Abstract
Background
Maternal depressive symptoms are a strong predictor of increases in depressive symptoms in offspring, yet knowledge of individual differences that may moderate the association between youth and maternal symptoms is still relatively scant. Youth genetic susceptibility to maternal depressive symptoms in particular is a nearly unexplored area of research.
Methods
This study used a multiwave prospective design and lagged hierarchical linear modeling analyses to examine whether youth 5-HTTLPR genotype moderated the longitudinal association between mother and youth depressive symptoms in a community sample (N = 241 youth). Maternal and youth symptoms were assessed every 3 months over 1 year (five waves of data).
Results
Youth 5-HTTLPR interacted with idiographic elevations in maternal depressive symptoms (elevations relative to mothers’ average level of symptoms) to predict prospective increases in youth symptoms 3 months later. Youth with the SS genotype experienced greatest increases in depressive symptoms when exposed to elevations in maternal symptoms. Youth 5-HTTLPR did not interact with maternal nomothetic elevations in depressive symptoms (severity of symptoms compared to the sample as a whole).
Conclusion
These findings advance knowledge on genetic susceptibility for intergenerational transmission of depression between mothers and their children.
Keywords: child/adolescent, depression, gene–environment, maternal child, mood disorders
INTRODUCTION
It is well documented that rates of depression among children with depressed mothers are significantly higher compared to other children.[1] Children of depressed parents, particularly mothers, are three to four times more likely to develop depression than children of nondepressed parents.[2] Maternal depression predicts early onset depression and greater depression chronicity among youth.[3]
There are likely multiple processes through which risk for depression is transmitted from mothers to children.[4] For example, youth may directly inherit risk for depression, or experience prenatal exposure to environments associated with maternal depression that may increase vulnerability to child depression. In addition to more biologically based mechanisms, researchers have also considered social mechanisms in the intergenerational transmission of depression. Interpersonal theorists have long since noted the effects of depressed individuals on each other.[5] Consistent with these theories, researchers have posited that exposure to depressed mothers’ affect, behavior, and cognitions may influence offspring’s vulnerability to depression.[4] Empirical studies show that clinical depression among youth occurs in proximity to maternal episodes of depression,[6] and elevations in maternal depressive symptoms are temporally associated with elevations in child depressive symptoms.[7–9]
Taken together, theory and research suggest that youth are especially at risk for increases in depressive symptoms during periods of exposure to elevations in maternal symptoms. Yet, not all youth experience depressive symptoms when exposed to increases in their mother’s depressive symptoms. It is therefore important to examine potential moderating variables of the association between mother and child depressive symptoms to identify factors that may influence youth vulnerability to maternal depression.
To date, research investigating moderators of the association between maternal depression and the development of youth depression is scant. There is some evidence that youth emotion regulation,[10] cognitive vulnerability,[7, 8] and coping skills [11] interact with maternal depressive symptoms or clinical levels of depression to predict increases in youth depressive symptoms. In addition to these psychosocial factors, recent studies demonstrating genetic sensitivity to the environment suggest that child genotype may be another important vulnerability factor that moderates the effects of maternal depression.[12] The majority of research investigating the role of molecular genetics within a Gene × Environment (G × E) model in the etiology of depression has focused on a polymorphism of the serotonin transporter gene (5-HTTLPR). Evidence suggests that variations in 5-HTTLPR influence individual differences in sensitivity to the environment, including stress and rearing environments.[13–15] Therefore, it is likely that youth carrying the short (S) allele of 5-HTTLPR (i.e., allele associated with greater sensitivity to the environment) experience greater increases in depressive symptoms when exposed to elevations in maternal symptoms.
However, youth genetic susceptibility to maternal depressive symptoms, as an important aspect of the environment within a G × E framework, is a virtually unexplored area of research. Therefore, the main objective of the current study was to investigate whether youth genotype, specifically 5-HTTLPR, enhances risk to increases in depressive symptoms in response to maternal depressive symptoms.
ROLE OF 5-HTTLPR IN SENSITIVITY TO ENVIRONMENT
Accumulating evidence suggests that 5-HTTLPR influences sensitivity, or reactivity, to the environment.[14] The S allele of this gene is associated with decreased transcriptional efficiency and reduced serotonin uptake compared to the long (L) allele.[16] A variety of studies show that the S allele in particular is linked to heightened sensitivity.[14, 17, 18] For example, among adults, the S allele is associated with greater negative affect in response to stress, and greater negative subjective appraisals of stressful events.[19, 20] Among youth, studies have shown that girls carrying the S allele of 5-HTTLPR exhibit elevated cortisol in response to a laboratory stressor,[21] and young children with the S allele exhibit greater observed negative emotionality provoked by stressful tasks.[22] Finally, neuroimaging studies of both adults and youth show exaggerated and faster amygdala activation in response to threat-related stimuli among S carriers compared to L carriers.[23–25]
5-HTTLPR risk alleles are also linked to poor social–emotional functioning in response to negative parenting behaviors.[15, 26, 27] However, only one study to our knowledge has specifically investigated whether 5-HTTLPR moderates the temporal association between mother and youth depressive symptoms.[9] This study provides preliminary evidence for this hypothesized effect; however, only concurrent associations between mother and youth depressive symptoms were examined over time, so the temporal direction of effects between maternal and youth depressive symptoms, as moderated by 5-HTTLPR, is unknown.
CURRENT STUDY
We sought to contribute to knowledge of the etiology of depression among youth by investigating whether youths’ genetic vulnerability moderates the longitudinal association between maternal depressive symptoms and their children’s later depressive symptoms. This study focused on offspring of mothers in particular, given that maternal depression is more strongly associated than paternal depression with offspring depression.[28]
We incorporated a multiwave design and assessed the prospective association between mother and youth depressive symptoms every 3 months over the course of 1 year (five waves of data). The study design and methodology allowed for more precision and power when examining youth genetic susceptibility to intergenerational transmission of depressive symptoms that would not be possible with cross-sectional or two time-point designs.[29] Additionally, and in contrast to prior multiwave studies that investigated only concurrent associations between mother and youth depressive symptoms, we investigated the temporal direction of these effects, and moderation by youths’ genotype, by explicitly testing whether youth would exhibit prospective increases following earlier exposure to elevations in maternal depressive symptoms.
Finally, the multiwave design of our study allowed for use of an idiographic approach to assessing maternal depressive symptoms, in contrast to a nomothetic approach.[30] Idiographically assessed maternal symptoms are conceptualized as deviations relative to a mother’s average level of depressive symptoms over the 1-year period, regardless of the mother’s rank-order position in the sample (i.e., within-person levels). The nomothetic approach is used when the severity of maternal depressive symptoms is assessed in comparison to the sample as a whole (i.e., between-person levels/rank order of level of stressors). Given that the 5-HTTLPR S allele is associated with increased sensitivity to the environment, it is likely that youth with a greater number of S alleles are particularly susceptible to deviations in their mothers’ typical level of symptoms (i.e., idiographic increases), above and beyond the level of severity of symptoms (nomothetic levels). Consistent with this hypothesis, Hankin et al.[30] only found support for a 5-HTTLPR × environment interaction predicting youth depressive symptoms when they used an idiographic approach to assess youth exposure to environmental stressors,[30] but not when they used a nomothetic approach that measured the rank order of stressors in comparison to the sample as a whole. Therefore, an idiographic G × E approach is likely a particularly meaningful and exacting method of conceptualizing and testing hypotheses.
We hypothesized that 5-HTTLPR in youth would moderate the effects of maternal depressive symptoms predicting later increases in youth depressive symptoms. Specifically and based on an additive genetic model, we hypothesized that youth carrying the S allele, especially those who are homozygous (SS genotype), would experience the greatest prospective increases in depressive symptoms following exposure to their mothers’ idiographic elevations in depressive symptoms.
METHOD
PARTICIPANTS
Children and adolescents were recruited by brief information letters sent home directly by participating school districts to families with a child in third, sixth, and ninth grades of public schools. The short letter stated that we were conducting a study on social and emotional development in children and adolescents and requested that interested participants call the laboratory to receive more detailed information on the study.
Two hundred sixty-two youth and their mothers agreed to participate in the study and were able to complete all baseline measures. Of these, 241 families completed one or more additional follow-up visits, providing sufficient data to be included in analyses. Youth ranged in age from 9 to 15 (M = 12.14, SD = 2.43). The sample was approximately evenly divided by sex (43% boys, 57% girls) and grade (30% third grade, 35% sixth grade, 35% ninth grade). The present sample was drawn from the general community of youth attending public schools, and was representative of both the broader population of the particular geographical area and school districts from which the sample was drawn. Ethnicity was as follows: Caucasian, 66%; African American, 7%; Latino, 8%; Asian/Pacific Islander, 4%; Other/Mixed ethnicity and race, 15%. Of the mothers, 72% were married, 11% were single, 14% were divorced, 2% were separated, and 1% was widowed. The median annual family income was $75,000 and 21% of the youth received free/reduced lunch at school.
PROCEDURES
The mother and youth visited the laboratory for the baseline assessment. Mothers provided informed written consent for their participation and for their child; youth provided written assent. The initial baseline assessment consisted of a battery of questionnaires completed by youth and mothers about the child and collection of youth DNA via saliva. Regular follow-up assessments were conducted every 3 months over a 1-year period (five waves of data) to assess mother and child depressive symptoms. The Institutional Review Board approved all procedures. Youth and mothers were reimbursed for participation at baseline and each follow-up.
MEASURES
Youth Depressive Symptoms
The Children’s Depression Inventory (CDI)[31] is a 27-item self-report questionnaire designed to assess youth depressive symptoms. Each item is rated on a scale from 0 to 2, with the total score ranging from 0 to 54. Higher scores indicate greater depressive symptoms. The CDI has been shown to have good reliability (test–retest and internal consistency) and good convergent validity in youth.[32, 33] The CDI was administered to youth at all five assessments, and internal consistency (α) in this sample was above .80 at all waves.
Maternal Depressive Symptoms
The Beck Depression Inventory (BDI)[34] is a 21-item self-report questionnaire designed to assess adult depressive symptoms. Each item is rated on a scale from 0 to 3, with the total score ranging from 0 to 63. Higher scores indicate greater depressive symptoms. The BDI has been shown to be a reliable and valid measure of depressive symptoms.[35] The BDI was administered to mothers at all five assessments, and internal consistency was above α = .85 across all waves.
Genotyping
At baseline, children provided buccal cells for DNA collection via Oragene® kits from DNA Genotek (Ottawa, Ontario, Canada), and genomic DNA was collected and isolated using standard salting out and solvent precipitation methods. The 5-HTTLPR alleles were assayed[36] and modified by using primers reported by Hu et al.[37] Samples were analyzed on an ABI PRISM (Applied Biosystems, Foster City, CA) 3130xl Sequencer. We used a biallelic approach to create three groups consisting of SS, SL, and LL genotypes. Prior G × E research has found no differences in results between biallelic and triallelic methods for genotyping 5-HTTLPR.[30]
STATISTICAL APPROACH
The primary hypotheses were evaluated using hierarchical mixed effects modeling[38] in which observations were nested within persons. At level 1, CDI scores (at Time T) were modeled as a function of an intercept, lagged CDI (CDI at time T-1) to control for levels of symptoms at the prior time point, and lagged maternal BDI (BDI at time T-1). Maternal BDI was also person-centered so that within-person (i.e., idiographic) fluctuations in depressive symptoms were analyzed. The intercept and the effect of BDI at level 1 were in turn predicted at level 2 by youth 5-HTTLPR genotype. We also entered between-person BDI scores (reflecting mothers’ average BDI levels over the course of the study), youth gender, and youth ethnicity at level 2 to control for these variables. Youth ethnicity was controlled for to address potential concerns about population stratification.[39] Restricted maximum likelihood estimation was used.
RESULTS
Means and standard deviations for youth and mother depressive symptoms at each time point are reported in Table 1. The average idiographic change for the BDI was .17 (SD = 4.90). At baseline, bivariate correlations revealed significant associations between mother and youth depressive symptoms (r=.18, P < .01), but no significant associations between 5-HTTLPR genotype and youth symptoms (r = .07, P = .26) or maternal symptoms (r = −.03, P = .63). A Pearson Chi-square test indicated that 5-HTTLPR allelic distributions did not significantly vary by ethnicity/race, χ2 (8) = 15.05, P = .06.
TABLE 1.
Means and standard deviations of maternal and youth depressive symptoms across the five waves
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| BDI | |||||
| M | 6.04 | 6.51 | 4.76 | 5.83 | 5.01 |
| SD | 6.52 | 8.80 | 6.11 | 6.97 | 7.87 |
| CDI | |||||
| M | 6.36 | 5.51 | 3.99 | 5.15 | 3.82 |
| SD | 5.51 | 5.65 | 4.17 | 5.20 | 4.06 |
BDI, Beck Depression Inventory; CDI, Children’s Depression Inventory.
YOUTH 5-HTTLPR × MOTHERS’ DEPRESSIVE SYMPTOMS PREDICTS PROSPECTIVE YOUTH SYMPTOMS
Analyses revealed that youth 5-HTTLPR significantly interacted with within-person idiographic BDI scores to predict prospective increases in CDI, b = 0.11, P < .05 (see Table 2). Follow-up analyses showed that for youth exposed to high levels of idiographic elevations in maternal depressive symptoms, there was a significant association between 5-HTTLPR genotype and youth depressive symptoms, such that the S allele was associated with greater CDI scores (b = 0.91, P < .05). For youth exposed to low levels of idiographic elevations in maternal symptoms, there was no association between 5-HTTLPR genotype and youth depressive symptoms (b=−0.12, P = .77).
TABLE 2.
5-HTTLPR × maternal depressive symptoms predicting prospective increases in youth depressive symptoms 3 months later over five waves (1 year)
| Predictor | b | SE | t | df |
|---|---|---|---|---|
| Fixed effects | ||||
| Level 1 | ||||
| Lagged CDI | 0.08 | 0.04 | 2.00* | 957 |
| Lagged within-person BDI | 0.01 | 0.12 | 0.06 | 957 |
| Level 2 | ||||
| 5-HTTLPR | 0.40 | 0.34 | 1.18 | 236 |
| Between-person BDI | 0.16 | 0.05 | 3.37** | 236 |
| 5-HTTLPR × lagged within-person BDI | 0.12 | 0.04 | 2.97* | 957 |
| Between-person BDI × lagged within-person BDI | −0.003 | 0.004 | −0.83 | 957 |
| Variance components | ||||
| Intercept | 10.22*** |
BDI, Beck Depression Inventory; CDI, Children’s Depression Inventory.
P < .05;
P < .01;
P < .001.
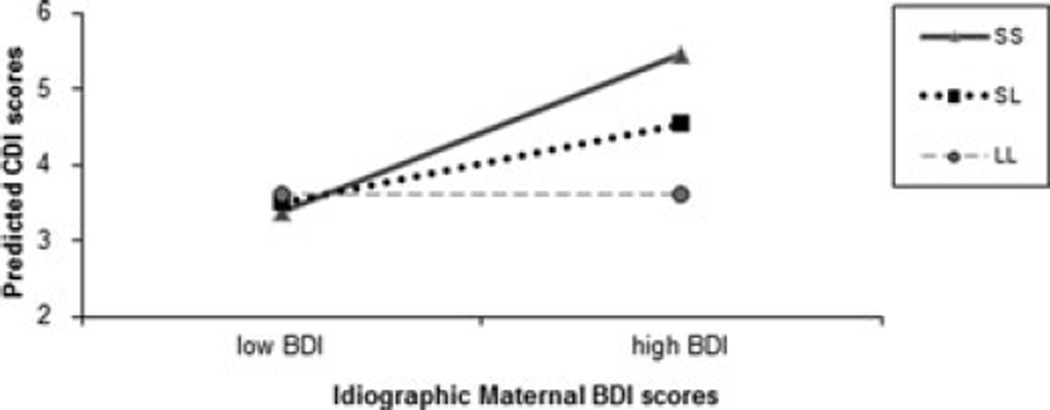
The significant 5-HTTLPR × ideographically ascertained BDI interaction is shown in Figure 1 with BDI depicted at 1 SD above and below the mean for idiographic levels of maternal depressive symptoms.
Figure 1.
Interaction between 5-HTTLPR and idiographic elevations in maternal depressive symptoms predicting youth depressive symptoms 3months later over time. S, short 5-HTTLPR allele; L, long 5-HTTLPR allele; CDI, Children’s Depression Inventory; BDI, Beck Depression Inventory.
Analyses were repeated for only the Caucasian youth in our sample to address any remaining concerns about population stratification. Results showed that the 5-HTTLPR still significantly interacted with idiographic increases in maternal depressive symptoms to predict youth symptoms for this subsample (b = 0.10, P < .05).
Given that our sample consisted of three cohorts of youth, we examined whether grade moderated findings. However, the interaction of grade × child 5-HTTLPR × idiographic maternal BDI increases was not significant (b=−0.01, P = .49).
Finally, we ran analyses using a nomothetic approach in which the rank order of maternal symptoms was assessed. To do this, we left maternal depressive symptoms uncentered in analyses. Consistent with our hypothesis and prior studies, results showed that the 5-HTTLPR × nomothetic maternal BDI levels were not significant (b = 0.03, P = .22).
DISCUSSION
Exposure to maternal depression is a powerful predictor of youth depression, yet relatively little is known about individual differences that may enhance youths’ vulnerability to depressive symptoms after experiencing increases in their mothers’ depressive symptoms. We hypothesized that certain measured child genes, especially 5-HTTLPR that has been shown to confer susceptibility to individuals’ environments, would interact with idiographic levels of maternal depressive symptoms to predict prospective increases in youth depressive symptoms. Results from the current study show that 5-HTTLPR moderates the effects of idiographic levels of maternal depressive symptoms on later elevations in youth depressive symptoms. Specifically, results showed that within-person increases in maternal depressive symptoms (i.e., idiographic increases) longitudinally predicted increases in youth depressive symptoms, especially among those youth with the SS genotype, and that this effect was observed even after controlling for mothers’ average level of depressive symptoms over the one-year course of the study. However, the severity of maternal depressive symptoms (i.e., nomothetic levels) did not interact with youth 5-HTTLPR genotype to predict depressive symptoms.
Results from this study contribute to knowledge on the intergenerational transmission of depression. These findings are consistent with a growing body of research demonstrating the temporal association between mother and child depression.[6–9] Prior multiwave studies examining associations between mother and youth depressive symptoms have examined only concurrent associations,[8, 9] including those using an idiographic approach.[7] Thus, the direction of effects were unclear based on prior studies, and could not clarify whether maternal depressive symptoms are an antecedent, concomitant, or consequence of child depressive symptoms. Findings from this study in which lagged analyses were used, provide strong evidence that maternal depressive symptoms precede increases in child symptoms, and likely contribute to prospective increases in youth symptoms, even after controlling for youths’ prior symptoms and average levels of mothers’ symptoms. In other words, findings suggest that youth exposed to idiographic increases in their mothers’ depressive symptoms are indeed at an increased risk for later experiencing elevated depressive symptoms themselves, especially when youth are genetically susceptible.
Although we found evidence supporting that maternal depressive symptoms are a precursor to child symptoms, findings from this study do not exclude alternative ways in which mother and child symptoms may be associated with each other. For example, maternal symptoms may also be contemporaneously associated with youth symptoms in response to external stressors, such as economic hardships, or marital conflict.[40, 41] The association between mother and child symptoms may also be bidirectional. Just as youth may be distressed in response to mothers’ behaviors associated with their depressive symptoms, mothers may also be distressed about their child’s internalizing symptoms.[41] It will be important for future work to investigate bidirectional and transactional associations between maternal and child depressive symptoms, and to examine how youth 5-HTTLPR genotype may moderate such potential transactional processes.
Results also extend prior findings on youth vulnerability factors for the intergenerational transmission of depression. Prior studies have found evidence that child traits and characteristics (e.g., cognitive vulnerability, emotion regulation skills)[7, 8, 10] interact with maternal depressive symptoms to predict youth symptoms. Our findings suggest that some youth may also be genetically more vulnerable to elevations in their mothers’ depressive symptoms. Findings are in line with research showing that 5-HTTLPR is associated with increased sensitivity to the environment, including sensitivity to parenting behaviors,[15, 27] as well as maternal depressive symptoms.[9]
The exact mechanisms through which 5-HTTLPR may confer risk for depression among youth exposed to idiographic elevations in mothers’ depression are still unclear. Theory and research suggest that 5-HTTLPR may be associated with increased sensitivity to the environment because it underlies the trait of negative emotionality,[14] which influences regulation of emotional information.[42] In particular, studies show that 5-HTTLPR is associated with atypical processing of emotional cues, such as attention biases to facial displays of emotion.[43] Depressed mothers likely exhibit elevated negative affect through verbal and nonverbal cues,[44, 45] and youth with the SS genotype may be more likely to over attend to this negative emotional information. This hypothesis is supported by recent evidence showing that youth 5-HTTLPR interacts with exposure to maternal depression to predict biased processing of affective faces.[46] Additionally, elevated maternal depressive symptoms are generally associated with problematic parenting behaviors, and SS youth may be more sensitive to parenting characterized by greater maternal irritability, criticism, and withdrawal.[44, 47] Future research will benefit from investigations of ways in which 5-HTTLPR may contribute to emotional processing biases and other mechanisms for susceptibility to intergenerational transmission of depression.
Confidence in the study’s findings is strengthened by the more rigorous multiwave study design used to test hypotheses that allowed for more statistical power.[48] Also, the multiwave design enabled an idiographic perspective and analytic approach, which is not possible in only cross-sectional or two time-point studies.
In addition to these strengths, the study has some limitations that can be the focus of future research. Only depressive symptoms, rather than clinical diagnoses of depression, were assessed in this study. Moreover, the depressive symptom scores for both mothers and youth fell mostly within the normal range. That is, youth average scores on the CDI were below the cutoff for clinical depression (raw score of 20),[31] and maternal average scores on the BDI were in the minimal range (raw score of 0–13) and consistent with nondepressed populations.[34] It is therefore unclear whether our findings generalize to clinically depressed populations. Future research is needed to examine whether the processes described in this study contribute to the intergenerational transmission of clinical depression. Additionally, our study focused on mothers given evidence showing larger effects for maternal depression on offspring compared to paternal depression.[28] However, less is known about the effects of fathers’ depression on offspring. It will be important to investigate whether parent gender influences the intergenerational transmission of depression, especially for genetically vulnerable youth.
Our study is also limited by a relatively small sample size, which may have limited power to detect accurate G × E effects.[50] However, the majority of prior G × E studied have used cross-sectional designs for which larger sample sizes may be necessary to achieve adequate power, whereas multiwave designs increase statistical power.[48] Furthermore, reviews have suggested that the likelihood of detecting the 5-HTTLPR × environment interaction in depression is associated with stronger, more psychometrically sound assessments of the environment.[51] Therefore, our study advances knowledge by using a novel conceptual approach that examines idiographic increases in maternal depressive symptoms, and a multiwave, longitudinal design. The rigorous assessment of the environment and use of repeated measures likely improved chances of detecting a G × E effect and addressed limitations of prior 5-HTTLPR × environment studies. However, future research is needed to test whether findings from this study are replicated in larger sample sizes.
Finally, this study examined the moderation effect of a single candidate gene. However, genes do not work in isolation.[52] Future studies are needed to test the possibility that the 5-HTTLPR may work in concert with other genes, such as others associated with the serotonergic system, to moderate the effect of maternal depression on offspring depression. Recently, pathway-based approaches have been developed which examine whether a group of related genes are associated with a trait of interest.[53] These approaches have promising applications for G × E studies, and may be a more powerful method for assessing genetic effects than those that examine a single genetic marker.
Acknowledgments
This work was supported by NIMH grant 5R01 MH077195 (Dr. Hankin and Dr. Young). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or National Institutes of Health.
REFERENCES
- 1.Goodman SH, Tulley E. Children of depressed mothers: implications for the etiology, treatment, and prevention of depression in children and adolescents. In: Abela JRZ, Hankin BL, editors. Handbook of Depression in Children and Adolescents. New York: Guilford Press; 2008. pp. 415–440. [Google Scholar]
- 2.England MJ, Sim IJ. Depression in Parents, Parenting, and Children: Opportunities to Improve Identification, Treatment, and Prevention. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 3.Hammen C, Brennan PA, Keenan-Miller D. Patterns of adolescent depression to age 20: the role of maternal depression and youth interpersonal dysfunction. J Abnorm Child Psychol. 2008;36:1189–1198. doi: 10.1007/s10802-008-9241-9. [DOI] [PubMed] [Google Scholar]
- 4.Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: a developmental model for understanding mechanisms of transmission. Psychol Rev. 1999;106:458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- 5.Joiner TE, Katz J. Contagion of depressive symptoms and mood: meta-analytic review and explanations from cognitive, behavioral, and interpersonal viewpoints. Clin Psychol. 1999;6:149–164. [Google Scholar]
- 6.Hammen C, Burge D, Adrian C. Timing of mother and child depression in a longitudinal study of children at risk. J Consult Clin Psychol. 1991;59:341–345. doi: 10.1037//0022-006x.59.2.341. [DOI] [PubMed] [Google Scholar]
- 7.Abela JRZ, Skitch SA, Adams P, Hankin BL. The timing of parent and child depression: a hopelessness theory perspective. J Clin Child Adolesc Psychol. 2006;35:253–263. doi: 10.1207/s15374424jccp3502_9. [DOI] [PubMed] [Google Scholar]
- 8.Flancbaum M, Oppenheimer CW, Abela JRZ, et al. The effects of rumination on the timing of maternal and child negative affect. J Clin Child and Adolesc Psychol. 2011;40:596–606. doi: 10.1080/15374416.2011.581615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibb BE, Benas JS, Grassia M, McGeary J. Children’s attentional biases and 5-HTTLPR genotype: potential mechanisms linking mother and child depression. J Clin Child Adolesc Psychol. 2009;38:415–426. doi: 10.1080/15374410902851705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silk JS, Shaw DS, Forbes EE, et al. Maternal depression and child internalizing: the moderating role of child emotion regulation. J Clin Child Adolesc Psychol. 2006;35:116–126. doi: 10.1207/s15374424jccp3501_10. [DOI] [PubMed] [Google Scholar]
- 11.Compas BE, Langrock AM, Keller G, et al. Children coping with parental depression: processes of adaptation to family stress. In: Goodman SH, Gotlib IH, et al., editors. Children of Depressed Parents: Alternative Pathways to Risk for Psychopathology. Washington, DC: American Psychological Association Press; 2002. pp. 227–252. (2002). [Google Scholar]
- 12.Dunn EC, Uddin M, Subramanian SV, et al. Research review: gene-environment interaction research in youth depression—a systematic review with recommendations for future research. J Child Psychol Psychiatry. 2011;52:1223–1238. doi: 10.1111/j.1469-7610.2011.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- 14.Caspi A, Hariri AR, Holmes A, et al. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hankin BL, Nederhof E, Oppenheimer CW, et al. Differential susceptibility in youth: evidence that 5-HTTLPR × positive parenting is associated with positive affect “for better and worse”. Transl Psychiatry. 2011;1:1–7. doi: 10.1038/tp.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- 17.McGuffin P, Alsabban S, Uher R. The truth about genetic variation in the serotonin transporter gene and response to stress and medication. Br J Psychiatry. 2011;198:464–471. doi: 10.1192/bjp.bp.110.085225. [DOI] [PubMed] [Google Scholar]
- 18.Thase ME. Neurobiological aspects of depression. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. 2nd ed. New York: Guilford Press; 2009. pp. 187–217. [Google Scholar]
- 19.Conway CC, Hammen C, Espejo EP, et al. Appraisals of stressful life events as a genetically-linked mechanism in the stress-depression relationship. Cogn Ther Res. 2012;36:338–347. [Google Scholar]
- 20.Gunthert KC, Connor TS, Armeli S, et al. Serotonin transporter gene polymorphism (5-HTTLPR) and anxiety reactivity in daily life: a daily process approach to gene-environment interaction. Psychosom Med. 2007;69:762–768. doi: 10.1097/PSY.0b013e318157ad42. [DOI] [PubMed] [Google Scholar]
- 21.Gotlib IH, Joorman J, Minor KL, Hallmayer J. HPA-axis reactivity: a mechanism underlying the associations among 5HT-TLPR, stress, and depression. Biol Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayden EP, Klein DN, Sheikh HI, et al. The serotonin transporter promoter polymorphism and childhood positive and negative emotionality. Emotion. 2010;10:696–702. doi: 10.1037/a0019527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertolino A, Arciero G, Rubino V, et al. Variation of human amygdala response during threatening stimuli as a function of 5-HTTLPR genotype and personality style. Biol Psychiatry. 2005;57:1517–1525. doi: 10.1016/j.biopsych.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 24.Furman D, Hamilton P, Joorman J, Gotlib I. Altered timing of amygdala activation during sad mood elaboration as a function of 5-HTTLPR. Soc Cogn Affect Neurosci. 2011;6:27–276. doi: 10.1093/scan/nsq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–404. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 26.Burkhouse KL, Gibb BE, Coles ME, et al. Serotonin transporter genotype moderates the link between children’s reports of over-protective parenting and their behavioral inhibition. J Abnorm Child Psychol. 2011;39:783–790. doi: 10.1007/s10802-011-9526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pluess M, Belsky J. Children’s differential susceptibility to effects of parenting. Fam Sci. 2010;1:14–25. [Google Scholar]
- 28.Connell AM, Goodman SH. The association between psychopathology in fathers versus mothers and children’s internalizing and externalizing behavior problems: a meta-analysis. Psychol Bull. 2002;128:746–773. doi: 10.1037/0033-2909.128.5.746. [DOI] [PubMed] [Google Scholar]
- 29.Curran PJ, Willoughby MT. Implications of latent trajectory models for the study of developmental psychopathology. Dev Psychopathol. 2003;15:581–612. doi: 10.1017/s0954579403000300. [DOI] [PubMed] [Google Scholar]
- 30.Hankin BL, Jenness J, Abela JRZ, Smolen A. Interaction of 5-HTTLPR and idiographic stressors predicts prospective depressive symptoms specifically among youth in a multi-wave design. J Clin Child Adolesc Psychol. 2011;40:572–585. doi: 10.1080/15374416.2011.581613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovacs M. Children’s Depression Inventory (CDI) Manual. New York: Multi-Health Systems; 1992. [Google Scholar]
- 32.Klein DN, Dougherty LR, Olino TM. Toward guidelines for evidence-based assessment of depression in children and adolescents. J Clin Child Adolesc Psychol. 2005;34:412–432. doi: 10.1207/s15374424jccp3403_3. [DOI] [PubMed] [Google Scholar]
- 33.Smucker MR, Craighead WE, Craighead LW, Green BJ. Normative and reliability data for the children’s depression inventory. J Abnorm Child Psychol. 1986;14:25–39. doi: 10.1007/BF00917219. (1986). [DOI] [PubMed] [Google Scholar]
- 34.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 35.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 36.Anchordoquy HC, McGeary C, Liu L, et al. Genotyping of three candidate genes following whole genome preamplification of DNA collected from buccal cells. Behav Genet. 2003;33:73–78. doi: 10.1023/a:1021007701808. [DOI] [PubMed] [Google Scholar]
- 37.Hu X, Oroszi G, Chun J, et al. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;9:8–16. doi: 10.1097/01.alc.0000150008.68473.62. (2005). [DOI] [PubMed] [Google Scholar]
- 38.Raudenbush SW, Bryk A, Cheong YF, et al. HLM 7: Linear and NonlinearModeling. Lincolnwood, IL: Scientific Software International, Inc; 2011. [Google Scholar]
- 39.Hutchison KE, Stallings M, McGeary J, Bryan A. Population stratification in the candidate gene study: fatal threat or red herring? Psychol Bull. 2004;130:66–79. doi: 10.1037/0033-2909.130.1.66. [DOI] [PubMed] [Google Scholar]
- 40.Conger RD, Conger KJ, Mathews LS, Elder GH. Pathways of economic influence on adolescent adjustment. Am J Community Psychol. 27:519–541. doi: 10.1023/A:1022133228206. [DOI] [PubMed] [Google Scholar]
- 41.Hammen C. The context of stress in families of children with depressed parents. In: Goodman S, Gotlib I, editors. Children of Depressed Parents: Mechanisms of Risk and Implications for Treatment. Washington, DC: American Psychological Association; 2002. pp. 175–199. [Google Scholar]
- 42.Watson D, Clark LA. Negative affectivity: the disposition to experience aversive emotional states. Psychol Bull. 1984;96:465–490. [PubMed] [Google Scholar]
- 43.Beevers CG, Wells TT, Ellis AJ, McGeary JE. Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. J Abnorm Psychol. 2009;118:670–681. doi: 10.1037/a0016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cummings EM, Davies PT. Maternal depression and child development. J Child Psychol Psychiatry. 1994;35:73–122. doi: 10.1111/j.1469-7610.1994.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 45.Foster CJE, Garber J, Durlak JA. Current and past maternal depression, maternal interaction behaviors, and children’s externalizing and internalizing symptoms. J Abnorm Child Psychol. 2008;36:527–537. doi: 10.1007/s10802-007-9197-1. [DOI] [PubMed] [Google Scholar]
- 46.Jacobs RH, Pine DS, Schoeny ME, et al. Maternal depressive history, teen 5HTTLPR genotype, and the processing of emotional faces: exploring mechanisms of risk. Behav Res Ther. 2011;49:80–84. doi: 10.1016/j.brat.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lovejoy MC, Graczyk PA, O’Hare E, Neuman G. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- 48.Snijders TAB, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. London: Sage; 1999. [Google Scholar]
- 49.Hankin BL, Fraley RC, Lahey BB, Waldman ID. Is depression best viewed as a continuous or discrete category? A taxometric analysis of childhood and adolescent depression in a population-based sample. J Abnorm Psychol. 2005;114:96–110. doi: 10.1037/0021-843X.114.1.96. [DOI] [PubMed] [Google Scholar]
- 50.Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment research in psychiatry. Am J Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Mol Psychiatry. 2010;15:18–21. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- 52.Schadt EE. Molecular networks as sensors and drivers of common human disease. Nature. 2009;461:218–223. doi: 10.1038/nature08454. [DOI] [PubMed] [Google Scholar]
- 53.Wang K, Li M, Hakonarson H. Analysing biological pathways in genome-wide association studies. Nat Rev Genet. 2010;11:843–854. doi: 10.1038/nrg2884. [DOI] [PubMed] [Google Scholar]