Abstract
A number of gustatory receptors have been proposed to underlie umami, the taste of l-glutamate, and certain other amino acids and nucleotides. However, the response profiles of these cloned receptors have not been validated against responses recorded from taste receptor cells that are the native detectors of umami taste. We investigated umami taste responses in mouse circumvallate taste buds in an intact slice preparation, using confocal calcium imaging. Approximately 5% of taste cells selectively responded to l-glutamate when it was focally applied to the apical chemosensitive tips of receptor cells. The concentration–response range for l-glutamate fell approximately within the physiologically relevant range for taste behavior in mice, namely 10 mm and above. Inosine monophosphate enhanced taste cell responses to l-glutamate, a characteristic feature of umami taste. Using pharmacological agents, ion substitution, and immunostaining, we showed that intracellular pathways downstream of receptor activation involve phospholipase C β2. Each of the above features matches those predicted by studies of cloned and expressed receptors. However, the ligand specificity of each of the proposed umami receptors [taste metabotropic glutamate receptor 4, truncated metabotropic glutamate receptor 1, or taste receptor 1 (T1R1) and T1R3 dimers], taken alone, did not appear to explain the taste responses observed in mouse taste cells. Furthermore, umami responses were still observed in mutant mice lacking T1R3. A full explanation of umami taste transduction may involve novel combinations of the proposed receptors and/or as-yet-undiscovered taste receptors.
Keywords: taste, receptor, signal transduction, glutamate, phospholipase, imaging, umami
Introduction
The perceived taste of glutamate, called umami, is believed to be distinct from sour, salty, sweet, and bitter (Lindemann et al., 2002). A characteristic feature of umami taste is its potentiation by purine (inosine, guanosine) nucleotide-5′-monophosphates, which also elicit an umami flavor on their own. The prototypic umami tastant monosodium glutamate (MSG) stimulates food intake in humans and other mammals and is widely used as a flavor enhancer in many cuisines in processed foods and in animal feed.
At least three G-protein-coupled receptors (GPCRs) have been proposed as taste receptors for glutamate. A taste-specific variant of a metabotropic glutamate receptor, taste-mGluR4, is expressed in rat taste buds from the posterior tongue (Chaudhari et al., 1996, 2000; Yang et al., 1999). When expressed in transfected cells, taste-mGluR4 responds to MSG and l-2-amino-4-phosphonobutyrate (l-AP-4) at taste-effective concentrations (Chaudhari et al., 2000). A heterodimer of two taste-specific GPCRs, taste receptor 1 (T1R1)+T1R3, when expressed in heterologous cells, also confers the ability to respond to glutamate and many non-umami amino acids. Additional receptors proposed for umami include a truncated mGluR1 (San Gabriel et al., 2005). Although these cloned receptors all appear to respond to umami stimuli when expressed in culture, which ones underlie native responses remains equivocal. Specifically, in contrast to behavioral responses, T1R1+T1R3 responds minimally to glutamate alone and instead requires a mix of glutamate and nucleotide for activation (Nelson et al., 2002). Conversely, taste-mGluR4 responds well to glutamate alone, but its response is unaltered by nucleotides (Chaudhari et al., 2000). Even genetic ablation studies of T1R receptors in mice have yielded conflicting data on umami taste (Damak et al., 2003; Zhao et al., 2003). Hence, the role of umami taste receptors has been inferred from indirect studies based on heterologous expression, afferent nerve recordings, and taste behavior.
Investigators have attempted to define umami receptor mechanisms using patch-clamp recordings or Ca2+ imaging on isolated taste buds with bath application of stimuli (Hayashi et al., 1996; Bigiani et al., 1997; Lin and Kinnamon, 1999; Lin et al., 2003). However, those findings are inconclusive because of the inability to confine chemical stimuli strictly to the apical, chemosensory tips of taste cells. The results are confounded by activating basolateral (nontaste) glutamate receptors and channels (Caicedo et al., 2000). In short, whether and how any of the proposed taste receptors can fully explain umami responses in native tissues (i.e., taste buds) has not yet been convincingly established.
There is general concurrence regarding glutamate taste transduction downstream of the umami taste receptors. Transduction for umami has been shown to involve the G-protein Gα-gustducin (He et al., 2004) and cAMP modulation (Abaffy et al., 2003). Knock-out (KO) of the phospholipase C (PLC) isoform expressed in taste cells, PLCβ2, leads to a striking deficit of afferent nerve response and taste behavior to umami stimuli. Collectively, umami transduction is believed to involve a GPCR-triggered modulation of [Ca2+]i and cAMP in taste sensory cells and subsequent release of transmitter to excite gustatory afferent fibers.
In the present study, we used a semi-intact tissue preparation in which it is possible to apply glutamate and other taste stimuli focally restricted to the apical chemosensitive tips of taste-bud cells and measure individual cellular responses with excellent time and spatial resolution. We show that responses to umami and amino acid tastants, at least in the posterior tongue, are surprisingly heterogeneous. The results suggest that more than a single receptor underlie umami detection in mouse taste buds.
Materials and Methods
Tissue preparation and functional imaging.
All experimental procedures were approved by the University of Miami Care and Use Committee. Animals were killed by exposure to CO2, followed by cervical dislocation. Tongues were removed and immersed in cold (4°C) Tyrode’s solution. We obtained lingual slices containing the vallate papilla from adult C57BL/6 mice (≥8 weeks old) and injected a calcium indicator dye into taste cells following similar procedures described by Caicedo et al. (2000, 2002). Briefly, the fluorescent Ca2+ indicator dye Calcium Green-1 dextran (CaGD) (1 mm in H2O, molecular weight, kDa; Invitrogen, Carlsbad, CA) was injected iontophoretically through a large-diameter-tip (40 μm) glass micropipette into the crypt surrounding the vallate papilla (−3.5 μA square pulses, 10 min). The CaGD-loaded tissue was sliced at 100 μm with a vibratome (VT1000S; Leica, Nussloch, Germany). Slices containing vallate taste buds were mounted on a glass coverslip coated with Cell-Tak (BD Biosciences, Franklin Lakes, NJ), put in a recording chamber, and superfused with Tyrode’s solution (30°C) at rate of 2 ml/min. Single glass micropipettes (2 μm tip diameter) were used to deliver taste stimuli directly for apical stimulation of a selected taste bud. Stimuli were ejected for 1 s with air pressure (1.5 psi) (PicoSpritzer; General Valve, Fairfield, NJ). Different pipettes were mounted for each different taste stimulus. All stimulus solutions contained 2 μm fluorescein to monitor stimulus application, duration, and concentration.
CaGD-loaded taste cells were viewed with a scanning laser confocal microscope using an argon laser (Fluoview; Olympus Optical, Melville, NY). Images were captured at 1.1 s intervals unless otherwise noted. Fluorometric signals are expressed as relative fluorescence change: ΔF/F = (F − F0)/F0, where F0 denotes the resting fluorescence level corrected for any bleaching that occurred during the recording. Using ΔF/F corrects for variations of baseline fluorescence, cell thickness, total dye concentration, and illumination (Helmchen, 2000). Peak ΔF/F constituted the response amplitude for statistical quantification. Taste buds could be stimulated repeatedly with a variety of taste stimuli, and viable responses could be collected from taste cells for up to 5 h.
Data analysis.
Statistical analyses of significance (paired Student’s t test, ANOVA with appropriate post hoc tests, or Fisher’s exact test, one tailed) were applied to peak ΔF/F to determine whether the changes in the response amplitudes to a given treatment were significant.
Reagents and solutions.
l-AP-4 was purchased from Tocris Bioscience (Ellisville, MO). All other chemicals, including monopotassium l-glutamate (MPG) and inosine 5′-monophosphate (IMP), were purchased from Sigma (St. Louis, MO). All tastants were freshly dissolved in Tyrode’s solution for each experiment. The standard medium consisted of Tyrode’s solution with the following composition (in mm): 130 NaCl, 5 KCl, 8 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, 10 sodium pyruvate, and 5 NaHCO3, pH 7.3 (318–323 mOsm). For Ca2+-free Tyrode’s solution, CaCl2 was removed, and 0.2 mm EGTA was added to the above composition. Stock solutions of phospholipase C inhibitor, U73122 (1-[6[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione), and endoplasmic reticulum Ca2+-ATPase inhibitor, thapsigargin, were dissolved in DMSO at 10 and 1 mm, respectively, and stored at −20°C.
Immunostaining.
To examine colocalization of PLCβ2-expressed cells and MPG-responsive cells, we immunostained lingual tissue slices after physiological recordings. Lingual slices from which we recorded Ca2+ responses were processed for immunohistochemistry using antibodies against PLCβ2 (rabbit polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA). Tissue slices were fixed with 4% paraformaldehyde in PBS for 20 min at room temperature. Slices were rinsed three times in PBS and blocked with PBS containing 1% goat serum, 3% bovine serum albumin, and 0.3% Triton X-100 for 2 h. Slices were incubated in primary antibody (1:1000) for 3 d at 4°C. Thereafter, slices were washed three times in PBS, incubated for 3 d in Alexa Fluor 594-conjugated secondary antibodies (1:500; Invitrogen), and then washed again three times in PBS.
To relocate cells from which recordings were taken, fixed and immunostained slices were imaged by laser scanning confocal microscope using argon (488 nm) and krypton (568 nm) lasers and emission filters for CaGD and Alexa Fluor 594. Dual-fluorescence images were matched with those obtained during the Ca2+ imaging recordings from the living tissue to relocate specific cells.
Results
Calcium responses to focally applied glutamate represent umami taste
l-Glutamate might be expected to elicit a variety of responses in taste cells because this amino acid is an umami taste stimulus, serves a number of metabolic roles, and may also be a synaptic transmitter in taste buds. To limit glutamate responses to those associated proximately with taste, we used a semi-intact lingual slice preparation, focally applied glutamate to the apical chemosensory tips of taste receptor cells, and imaged intracellular [Ca2+] changes in taste cells with confocal scanning microscopy (Caicedo et al., 2000; Richter et al., 2003). Focal application of MSG (500 mm) produced calcium responses (Δ[Ca2+]i) in a fraction of taste cells (Fig. 1A,B). Some, but not all, MSG-responsive cells also responded to focally applied NaCl (500 mm) because Na+ is itself a taste stimulus (i.e., salty) and elicits taste responses in taste cells (Fig. 1A) (Caicedo et al., 2002). In an attempt to identify a chemical stimulus that would selectively stimulate glutamate taste receptors without confounding salty responses, we compared responses evoked by focal application of MSG, NaCl, MPG, potassium gluconate, and KCl. The results indicated that MPG reliably evoked taste responses to the glutamate moiety and were least likely to be confounded with salty (i.e., Na+) or other taste responses (Fig. 1). Focal stimulation with KCl (data not shown) or with potassium gluconate did not elicit a change of [Ca2+]i, further validating the taste specificity of the glutamate responses. [Gluconate does not itself appear to elicit a taste (Delwiche et al., 1999).] Furthermore, whereas focal KCl and potassium gluconate did not elicit a response in MPG-responsive cells, NH4 glutamate did (data not shown). Thus, all additional experiments were conducted using MPG as an umami stimulus. (We did not distinguish between cells that responded only to glutamate and those that responded to glutamate and Na+.) We note that MPG has also been used in experiments using nerve recordings and behavioral assays and has been shown to be an effective umami tastant (Sako et al., 2003).
Figure 1.
Taste cell responses (ΔCa2+) evoked by umami and salty taste stimuli, recorded in a slice preparation of the mouse circumvallate papilla. Taste cells were stimulated sequentially with four salt compounds, all at 500 mm. A, Sequential responses of one taste cell. NaCl, MSG, MPG, and potassium gluconate were applied focally and transiently onto the chemosensitive apical tip of taste buds, as shown by short bars below the traces. The Na+ (i.e., NaCl, MSG traces) and the glutamate (i.e., MSG, MPG traces) moieties both evoked responses in this cell. B, The same sequence of taste stimuli, applied to another taste cell, produced Ca2+ responses from the glutamate moiety alone.
Focally applied MPG (500 mm) evoked transient [Ca2+]i increases in 4.2% of taste cells in the circumvallate papilla (34 of 804 cells obtained from 28 mice). This fraction is lower than the 11% of cells reported previously to respond to monosodium glutamate (Caicedo et al., 2002). However, that previous study stimulated taste cells with MSG and thus could not selectively distinguish umami versus salty taste responses. The mean amplitude of Ca2+ responses (ΔF/F) evoked by 500 mm MPG was 7.4 ± 1.5% (mean ± SE; n = 34 cells).
We determined concentration–response relationships for MPG and found the threshold value at or near 10 mm (Fig. 2B, open circles). This value is similar to threshold concentrations of glutamate required for afferent nerve responses or behavioral assays (Yamamoto et al., 1991; Bachmanov et al., 2000; Inoue et al., 2004). We could not apply MPG at concentrations much over 500 mm and did not establish a saturating dose for the concentration–response relationships. Thus, it was not possible to derive EC50 values (Fig. 2). Importantly, applying concentrations of glutamate (>30 mm) did not recruit Ca2+ responses in additional taste cells. This suggests that the Ca2+ responses we recorded did not represent nonspecific activation of taste-bud cells and instead reflected selective stimulation of a specific subpopulation of glutamate-responsive taste receptor cells.
Figure 2.
Glutamate responses are enhanced by coapplication of IMP, consistent with umami taste stimulation. A, Responses evoked by MPG (30 mm) were enhanced by coapplication of IMP (1 mm). IMP alone did not induce a detectable Ca2+ response (middle trace). B, Concentration–response relationship for glutamate in the presence (filled circles) or absence (open circles) of 1 mm IMP (means ± SE; n = 4 cells)
Behavioral and afferent nerve responses to glutamate are synergistically enhanced by the presence of 5′-ribonucleotides, a characteristic feature of umami taste (Kuninaka, 1960). Thus, we tested whether glutamate-induced Ca2+ responses were altered by IMP, as expected for umami signals. MPG (10–500 mm) was focally applied with or without 1 mm IMP to the apical taste pore of circumvallate taste buds in slices. Glutamate-induced [Ca2+]i responses were enhanced in the presence of IMP, and the concentration–response relationship appeared to be left-shifted ∼½ log unit (Fig. 2A,B). (Without data for saturating responses, we could not establish whether this shift represented synergy, per se, between IMP and MPG.) When we applied 1 mm IMP alone, it did not induce [Ca2+]i responses.
Responses to umami stimuli represent phospholipase-induced Ca2+ release
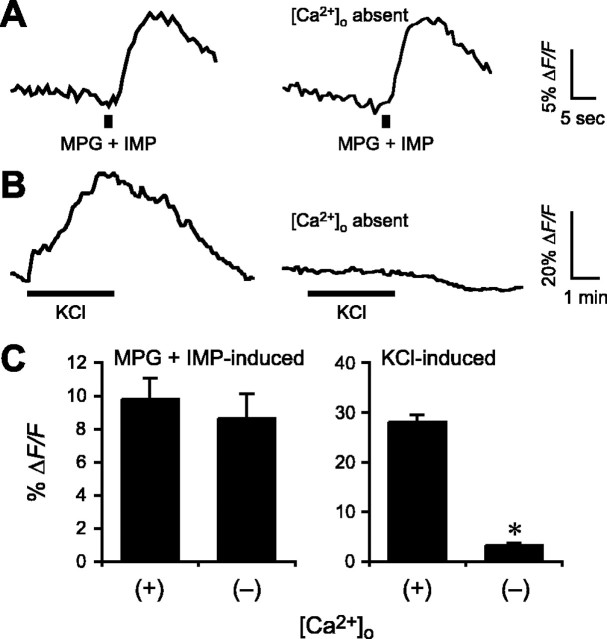
We next investigated the Ca2+-mobilizing pathway activated by umami stimuli in taste cells. First, we examined responses in the acute absence of extracellular Ca2+ by bathing slices in a Ca2+-free Tyrode’s solution (with EGTA) 2 min before focal glutamate stimulation. As shown in Figure 3, A and C, responses evoked by monopotassium glutamate (plus IMP) were not significantly changed relative to those in the presence of extracellular Ca2+ (control, ΔF/F = 9.8 ± 1.3%; Ca2+-free, ΔF/F = 8.7 ± 1.5%; n = 9). In contrast, depolarization-evoked Ca2+ responses, elicited by perfusing the slice with 50 mm KCl [which allows Ca2+ influx through voltage-gated cation channels (Richter et al., 2004)] were nearly completely abolished in the absence of extracellular Ca2+ under parallel treatments (control, ΔF/F = 28.1 ± 1.5%; Ca2+-free, ΔF/F = 3.2 ± 0.5%; n = 12; p < 0.001) (Fig. 3B,C). These results are consistent with umami taste transduction involving release of intracellular Ca2+, not Ca2+ influx.
Figure 3.
Taste responses elicited by glutamate are unaffected by removing extracellular Ca2+. A, MPG (200 mm) plus IMP (1 mm) was focally applied in medium containing Ca2+ (left trace) or in the absence of Ca2+ (Ca-free medium with 0.2 mm EGTA; right trace). B, Responses elicited by depolarization (bath-applied KCl, 50 mm) and influx of Ca2+ through voltage-dependent Ca2+ channels were abolished in the absence of [Ca2+]o. C, Mean amplitudes of MPG-induced responses in the presence or absence of [Ca2+]o (mean ± SE; *p ≤ 0.001).
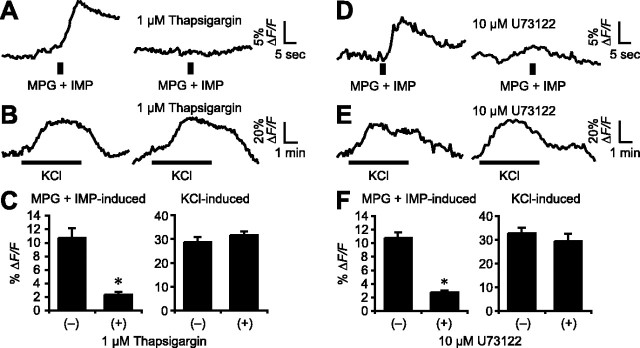
To test directly whether glutamate-evoked Ca2+ responses originated from intracellular stores, we depleted Ca2+ stores with thapsigargin (1 μm, 20 min), a selective inhibitor of endoplasmic reticulum Ca2+-ATPase (Thastrup et al., 1990). After thapsigargin treatment, Ca2+ responses evoked by MPG (plus IMP) were diminished by 73% (control, ΔF/F = 10.7 ± 1.5%; thapsigargin, ΔF/F = 2.3 ± 0.9%; n = 4; p ≅ 0.01) (Fig. 4A,C). In contrast, depolarization (KCl)-induced responses were not affected by thapsigargin (control, ΔF/F = 28.8 ± 2.0%; thapsigargin, ΔF/F = 31.7 ± 1.5%; n = 8) (Fig. 4B,C). These data strongly support the notion that glutamate taste mechanisms involve intracellular Ca2+ release.
Figure 4.
Ca2+ response elicited by glutamate involves intracellular Ca2+ stores and phospholipase C. A, Responses evoked by MPG (200 mm) plus IMP (1 mm; left) were blocked by thapsigargin (1 μm; right). B, Responses evoked by depolarization (50 mm KCl) were unaffected by thapsigargin. C, Mean amplitudes of the responses in the presence or absence of thapsigargin (mean ± SE; *p ≅ 0.01). D, Responses to MPG plus IMP in another cell were inhibited by U73122 (10 μm; right). E, Control from same preparation; depolarization-evoked responses were unaffected by U73122. F, Mean amplitudes of the responses in the presence or absence of U73122 (mean ± SE; *p ≅ 0.003).
Many sweet, bitter, and umami stimuli are believed to trigger the release of stored Ca2+ by activating PLCβ2 (Rossler et al., 1998; Huang et al., 1999; Ogura and Kinnamon, 1999; Zhang et al., 2003). To directly test whether umami-elicited Ca2+ responses stem from PLC activation, we used a nonselective PLC inhibitor, U73122 (Bleasdale et al., 1990; Thompson et al., 1991; Salari et al., 1993). After incubation with 10 μm U73122 for 15 min, responses elicited by MPG (plus IMP) were inhibited 74% (control, ΔF/F = 10.8 ± 0.8%; U73122, ΔF/F = 2.8 ± 1.7%; n = 4; p ≅ 0.003) (Fig. 4D,F). In contrast, depolarization (KCl)-induced responses were not significantly altered by treatment with U73122 (control, ΔF/F = 32.7 ± 2.3%; U73122, ΔF/F = 29.3 ± 3.2%; n = 6) (Fig. 4E,F).
Last, to correlate umami taste responses with the presence of PLCβ2 on an individual cell basis, lingual slices from which successful recordings of MPG (plus IMP) responses were taken were immunostained for the presence of PLCβ2. After fixation and immunostaining, recorded cells were relocated by their CaGD dye fluorescence (which remained throughout the immunostaining) and by their position within the lingual slice. MPG-responsive cells were immunopositive for PLCβ2 (Fig. 5), confirming the direct and immediate association between umami taste mechanisms and the presence of PLCβ2. The specificity of this anti-PLCβ2 antibody was confirmed with the use of tissue from PLCβ2 knock-out mice (Jiang et al., 1997). No fluorescent signals were evident in taste cells from the knock-out mice, processed in parallel with wild-type (WT) tissues (Kim et al., 2006).
Figure 5.
Umami-responsive cells express PLCβ2. A, Micrograph of a living slice preparation showing three cells filled with CaGD in two taste buds (dotted outlines). One of these cells (asterisk) responded to MPG plus IMP (next). The micrograph is pseudocolored to show CaGD fluorescence during live imaging (colors indicate pixel intensity mapping) (cf. Caicedo et al., 2000). A layer of CaGD fluorescent dye (dashed line) adheres to the surface epithelium and marks the mucosal boundary of the epithelium. B, The cell marked by the asterisk in A responded to focal stimulation of the apical tip of the taste bud with 200 mm MPG plus 1 mm IMP. None of the other CaGD-filled cells in the taste buds responded to MPG plus IMP. C–E, After immunostaining the preparation for PLCβ2, the same cell marked by the asterisk in A was relocated. C, The CaGD signal (green) is still visible in the three taste cells after immunostaining. D, PLCβ2 immunoreactivity (red) is present in many taste cells, including the recorded cell (asterisk). E, Signals from the PLCβ2 immunostaining and CaGD channels are merged. Many taste cells other than the recorded cell contain either PLCβ2 or CaGD, but not both. Scale bars, 10 μm.
We were unable to obtain Ca2+ responses to umami stimuli in taste cells from mutant mice lacking PLCβ2 tested in parallel experiments. Of 78 taste cells from PLCβ2-KO mice tested with glutamate plus IMP, none generated responses. This differs significantly from the incidence of umami-responsive cells in wild-type mice (i.e., 34 of 804 cells, see above; p < 0.05, Fisher’s exact test). Although it is possible that there are alterations in taste receptor cells from the KO mice other than just the absence of PLCβ2, this result is consistent with a central role of PLCβ2 in umami transduction.
Collectively, the above data confirm that the signals evoked by umami stimuli in mouse vallate taste cells represent PLC-mediated release of stored Ca2+.
Umami taste stimuli other than glutamate
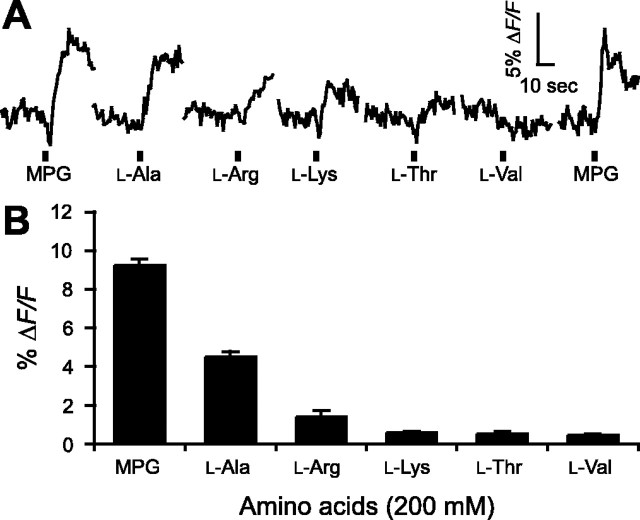
The T1R1+T1R3 heterodimer has been proposed to be the sole receptor underlying umami and amino acid responses in mice (Zhao et al., 2003). This receptor dimer, when transiently expressed in HEK293 cells, induces Ca2+ responses to glutamate plus IMP but, surprisingly, also to a broad range of amino acids not normally associated with umami taste (Nelson et al., 2002). We asked whether glutamate and other amino acids produced similar responses in native taste tissue of mice. We identified umami-responsive cells in circumvallate slices as described above and then focally stimulated these taste buds sequentially with five other l-amino acids identified to be effective at T1R1+T1R3, each at 200 mm, in the presence of 1 mm IMP. As shown in Figure 6, A and B, l-Ala induced a small but significant [Ca2+]i increase in most glutamate-sensitive cells (MPG, ΔF/F = 9.2 ± 0.4%; l-Ala, 4.5 ± 0.3%; n = 4). Conversely, none of the other tested amino acids, l-Arg, l-Lys, l-Thr, or l-Val (each at 200 mm with 1 mm IMP), induced significant responses in glutamate-responsive circumvallate taste cells, nor did we did find responses to these amino acids (l-Arg, l-Lys, l-Thr, or l-Val) in glutamate-insensitive cells. The results suggest that the functional properties of native taste cells in the circumvallate papilla do not agree well with the response profiles reported from heterologous expression systems.
Figure 6.
Taste cell responses evoked by focal application of MPG and other amino acids. A, Examples of responses from a taste cell after focal application of 200 mm: MPG, l-Ala, l-Arg, l-Lys, l-Thr, and l-Val, all containing 1 mm IMP. B, Mean ± SE amplitude of responses evoked by these stimuli (n = 4 cells).
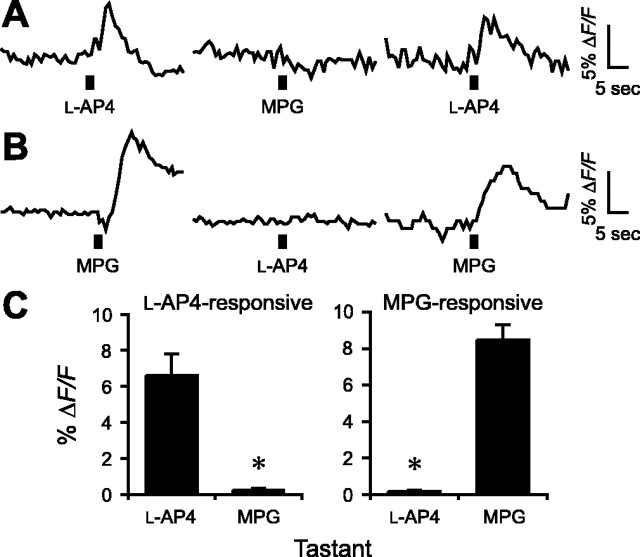
The glutamate analog l-AP-4 is an agonist for metabotropic glutamate receptors, (specifically, mGluR4, mGluR6, mGluR7, and mGluR8). Furthermore, conditioned taste aversion experiments suggest that l-AP-4 produces a taste with marked similarity to glutamate in rats (Chaudhari et al., 1996; Stapleton et al., 1999) and mice (Nakashima et al., 2001). In transiently or stably expressing cell lines or Xenopus oocytes, l-AP-4 also activates truncated taste-mGluR4, truncated mGluR1, and T1R1+T1R3 dimers (Chaudhari et al., 2000; Nelson et al., 2002; San Gabriel et al., 2005). In combination, these results have suggested that l-AP-4 mimics the taste of MSG, at least in part by activating the same taste receptors as umami compounds (Delay et al., 2004). To test this interpretation directly, we focally applied glutamate and l-AP-4 sequentially to circumvallate taste buds. l-AP-4 (10 mm with 1 mm IMP) evoked transient Ca2+ responses in some taste cells but not in those that responded to glutamate (200 mm with 1 mm IMP) (l-AP-4, ΔF/F = 6.8 ± 1.8%; MPG, ΔF/F = 0.2 ± 0.2%; n = 8; p ≅ 0.01) (Fig. 7A,C). Conversely, glutamate-sensitive cells did not respond to l-AP-4 (l-AP-4, ΔF/F = 0.2 ± 0.1%; MPG, ΔF/F = 8.5 ± 0.9%; n = 5; p < 0.001) (Fig. 7B,C). These data suggest that separate receptors, found on separate cells, generate our Ca2+ responses to MPG and l-AP-4. In cells that respond strongly to each agonist, we cannot rule out the possibility of subthreshold responses to the other agonist. Nevertheless, as in the case of amino acids (above), these results emphasize that responses to umami tastants in native taste tissues are highly heterogeneous and vary markedly from those described for the proposed umami receptors taken individually, taste-mGluR4, truncated mGluR1, and T1R1+T1R3.
Figure 7.
MPG-sensitive and l-AP-4-sensitive taste cells represent different cell populations. l-AP-4 (25 mm) and MPG (100 mm) (each containing 1 mm IMP) were applied sequentially to the apical tips of taste buds in the slice preparation. A, One taste cell responds to l-AP-4 but not to MPG (l-AP-4 responsive). B, Another cell responds to MPG but not to l-AP-4 (MPG-responsive). C, Summary of responses to focal application of l-AP-4 and MPG for these two populations of taste cells (means ± SE; *p ≅ 0.01 for l-AP-4-responsive cells, n = 8 cells; *p < 0.001 for MPG-responsive cells, n = 5 cells).
Glutamate-induced responses in taste cells from T1R3 knock-out mice
T1R3 has been proposed as an obligate member of the umami taste receptor dimer (Zhao et al., 2003). However, others have challenged those findings and concluded that multiple umami taste receptors exist (Damak et al., 2003). To test whether multiple receptors might be involved in umami taste mechanisms, we compared glutamate-induced responses in circumvallate taste cells in lingual slices from WT mice and mutant mice lacking T1R3 (T1R3-null). MPG (plus IMP) induced Ca2+ responses in both WT and T1R3-null mice. However, in slices tested blindly and in parallel, the amplitude of glutamate responses in T1R3-null taste cells were smaller than in WT taste cells (WT, ΔF/F = 9.1 ± 0.9%, n = 7; T1R3-null, 5.4 ± 0.7%, n = 6; p ≅ 0.01) (Fig. 8). The incidence of umami-responsive taste cells was not noticeably different between the two genotypes. By way of control, there were no significant differences between the incidence or the amplitude of depolarization (KCl)-induced responses in WT and T1R3-null mice (WT, ΔF/F = 30.9 ± 1.3%, n = 7; T1R3-KO, 25.9 ± 2.9%, n = 5; p ≅ 0.11).
Figure 8.

Umami stimulation evokes responses in circumvallate taste cells from mutant mice lacking T1R3. A, Superimposed recordings of responses from five different taste cells after MPG (500 mm) plus IMP (1 mm) stimulation. Recordings from WT mice are shown at left and from T1R3-null (T1R3-KO) mice at right. B, Control responses to KCl-depolarization recorded from taste cells from wild-type mice (left) and T1R3-null mice (right). C, Summary; mean amplitudes of responses in taste cells from wild-type and T1R3-null cells (mean ± SE; *p ≅ 0.01, n = 7 or 6 for MPG-responsive cells in wild-type or T1R3 knock-out mice, respectively; n = 7 or 5 for KCl-responsive cells in wild-type or T1R3 knock-out mice, respectively).
Discussion
The aim of this study was to investigate umami taste responses in native taste cells in the mouse to compare results with the responses of cloned taste receptors expressed in heterologous cells (HEK293, CHO, and others). We used a preparation, lingual slices from vallate papilla, that allows one to apply tastants selectively to the apical chemosensory tips of taste cells while avoiding stimulating nontaste cells and basolateral regions of taste buds. We identified a small population (∼5%) of taste cells in the circumvallate papillae that selectively respond to l-glutamate. This compares with our previous estimates, in the same preparation, of 28% cells that respond to bitter and 14% to sweet stimuli (Caicedo et al., 2002). The threshold concentration of l-glutamate needed to activate a Ca2+ response in taste cells was 10 mm. This is consistent with reports on behavioral analysis and sensory nerve recordings in mice (Yamamoto et al., 1991; Bachmanov et al., 2000; Inoue et al., 2004). As expected for umami taste, inosine 5′-monophosphate enhanced the l-glutamate-evoked Ca2+ responses of taste cells.
Studying taste responses to l-glutamate is complicated by the necessity to apply a salt of glutamic acid yet avoid stimulating acid (sour) or salty taste mechanisms. Monosodium glutamate is conventionally used as the prototypic umami taste stimulus, but this itself introduces a possible confound, Na+ taste (i.e., salty). We avoided these issues in the present study by using MPG as the taste stimulus. MPG elicits umami taste and has been used by others to investigate nerve responses to umami taste stimuli (Sako and Yamamoto, 1999; He et al., 2004). Although, as one might expect, K+ is capable of depolarizing taste cells when bath applied to basolateral regions of the taste bud (Caicedo et al., 2000; Richter et al., 2004), focal application of K gluconate or KCl to the taste pore did not. Hence, we inferred that MPG only yielded responses to the anion moiety (i.e., glutamate) in taste cells. Parenthetically, in humans, potassium salts such as KCl elicit bitter–salty taste. One might anticipate that there would be a population of taste cells that are stimulated by focally applied K+ salts. Such cells, if they exist in mouse circumvallate papillae, did not figure in the present report.
The magnitude of nucleotide potentiation in whole-nerve and single-fiber recordings varies considerably between the chorda tympani (CT) and glossopharyngeal (GL) nerves, even within a single species (Ninomiya et al., 1993, 2000). Gurmarin, a peptide that inhibits sweet taste in rodents, presumably by interaction with taste receptors, also inhibits umami signals preferentially in the CT (Yamamoto et al., 1991; Ninomiya et al., 1993, 2000; Sako and Yamamoto, 1999). Collectively, the nerve recording data suggest that responses to MSG differ significantly between the anterior (CT innervation) and posterior (GL innervation) lingual taste fields. Our findings demonstrate heterogeneity in the responses to various umami stimuli in individual taste cells, at least in the posterior tongue.
Our results suggest that glutamate-induced increases of [Ca2+]i arise principally via mobilization from intracellular stores because responses were essentially unperturbed by depletion of extracellular Ca2+. Conversely, responses were abolished by pretreatment with thapsigargin or U73122, demonstrating the need for Ca2+ stores and the involvement of a phospholipase C (likely PLCβ2) in umami taste transduction. These results provide direct evidence of the existence of a functional umami receptor coupled to Ca2+ mobilization in taste receptor cells and explains the loss of behavioral and afferent responses to umami in PLCβ2 knock-out mice (Zhang et al., 2003). We note that neither thapsigargin nor U73122 totally eliminated Ca2+ responses evoked by umami stimuli. Whether this represents minor additional pathways remains unresolved.
Previously, it was reported that the heterodimer receptor T1R1+T1R3 is necessary and sufficient to elicit taste sensitivity to l-glutamate or to a number of other amino acids, as well as to l-AP-4 (Zhao et al., 2003). This interpretation was based on the observation that behavioral and neural responses to umami were absent in mice genetically lacking either T1R1 or T1R3. In contrast, an independently produced T1R3 knock-out mouse was reported to have reduced but still detectable responses to umami stimuli (Damak et al., 2003). Both of these reports used assays (behavioral and neural activity) that are one or more synapses downstream from the cells primarily affected by the genetic ablation: taste receptor cells. However, when we examined taste cells in T1R3-null mice, we found unequivocal evidence for umami-evoked Ca2+ responses that persist, albeit with a decreased amplitude. Our data strongly suggest that the T1R1+T1R3 dimer is not the sole umami taste receptor in the mouse. Furthermore, there are several discrepancies between the response profile of T1R1+T1R3 (in HEK293 cells) and of native taste cells. First, mouse T1R1+T1R3 is reported to be unresponsive to glutamate (even as high as 200 mm) unless IMP is also present (Nelson et al., 2002). In contrast, we obtained measurable cellular responses to 30 mm glutamate alone, similar to the response of behaving animals. Second, although T1R1+T1R3 expressed in HEK293 cells responds to glutamate and a very broad range of amino acids (Nelson et al., 2002), our physiological studies in circumvallate slices show that glutamate-sensitive taste cells do not respond to l-Lys, l-Thr, and l-Val (all of which were effective on heterologously expressed T1R1+T1R3). Not surprisingly, many amino acids are thought to produce taste qualities distinct from umami, e.g., bitter for l-Val and sweet for l-Ala (Pritchard and Scott, 1982; Iwasaki et al., 1985).
In cDNA profiling experiments (Max et al., 2001) and in situ hybridization analyses (Kim et al., 2003), T1R1 and T1R3 were expressed independently of each other in substantial numbers of cells, especially in the vallate taste buds. The findings suggest that the T1R1+T1R3 pairing is not obligatory in native cells and that umami responses may stem from additional receptor types. Furthermore, although l-AP-4 and MSG are both umami stimuli, rats also easily discriminate between these tastants (Delay et al., 2004), indicating that some taste receptors may exist that are activated by one but not the other ligand, consistent with our findings (Fig. 7). Again, the implication is that umami responses may originate from more than a single type of receptor or receptor combination. Indeed, taste would not be unique in possessing such redundancy of receptors. Most mammalian sensory systems include more than a single receptor capable of responding to a given stimulus, whether these are multiple opsins (responding to a given wavelength of light), multiple odorant receptors (responding to a single odorant), or multiple receptors in peripheral nociceptors (responding to H+ or ATP from tissue damage).
In summary, our results demonstrate that umami taste responses in native circumvallate taste cells are not concordant with the ligand specificities of any single proposed umami receptor: taste-mGluR4, T1R1+T1R3 dimers, or truncated mGluR1. We surmise that a full explanation of umami taste transduction may involve novel combinations of the receptors proposed to date and/or as-yet-undiscovered taste receptors (Ninomiya et al., 2000; Nakashima et al., 2001; Damak et al., 2003).
Footnotes
This work was supported by National Institutes of Health–National Institute on Deafness and Other Communication Disorders Grants R01 003155 (R.F.M.), R01 006308 (N.C.), and R01 000374 (S.D.R.) and the International Glutamate Technical Committee. We thank Dr. Dianqing Wu (University of Connecticut Health Center, Farmington, CT) for providing mutant mice lacking PLCβ2.
References
- Abaffy T, Trubey KR, Chaudhari N (2003). Adenylyl cyclase expression and modulation of cAMP in rat taste cells. Am J Physiol Cell Physiol 284:C1420–C1428. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK (2000). Intake of umami-tasting solutions by mice: a genetic analysis. J Nutr 130:935S–941S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigiani A, Delay RJ, Chaudhari N, Kinnamon SC, Roper SD (1997). Responses to glutamate in rat taste cells. J Neurophysiol 77:3048–3059. [DOI] [PubMed] [Google Scholar]
- Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S (1990). Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther 255:756–768. [PubMed] [Google Scholar]
- Caicedo A, Jafri MS, Roper SD (2000). In situ Ca2+ imaging reveals neurotransmitter receptors for glutamate in taste receptor cells. J Neurosci 20:7978–7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Kim K-N, Roper SD (2002). Individual mouse taste cells respond to multiple chemical stimuli. J Physiol (Lond) 544:501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Yang H, Lamp C, Delay E, Cartford C, Than T, Roper S (1996). The taste of monosodium glutamate: membrane receptors in taste buds. J Neurosci 16:3817–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Landin AM, Roper SD (2000). A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci 3:113–119. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF (2003). Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301:850–853. [DOI] [PubMed] [Google Scholar]
- Delay ER, Sewczak GM, Stapleton JR, Roper SD (2004). Glutamate taste: discrimination between the tastes of glutamate agonists and monosodium glutamate in rats. Chem Senses 29:291–299. [DOI] [PubMed] [Google Scholar]
- Delwiche JF, Halpern BP, Desimone JA (1999). Anion size of sodium salts and simple taste reaction times. Physiol Behav 66:27–32. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Zviman MM, Brand JG, Teeter JH, Restrepo D (1996). Measurement of membrane potential and [Ca2+]i in cell ensembles: application to the study of glutamate taste in mice. Biophys J 71:1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Yasumatsu K, Varadarajan V, Yamada A, Lem J, Ninomiya Y, Margolskee RF, Damak S (2004). Umami taste responses are mediated by α-transducin and α-gustducin. J Neurosci 24:7674–7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F (2000). Calibration of fluorescent calcium indicators. In: Imaging neurons: a laboratory manual, Chap 32 (Yuste R, Lanni F, Konnerth A, eds) , Cold Spring Harbor, NY: Cold Spring Harbor Laboratory. [DOI] [PubMed]
- Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF (1999). Gγ13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci 2:1055–1062. [DOI] [PubMed] [Google Scholar]
- Inoue M, Beauchamp GK, Bachmanov AA (2004). Gustatory neural responses to umami taste stimuli in C57BL/6ByJ and 129P3/J mice. Chem Senses 29:789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K, Kasahara T, Sato M (1985). Gustatory effectiveness of amino acids in mice: behavioral and neurophysiological studies. Physiol Behav 34:531–542. [DOI] [PubMed] [Google Scholar]
- Jiang H, Kuang Y, Wu Y, Xie W, Simon MI, Wu D (1997). Roles of phospholipase C β2 in chemoattractant-elicited responses. Proc Natl Acad Sci USA 94:7971–7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-W, Roberts C, Maruyama Y, Berg S, Roper SD, Chaudhari N (2006). Faithful expression of GFP from the PLCβ2 promoter in a functional class of taste receptor cells. Chem Senses in press. [DOI] [PubMed]
- Kim M-R, Kusakabe Y, Miura H, Shindo Y, Ninomiya Y, Hino A (2003). Regional expression patterns of taste receptors and gustducin in the mouse tongue. Biochem Biophys Res Commun 312:500–506. [DOI] [PubMed] [Google Scholar]
- Kuninaka A (1960). Studies on taste of ribonucleic acid derivatives. J Agric Chem Soc Jpn 34:487–492. [Google Scholar]
- Lin W, Kinnamon SC (1999). Physiological evidence for ionotropic and metabotropic glutamate receptors in rat taste cells. J Neurophysiol 82:2061–2069. [DOI] [PubMed] [Google Scholar]
- Lin W, Ogura T, Kinnamon SC (2003). Responses to di-sodium guanosine 5′-monophosphate and monosodium l-glutamate in taste receptor cells of rat fungiform papillae. J Neurophysiol 89:1434–1439. [DOI] [PubMed] [Google Scholar]
- Lindemann B, Ogiwara Y, Ninomiya Y (2002). The discovery of umami. Chem Senses 27:843–844. [DOI] [PubMed] [Google Scholar]
- Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF (2001). Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet 28:58–63. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Katsukawa H, Sasamoto K, Ninomiya Y (2001). Behavioral taste similarities and differences among monosodium L-glutamate and glutamate receptor agonists in C57BL mice. J Nutr Sci Vitaminol (Tokyo) 47:161–166. [DOI] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS (2002). An amino-acid taste receptor. Nature 416:199–202. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Kajiura H, Mochizuki K (1993). Differential taste responses of mouse chorda tympani and glossopharyngeal nerves to sugars and amino acids. Neurosci Lett 163:197–200. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Nakashima K, Fukuda A, Nishino H, Sugimura T, Hino A, Danilova V, Hellekant G (2000). Responses to umami substances in taste bud cells innervated by the chorda tympani and glossopharyngeal nerves. J Nutr 130:950S–953S. [DOI] [PubMed] [Google Scholar]
- Ogura T, Kinnamon SC (1999). IP3-independent release of Ca2+ from intracellular stores: a novel mechanism for transduction of bitter stimuli. J Neurophysiol 82:2657–2666. [DOI] [PubMed] [Google Scholar]
- Pritchard TC, Scott TR (1982). Amino acids as taste stimuli. Brain Res 253:81–92. [DOI] [PubMed] [Google Scholar]
- Richter TA, Caicedo A, Roper SD (2003). Sour taste stimuli evoke Ca2+ and pH responses in mouse taste cells. J Physiol (Lond) 547:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter TA, Dvoryanchikov GA, Chaudhari N, Roper SD (2004). Acid-sensitive two-pore domain potassium (K2P) channels in mouse taste buds. J Neurophysiol 92:1928–1936. [DOI] [PubMed] [Google Scholar]
- Rossler P, Kroner C, Freitag J, Noe J, Breer H (1998). Identification of a phospholipase C β subtype in rat taste cells. Eur J Cell Biol 77:253–261. [DOI] [PubMed] [Google Scholar]
- Sako N, Yamamoto T (1999). Analyses of taste nerve responses with special reference to possible receptor mechanisms of umami taste in the rat. Neurosci Lett 261:109–112. [DOI] [PubMed] [Google Scholar]
- Sako N, Tokita K, Sugimura T, Yamamoto T (2003). Synergistic responses of the chorda tympani to mixtures of umami and sweet substances in rats. Chem Senses 28:261–266. [DOI] [PubMed] [Google Scholar]
- Salari H, Bramley A, Langlands J, Howard S, Chan-Yeung M, Chan H, Schellenberg R (1993). Effect of phospholipase C inhibitor U-73122 on antigen-induced airway smooth muscle contraction in guinea pigs. Am J Respir Cell Mol Biol 9:405–410. [DOI] [PubMed] [Google Scholar]
- San Gabriel AM, Uneyama H, Torii K, Yoshie S (2005). Functional characterization of a rat mGluR1 variant from vallate papillae. Chem Senses 30:A195. [DOI] [PubMed] [Google Scholar]
- Stapleton JR, Roper SD, Delay ER (1999). The taste of monosodium glutamate (MSG), l-aspartic acid, and N-methyl-d-aspartate (NMDA) in rats: are NMDA receptors involved in MSG taste? Chem Senses 24:449–457. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP (1990). Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci USA 87:2466–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Mostafapour SP, Denlinger LC, Bleasdale JE, Fisher SK (1991). The aminosteroid U-73122 inhibits muscarinic receptor sequestration and phosphoinositide hydrolysis in SK-N-SH neuroblastoma cells. J Biol Chem 266:23856–23862. [PubMed] [Google Scholar]
- Yamamoto T, Matsuo R, Fujimoto Y, Fukunaga I, Miyasaka A, Imoto T (1991). Electrophysiological and behavioral studies on the taste of umami substances in the rat. Physiol Behav 49:919–925. [DOI] [PubMed] [Google Scholar]
- Yang H, Wanner IB, Roper SD, Chaudhari N (1999). An optimized method for in situ hybridization with signal amplification that allows the detection of rare mRNAs. J Histochem Cytochem 47:431–446. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ (2003). Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112:293–301. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS (2003). The receptors for mammalian sweet and umami taste. Cell 115:255–266. [DOI] [PubMed] [Google Scholar]