Abstract
There is emerging evidence that two parallel lines of gustatory information are generated in taste buds. One pathway leads to higher cortical centers and is involved in discriminating basic taste qualities (sweet, bitter, sour, salty, umami) and perceiving flavors. The other pathway may conduct information involved in physiological reflexes such as swallowing, salivation, and cephalic phase digestion. If this notion is true, the existence of two populations of taste bud cells that have different functional characteristics may lie at the origins of the two pathways. This speculative concept is explored in this review of taste signal processing in mammalian taste buds.
Keywords: Taste transmitters, ATP, Serotonin, 5-HT, Noradrenalin, Pannexin 1, Cephalic phase digestion, Taste receptor cells, Taste bud synapses
1. Introduction: are there parallel pathways for taste?
Taste buds are the peripheral sensory organs of gustation. These sensory structures have the task of monitoring the chemical environment of the oral cavity and particularly of sensing which ingested foods are palatable, which are toxic, which are aversive, which are nutritive, etc. In short, taste buds help oversee the first stage of energy balance, food intake. Taste buds also contribute to our quality of life by parsing food chemicals into the gustatory qualities of sweet, salty, sour, bitter, umami and perhaps other tastes (e.g., fatty, astringent, metallic).
Signals generated by taste buds are transmitted to higher centers in the brain possibly via two or more parallel streams of information [1,2]. For instance, evidence is emerging that information leading to the perceptions of sweetness, sourness, saltiness, etc. may follow a somewhat different pathway than information that elicits cephalic phase digestive processes, swallowing, salivation, and other reflexes. The discrimination of basic taste qualities in primates is believed to take place in the primary gustatory cortex [3]. However, this generalization is species-dependent. For example, in rodents the function of the primary gustatory cortex is not so evident: lesions to this area in rats produce no obvious impairment in taste discrimination [4]. In primates, gustatory information passes from the primary gustatory cortex to association cortices, primarily the orbitofrontal cortex where it contributes to the perception of flavor [5]. In contrast to taste quality discrimination, there may be a quite separate information stream to other brain centers that control physiological reflexes such as cephalic phase digestion and reflex salivation. Researchers believe this latter pathway involves neurons in the reticular formation and oromotor nuclei; higher order central connections are not yet known in much detail.
Presently it is only speculation that the information for taste qualities versus signals for taste-evoked cephalic phase reflexes follow separate and parallel lines. These putative two streams of information are initiated in the peripheral sensory organs of gustation, taste buds. Evidence for the existence of two parallel information streams in taste comes from anatomical studies. For instance, Whitehead and his colleagues have recently shown that mouse gustatory sensory ganglion cells in the geniculate ganglion can be divided into two groups [2]. One group projects to the nucleus of the solitary tract (NTS) and then to the parabrachial nucleus en route to higher cortical centers (vide ante); the other projects to the NTS and then to the reticular formation (Fig. 1).
Fig. 1.
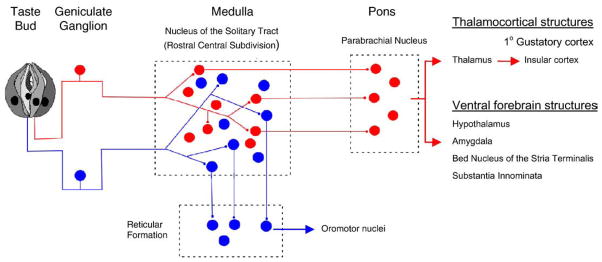
Parallel neuronal pathways for information from taste buds to higher brain centers. In rodents, neurons in the parabrachial nucleus project to thalamocortical and ventral forebrain structures. (In primates, however, signals from the nucleus of the solitary tract travel directly to the thalamus). Discrimination of the basic taste qualities is believed to be take place in the primary gustatory cortex, at least in primates [3]. Neurons that project to the reticular formation (blue) initiate physiological reflexes, including those involved in cephalic phase digestion, triggered by taste stimulation. Central pathways for these cephalic phase reflexes are not known in great detail. Modified from [1,2].
If there are indeed two parallel pathways, the question remains, how are these pathways established within the taste bud? This is the topic of the following pages. I will review recent information from a number of laboratories including our results that have yielded a good understanding of at least some aspects of signal processing in mammalian taste buds. I will conclude with a highly speculative scheme that purports to show how two different cell types in taste buds generate two parallel information pathways.
2. Taste bud cell types
Understanding signal processing in the peripheral organs of taste begins with the appreciation that there are multiple cell types in taste buds. It has been known for over a hundred years that there are at least three types of taste cells, as distinguished by histological staining—so-called dark cells, light cells, and basal cells (reviewed by [6,7]). These morphological distinctions were refined with electron microscopy and ultimately 4 major categories of taste cells have been distinguished—Types I, II, III, and IV [8,9]. Moreover, ultrastructural features of the Type III cells indicated that only these cells form synapses with axons [8,10–14]. Thus, Type III cells were held to generate the signal output for taste information. Immunostaining, in situ hybridization, and genetically engineered mice further refined our knowledge about taste cell types and have allowed researchers to begin to assign functions to these cells. For instance, Type I cells immunostain for an ecto-ATPase, NTPDase2 [15], and a glutamate transporter, GLAST [16], suggesting they are glial-like cells involved with terminating neurotransmitter actions. Type II cells co-express G protein-coupled taste receptors and their downstream effectors (principally gustducin, class β2 phospholipase C [PLCβ2], Type 3 inositol triphosphase receptors [IP3R3], and class M5 transient receptor potential channels [TRPM5] [17–19]), implying that these cells are sensory receptor cells. Type III cells are immunopositive for molecules associated with excitable cells, neuro-transmitters, and synapses (principally SNAP25, neural cellular adhesion molecule (NCAM), and serotonin [14,20,21]). These findings are consistent with the presence of synapses on Type III cells, as had been found in electron micrographs. Type IV cells express the developmental signaling gene, sonic hedgehog, underscoring their role as proliferating basal cells [22]. Table 1 summarizes data for Types II and III cells.
Table 1.
Key properties of Receptor (Type II) Cells and Presynaptic (Type III) Cells in taste buds (see text for references).
| Receptor cells | Presynaptic cells | ||
|---|---|---|---|
| Express | T1R or T2R taste receptors | Express | Voltage-gated Ca channels |
| PLCβ2 | Synaptic proteins (SNAP-25) | ||
| IP3R3 | NCAM | ||
| TRPM5 | |||
| Respond to sweet, bitter, or umami taste compounds with intracellular Ca2+ release | Respond to K+ depolarization with an influx of Ca2+ | ||
| Secrete ATP via pannexin 1 gap junction hemichannels in response to taste stimulation | Release 5-HT or 5-HT plus noradrenalin in response to stimulation | ||
| Possess synapses | |||
| Respond to ATP stimulation | |||
Collectively, the descriptions of Types II and III cells raised the awkward question of how taste-evoked signals from Type II cells could be transmitted to gustatory afferent fibers in view of the fact that these cells have no synapses, as defined by the conventional ultrastructural criteria of vesicle clusters and presynaptic and post-synaptic membrane densities [18]. And conversely, what information could Type III cells be sending across their synapses?
3. Functional imaging and determining the roles of taste bud cells
An understanding of the roles of the different types of taste cells was considerably advanced by functional characterization using a combination of Ca2+ imaging, transgenic mice, and molecular profiling with single cell RT-PCR. DeFazio et al. [23] showed that Type II cells respond to sweet and bitter taste stimulation. These researchers also showed that taste-responsive Type II cells express G protein-coupled taste receptors, PLCβ2, TRPM5, and IP3R3. In contrast, other cells isolated from taste buds were taste-unresponsive but generated robust Ca2+ signals in response to depolarization with KCl. These cells express voltage-gated Ca channels, the synaptic snare protein SNAP25, and NCAM. These data solidified the distinction of two functionally separate cell types. The cells were termed Receptor Cells (Type II cells) and Presynaptic Cells (Type III cells) to reflect their functions (Table 1).
Tomchik et al. [24] subsequently conducted imaging studies on semi-intact taste buds in slices of mouse lingual tissue. They showed that responses of Receptor Cells and Presynaptic Cells were very different from each other. In these experiments, Tomchik et al. [24] identified Receptor and Presynaptic cells by the presence of Green Fluorescent Protein (GFP) in transgenic mice engineered to express GFP either in one or the other of these cell types [24,25]. Receptor Cells responded only to one of three different taste qualities: sweet, bitter, or umami compounds. This result corresponded nicely with previous molecular studies indicating that taste cells expressed either T2Rs (bitter taste receptors) or T1Rs (sweet and umami taste receptors), but not both [26]. The data also corresponded well with the imaging studies on isolated taste cells [23] where Receptor Cells had also been taste-responsive. Responses of Presynaptic Cells in situ (i.e., in the lingual slice preparation), however, markedly differed from responses of these same cells when isolated from taste buds and studied in vitro. Presynaptic Cell responses also differed from responses of Receptor Cells. Namely, Presynaptic Cells in situ responded broadly, sometimes to all 5 taste qualities tested (sweet, sour, salty, bitter, umami). These cells also respond to depolarization with KCl. In vitro, Presynaptic cells only responded to KCl depolarization and to acid (sour) stimulation [27].
The explanation to the broad responsiveness of Type III Cells in situ and a resolution to the question of why data from Presynaptic Cells in isolation [23,27] differs from recordings in situ [24] requires information from studies designed to identify taste bud transmitters (below). In brief, the resolution is that there is cell-to-cell synaptic communication between Receptor and Presynaptic Cells: Presynaptic Cells receive excitatory input from Receptor cells and thus are indirectly excited by several taste compounds.
4. Taste bud neurotransmitters
Over the decades, many neurotransmitter candidates have been put forward for taste buds. However, only recently has there been definitive proof of certain ones. Specifically, Finger et al. [28] showed that ATP is likely a transmitter released by taste buds in response to gustatory stimulation. Mutant mice lacking P2X receptors on gustatory afferent fibers were unresponsive to taste stimulation. This lack of taste responses in the mutant mice was interpreted to mean that taste buds normally communicate synaptically to sensory afferent fibers via ATP, but when the postsynaptic receptors for ATP are missing, taste information is not conveyed to these fibers. These researchers also measured taste-evoked ATP release in a superfusate from strips of lingual tissue containing taste buds. Subsequently, Huang et al. [29] and Romanov et al. [30] used cellular ATP-sensitive biosensors to show definitively that isolated Receptor (Type II) Cells secreted ATP in response to taste stimulation. Moreover, ATP release was mediated by a novel, unexpected mechanism: secretion through gap junction hemichannels, likely to be pannexin 1 [29,30].
5. Identifying taste neurotransmitters, an aside on techniques
The above findings identifying ATP as a taste transmitter were greatly advanced by using a new approach in taste studies, biosensor cells. Biosensor cells are cell lines genetically engineered to express high-affinity neurotransmitter receptors. For instance, Huang et al. [25,27,29,31] used CHO cells expressing neurotransmitter receptors coupled to Ca2+ mobilization to measure biosensor responses with Ca2+ imaging methodology. Thus, CHO cells expressing 5-HT2c receptors reliably responded to low concentrations of serotonin (EC50=9 nM). Similarly, CHO cells expressing P2X2/P2X3 receptors were sensitive reporters for ATP (EC50=380 nM). Although CHO cells have endogenous P2Y receptors (vide infra), when transfected with P2X2/P2X3 receptors, CHO cells become much more sensitive to ATP. This makes them highly responsive ATP sensors. CHO cells expressing α1A adrenergic receptors were biosensors for noradrenalin release (EC50 for noradrenalin=117 nM). Finally, Huang et al. [25] used CHO cells expressing combinations of neurotransmitter receptors, such as 5-HT2c plus α1A to detect release of serotonin and noradrenalin. Selective receptor antagonists for 5-HT2c or to α1A receptors distinguished which expressed receptors were activated on the dual biosensor cells. Such dual biosensor cells allowed Huang et al. [25] to show unambiguously that some Presynaptic (Type III) Cells co-release 5-HT and noradrenalin.
To identify transmitters, CHO biosensor cells loaded with the Ca2+-sensitive dye, Fura 2, are manipulated towards and pressed against isolated taste buds or single taste cells, using a fire-polished glass micropipette (Fig. 2). The CHO biosensor cell is held onto the micro-pipette with gentle suction.
Fig. 2.
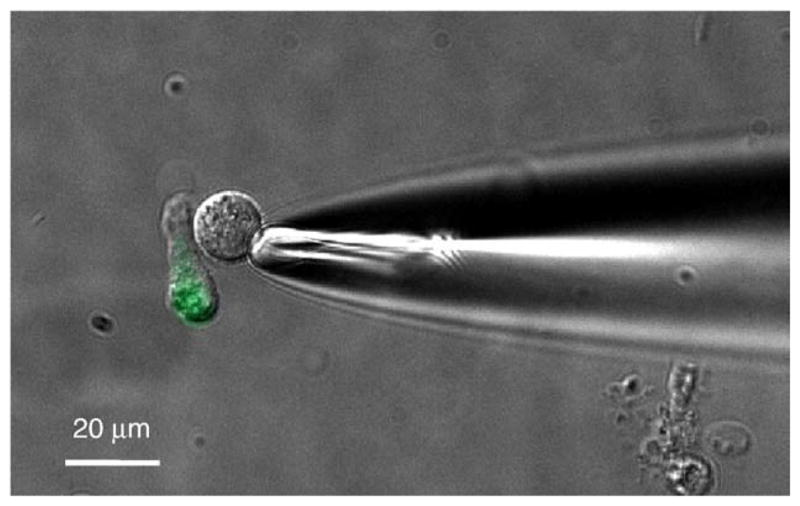
CHO cells stably expressing high affinity receptors for neurotransmitters constitute effective biosensors for identifying taste transmitters. This micrograph shows a biosensor cell, held on the tip of a glass micropipette and apposed to a Presynaptic Cell isolated from a mouse vallate papilla. Presynaptic taste cells isolated from transgenic mice that express GFP on the GAD67 promoter fluoresce green [24], allowing confident identification of this cell type, as shown here. Presynaptic cells, when depolarized by KCl, secrete serotonin, and in some cases (~33%) co-secrete noradrenalin with serotonin. (No cells were found with depolarization-evoked noradrenalin alone). Modified from [25].
Bath-applied taste stimuli or KCl depolarization, neither of which alone stimulates Ca2+ transients in CHO biosensor cells, can be used to activate taste buds or taste cells. If the CHO biosensor is positioned at or near release sites for neurotransmitters, the biosensor cell detects the release and generates a Ca2+ signal (αCa2+). By loading taste buds or taste cells with Fura 2, it is possible to measure concurrently the stimulus-evoked Ca2+ transients in taste cells followed after a brief latency by CHO biosensor cell Ca2+ signals evoked by the secretion of transmitter from the taste cell (Fig. 3).
Fig. 3.
Ca2+ imaging of a serotonin biosensor cell closely apposed to an isolated Presynaptic Cell (such as in Fig. 2). The Presynaptic Cell (Pre) and the biosensor cell (5HT-Bio) were both loaded with the Ca2+-sensitive dye, Fura 2. Depolarizing the Presynaptic Cell with 50 mM KCl (bar below traces) generated a robust signal in the Presynaptic Cell due to Ca2+ influx. This was followed after a brief latency by a 5-HT biosensor cell response, indicating depolarization-evoked release of 5-HT from the Presynaptic Cell. KCl had no direct effect on the biosensor cell. Signals were only observed when the biosensor cell was pressed against the Presynaptic Cell. Modified from [29].
Important controls for using CHO biosensor cells as detectors for taste-evoked neurotransmitter release include that the biosensors themselves do not respond directly to any of the taste test stimuli or to KCl depolarization. Further, biosensor responses must be blocked by appropriate pharmacological antagonists (i.e., specific for the expressed receptors). Thus, for example, 5-HT biosensor responses generated by taste-evoked secretion of serotonin were blocked by mianserin, an antagonist of the 5-HT2c receptors expressed on the CHO biosensors. This is an important control because CHO cells themselves express certain endogenous receptors (http://www.tumor-gene.org/cgi-bin/GPCR/by_cell_line.cgi) which could complicate the identification of transmitters released by the taste cells. It is critical to distinguish the origin of the Ca2+ signals in biosensor cells as stemming from the expressed receptors and not from unidentified endogenous receptors. Because ATP is a taste transmitter [28–30], a particularly problematic endogenous receptor on CHO cells is a P2Y receptor. Endogenous purinoceptors can be eliminated by incubating CHO biosensor cells in a high concentration of ATP (e.g., 500 μM) for prolonged periods prior to using the cells as biosensors. This desensitizes endogenous CHO purinoceptors for several hours (see supporting information in [29]). (Needless to say, in experiments using biosensors to detect ATP release, CHO biosensor cells are not pre-treated with ATP to desensitize their expressed P2X or endogenous P2Y purinoceptors!). All the above controls have been stringently conducted in the course of identifying ATP, serotonin, and noradrenalin as taste transmitters with CHO biosensor cells.
Using cells expressing high-affinity receptors to identify neurotransmitters secreted by cells and tissues is not a new concept. In 1991, Tachibana and Okada [32] exploited endogenous glutamate receptors of catfish horizontal cells to probe goldfish retinal bipolar cells. They patch-clamped both cell types and measured depolarization-evoked glutamate release from the bipolar neurons. In an innovative approach to identifying components of complex mixtures from column separations, Shear et al. [33] used rat PC12 cells (Ca2+ imaging) or xenopus oocytes expressing high-affinity receptors (two electrode voltage clamp) to detect biologically active components such as acetylcholine or serotonin. Rat PC12 cells expressing endogenous P2X2 purinoceptors have also been used to detect ATP secretion from rat pancreatic β cells [34] and rabbit macula densa cells [35]. Commercially-available HEK293H-CNG cells stably expressing human glucagon receptor (BD Biosciences) have been used to measure glucagon release from pancreatic α cells [36]. Hayashi et al. [37] summarized methods for using PC12 cells or HEK cells expressing P2X2 receptors as ATP biosensors. More recently, Romanov et al. [30] used Ca2+ imaging and endogenous purinoceptors of COS-1 cells to identify ATP secreted from taste bud cells, similar to how Huang et al. [29] had used CHO cells expressing P2X2/P2X3 receptors as ATP biosensors for the same taste cells. CHO biosensors genetically engineered as ATP biosensors have also been used to show ATP secretion from supporting cells in cochlear organotypic cultures [38]. Finally, as summarized above, CHO cells expressing high affinity 5-HT and adrenergic receptors were used to identify serotonin and noradrenalin co-secretion from taste bud cells [25]. In short, there is a small but growing literature supporting the importance and utility of cellular biosensors for identifying neuro-transmitters secreted from cells and tissues of interest.
6. Cell-to-cell communication in taste buds
Huang et al. [29] showed that ATP, in addition to its proposed role as a transmitter from taste buds onto primary afferent fibers, excites adjacent Presynaptic (Type III) Cells. When stimulated by ATP, Pre-synaptic Cells release serotonin, or serotonin plus noradrenalin [25]. Thus, taste stimulation directly activates Receptor Cells and indirectly, via ATP, excites Presynaptic Cells.
Because of the unique mechanism for secretion of ATP through pannexin 1 gap junction hemichannels in the plasma membrane of Receptor Cells, it is now understandable that these cells do not form morphologically identifiable synapses. ATP diffuses from gap junction hemichannels on Receptor Cells to nearby targets, adjacent afferent fibers and Presynaptic Cells. Type I taste bud cells express ecto-ATPase (NTPDase2) [15] which very effectively cleaves ATP to ADP and may serve to limit the diffusion of ATP secreted from Receptor Cells. Furthermore, Type I taste bud cells possess lamellar processes that enwrap the other taste bud cells [39], further reducing ATP diffusion and possibly limiting purinergic transmission within the taste bud to very short distances.
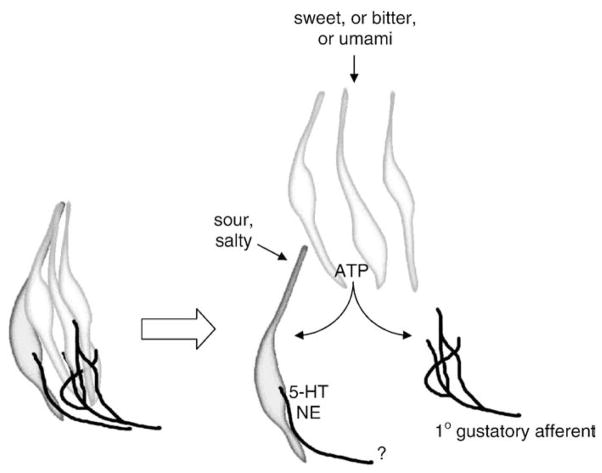
Cell-to-cell communication between Receptor and Presynaptic Cells via ATP can explain the in situ taste response patterns of these cells, such as those recorded in the lingual slice preparation. As stated above, sweet, bitter, and umami taste receptors are uniquely expressed in Receptor Cells [26]. Correspondingly, Receptor Cells are activated by sweet, bitter, or umami taste stimulation; that is, they are “tuned” to individual tastants. Presynaptic Cells, which do not express receptors for these taste qualities, are nonetheless indirectly excited by these taste stimuli via ATP released from Receptor Cells. Convergent information from multiple different Receptor Cells impinges onto Presynaptic Cells. In addition, Presynaptic Cells are themselves directly activated by salty and sour [24,27]. Consequently, Presynaptic Cells are broadly tuned and respond to taste bud stimulation from multiple taste qualities [24]. Presynaptic Cells represent a point of convergent input from Receptor Cells. It is possible that small clusters of Receptor Cells are closely apposed to Presynaptic Cells and form a gustatory signaling unit [40]. This hypothesis remains to be tested, however. Fig. 4 summarizes this notion.
Fig. 4.
Schematic diagram of hypothesized signal processing in mammalian taste buds. At left is shown a postulated taste bud processing unit, consisting of a cluster of Receptor (Type II) Cells, a Presynaptic (Type III) Cell, and their nerve innervation. For simplicity, only 1 Presynaptic Cell is illustrated. Type I ensheathing glial-like cells that may surround the cluster and limit ATP diffusion have also been omitted for clarity. At the right is shown the same cluster, but expanded to illustrate cell-to-cell communication mediated by taste-evoked ATP. Taste excitation by sweet, bitter or umami compounds stimulate Receptor cells to secrete ATP and activate 1° gustatory afferent fibers. Taste-evoked ATP also excites Presynaptic Cells which form synapses with as-yet unidentified fibers (shown by “?”). Sour and salty stimuli act directly on Presynaptic Cells [27]. Presynaptic Cells release serotonin and noradrenalin, perhaps as neurocrine transmitters at their synapses, or as paracrine transmitters acting within the confines of the taste bud, or both.
It is intriguing that recordings from geniculate ganglion cells that innervate fungiform taste buds show a rough division into “specialists” (ganglion cells that respond to relatively few taste stimuli)and “generalists” (ganglion cells that respond somewhat broadly to taste stimuli) [41]. It is tempting to speculate that these two classes correspond to gustatory ganglion cells that innervate Receptor and Presynaptic Cells, respectively.
7. Two parallel signal pathways from taste buds
There may be two signal outputs for taste buds, as shown in Figs. 1 and 4. Primary afferent fibers are believed to receive signals directly from Receptor Cells. We do not yet know much about the fibers that synapse with Presynaptic Cells, hence the question mark in Fig. 4. One might speculate that these fibers represent a second, parallel output from taste buds, perhaps an aminergic and/or noradrenergic pathway that parallels the purinergic pathway of the 1° gustatory afferent fibers shown in Fig. 4. Might this represent a pathway that projects to the reticular formation, as shown in Fig. 1? Needless to say, this notion is completely speculative, but testable. For instance, a serious reservation to this conjecture that will need to be resolved is that the anatomical data refer to findings from nerve fibers innervating the anterior tongue (fungiform taste buds), but the physiological data for cell types and their functions are mainly derived from studies on taste buds located in the posterior tongue (mainly vallate papillae). There are major differences in the gustatory responses between taste buds on the anterior and posterior tongue, and possibly even in cell-to-cell communication within fungiform versus vallate taste buds. To resolve these problems and vindicate (or repudiate) the hypothesis will at a minimum require (1) identifying the source of fibers that form synapses onto Presynaptic Cells, (2) determining whether the transmitter at those synapses is serotonin, noradrenalin, or some as yet unidentified compound, and (3) discovering whether cell-to-cell communication and synaptic transmission between taste bud cells are similar in vallate, foliate, and fungiform papillae.
References
- 1.Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev. 2005;4:143–91. doi: 10.1177/1534582305280031. [DOI] [PubMed] [Google Scholar]
- 2.Zaidi FN, Todd K, Enquist L, Whitehead MC. Types of taste circuits synaptically linked to a few geniculate ganglion neurons. J Comp Neurol. 2008;511:753–72. doi: 10.1002/cne.21869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rolls ET. Sensory processing in the brain related to the control of food intake. Proc Nutr Soc. 2007;66:96–112. doi: 10.1017/S0029665107005332. [DOI] [PubMed] [Google Scholar]
- 4.Lasiter PS, Deems DA, Oetting RL, Garcia J. Taste discriminations in rats lacking anterior insular gustatory neocortex. Physiol Behav. 1985;35:277–85. doi: 10.1016/0031-9384(85)90350-6. [DOI] [PubMed] [Google Scholar]
- 5.Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol. 2008;86:216–44. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Roper SD. The cell biology of vertebrate taste receptors. Annu Rev Neurosci. 1989;12:329–53. doi: 10.1146/annurev.ne.12.030189.001553. [DOI] [PubMed] [Google Scholar]
- 7.Reutter K, Witt M. Morphology of vertebrate taste organs and their nerve supply. In: Simon SA, Roper SD, editors. Mechanisms of Taste Transduction. CRC; 1993. pp. 29–82. [Google Scholar]
- 8.Takeda M, Hoshino T. Fine structure of taste buds in the rat. Arch Histol Jpn. 1975;37:395–413. doi: 10.1679/aohc1950.37.395. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto S, Yamamoto K, Yoshizuka M, Yokoyama M. Pre- and postnatal development of rabbit foliate papillae with special reference to foliate gutter formation and taste bud and serous gland differentiation. Microsc Res Tech. 1993;26:120–32. doi: 10.1002/jemt.1070260205. [DOI] [PubMed] [Google Scholar]
- 10.Takeda M. An electron microscopic study on the innervation in the taste buds of the mouse circumvallate papillae. Arch Histol Jpn. 1976;39:257–69. doi: 10.1679/aohc1950.39.257. [DOI] [PubMed] [Google Scholar]
- 11.Murray RG. The ultrastructure of taste buds. In: Friedemann I, editor. The ultrastructure of sensory organs. Amsterdam: North-Holland Pub. Co; 1973. pp. 1–81. [Google Scholar]
- 12.Seta Y, Toyoshima K. Three-dimensional structure of the gustatory cell in the mouse fungiform taste buds: a computer-assisted reconstruction from serial ultrathin sections. Anat Embryol (Berl) 1995;191:83–8. doi: 10.1007/BF00186781. [DOI] [PubMed] [Google Scholar]
- 13.Murray RG. The mammalian taste bud type III cell: a critical analysis. J Ultrastruct Mol Struct Res. 1986;95:175–88. doi: 10.1016/0889-1605(86)90039-x. [DOI] [PubMed] [Google Scholar]
- 14.Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9”. 5, and serotonin. J Comp Neurol. 2001;440:97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- 15.Bartel DL, Sullivan SL, Lavoie EG, Sevigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 2006;497:1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawton DM, Furness DN, Lindemann B, Hackney CM. Localization of the glutamate-aspartate transporter, GLAST, in rat taste buds. Eur J Neurosci. 2000;12:3163–71. doi: 10.1046/j.1460-9568.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- 17.Miyoshi MA, Abe K, Emori Y. IP(3) receptor type 3 and PLCbeta2 are co-expressed with taste receptors T1R and T2R in rat taste bud cells. Chem Senses. 2001;26:259–65. doi: 10.1093/chemse/26.3.259. [DOI] [PubMed] [Google Scholar]
- 18.Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J Comp Neurol. 2004;468:311–21. doi: 10.1002/cne.10963. [DOI] [PubMed] [Google Scholar]
- 19.Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang R, Stoick CL, Kinnamon JC. Synaptobrevin-2-like immunoreactivity is associated with vesicles at synapses in rat circumvallate taste buds. J Comp Neurol. 2004;471:59–71. doi: 10.1002/cne.20021. [DOI] [PubMed] [Google Scholar]
- 21.Dvoryanchikov G, Tomchik SM, Chaudhari N. Biogenic amine synthesis and uptake in rodent taste buds. J Comp Neurol. 2007;505:302–13. doi: 10.1002/cne.21494. [DOI] [PubMed] [Google Scholar]
- 22.Miura H, Kusakabe Y, Sugiyama C, Kawamatsu M, Ninomiya Y, Motoyama J, et al. Shh and Ptc are associated with taste bud maintenance in the adult mouse. Mech Dev. 2001;106:143–5. doi: 10.1016/s0925-4773(01)00414-2. [DOI] [PubMed] [Google Scholar]
- 23.DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, et al. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 2006;26:3971–80. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;27:10840–8. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang YA, Maruyama Y, Roper SD. Norepinephrine is coreleased with serotonin in mouse taste buds. J Neurosci. 2008;28:13088–93. doi: 10.1523/JNEUROSCI.4187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–90. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 27.Huang YA, Maruyama Y, Stimac R, Roper SD. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J Physiol. 2008;586:2903–12. doi: 10.1113/jphysiol.2008.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–9. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 29.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA. 2007;104:6436–41. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–67. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, et al. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005;25:843–7. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tachibana M, Okada T. Release of endogenous excitatory amino acids from ON-type bipolar cells isolated from the goldfish retina. J Neurosci. 1991;11:2199–208. doi: 10.1523/JNEUROSCI.11-07-02199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shear JB, Fishman HA, Allbritton NL, Garigan D, Zare RN, Scheller RH. Single cells as biosensors for chemical separations. Science. 1995;267:74–7. doi: 10.1126/science.7809609. [DOI] [PubMed] [Google Scholar]
- 34.Hazama A, Hayashi S, Okada Y. Cell surface measurements of ATP release from single pancreatic beta cells using a novel biosensor technique. Pflugers Arch. 1998;437:31–5. doi: 10.1007/s004240050742. [DOI] [PubMed] [Google Scholar]
- 35.Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, et al. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA. 2003;100:4322–7. doi: 10.1073/pnas.0736323100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cabrera O, Jacques-Silva MC, Speier S, Yang SN, Kohler M, Fachado A, et al. Glutamate is a positive autocrine signal for glucagon release. Cell Metab. 2008;7:545–54. doi: 10.1016/j.cmet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayashi S, Hazama A, Dutta AK, Sabirov RZ, Okada Y. Detecting ATP release by a biosensor method. Sci STKE. 2004;2004:l14. doi: 10.1126/stke.2582004pl14. [DOI] [PubMed] [Google Scholar]
- 38.Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci USA. 2008;105:18770–5. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pumplin DW, Yu C, Smith DV. Light and dark cells of rat vallate taste buds are morphologically distinct cell types. J Comp Neurol. 1997;378:389–410. doi: 10.1002/(sici)1096-9861(19970217)378:3<389::aid-cne7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 40.Roper SD. Signal transduction and information processing in mammalian taste buds. Pflugers Arch. 2007;454:759–76. doi: 10.1007/s00424-007-0247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breza JM, Curtis KS, Contreras RJ. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol. 2006;95:674–85. doi: 10.1152/jn.00793.2005. [DOI] [PubMed] [Google Scholar]