neurochem. 2007;103:2327–2341. doi: 10.1111/j.1471-4159.2007.04910.x. [DOI] [PubMed] [Google Scholar]
- 18.Liour SS, Yu RK. Differential effects of three inhibitors of glycosphingolipid biosynthesis on neuronal differentiation of embryonal carcinoma stem cells. Neurochem Res. 2002;27:1507–1512. doi: 10.1023/a:1021652506370. [DOI] [PubMed] [Google Scholar]
- 19.Nakatani Y, Yanagisawa M, Suzuki Y, Yu RK. Characterization of GD3 ganglioside as a novel biomarker of mouse neural stem cells. Glycobiology. 2010;20:78–86. doi: 10.1093/glycob/cwp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liour SS, Kapitonov D, Yu RK. Expression of gangliosides in neuronal development of P19 embryonal carcinoma stem cells. J Neurosci Res. 2000;62:363–373. doi: 10.1002/1097-4547(20001101)62:3<363::AID-JNR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 21.Liour SS, Kraemer SA, Dinkins MB, Su CY, Yanagisawa M, Yu RK. Further characterization of embryonic stem cell-derived radial glial cells. Glia. 2006;53:43–56. doi: 10.1002/glia.20257. [DOI] [PubMed] [Google Scholar]
- 22.Wang G, Silva J, Krishnamurthy K, Tran E, Condie BG, Bieberich E. Direct binding to ceramide activates protein kinase Czeta before the formation of a pro-apoptotic complex with PAR-4 in differentiating stem cells. J Biol Chem. 2005;280:26415–26424. doi: 10.1074/jbc.M501492200. [DOI] [PubMed] [Google Scholar]
- 23.Bieberich E, Freischutz B, Suzuki M, Yu RK. Differential effects of glycolipid biosynthesis inhibitors on ceramide-induced cell death in neuroblastoma cells. J Neurochem. 1999;72:1040–1049. doi: 10.1046/j.1471-4159.1999.0721040.x. [DOI] [PubMed] [Google Scholar]
- 24.Bieberich E, Kawaguchi T, Yu RK. N-acylated serinol is a novel ceramide mimic inducing apoptosis in neuroblastoma cells. J Biol Chem. 2000;275:177–181. doi: 10.1074/jbc.275.1.177. [DOI] [PubMed] [Google Scholar]
- 25.Bieberich E, MacKinnon S, Silva J, Yu RK. Regulation of apoptosis during neuronal differentiation by ceramide and b-series complex gangliosides. J Biol Chem. 2001;276:44396–44404. doi: 10.1074/jbc.M107239200. [DOI] [PubMed] [Google Scholar]
- 26.Bieberich E, MacKinnon S, Silva J, Noggle S, Condie BG. Regulation of cell death in mitotic neural progenitor cells by asymmetric distribution of prostate apoptosis response 4 (PAR-4) and simultaneous elevation of endogenous ceramide. J Cell Biol. 2003;162:469–479. doi: 10.1083/jcb.200212067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bieberich E. Integration of glycosphingolipid metabolism and cell-fate decisions in cancer and stem cells: review and hypothesis. Glycoconj J. 2004;21:315–327. doi: 10.1023/B:GLYC.0000046274.35732.47. [DOI] [PubMed] [Google Scholar]
- 28.Krishnamurthy K, Wang G, Silva J, Condie BG, Bieberich E. Ceramide Regulates Atypical PKC{zeta}/{lambda}-mediated Cell Polarity in Primitive Ectoderm Cells: A NOVEL FUNCTION OF SPHINGOLIPIDS IN MORPHOGENESIS. J Biol Chem. 2007;282:3379–3390. doi: 10.1074/jbc.M607779200. [DOI] [PubMed] [Google Scholar]
- 29.Krishnamurthy K, Dasgupta S, Bieberich E. Development and characterization of a novel anti-ceramide antibody. J Lipid Res. 2007;48:968–975. doi: 10.1194/jlr.D600043-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Bieberich E. Smart drugs for smarter stem cells: making SENSe (sphingolipid-enhanced neural stem cells) of ceramide. Neurosignals. 2008;16:124–139. doi: 10.1159/000111558. [DOI] [PubMed] [Google Scholar]
- 31.Bieberich E. Ceramide signaling in cancer and stem cells. Future Lipidol. 2008;3:273–300. doi: 10.2217/17460875.3.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang G, Krishnamurthy K, Chiang YW, Dasgupta S, Bieberich E. Regulation of neural progenitor cell motility by ceramide and potential implications for mouse brain development. J Neurochem. 2008;106:718–733. doi: 10.1111/j.1471-4159.2008.05451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang G, Silva J, Dasgupta S, Bieberich E. Long-chain ceramide is elevated in presenilin 1 (PS1M146V) mouse brain and induces apoptosis in PS1 astrocytes. Glia. 2008;56:449–456. doi: 10.1002/glia.20626. [DOI] [PubMed] [Google Scholar]
- 34.Wang G, Krishnamurthy K, Umapathy NS, Verin AD, Bieberich E. The carboxyl-terminal domain of atypical protein kinase Czeta binds to ceramide and regulates junction formation in epithelial cells. J Biol Chem. 2009;284:14469–14475. doi: 10.1074/jbc.M808909200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanai J, Doetchman T, Laufer N, Maslaton J, Mor-Yosef S, Safran A, Shani M, Sofer D. Embryonic cultures but not embryos transplanted to the mouse's brain grow rapidly without immunosuppression. Int J Neurosci. 1995;81:21–26. doi: 10.3109/00207459509015295. [DOI] [PubMed] [Google Scholar]
- 36.Wakitani S, Takaoka K, Hattori T, Miyazawa N, Iwanaga T, Takeda S, Watanabe TK, Tanigami A. Embryonic stem cells injected into the mouse knee joint form teratomas and subsequently destroy the joint. Rheumatology (Oxford) 2003;42:162–165. doi: 10.1093/rheumatology/keg024. [DOI] [PubMed] [Google Scholar]
- 37.Teramoto K, Hara Y, Kumashiro Y, Chinzei R, Tanaka Y, Shimizu-Saito K, Asahina K, Teraoka H, Arii S. Teratoma formation and hepatocyte differentiation in mouse liver transplanted with mouse embryonic stem cell-derived embryoid bodies. Transplant Proc. 2005;37:285–286. doi: 10.1016/j.transproceed.2004.12.120. [DOI] [PubMed] [Google Scholar]
- 38.Swijnenburg RJ, Tanaka M, Vogel H, Baker J, Kofidis T, Gunawan F, Lebl DR, Caffarelli AD, de Bruin JL, Fedoseyeva EV, Robbins RC. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation. 2005;112:I166–172. doi: 10.1161/CIRCULATIONAHA.104.525824. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Pernaute R, Studer L, Ferrari D, Perrier A, Lee H, Vinuela A, Isacson O. Long-term survival of dopamine neurons derived from parthenogenetic primate embryonic stem cells (cyno-1) after transplantation. Stem Cells. 2005;23:914–922. doi: 10.1634/stemcells.2004-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim D, Gu Y, Ishii M, Fujimiya M, Qi M, Nakamura N, Yoshikawa T, Sumi S, Inoue K. In vivo functioning and transplantable mature pancreatic islet-like cell clusters differentiated from embryonic stem cell. Pancreas. 2003;27:e34–41. doi: 10.1097/00006676-200308000-00021. [DOI] [PubMed] [Google Scholar]
- 41.Fujikawa T, Oh SH, Pi L, Hatch HM, Shupe T, Petersen BE. Teratoma formation leads to failure of treatment for type I diabetes using embryonic stem cell-derived insulin-producing cells. Am J Pathol. 2005;166:1781–1791. doi: 10.1016/S0002-9440(10)62488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fong SP, Tsang KS, Chan AB, Lu G, Poon WS, Li K, Baum LW, Ng HK. Trophism of neural progenitor cells to embryonic stem cells: neural induction and transplantation in a mouse ischemic stroke model. J Neurosci Res. 2007;85:1851–1862. doi: 10.1002/jnr.21319. [DOI] [PubMed] [Google Scholar]
- 43.Choi D, Oh HJ, Chang UJ, Koo SK, Jiang JX, Hwang SY, Lee JD, Yeoh GC, Shin HS, Lee JS, Oh B. In vivo differentiation of mouse embryonic stem cells into hepatocytes. Cell Transplant. 2002;11:359–368. [PubMed] [Google Scholar]
- 44.Bielby RC, Boccaccini AR, Polak JM, Buttery LD. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng. 2004;10:1518–1525. doi: 10.1089/ten.2004.10.1518. [DOI] [PubMed] [Google Scholar]
- 45.Arnhold S, Klein H, Semkova I, Addicks K, Schraermeyer U. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest Ophthalmol Vis Sci. 2004;45:4251–4255. doi: 10.1167/iovs.03-1108. [DOI] [PubMed] [Google Scholar]
- 46.Baker M. Stem cells: Fast and furious. Nature. 2009;458:962–965. doi: 10.1038/458962a. [DOI] [PubMed] [Google Scholar]
- 47.Leor J, Gerecht S, Cohen S, Miller L, Holbova R, Ziskind A, Shachar M, Feinberg MS, Guetta E, Itskovitz-Eldor J. Human embryonic stem cell transplantation to repair the infarcted myocardium. Heart. 2007;93:1278–1284. doi: 10.1136/hrt.2006.093161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv Cancer Res. 2008;100:133–158. doi: 10.1016/S0065-230X(08)00005-5. [DOI] [PubMed] [Google Scholar]
- 49.Lee AS, Tang C, Cao F, Xie X, van der Bogt K, Hwang A, Connolly AJ, Robbins RC, Wu JC. Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle. 2009;8:2608–2612. doi: 10.4161/cc.8.16.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fong CY, Gauthaman K, Bongso A. Teratomas from pluripotent stem cells: A clinical hurdle. J Cell Biochem. 2010 doi: 10.1002/jcb.22775. [DOI] [PubMed] [Google Scholar]
- 51.Kuznetsov S, Cherman N, Gehron Robey P. In Vivo Bone Formation by Progeny of Human Embryonic Stem Cells. Stem Cells Dev. 2010 doi: 10.1089/scd.2009.0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang NK, Tosi J, Kasanuki JM, Chou CL, Kong J, Parmalee N, Wert KJ, Allikmets R, Lai CC, Chien CL, Nagasaki T, Lin CS, Tsang SH. Transplantation of reprogrammed embryonic stem cells improves visual function in a mouse model for retinitis pigmentosa. Transplantation. 2010;89:911–919. doi: 10.1097/TP.0b013e3181d45a61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50(Suppl):S91–96. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 55.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 56.Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO Rep. 2004;5:777–782. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merrill AH, Jr, Schmelz EM, Dillehay DL, Spiegel S, Shayman JA, Schroeder JJ, Riley RT, Voss KA, Wang E. Sphingolipids--the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol Appl Pharmacol. 1997;142:208–225. doi: 10.1006/taap.1996.8029. [DOI] [PubMed] [Google Scholar]
- 58.Edsall LC, Pirianov GG, Spiegel S. Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J Neurosci. 1997;17:6952–6960. doi: 10.1523/JNEUROSCI.17-18-06952.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fyrst H, Saba JD. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat Chem Biol. 2010;6:489–497. doi: 10.1038/nchembio.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bieberich E, Hu B, Silva J, MacKinnon S, Yu RK, Fillmore H, Broaddus WC, Ottenbrite RM. Synthesis and characterization of novel ceramide analogs for induction of apoptosis in human cancer cells. Cancer Lett. 2002;181:55–64. doi: 10.1016/s0304-3835(02)00049-6. [DOI] [PubMed] [Google Scholar]
- 61.Osinde M, Mullershausen F, Dev KK. Phosphorylated FTY720 stimulates ERK phosphorylation in astrocytes via S1P receptors. Neuropharmacology. 2007;52:1210–1218. doi: 10.1016/j.neuropharm.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 62.Coelho RP, Payne SG, Bittman R, Spiegel S, Sato-Bigbee C. The immunomodulator FTY720 has a direct cytoprotective effect in oligodendrocyte progenitors. J Pharmacol Exp Ther. 2007;323:626–635. doi: 10.1124/jpet.107.123927. [DOI] [PubMed] [Google Scholar]
- 63.Saini HS, Coelho RP, Goparaju SK, Jolly PS, Maceyka M, Spiegel S, Sato-Bigbee C. Novel role of sphingosine kinase 1 as a mediator of neurotrophin-3 action in oligodendrocyte progenitors. J Neurochem. 2005;95:1298–1310. doi: 10.1111/j.1471-4159.2005.03451.x. [DOI] [PubMed] [Google Scholar]
- 64.Hojjati MR, Li Z, Jiang XC. Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochim Biophys Acta. 2005;1737:44–51. doi: 10.1016/j.bbalip.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 65.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou H, Summers SA, Birnbaum MJ, Pittman RN. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J Biol Chem. 1998;273:16568–16575. doi: 10.1074/jbc.273.26.16568. [DOI] [PubMed] [Google Scholar]
- 67.Jung CG, Kim HJ, Miron VE, Cook S, Kennedy TE, Foster CA, Antel JP, Soliven B. Functional consequences of S1P receptor modulation in rat oligodendroglial lineage cells. Glia. 2007;55:1656–1667. doi: 10.1002/glia.20576. [DOI] [PubMed] [Google Scholar]
- 68.Hsieh HL, Wu CB, Sun CC, Liao CH, Lau YT, Yang CM. Sphingosine-1-phosphate induces COX-2 expression via PI3K/Akt and p42/p44 MAPK pathways in rat vascular smooth muscle cells. J Cell Physiol. 2006;207:757–766. doi: 10.1002/jcp.20621. [DOI] [PubMed] [Google Scholar]
- 69.Wong RC, Tellis I, Jamshidi P, Pera M, Pebay A. Anti-apoptotic effect of sphingosine-1-phosphate and platelet-derived growth factor in human embryonic stem cells. Stem Cells Dev. 2007;16:989–1001. doi: 10.1089/scd.2007.0057. [DOI] [PubMed] [Google Scholar]
- 70.Arboleda G, Morales LC, Benitez B, Arboleda H. Regulation of ceramide-induced neuronal death: cell metabolism meets neurodegeneration. Brain Res Rev. 2009;59:333–346. doi: 10.1016/j.brainresrev.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 71.Bourbon NA, Sandirasegarane L, Kester M. Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J Biol Chem. 2002;277:3286–3292. doi: 10.1074/jbc.M110541200. [DOI] [PubMed] [Google Scholar]
- 72.Stoica BA, Movsesyan VA, Lea PMt, Faden AI. Ceramide-induced neuronal apoptosis is associated with dephosphorylation of Akt, BAD, FKHR, GSK-3beta, and induction of the mitochondrial-dependent intrinsic caspase pathway. Mol Cell Neurosci. 2003;22:365–382. doi: 10.1016/s1044-7431(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 73.Osawa Y, Uchinami H, Bielawski J, Schwabe RF, Hannun YA, Brenner DA. Roles for C16-ceramide and sphingosine 1-phosphate in regulating hepatocyte apoptosis in response to tumor necrosis factor-alpha. J Biol Chem. 2005;280:27879–27887. doi: 10.1074/jbc.M503002200. [DOI] [PubMed] [Google Scholar]
- 74.Fernandez-Marcos PJ, Abu-Baker S, Joshi J, Galvez A, Castilla EA, Canamero M, Collado M, Saez C, Moreno-Bueno G, Palacios J, Leitges M, Serrano M, Moscat J, Diaz-Meco MT. Simultaneous inactivation of Par-4 and PTEN in vivo leads to synergistic NF-{kappa}B activation and invasive prostate carcinoma. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0813055106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee TJ, Lee JT, Kim SH, Choi YH, Song KS, Park JW, Kwon TK. Overexpression of Par-4 enhances thapsigargin-induced apoptosis via down-regulation of XIAP and inactivation of Akt in human renal cancer cells. J Cell Biochem. 2008;103:358–368. doi: 10.1002/jcb.21642. [DOI] [PubMed] [Google Scholar]
- 76.Diaz-Meco MT, Abu-Baker S. The Par-4/PTEN connection in tumor suppression. Cell Cycle. 2009;8:2518–2522. doi: 10.4161/cc.8.16.9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee TJ, Jang JH, Noh HJ, Park EJ, Choi KS, Kwon TK. Overexpression of Par-4 sensitizes TRAIL-induced apoptosis via inactivation of NF-kappaB and Akt signaling pathways in renal cancer cells. J Cell Biochem. 2010;109:885–895. doi: 10.1002/jcb.22504. [DOI] [PubMed] [Google Scholar]
- 78.Sun B, Lu C, Zhou GP, Xing CY. Suppression of Par-4 Protects Human Renal Proximal Tubule Cells from Apoptosis Induced by Oxidative Stress. Nephron Exp Nephrol. 2010;117:e53–e61. doi: 10.1159/000320594. [DOI] [PubMed] [Google Scholar]
- 79.Goswami A, Ranganathan P, Rangnekar VM. The phosphoinositide 3-kinase/Akt1/Par-4 axis: a cancer-selective therapeutic target. Cancer Res. 2006;66:2889–2892. doi: 10.1158/0008-5472.CAN-05-4458. [DOI] [PubMed] [Google Scholar]
- 80.Hancock CR, Wetherington JP, Lambert NA, Condie BG. Neuronal differentiation of cryopreserved neural progenitor cells derived from mouse embryonic stem cells. Biochem Biophys Res Commun. 2000;271:418–421. doi: 10.1006/bbrc.2000.2631. [DOI] [PubMed] [Google Scholar]
- 81.Okabe S, Forsberg-Nilsson K, Spiro AC, Segal M, McKay RD. Development of neuronal precursor cells and functional postmitotic neurons from embryonic stem cells in vitro. Mech Dev. 1996;59:89–102. doi: 10.1016/0925-4773(96)00572-2. [DOI] [PubMed] [Google Scholar]
- 82.Ruhparwar A, Bara C, Kofidis T, Ruebesamen N, Karck M, Martin U, Haverich A. In vivo detection of integration of grafted cells after myocardial transplantation. Zentralbl Chir. 2006;131:420–424. doi: 10.1055/s-2006-949537. [DOI] [PubMed] [Google Scholar]
- 83.Ruhparwar A, Kofidis T, Ruebesamen N, Karck M, Haverich A, Martin U. Intra-vital fluorescence microscopy for intra-myocardial graft detection following cell transplantation. Int J Cardiovasc Imaging. 2005;21:569–574. doi: 10.1007/s10554-005-0654-z. [DOI] [PubMed] [Google Scholar]
- 84.Xian HQ, McNichols E, St Clair A, Gottlieb DI. A subset of ES-cell-derived neural cells marked by gene targeting. Stem Cells. 2003;21:41–49. doi: 10.1634/stemcells.21-1-41. [DOI] [PubMed] [Google Scholar]
- 85.Xian H, Gottlieb DI. Dividing Olig2-expressing progenitor cells derived from ES cells. Glia. 2004;47:88–101. doi: 10.1002/glia.20010. [DOI] [PubMed] [Google Scholar]
- 86.Paugh SW, Payne SG, Barbour SE, Milstien S, Spiegel S. The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS Lett. 2003;554:189–193. doi: 10.1016/s0014-5793(03)01168-2. [DOI] [PubMed] [Google Scholar]
- 87.Loveridge C, Tonelli F, Leclercq T, Lim KG, Long JS, Berdyshev E, Tate RJ, Natarajan V, Pitson SM, Pyne NJ, Pyne S. The sphingosine kinase 1 inhibitor 2-(P-hydroxyanilino)-4-(P-chlorophenyl)thiazole induces proteasomal degradation of sphingosine kinase 1 in mammalian cells. J Biol Chem. 2010 doi: 10.1074/jbc.M110.127993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berdyshev EV, Gorshkova I, Skobeleva A, Bittman R, Lu X, Dudek SM, Mirzapoiazova T, Garcia JG, Natarajan V. FTY720 inhibits ceramide synthases and up-regulates dihydrosphingosine 1-phosphate formation in human lung endothelial cells. J Biol Chem. 2009;284:5467–5477. doi: 10.1074/jbc.M805186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tonelli F, Lim KG, Loveridge C, Long J, Pitson SM, Tigyi G, Bittman R, Pyne S, Pyne NJ. FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer cells. Cell Signal. 2010;22:1536–1542. doi: 10.1016/j.cellsig.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lahiri S, Park H, Laviad EL, Lu X, Bittman R, Futerman AH. Ceramide Synthesis Is Modulated by the Sphingosine Analog FTY720 via a Mixture of Uncompetitive and Noncompetitive Inhibition in an Acyl-CoA Chain Length-de pend ent Manner. J Biol Chem. 2009;284:16090–16098. doi: 10.1074/jbc.M807438200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kasai N, Yu RK. The monoclonal antibody A2B5 is specific to ganglioside GQ1c. Brain Res. 1983;277:155–158. doi: 10.1016/0006-8993(83)90918-6. [DOI] [PubMed] [Google Scholar]
- 92.Kim SU, Moretto G, Lee V, Yu RK. Neuroimmunology of gangliosides in human neurons and glial cells in culture. J Neurosci Res. 1986;15:303–321. doi: 10.1002/jnr.490150303. [DOI] [PubMed] [Google Scholar]
- 93.Rao MS, Mayer-Proschel M. Glial-restricted precursors are derived from multipotent neuroepithelial stem cells. Dev Biol. 1997;188:48–63. doi: 10.1006/dbio.1997.8597. [DOI] [PubMed] [Google Scholar]
- 94.Rao MS, Noble M, Mayer-Proschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci U S A. 1998;95:3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Herrera J, Yang H, Zhang SC, Proschel C, Tresco P, Duncan ID, Luskin M, Mayer-Proschel M. Embryonic-derived glial-restricted precursor cells (GRP cells) can differentiate into astrocytes and oligodendrocytes in vivo. Exp Neurol. 2001;171:11–21. doi: 10.1006/exnr.2001.7729. [DOI] [PubMed] [Google Scholar]
- 96.Noble M, Proschel C, Mayer-Proschel M. Getting a GR(i)P on oligodendrocyte development. Dev Biol. 2004;265:33–52. doi: 10.1016/j.ydbio.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 97.Levi G, Gallo V, Ciotti MT. Bipotential precursors of putative fibrous astrocytes and oligodendrocytes in rat cerebellar cultures express distinct surface features and “neuron-like” gamma-aminobutyric acid transport. Proc Natl Acad Sci U S A. 1986;83:1504–1508. doi: 10.1073/pnas.83.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schnitzer J, Schachner M. Cell type specificity of a neural cell surface antigen recognized by the monoclonal antibody A2B5. Cell Tissue Res. 1982;224:625–636. doi: 10.1007/BF00213757. [DOI] [PubMed] [Google Scholar]
- 99.Abney ER, Williams BP, Raff MC. Tracing the development of oligodendrocytes from precursor cells using monoclonal antibodies, fluorescence-activated cell sorting, and cell culture. Dev Biol. 1983;100:166–171. doi: 10.1016/0012-1606(83)90207-5. [DOI] [PubMed] [Google Scholar]
- 100.Raff MC, Abney ER, Miller RH. Two glial cell lineages diverge prenatally in rat optic nerve. Dev Biol. 1984;106:53–60. doi: 10.1016/0012-1606(84)90060-5. [DOI] [PubMed] [Google Scholar]
- 101.Saneto RP, de Vellis J. Characterization of cultured rat oligodendrocytes proliferating in a serum-free, chemically defined medium. Proc Natl Acad Sci U S A. 1985;82:3509–3513. doi: 10.1073/pnas.82.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lubetzki C, Goujet-Zalc C, Gansmuller A, Monge M, Brillat A, Zalc B. Morphological, biochemical, and functional characterization of bulk isolated glial progenitor cells. J Neurochem. 1991;56:671–680. doi: 10.1111/j.1471-4159.1991.tb08202.x. [DOI] [PubMed] [Google Scholar]
- 103.Kalyani A, Hobson K, Rao MS. Neuroepithelial stem cells from the embryonic spinal cord: isolation, characterization, and clonal analysis. Dev Biol. 1997;186:202–223. doi: 10.1006/dbio.1997.8592. [DOI] [PubMed] [Google Scholar]
- 104.Amat JA, Farooq M, Ishiguro H, Norton WT. Cells of the oligodendrocyte lineage proliferate following cortical stab wounds: an in vitro analysis. Glia. 1998;22:64–71. doi: 10.1002/(sici)1098-1136(199801)22:1<64::aid-glia6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 105.Bansal R, Winkler S, Bheddah S. Negative regulation of oligodendrocyte differentiation by galactosphingolipids. J Neurosci. 1999;19:7913–7924. doi: 10.1523/JNEUROSCI.19-18-07913.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gensert JM, Goldman JE. Heterogeneity of cycling glial progenitors in the adult mammalian cortex and white matter. J Neurobiol. 2001;48:75–86. [PubMed] [Google Scholar]
- 107.Wilson HC, Onischke C, Raine CS. Human oligodendrocyte precursor cells in vitro: phenotypic analysis and differential response to growth factors. Glia. 2003;44:153–165. doi: 10.1002/glia.10280. [DOI] [PubMed] [Google Scholar]
- 108.Dasgupta S, Everhart MB, Bhat NR, Hogan EL. Neutral monoglycosylceramides in rat brain: occurrence, molecular expression and developmental variation. Dev Neurosci. 1997;19:152–161. doi: 10.1159/000111201. [DOI] [PubMed] [Google Scholar]
- 109.Sells SF, Wood DP, Jr, Joshi-Barve SS, Muthukumar S, Jacob RJ, Crist SA, Humphreys S, Rangnekar VM. Commonality of the gene programs induced by effectors of apoptosis in androgen-dependent and -independent prostate cells. Cell Growth Differ. 1994;5:457–466. [PubMed] [Google Scholar]
- 110.Guo Q, Fu W, Xie J, Luo H, Sells SF, Geddes JW, Bondada V, Rangnekar VM, Mattson MP. Par-4 is a mediator of neuronal degeneration associated with the pathogenesis of Alzheimer disease. Nat Med. 1998;4:957–962. doi: 10.1038/nm0898-957. [DOI] [PubMed] [Google Scholar]
- 111.Azmi AS, Wang Z, Burikhanov R, Rangnekar VM, Wang G, Chen J, Wang S, Sarkar FH, Mohammad RM. Critical role of prostate apoptosis response-4 in determining the sensitivity of pancreatic cancer cells to small-molecule inhibitor-induced apoptosis. Mol Cancer Ther. 2008;7:2884–2893. doi: 10.1158/1535-7163.MCT-08-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao Y, Rangnekar VM. Apoptosis and tumor resistance conferred by Par-4. Cancer Biol Ther. 2008;7:1867–1874. doi: 10.4161/cbt.7.12.6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang G, Silva J, Krishnamurthy K, Bieberich E. A novel isoform of prostate apoptosis response 4 (PAR-4) that co-distributes with F-actin and prevents apoptosis in neural stem cells. Apoptosis. 2006;11:315–325. doi: 10.1007/s10495-006-3979-8. [DOI] [PubMed] [Google Scholar]
- 114.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 115.Sim-Selley LJ, Goforth PB, Mba MU, Macdonald TL, Lynch KR, Milstien S, Spiegel S, Satin LS, Welch SP, Selley DE. Sphingosine-1-Phosphate Receptors Mediate Neuromodulatory Functions in the Cns. J Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.06202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Taha TA, Argraves KM, Obeid LM. Sphingosine-1-phosphate receptors: receptor specificity versus functional redundancy. Biochim Biophys Acta. 2004;1682:48–55. doi: 10.1016/j.bbalip.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 117.Mao C, Obeid LM. Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim Biophys Acta. 2008;1781:424–434. doi: 10.1016/j.bbalip.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qin J, Berdyshev E, Goya J, Natarajan V, Dawson G. Neurons and oligodendrocytes recycle sphingosine 1-phosphate to ceramide: significance for apoptosis and multiple sclerosis. J Biol Chem. 2010;285:14134–14143. doi: 10.1074/jbc.M109.076810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Coelho RP, Saini HS, Sato-Bigbee C. Sphingosine-1-phosphate and oligodendrocytes: from cell development to the treatment of multiple sclerosis. Prostaglandins Other Lipid Mediat. 2010;91:139–144. doi: 10.1016/j.prostaglandins.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 120.Miron VE, Jung CG, Kim HJ, Kennedy TE, Soliven B, Antel JP. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann Neurol. 2008;63:61–71. doi: 10.1002/ana.21227. [DOI] [PubMed] [Google Scholar]
- 121.Miron VE, Schubart A, Antel JP. Central nervous system-directed effects of FTY720 (fingolimod) J Neurol Sci. 2008;274:13–17. doi: 10.1016/j.jns.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 122.Lee CW, Choi JW, Chun J. Neurological S1P signaling as an emerging mechanism of action of oral FTY720 (Fingolimod) in multiple sclerosis. Arch Pharm Res. 2010;33:1567–1574. doi: 10.1007/s12272-010-1008-5. [DOI] [PubMed] [Google Scholar]