Abstract
Rapid changes in mitochondrial DNA allele frequency between generations have been explained by an ‘mtDNA bottleneck’ in the germ line, and mtDNA aggregates, or nucleoids have been recently proposed to drive such a bottleneck. Now, a new study finds a sharp reduction in mtDNA content in the germ line and suggests that such reduction alone may account for the bottleneck effect.
At conception, we receive about 100,000 copies of mitochondrial DNA (mtDNA) that populate the egg. Intriguingly, all these copies are usually identical. How and why does Nature maintain such extraordinary uniformity? Since mitochondrial genomes in a cell are manifold, they can compensate each others’ defects and therefore tolerate mutations. Thus, mtDNA in the germ line should be highly prone to quietly accumulating detrimental mutations, one genome at a time, until the genetic pool of the population is critically contaminated. Fortunately, this does not happen because mitochondrial genomes populating an oocyte do not evolve independently: instead they all usually descend from a single mtDNA molecule that existed just several generations before. Such a molecule in most cases is free of mutations.. If however this mtDNA molecule does carry a pathogenic mutation, the corresponding oocyte would receive a high percentage of pathogenic mtDNA, and probably would be eliminated (1). In the worst case, an individual inheriting mutant mtDNA would develop mitochondrial disease and probably would not live to transmit the mutant mtDNA to progeny. In either case the mtDNA genetic pool is kept clean (2). This tight mode of inheritance is thought to depend on a ‘mitochondrial bottleneck’, though details of how exactly the bottleneck is created have been unknown. In this issue, Patrick Chinnery and colleagues now report that the mitochondrial bottleneck is driven by a reduction of mtDNA content in the germ line (3). Paradoxically, a study from Cao et al. published nearly a year ago came to the opposite conclusion that the bottleneck occurs without reduction of mtDNA content in germ cells4. What are the reasons for the discrepancy, and what does this mean?
Cellular mtDNA population and genetic drift
Each cell of the body contains a large number of mtDNA molecules that, from a population genetics standpoint, constitutes a population. mtDNA molecules reproduce (replicate) and ‘die’ when mitochondria are recycled or segregate into a cell that leaves the lineage. As with any population, mtDNA molecules in a cell are subject to genetic drift, that is, stochastic change in the fractions of individuals of different ancestry. Sufficiently rapid genetic drift eventually causes individuals of one single ancestry to take over the whole population5. This is exactly what is needed to establish the recent common ancestry of mtDNA molecules populating an oocyte and thus prevent the quiet spread of mitochondrial disease mentioned above. However, the rate of genetic drift (per generation) is inversely proportional to the size of the population. In the huge population of 100,000 mtDNA that we receive from our mothers, genetic drift would be hopelessly slow. To boost the genetic drift we need a bottleneck—a stage in germ line development with low numbers of mtDNA molecules per cell. Indeed, in early development, the germ line exists as primordial germ cells (PGCs), which presumably contain relatively few mtDNA molecules. In mice, it has been estimated that to account for the observed genetic drift, PGCs should contain very few mtDNA copies, approximately 200 each6.
The first direct measurements of PGC mtDNA content brought a surprise: PGCs contain about 2,000 mtDNA copies (4). This still may be called a bottleneck considering that the copy number at other stages of the female germ line is much higher, but 2,000 is tenfold too many copies to sustain the observed genetic drift (6) (Fig. 1a). To explain this discrepancy, Cao et al. invoked nucleoids, hypothetical aggregates of several mtDNA molecules that segregate as a single unit (7). If all mtDNA of each nucleoid are of the same ancestry, then nucleoids, not individual mtDNA molecules, are the members of the cellular mtDNA population. This would imply a much narrower effective bottleneck, as measured by the number of segregation units (nucleoids), rather than mtDNA molecules (Fig. 1b), and proportionally faster drift.
Figure 1.
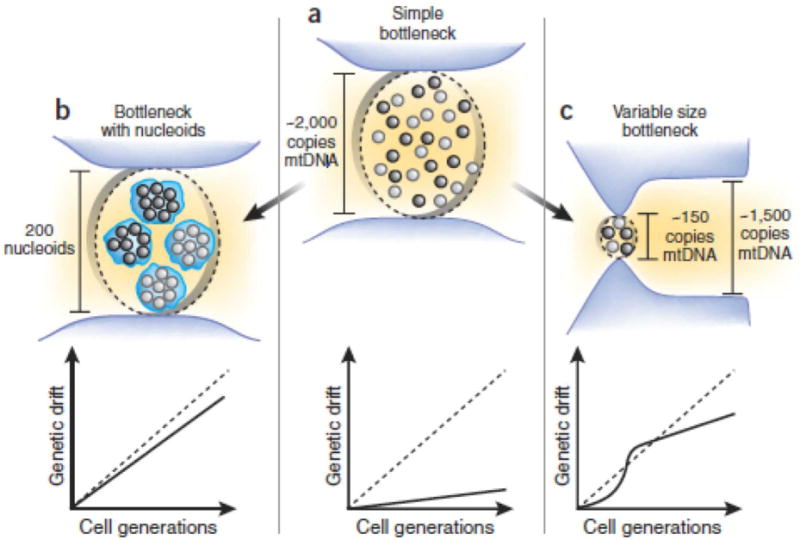
(a) The problem: A simple model of the mitochondrial bottleneck contains too many mtDNA molecules and creates less estimated genetic drift (solid line) than experimentally observed genetic drift (dotted line). (b) Assuming aggregation of mtDNA molecules into nucleoids results in a much tighter effective bottleneck and thus accelerates the estimated drift to fit observations (4). (c) Alternatively, observed drift can be accounted for by a variable bottleneck with temporal reduction of copy number as described in this issue 3.
Variable bottleneck and more
In contrast, Cree et al. (3) now suggest that nucleoids are not necessarily needed, and propose that rapid genetic drift in the germ line can be explained by a combination of several other factors. First, the authors used Stella-GFP protein, a marker of the PGC line with improved specificity, as compared to alkaline phosphatase histochemistry, that have been previously used to examine PGC mtDNA copy number4, to demonstrate that the mtDNA content of PGCs varies with time and has a sharp minimum in early PGCs just before mtDNA replication in the embryo is initiated (Fig. 1c). This bottleneck narrowing gives genetic drift a boost, though because the minimum is short-lived, the overall drift is increased only moderately, about 2.5-fold. Second, Unlike previous studies (4, 6), which used Wright’s equation8 to estimate genetic drift, Cree et al. employed a computer simulation of a variable size bottleneck that cannot be described by Wright’s equation. Notably, in the constant size portion of the bottleneck, where Wright’s equation is applicable, the simulation by Cree et al. predicted a ~2.5-fold higher drift rate than that estimated by using Wright’s equation. The exact reason for this difference is unclear and needs further investigation. These two factors, the variable size bottleneck and the higher drift rate generated by the simulation, can account for most of the observed excess of genetic drift. Furthermore, observations by Cree et al. point to other factors that may be boosting genetic drift. In particular, mtDNA copy number distribution in early PGCs is impressively wide, spanning about two orders of magnitude (20–2,000 mtDNA copies per cell). Thus, the germ line bottleneck may be more accurately represented by a superposition of multiple bottlenecks of different width (each corresponding to a particular germ cell lineage), which should result in a higher perceived genetic drift.
Although the current study suggests that the mtDNA bottleneck may be accounted for without invoking nucleoids3, this does not preclude their involvement. Rather, it seems likely that multiple factors, including those discussed above, may be driving the mitochondrial bottleneck. Further investigation into this complex system is needed in order to clarify the nature of the mtDNA bottleneck.
References
- 1.Krakauer DC, Mira A. Nature. 1999;400:125–126. doi: 10.1038/22026. [DOI] [PubMed] [Google Scholar]
- 2.Bergstrom CT, Pritchard J. Genetics. 1998;149:2135–2146. doi: 10.1093/genetics/149.4.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cree LM, et al. Nat Genet. 2008;40:249–254. doi: 10.1038/ng.2007.63. [DOI] [PubMed] [Google Scholar]
- 4.Cao L, et al. Nat Genet. 2007;39:386–390. doi: 10.1038/ng1970. [DOI] [PubMed] [Google Scholar]
- 5.Avise J, Neigel J, Arnold JJ. Mol Evol. 1984;20:99–105. doi: 10.1007/BF02257369. [DOI] [PubMed] [Google Scholar]
- 6.Jenuth JP, Peterson AC, Fu K, Shoubridge EA. Nat Genet. 1996;14:146–151. doi: 10.1038/ng1096-146. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs HT, Lehtinen SK, Spelbrink JN. Bioessays. 2000;22:564–572. doi: 10.1002/(SICI)1521-1878(200006)22:6<564::AID-BIES9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Wright S. Evolution and the genetics of populations. University of Chicago Press; Chicago: 1969. [Google Scholar]


