Abstract
Neuroscientists are now coming to appreciate that a significant degree of information processing occurs in the peripheral sensory organs of taste prior to signals propagating to the brain. Gustatory stimulation causes taste bud cells to secrete neurotransmitters that act on adjacent taste bud cells (paracrine transmitters) as well as on primary sensory afferent fibers (neurocrine transmitters). Paracrine transmission, representing cell-cell communication within the taste bud, has the potential to shape the final signal output that taste buds transmit to the brain. The following paragraphs summarize current thinking about how taste signals generally, and umami taste in particular, are processed in taste buds.
Keywords: taste, neurotransmitters, serotonin, ATP, norepinephrine, synaptic transmission
Taste Cell Morphotypes
A discussion of information processing in taste buds begins with the observation that taste buds are populated by different types of cells. From the earliest microscopic studies, taste buds have been described as having “dark” and “light” cells, depending on the intensity of histological staining. These were variously termed receptor cells and supporting cells but with no strong evidence for either categorization (for reviews of the earlier literature on correlating taste cell morphotypes and function, see Reutter1 and Roper2). With the advent of modern electron microscopy, the classification of taste cell morphotypes was refined by defining four categories: Type I (formerly dark cells); Type II (formerly light cells), Type III (cells that when stained with routine histological methods appeared dark but that when observed in electron micrographs possessed synaptic contacts); and Type IV (basal cells believed to be stem or progenitor cells). However, these classifications are not hard and fast, and the specific features characterizing each morphotype vary somewhat from species to species.
A major breakthrough in understanding the functional categories of the different types of taste bud cells was made by using immunohistochemistry to identify which proteins were present.3 However, initial attempts to correlate proteins with the morphotypes did not consistently result in a clear-cut segregation into Type I, II, III, and IV cells.
The issue of taste cell types was largely resolved by studies that combine physiological tests on isolated taste cells with immunostaining and single cell RT-PCR to correlate structure with function. These investigations unambiguously resolved that there are two major classes of taste-sensitive cells in mammalian taste buds. Type II cells express G protein-coupled receptors (GPCRs) and downstream effectors for sweet, bitter, and umami taste compounds and are directly stimulated by these tastants.4–6 Accordingly, these cells can be termed receptor cells. In contrast, Type III cells possess ultrastructural features of synapses (vesicle clusters and synaptic membrane thickenings), express proteins normally associated with synapses, and display typical neuronal features, such as depolarization-induced Ca2+ influx.3,7 Thus, we have termed these cells presynaptic taste cells. Presynaptic (Type III) cells do not express taste GPCRs. Presynaptic cells directly respond to sour and salty taste stimulation, and as will be explained below, only indirectly to sweet, bitter, and umami tastes. Type I cells express an ecto-ATPase and may be involved in degrading a neurotransmitter (ATP) secreted by Type II cells.8 Type I cells may also be involved in salt taste, but the details remain to be firmly established.9 Table 1 summarizes important features of Type I, II, and III taste bud cells.
TABLE 1. Key Features of Receptor, Presynaptic, and Glial-like Taste Cells.
| Taste cell type | Characteristic proteins (immunostaining, RT-PCR) | Function |
|---|---|---|
| Glial-like (Type I) cells | GLASTa |
|
| NTPD2 (ecto-ATPase) | ||
| Receptor (Type II) cells | Taste GPCRs (e.g., T1Rs, T2Rs) |
|
| PLCβ2 | ||
| IP3R3 | ||
| TRPM5 | ||
| Presynaptic (Type III) cells | SNAP25 |
|
| NCAM | ||
| GAD67 (not all Type III cells) | ||
| AADC | ||
| Voltage-gated Ca2+ channels | ||
| PKD2L1 | ||
| Serotonin (not all Type III cells) |
Cell-Cell Interactions
The above observations that receptor (Type II) cells express GPCR taste receptors but lack synapses, and that presynaptic (Type III) cells express synaptic proteins, have synapses, and manifest stimulus-evoked Ca2+ influx are consistent with the hypothesis that cell–cell interactions in taste buds are involved in signal processing. This suggestion was put forth long ago by K. Reutter1 in his treatise on the ultrastructure of taste buds in a teleost fish, Ameiurur nebulosus (bullhead). Reutter observed ultrastructural evidence for synapses between taste cells. Some years later, Ewald and Roper12,13 impaled pairs of cells in the large taste buds of Necturus maculosus and showed that electrical stimulation of one cell evoked responses in the other cell of the pair, confirming the existence of cell–cell communication. Interestingly, stimulation of the one taste cell evoked hyperpolarizing responses in the follower cell, and then only after repetitive stimulation. The slow, long-lasting hyper-polarization in the follower cell was mimicked approximately by bath-applying serotonin, presaging aminergic paracrine modulation within the taste bud as proposed by Kaya and colleagues14 for mammalian taste buds.
In addition, Reutter1 wrote that taste bud cells also communicated synaptically with afferent nerve terminals, based on his and others' morphological findings. This, too, previewed what is now believed to occur during signal processing, as described below.
Synaptic Transmitters in Taste Buds
To clarify and consolidate the above concepts, we and others have set about identifying synaptic transmitters associated with each of the cell–cell interactions. Nagai and coworkers15 reviewed evidence for several possible taste bud neurotransmitters, among which serotonin (5-HT) was a leading candidate. Ewald and Roper12 and Herness and colleagues14 concluded that 5-HT was a paracrine transmitter in taste buds, possibly in addition to its hypothesized role as a taste transmitter onto gustatory primary afferent fibers.16 Since then, Finger and colleagues17 showed that isolated strips of lingual epithelium containing taste buds secreted ATP, and the ATP acted on purinoceptors of subclass X2 (p2X2) of gustatory afferent fibers. The crux of their evidence for the latter was that mutant mice lacking P2X2 and P2X3 receptors had marked taste losses. Dvoryanchikov et al. also used isolated strips of lingual epithelium to show that taste buds take up and resecrete norepinephrine (NE).18 We have used Chinese hamster ovary cells expressing high affinity neurotransmitter receptors as biosensors to probe isolated taste buds and isolated taste cells for taste transmitter release.19–21 This has allowed us to refine transmitter identification and localize which cells secrete what transmitters. This work has conclusively demonstrated that taste stimulation causes receptor (Type II) cells to secrete ATP. ATP secretion is independent of extracellular Ca2+, but this does not mean that the release is Ca-independent. Indeed, ATP secretion from receptor cells is consistent with mobilization of intracellular Ca2+ evoked by stimulating taste GPCRs on those cells. In contrast, presynaptic (Type III) cells release 5-HT and NE in response to stimulation by ATP, KCl depolarization, or sour taste (acids).19–22 5-HT and NE release evoked by KCl depolarization and acid (sour) taste depends on Ca2+ influx, suggesting conventional vesicular exocytosis. However, ATP stimulates 5-HT and NE secretion in the absence of extracellular Ca2+, suggesting intracellular Ca2+ mobilization also triggers transmitter release from presynaptic cells. These specifics remain to be established.
Taking all the above findings together, our understanding of cell–cell communication in the taste bud can now be refined (Fig. 1). There is some ambiguity about the postsynaptic targets for 5-HT and NE. These details are under investigation in a number of laboratories.
Figure 1.
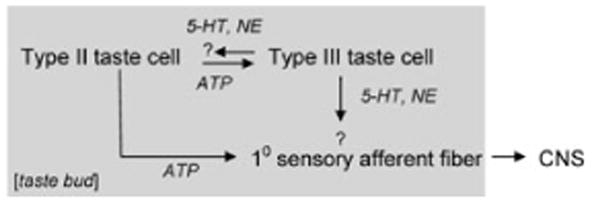
Schematic drawing of signal transfer and synaptic transmitters in taste buds. The gray shaded area shows events taking place within the taste bud. The precise targets for 5-HT and NE are not known with confidence, as represented here by question marks.
Inconsistencies, Anomalies, and Ambiguities
Taste stimulation causes receptor (Type II) taste cells to secrete ATP, but these cells do not possess synapses or synaptic proteins. Then how is ATP secreted? Huang and colleagues21 and Romanov and co-workers23 revealed that taste cells secrete ATP via gap junction hemichannels, presumably gated open by increased intracellular Ca2+ and membrane depolarization. Huang and colleagues21 specifically showed that ATP was secreted through pannexin 1 hemichannels in receptor cells. This finding resolves the longstanding problem of how transmitter release can occur from cells that lack conventional synaptic structures, such as vesicle clusters and presynaptic densities, and that do not express synaptic proteins (SNARE proteins), such as SNAP-25.
The postsynaptic sites for 5-HT and NE remain ambiguous. Ewald and Roper12 and later Herness et al.14 provided evidence that cells within the taste bud were themselves postsynaptic targets. However, Finger and collaborators17 showed that there was no difference between taste-evoked behaviors of wild-type and mutant mice lacking one type of serotonin receptor (5-HT3). The mutant mice were able to detect sweet, bitter, sour, and umami taste stimuli. This finding has erroneously been interpreted as indicating 5-HT is not involved in taste signaling. There are at least 14 different 5-HT receptors, leaving open the possibility that receptors other than 5-HT3 mediate serotonergic mechanisms in taste buds. The specific targets for the 5-HT and NE secreted by presynaptic taste cells have yet to be identified.
Huang and colleagues recently showed that presynaptic taste cells secrete NE when they are stimulated with a sour tastant (acetic acid) or depolarized with KCl.22 Further, in some presynaptic cells, NE is coreleased with 5-HT. All presynaptic cells tested appear to release 5-HT upon stimulation, but only about 33% cosecrete NE. This suggests that only a limited subset of presynaptic cells coreleases both 5-HT and NE. Alternatively, all presynaptic cells might cosecrete 5-HT and NE upon stimulation, but NE is released only at focal, synaptic sites, whereas 5-HT is secreted widely from the cell surface. Indeed, if 5-HT is a paracrine neurotransmitter, widespread release from the basolateral surface might be understandable. Either of these two scenarios would explain the results obtained with cellular biosensors. In the latter case, the chance of the biosensor being immediately adjacent to a focal, synaptic 5-HT/NE corelease site would be 33%. These refined questions about location of release sites have not yet been studied.
A curiosity about NE secretion is that taste bud cells, including presynaptic cells, do not synthesize NE. Presynaptic cells lack the biosynthetic enzymes for NE, tyrosine hydroxylase and dopamine β-hydroxylase.18 Presynaptic cells apparently obtain NE by uptake via the transporter, NET, then repackage and secrete NE. The source of NE for presynaptic cell uptake may be the surrounding aminergic (presumably autonomic) nerve fibers.
Last, the identification of ATP as the neurotransmitter between receptor taste cells and primary sensory afferents rests heavily on the finding that mutant mice lacking P2X2 and P2X3 receptors do not respond to taste stimulation.17 However, recent studies reveal that these mutant mice also are deficient in an earlier step in afferent transmission: neurotransmitter secretion from receptor cells is defective.24 Thus, activity in purinergic afferent fibers, cell–cell communication mediated by ATP within the taste bud,21 and release of neurotransmitter(s) in addition to ATP could all be compromised in P2X2/X3 double-knockout mice. Consequently, the new findings weaken the argument that ATP alone acts postsynaptically.
The Final Model, Work Still in Progress
Despite wrinkles in some of the details, a fairly consistent model for cell–cell signal processing in taste buds has emerged over the past few years. Tests in semi-intact preparations of slices of lingual tissue containing taste buds have significantly supported this model.25 The findings include that one class of taste bud cells, receptor (Type II) cells, is tuned to a single category of taste—either sweet, bitter, or umami. A given receptor cell expresses GPCR taste receptors for only one of these three classes, and for the most part, receptor cells respond to taste stimulation by only one of these taste qualities. In contrast, presynaptic (Type III) cells are stimulated by ATP secreted from receptor cells. Hence, presynaptic cells represent a point of convergent signals. Consequently, a single presynaptic cell responds broadly to sweet, bitter, and umami.25 Further, presynaptic cells themselves are excited by sour (acid) taste and salt (NaCl). Thus, the presynaptic cell appears to integrate information about several tastants. Recent findings by Vanderbeuch and colleagues9 suggest that Type I cells may be important in Na+ taste. Interactions, if any, between Type I taste cells and other taste cells remains to be investigated. The final working model, then, becomes as shown in Figure 2.
Figure 2.
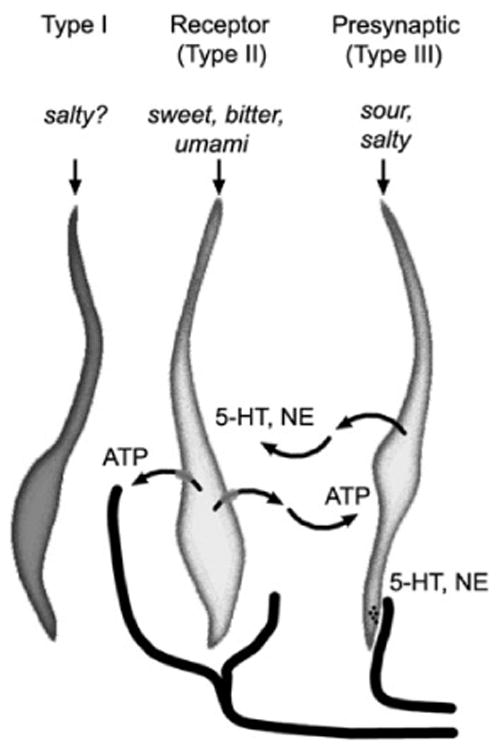
Cell–cell communication within the taste bud. Receptor (Type II) cells secrete ATP through pannexin 1 hemichannels. ATP acts on primary gustatory afferent fibers as well as on adjacent presynaptic (Type III) taste cells. Presynaptic taste cells release 5-HT and NE, possibly at synaptic sites as well as widely from basolateral membrane sites. The latter may represent paracrine transmission, whereas synaptic release would be consistent with neurocrine transmission.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.Reutter K. Taste organ in the bullhead (Teleostei) Adv Anat Embryol Cell Biol. 1978;55:3–94. doi: 10.1007/978-3-642-67008-4. [DOI] [PubMed] [Google Scholar]
- 2.Roper SD. The cell biology of vertebrate taste receptors. Annu Rev Neurosci. 1989;12:329–353. doi: 10.1146/annurev.ne.12.030189.001553. [DOI] [PubMed] [Google Scholar]
- 3.Yee CL, Yang R, Bottger B, et al. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol. 2001;440:97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- 4.Clapp TR, Medler KF, Damak S, et al. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP- 25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clapp TR, Yang R, Stoick CL, et al. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J Comp Neurol. 2004;468:311–321. doi: 10.1002/cne.10963. [DOI] [PubMed] [Google Scholar]
- 6.DeFazio RA, Dvoryanchikov G, Maruyama Y, et al. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medler KF, Margolskee RF, Kinnamon SC. Electrophysiological characterization of voltage-gated currents in defined taste cell types of mice. J Neurosci. 2003;23:2608–2617. doi: 10.1523/JNEUROSCI.23-07-02608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartel DL, Sullivan SL, Lavoie EG, et al. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 2006;497:1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungi-form taste cells in mouse. BMC Neurosci. 2008;9:1. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawton DM, Furness DN, Lindemann B, Hackney CM. Localization of the glutamate-aspartate transporter, GLAST, in rat taste buds. Eur J Neurosci. 2000;12:3163–3171. doi: 10.1046/j.1460-9568.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- 11.Caicedo A, Jafri MS, Roper SD. In situ Ca2+ imaging reveals neurotransmitter receptors for glutamate in taste receptor cells. J Neurosci. 2000;20:7978–7985. doi: 10.1523/JNEUROSCI.20-21-07978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ewald DA, Roper SD. Bidirectional synaptic transmission in Necturus taste buds. J Neurosci. 1994;14:3791–3804. doi: 10.1523/JNEUROSCI.14-06-03791.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewald DA, Roper SD. Intercellular signaling in Necturus taste buds: chemical excitation of receptor cells elicits responses in basal cells. J Neurophysiol. 1992;67:1316–1324. doi: 10.1152/jn.1992.67.5.1316. [DOI] [PubMed] [Google Scholar]
- 14.Kaya N, Shen T, Lu SG, et al. A paracrine signaling role for serotonin in rat taste buds: expression and localization of serotonin receptor subtypes. Am J Physiol Regul Integr Comp Physiol. 2004;286:R649–R658. doi: 10.1152/ajpregu.00572.2003. [DOI] [PubMed] [Google Scholar]
- 15.Nagai T, Kim DJ, Delay RJ, Roper SD. Neuromodulation of transduction and signal processing in the end organs of taste. Chem Senses. 1996;21:353–365. doi: 10.1093/chemse/21.3.353. [DOI] [PubMed] [Google Scholar]
- 16.Esakov AI, Golubtsov KV, Solov'eva NA. Significance of serotonin in the activity of the taste receptor apparatus of the frog Rana temporaria. Zh Evol Biokhim Fiziol. 1983;19:62–67. [PubMed] [Google Scholar]
- 17.Finger TE, Danilova V, Barrows J. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 18.Dvoryanchikov G, Tomchik SM, Chaudhari N. Biogenic amine synthesis and uptake in rodent taste buds. J Comp Neurol. 2007;505:302–313. doi: 10.1002/cne.21494. [DOI] [PubMed] [Google Scholar]
- 19.Huang YA, Maruyama Y, Stimac R, Roper SD. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J Physiol. 2008;586:2903–2912. doi: 10.1113/jphysiol.2008.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang YJ, Maruyama Y, Lu KS, et al. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005;25:843–847. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang YJ, Maruyama Y, Dvoryanchikov G, et al. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang AL, Maruyama Y, Roper SD. Norepinephrine is coreleased with serotonin in mouse taste buds. J Neurosci. 2008;28:13088–13093. doi: 10.1523/JNEUROSCI.4187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romanov RA, Rogachevskaja OA, Bystrova MF, et al. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roper SD, Huang AL, Stone LM, et al. Knocking out P2X receptors prevents ATP release from taste buds. Chem Senses. 2008;33:S127–S128. [Google Scholar]
- 25.Tomchik SM, Berg S, Kim JW, et al. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;27:10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


