Abstract
The decision to admit a patient to the hospital after an emergency department (ED) visit is expensive, frequently not evidence-based, and variable. Outpatient critical pathways are a promising approach to reduce hospital admission after emergency care. Critical pathways exist to risk-stratify patients for potentially serious diagnoses (e.g. acute myocardial infarction) or evaluate response to therapy (e.g. community acquired pneumonia) within a short time period (i.e. less than 36 hours), to determine if further hospital-based acute care is needed. Yet, such pathways are variably used while many patients are admitted for conditions for which they could be treated as outpatients. In this article, the authors propose a model of post-ED critical pathways, describe their role in emergency care, list common diagnoses that are amenable to critical pathways in the outpatient setting, and propose a research agenda to address barriers and solutions to increase the use of outpatient critical pathways. If emergency providers are to routinely conduct rapid evaluations in outpatient or observation settings, they must have several conditions at their disposal: 1) evidence-based tools to accurately risk-stratify patients for protocolized care, 2) systems of care that reliably facilitate workup in the outpatient setting, and 3) a medical environment conducive to non-inpatient pathways, with aligned risks and incentives among patients, providers, and payers. Increased use of critical pathways after emergency care is a potential way to improve the value of emergency care.
INTRODUCTION
The cost of health care is a national priority, and emergency care is cited as an important cost driver by politicians and the mainstream media.1 Approximately half of hospitalizations are admitted though the emergency department (ED), and hospital care accounts for one-third of U.S. national health expenditures -- $789 billion in 2010.2,3 Therefore, while the direct costs of emergency care account for a small proportion of U.S. health care expenditures,4 emergency providers directly influence a much larger proportion of U.S. health care costs. Hospital admission is the single most costly decision that emergency providers make. Because there is variation in admission rates between emergency providers and between EDs, it may be feasible to reduce hospital admission rates from some EDs and by some providers without affecting outcomes.5,6,7
A promising approach to reduce hospital admissions is to use critical pathways to improve the efficiency of evaluations for patients with urgent, post-ED care needs that may not require a hospital admission. Critical pathways serve to risk-stratify patients for potentially serious diagnoses (e.g. acute myocardial infarction [AMI]) or response to therapy (e.g. community acquired pneumonia) within a short time period (i.e. less than 36 hours), to determine if further hospital-based acute care is needed.8,9 Evidence-based diagnostic and treatment pathways exist for a variety of conditions, allowing clinicians to identify patients safe for discharge with expedited outpatient workups, or after a period of observation. These pathways are variably used, while many patients are admitted for conditions for which they could be treated as outpatients.5,10,11 Barriers to the use of outpatient critical pathways include patient factors such as individual patient psychosocial needs, provider factors such as a lack of shared decision-making between patient and provider and provider discomfort with risk (both risk to patients and medico-legal risk), and system factors such as the lack of outpatient care to handle the specific needs of a critical pathway in a timely fashion and misaligned financial incentives. Increasing the use of critical pathways after emergency care is a potential way to improve the value of emergency care.
This article reports the results of a working group charged to address methods to improve value in post-ED transitions that was part of the consensus conference, “Quality and Efficiency of Emergency Care Across the Continuum: A Systems Approach,” sponsored by the American College of Physicians, the Society for Academic Emergency Medicine, and the Agency for Health Care Research and Quality and held in Boston in October 2009. In this article, we describe a model of post-ED critical pathways, describe their role in emergency care, list common diagnoses that are amenable to critical pathways in the outpatient setting, and propose a research agenda to address barriers and solutions to increasing the use of outpatient critical pathways.
Conceptual Model of Outpatient Critical Pathways after Emergency Care
Emergency providers face uncertainty when determining the appropriate disposition for patients who do not have a clear indication for hospital care, but still have a risk of poor outcome without further timely evaluation. As described in the Model of the Clinical Practice of Emergency Medicine, assessing undifferentiated signs and symptoms is a core task of the emergency physician (EP): “an emergency physician’s frame of reference in a patient encounter is fundamentally related to the actual, apparent, or potential acuity of the patient’s condition.”12 For example, many patients with chest pain who are not diagnosed with AMI by electrocardiogram (ECG) or cardiac enzymes are still at risk of acute coronary syndrome and adverse outcomes within a short period of time.13
The disposition for patients with diagnostic or therapeutic uncertainty after an emergency visit is determined according to local standards of care, the availability of post-ED care, patient preferences, and the EP’s assessment of and tolerance for risk. Medical decisions that are based on provider preference often show the greatest degree of variability.6,7,14 Despite evidence that many patients with diagnostic or therapeutic uncertainty can undergo definitive care as an outpatient or in observation care, many emergency providers default to the path of perceived caution: admitting most such patients.10 Standardizing care through the use of critical pathways is a potential method to reduce variability in these equivocal decisions, decrease malpractice risk, and shift the standard of care to a lower cost venue while maintaining high quality. In order for emergency providers to routinely conduct rapid evaluations in outpatient or observation settings, they must have several conditions at their disposal: 1) evidence-based tools to accurately risk-stratify patients for protocolized care, 2) systems of care that reliably facilitate workup in the outpatient setting, and 3) a medical environment conducive to non-inpatient pathways, with aligned risks and incentives among patients, providers, and payers. This is further detailed in Figure 1.
Figure 1.
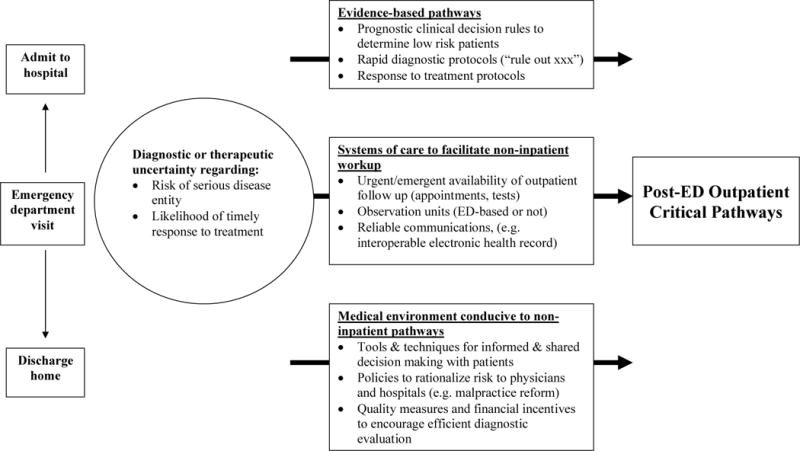
Conceptual Model of Critical Pathways after Emergency Care
Clinical conditions prevalent in the ED and potentially amenable to outpatient critical pathways are listed in Table 1. We identified conditions with three characteristics: 1) a large number of ED visits, 2) a significant proportion of patients are admitted, and 3) there are evidence-based critical pathways that could be conducted in an ED observation unit or outpatient setting.
Table 1.
Emergency Clinical Conditions Amenable to Post Emergency Department Outpatient Critical Pathways
| Condition | Annual ED visits (2006)* | % Admitted* | % Discharged* | Selected prognostic tools/clinical decision rules | Evidence-based evaluation and/or treatment pathways (OBS or Outpatient) | Issues associated with urgent/emergent availability of outpatient follow-up |
|---|---|---|---|---|---|---|
| Abdominal Pain [CCS 251 Abdominal Pain] | 4,533,940 | 3.4 | 96.6 | Clinical decision rule for identifying children at low risk for appendicitis.58 Pediatric appendicitis score.59 The Alvarado Score.60 Clinical decision rule for risk of ectopic pregnancy.61 | Observation unit protocols.62,63 | Availability of outpatient nurse or physician follow-up/support. Communication with the provider who will care for the patient. |
| Chest Pain [CCS 102 Nonspecific Chest Pain] | 3,748,198 | 19.5 | 80.5 | TIMI Risk Score,64 GRACE risk score,65 Vancouver Chest Pain Rule,66 Quantitative Pretest Probability Assessment,67 Goldman Rule.68 | Institute for Clinical Systems Improvement Guideline for Chest Pain and ACS, Observation of Cocaine Chest Pain,69 CT Coronary Angiogram,70 6 hour rule-out protocol,71 Erlanger Protocol,72 Observation Unit Evaluation,73 Myocardial perfusion imaging reduces unnecessary chest pain admissions.74 | Lack of availability of urgent outpatient functional testing can lead to increased utilization of OBS or inpatient stays |
| Cellulitis [CCS 197 Skin and Subcutaneous Tissue Infections] | 3,018,547 | 13.5 | 86.5 | Management of skin/soft tissue infections - IDSA,75 Observation unit literature.76 | Cost and availability of medication at discharge | |
| Community Acquired Pneumonia [CCS 122 Pneumonia] | 1,767,629 | 52.7 | 47.3 | BTS prediction rule,77 ATS prediction rule,78 Pneumonia Severity Index,79 Clinical prediction rule for pulmonary infiltrates.80 | Pneumonia care process model (Intermountain Health).81 | Cost of obtaining antibiotics for uninsured or underinsured patients. |
| Asthma [CCS 128 Asthma] | 1,728,051 | 17.0 | 83.0 | Predicting the need for hospital admission in children with acute asthma,82 assessment of severity measures for asthma.83 | Asthma guidelines (ICSI), observation unit literature.84 | Cost and availability of medication at discharge |
| Chronic Obstructive Pulmonary Disease (COPD) [CCS 127 COPD and Bronchiectasis] | 1,694,894 | 26.9 | 73.1 | Outcome predictors after ED evaluation.85 | COPD Guidelines (AHRQ) ACCP Guidelines,86 Hospital at Home for COPD.87 | Availability of outpatient nurse or physician follow-up/support |
| Congestive Heart Failure (CHF) [CCS 108 CHF; Non-hypertensive] | 1,024,970 | 81.0 | 19.0 | Prediction rule to identify low-risk patients with heart failure,88 acute heart failure index,89 others.90,91,92 | Observation unit literature. 93,94,95 | Availability of ECHO in the OBS or outpatient setting. |
| Syncope [ICD-9-CM 780.2] | 992,713 | 23.8% | 76.2% | San Francisco Rule,96 OESIL (Osservatorio Epidemiologico sulla Sincope nel Lazio).97 | ACEP Clinical Guideline,98 SEEDS,99 ED Syncope Protocol.100 | Availability of ECHO in the OBS or outpatient setting. |
| Upper GI Bleed [CCS 153 Gastrointestinal Hemorrhage] | 566,424 | 51.4 | 48.6% | Rockall Score,101 Blatchford Score.102 | Outpatient management of UGIB,103 Clinical guideline addressing hospital LOS need for UGIB.104 | Availability of endoscopy in the ED or observation unit. |
| Atrial Fibrillation & Atrial Flutter [ICD-9-CM Diagnosis 427.31 & 427.32] | 437,915 | 65.7 | 34.3 | CHADS2,105 SPAF,106 Framingham,107 VanWalraven.108 | Observation unit pathway,109 8 hour OBS Protocol,23 Ottawa Protocol.110 | Cost of obtaining LMWH (uninsured, public aid), availability of urgent outpatient physician follow-up |
| Transient Ischemic Attack (TIA) [CCS† 112 Transient Cerebral Ischemia] | 275,031 | 60.3 | 39.7 | ABCD(2) Score.111 | Aggressive early treatment after TIA: EXPRESS,112 observation unit pathway.24 | Availability of neurology consultation in the ED, availability of expedited TIA outpatient workup or neurology evaluation. |
| Pyelonephritis [ICD-9-CM Diagnosis 590.1, 590.10, 590.11 Acute Pyelonephritis] | 146,693 | 32.1 | 67.9 | Prediction of treatment failure and mortality.113 | Observation unit management.114 | Cost and availability of medication at discharge |
| Pulmonary Embolus (PE) [ICD-9-CM 415.19 Pulmonary Embolism/Infarct NEC] | 132,807 | 93.2 | 6.6 | Geneva,115 Pisa,116 Wells,117 Charlotte,118 PE Rule out Criteria,119 PE Severity Index.120 | DVT and PE policies and guidelines.121,122 | Cost of obtaining LMWH (uninsured, public aid), availability of urgent outpatient physician follow-up. LMWH education |
| Deep Venous Thrombosis (DVT) [ICD-9-CM 453.40-453.42 Venous embolism and thrombosis of deep vessels of lower extremity] | 118,357 | 63.2 | 36.8 | Wells Criteria.123 | Institute for Clinical Systems Improvement: Venous Thromboembolism Guideline, Cochrane Review: Home vs. inpatient treatment for DVT,124 DVT and PE Management (AHRQ).125 | Availability of diagnostic services in the ED (venous US), Availability of outpatient diagnostic services (venous US), Cost of obtaining LMWH, LMWH education |
ED visit and admission data are from the Agency for Healthcare Research and Quality Nationwide Emergency Department Sample from 2006, available at http://hcupnet.ahrq.gov/
ACS = acute coronary syndrome, CCS = Clinical classification system, LMWH = low molecular weight heparin, OBS = observation, US = ultrasound
Barriers to Implement Post-ED Critical Pathways: Opportunities for Research
Evidence-based Tools to Identify Patients Safe for Outpatient Critical Pathways
Prognostic tools have been developed to stratify patients by their short-term risk for adverse events, which allow emergency providers to determine which conditions can be safely and appropriately treated as outpatients or in observation. Table 1 lists selected clinical decision rules for conditions that are potentially eligible for an outpatient critical pathway after an ED visit. Some tools, such as the Pneumonia Severity Index, may be best used to determine safety for discharge and rapid outpatient follow-up, as the evaluation for pneumonia does not require a specific set of diagnostic tests.15 Other tools, such as the ABCD2 score for transient ischemic attack (TIA), can be of use in deciding which patients are appropriate to receive testing in an outpatient TIA critical pathway, as only patients at very low risk of a post-TIA stroke would be appropriate for discharge.16 Among the conditions identified as potentially amenable to a critical pathway, there are few prediction tools with the accuracy to reliably determine who can be discharged from the ED with less than 1% chance of a serious short-term adverse event – the degree of risk to which many emergency providers feel held.17 If a validated risk-stratification tool is not available (e.g. syncope), critical pathways may be more appropriately performed during observation, as diagnostic tests can be performed to further risk-stratify a patient (e.g. cardiac monitoring and ECG for patients with syncope). Research is needed to develop new clinical decision rules and test them in diverse settings, as few clinical decision rules for emergency conditions have been validated and proven clinical effective.
Evidence-Based Critical Pathways
The use of non-inpatient evidence-based critical pathways for patients after emergency care was first developed in ED observation units, and now is supported by peer-reviewed studies and decades of clinical experience.18 The concept of observation care was built on early studies of chest pain centers designed to rule out acute coronary syndromes in low-risk patients and provide subsequent risk stratification while avoiding costly hospital admission.19,20,21 Based on this model, further clinical investigation has attempted to widen the scope of clinical entities suitable for observation care. Observation pathways have demonstrated clinical diagnostic or therapeutic equivalence to inpatient admission for more than 10 distinct clinical conditions including cocaine-associated chest pain,22 acute onset atrial fibrillation,23 TIA,24 acute decompensated heart failure,25 and others.18 The development and validation of additional evidence-based critical pathways for observation and outpatient settings is a research priority.
Systems of Care: Outpatient Pathways
Outpatient critical pathways have proven and theoretical benefits, but their successful execution is dependent on the systems of care that support their use. In order to safely and rapidly complete an evaluation in the outpatient setting, the emergency provider must have confidence that the patient will reliably undergo a specific set of tests, attend follow-up appointments, and have reliable communication between the patient, the diagnostic center, and the follow-up provider in a defined time period. All of these elements most commonly exist in integrated health care systems, specific examples of which have been cited as models of excellence (e.g., Intermountain Healthcare).26 However, most patients in the United States are not cared for in such well-coordinated systems. Creating and maintaining reliable delivery systems to realize the benefits of critical pathways will require new policies that align incentives and reward more efficient, cost-effective delivery across the continuum of outpatient care. Accountable care organizations, a prominent concept in the new Patient Protection and Affordable Care Act (ACA), are designed to do just that.27 One measure of accountable care organizations should be the availability of reliable outpatient critical pathways for diagnosis and prognosis after emergency care.
Barriers to rapid expedited outpatient workup include patients’ lack of insurance or underinsurance, lack of availability of primary care providers to coordinate and follow up on diagnostic testing and re-evaluation, and patient difficulties navigating the fragmented health care system. First, the patient must have timely and reliable follow-up with a physician who can order, obtain, and interpret the results of needed diagnostic tests. Scheduling outpatient visits during the ED visit increases the likelihood that follow-up care will occur, but is not possible in many systems outside of regular business hours.28 Even diligent patients who seek outpatient appointments after an ED visit find timely follow-up difficult; in one study, 37% of patients with private insurance and 66% of patients with Medicaid were unable to obtain an outpatient clinic appointment within the week following an ED visit.29 This is a real concern for emergency providers, as a disproportionate number of patients who lack insurance or have public insurance use the ED for care.30 Second, a nationwide shortage of primary care physicians is a barrier to outpatient evaluations even for insured patients.31 Although health care reform should decrease the number of uninsured and may improve payments from Medicaid, these changes will take time. Third, arranging expedited outpatient care depends on resources available in the community and differs between patients in a single ED. Coordination with outpatient services is simply unavailable when much emergency care is delivered: nights and weekends. Finally, without systems of expedited outpatient care, responsibility often falls to the patient, who is given verbal and written discharge instructions at the completion of their ED visit, which they may not understand.32
Systems of Care: Observation Care
In cases where expedited outpatient evaluation is inappropriate or unavailable, patients in need of rapid diagnosis or prognosis after emergency care can be observed as an alternative to inpatient admission. Observation care can be delivered in multiple settings; after emergency care it is most frequently delivered in an ED observation unit (EDOU) or on a hospital ward. On average, about 80% of patients managed in an EDOU can be safely discharged, while the remainder require further inpatient hospitalization.18 Despite evidence of clinical effectiveness, observation care is not completely integrated with emergency care across the United States. In 2007 it was estimated that only 36% of EDs had an observation unit: half of these are administratively part of the ED, while the rest are part of the inpatient hospital.33 As a result, there is a significant gap between the existing capacity of EDs to care for observation patients and the potential need. Identifying the reasons for this gap and deploying interventions to eliminate it are important research priorities.
The Medical Environment
In order for emergency providers to conduct diagnostic and prognostic critical pathways outside of the hospital setting, the medical environment needs to be receptive to them. Currently, barriers include a lack of shared decision making, a medico-legal environment where providers perceive excessive risk associated with non-inpatient care, and payment policies that incentivize inpatient over outpatient care. Yet, there are reasonable medical and policy approaches to deal with each of these.
Tools and Techniques for Informed and Shared Decision Making with Patients
Involving patients in the decision of whether a rapid evaluation should occur as an inpatient, during observation, or as an outpatient has the potential to increase the proportion of such evaluations that are done in the outpatient setting. With the exception of one study,34 none of the prediction tools or evidence-based evaluation pathways in Table 1 were designed with the intent of engaging patients in shared decision making regarding their care. The traditional paternalistic health care model, characterized by doctors playing the dominant role, is evolving toward a patient-centered model that emphasizes patient autonomy, informed consent, and patient empowerment.35 Shared decision making was identified as a priority research area in the Institute of Medicine report on comparative effectiveness research,36 and is a key priority in the ACA.37 Shared decision making has several core attributes: involvement of both the patient and doctor, a sharing of information by both parties, both parties taking steps to build a consensus about the preferred treatment, and reaching an agreement about which treatment to implement.38 The overall goal of shared decision making is to achieve evidence-based patient choice.38
One example of a shared decision making approach to rapid post-ED evaluations is a recent randomized trial assessing the effect of a quantitative pretest probability calculator in ED patients with low-risk chest pain.34 In this trial, 400 patient and clinician pairs were randomized to either the intervention (pretest probability printout provided to both patient and clinician), or control group (no printout). There was no difference in the rate of missed or delayed acute coronary syndrome between groups, and patients randomized to the intervention group were less likely to be hospitalized. A follow-up survey revealed that a greater proportion of patients randomized to the intervention group reported feeling “very satisfied” with the clinician’s explanation of the problem. Recent trials on shared decision making for communicating cardiovascular disease risk,39 and use of antibiotics for acute respiratory infections40 in the primary care setting have reported increased patient satisfaction and decreased resource use in low-risk patients. These studies suggest that patients’ risk tolerance is significantly higher than that of physicians, and that low-risk patients can safely choose less resource-intensive care options, such as a rapid outpatient evaluation, when properly engaged. Other diagnoses with post-ED outpatient critical pathways such as TIA or acute onset atrial fibrillation should also be amenable to a shared decision making model. In each of these conditions, alternative pathways for evaluation and management exist (which are accompanied by a range of possible risks and outcomes for the patient), and well-developed prediction tools to estimate the short-term risk of an adverse event are available. For each condition, research is needed to determine if communicating the risks and benefits of each potential management option using a quantitative, patient-centered approach may empower patients to participate in their care, increase patient knowledge and trust in the physician, and safely limit resource use in low risk patients.
Policies to Rationalize Risk to Physicians and Hospitals
The first hurdle in creating an environment where patients and physicians utilize rapid outpatient evaluations understandably revolves around the patients, their preferences, and their tolerance for perceived risk. But the second hurdle is provider preference and perceived risk. The practice of emergency medicine is fraught with frequent assessments of clinical risk and benefit, judgments of probability, and associated liability for mistakes.41 Weighing what is best for the patient given an assumed probability of disease, against the smaller probability of a poor outcome or inaccurate assessment, often balances in favor of doing more rather than less.42 The patients who emergency providers worry the most about at the end of their shifts are those who are discharged -- hospitalization is often seen as the safest alternative for the physician and patient if there is any unanswered question.
If EPs are to use outpatient critical pathways as indicated by the evidence, specific actions are needed to reduce their perceptions of risk associated with outpatient care. First, scientifically validated pathways of care can help improve risk assessments and reduce the associated risk. Second, shared decision-making models of care may help reduce perceived risk by increasing the quality of the physician-patient relationship and by empowering patients to actively participate in the choice of disposition. Finally, a reliable system of medical justice that acknowledges clinical guidelines when determining standards of care could help reduce physicians’ perceived risk. Given the lack of sensitivity and specificity of our current medical malpractice system, it is difficult for physicians to predict what actions or inactions will fall within the standard of care.41,43 Clinical practice guidelines, which often define critical pathways, may reduce regional practice variability and increase legal predictability by setting a standard that is based on following best evidence prospectively, rather than based on local expert witnesses retrospectively.44
Quality Measures and Financial Incentives to Encourage Efficient Diagnostic Evaluation
Creating and maintaining delivery systems that can accomplish outpatient critical pathways after ED care also will require policies that align financial incentives and reward more efficient care across the continuum. Performance measurement and payment reform are two tools that can help with this transformation.
Traditional medical education modalities have been ineffective at improving the quality of medical care.45 Performance measurement and quality improvement can be more effective strategies46 that have led to marked improvements in care for cardiology, oncology, and surgery patients.47,48,49 Performance measures should be developed to compare EDs’ use of hospitalization, compared to outpatient care for common emergency conditions such as chest pain, TIA, and pneumonia. These will need to be balanced with measures of post-ED adverse events, such as missed MI, stroke, and rehospitalization, respectively. Current validated performance measures target potentially avoidable hospital admissions for chronic conditions sensitive to primary care (e.g. asthma),50 or care after an inpatient stay (e.g. 30-day readmission after congestive heart failure),51 and may indirectly increase the use of outpatient and observation pathways as a means to avoid hospitalization. Yet, there are currently no validated performance measures designed to evaluate the use of outpatient critical pathways after emergency care. The Society of Chest Pain Centers has proposed several unadjusted metrics to compare the use of observation to admission for patients with chest pain,52 but before being used to publicly compare EDs, or for performance-based reimbursement, such measures will need to adequately risk-adjust for patient disease severity and comorbidity.53 Key stakeholders, such as the National Quality Forum, could stimulate the development and testing of quality measures for post-ED critical pathways by the topic to their efficiency portfolio. Such measures could help align incentives, as they would encourage hospital systems to develop the systems to support outpatient critical pathways, and emergency providers to use them.
Robust economic analyses of post-ED critical pathways are also needed. Observation care in an EDOU has been compared to inpatient hospitalization for multiple conditions and found to be cost efficient,18 but questions remain regarding real resource utilization and potential societal cost savings due to the efficiency of the evaluation. Supplier induced demand is an economic concept that may apply to observation care. The availability of observation beds may lead to use that would not have otherwise occurred in the absence of that supply.6,7 The result: although more patients are admitted to observation, it is not clear that more patients are discharged. One institution, Cook County Hospital in Chicago, found that opening an observation unit appeared to reduce inpatient general medical admissions, but they did not look at the effect on ED discharges.54 Second, significant fixed costs are needed to maintain a distinct observation unit: 6 to 20 beds and dedicated nursing staff.18 A true cost-effectiveness analysis of observation care requires accounting for these beds, nurses, and physician time. Third, the cost-effectiveness of observation care is dependent on the author’s perspective. Patients may accrue cost efficiencies that are not accounted for by hospital cost accounting systems. For example, the increase in the efficiency of an EDOU evaluation vs. an equivalent outpatient evaluation reduces the time the patient must spend away from other societal responsibilities.
Research is needed to determine how best to align payment policies with efficient pathways of care for post-ED evaluations. Medicare payment policies have traditionally incentivized hospitals to conduct post-ED evaluations during hospital admissions, as the hospital received generous facility payments for the inpatient stay. Ancillary tests, such as diagnostic imaging, when performed in inpatient settings receive a significant premium over identical outpatient exams.55 The economic and operational incentives on post-ED rapid evaluation depend on each hospital’s clientele and occupancy. In a hospital with frequent crowding, where beds can be filled with high-margin elective cases, ED observation care may be profitable, as it frees up inpatient beds. Alternatively, in a hospital that is under-census, ED observation may be economically challenging, as it siphons off potential admissions. Several recent payment reforms may have changed this dynamic. First, Medicare’s recovery audit contractor program has brought more attention to these admission decisions, as hospitals can lose large sums by inappropriately labeling short stay admissions as inpatient status.56 Additionally, the premium for inpatient imaging has been reduced or removed. In response, hospitals have expanded the use of observation care in the inpatient setting, classifying many patients with short stays on regular hospital wards as observation care.57 There is no limit to the length of stay in observation, and the rate of observation visits over 48 hours has doubled in recent years.57 Because observation charges are billed as outpatient care under Medicare Part B, and as observation stays do not count toward the three day hospitalization requirement for subsequent skilled nursing facility eligibility, observation patients are subject to potentially higher out-of-pocket costs than if they were admitted (e.g., Medicare Part B co-pays, medication charges, etc.). Patient advocacy groups, such as the American Association of Retired Persons, are advocating for the elimination of hospital-based observation care.57 Further research is needed to study the effects of these complex payment policies on patients, hospitals, and health care systems with a goal of aligning incentives to collaborate and create alternative outpatient delivery pathways that can provide the same quality of care at a lower cost.
Research Agenda for Outpatient Critical Pathways
Research is needed to address clinical, systems, and policy questions around the use of critical pathways for diagnosis and treatment after emergency care. Developing new clinical prediction tools, assessing the prognostic accuracy of these tools, and investigating effective methods to facilitate uptake into clinical practice are all important priorities. Research is needed to translate the shared decision making model to the ED and to design effective decision aids and test their effects on the quality of the decision making process, safety, and cost of care for common emergency conditions. Development of critical pathways and evaluation of these pathways to determine their reliability in different practice settings, and their potential effect on patient safety and resource use, is needed. Research on how to best encourage physician adoption of critical pathways is equally important, as physicians’ perceptions of loss of autonomy from standardized care is a potential barrier to implementation of critical pathways. Finally, research to evaluate the comparative effectiveness of critical pathways in different settings (inpatient vs. observation; observation vs. outpatient) is needed for many conditions listed in Table 1. Such studies should investigate the effect of critical pathways on costs and quality for the studied condition, as well as on ED and inpatient crowding and operations. Although to date there has been limited interest in this research, it increasingly has resonance for specific conditions at appropriate National Institutes and AHRQ under the comparative effectiveness paradigm.36
CONCLUSIONS
Post-ED critical pathways hold significant promise for improving the value of emergency care. A robust research agenda is needed to create and refine evidence-based pathways, identify and eliminate system barriers to widespread implementation, and highlight required policy changes that will make the medical environment more conducive to post-ED critical pathways. Given today’s substantial variation in resource use for the evaluation and management of conditions that may be appropriate for outpatient critical pathways, aggressive pursuit of this research agenda is an important priority. We believe the conceptual model of critical pathways after emergency care presented here will be a useful tool for guiding a systematic approach to this research agenda.
Acknowledgments
This is a product of “Quality and Efficiency of Emergency Care Across the Continuum: A Systems Approach” consensus conference, sponsored by ACEP, SAEM, and AHRQ and held in Boston in October 2009.
Funding for this conference was made possible [in part] by 1R13HS018114-01 from the Agency for Healthcare Research and Quality (AHRQ). The views expressed in written conference materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government.
Footnotes
Disclosures:
- Dr. Schuur did not receive any funding to produce this paper. He receives grant funding from the Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers) for research on observation care. Additionally he receives grant funding from AHRQ for unrelated work. He is a member of the Primary Care and Emergency Medicine Scientific Advisory Board for United Health Care.
- Dr. Baugh is a co-investigator for an ongoing study involving the use of observation for patients with syncope, which is funded by the National Heart, Lung and Blood Institute.
- Dr. Hess is a recipient of the Fellow-to-Faculty transition award from the American Heart Association, the Society for Academic Emergency Medicine, and the Emergency Medicine Foundation and also receives grant funding from the Foundation for Informed Medical Decision Making.
- Dr. Pines receives grant funding from the Robert Wood Johnson Foundation and Abbott Point-of-Care for unrelated work.
- Dr. Asplin did not receive any funding to produce this paper.
References
- 1.Jones KB. Advertising emergency wait times raise health costs. The Salt Lake Tribune. Available at: http://www.sltrib.com/sltrib/opinion/50054001-82/costs-health-patients-hospital.html.csp. Accessed Mar 14, 2011.
- 2.DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. National Hospital Discharge Survey. Natl Health Stat Rep. 2006;2008:1–20. [PubMed] [Google Scholar]
- 3.Truffer CJ, Keehan S, Smith S, et al. Health spending projections through 2019: the recession’s impact continues. Health Affairs. 2010;29:522–9. doi: 10.1377/hlthaff.2009.1074. [DOI] [PubMed] [Google Scholar]
- 4.Kellermann AL. Calculating the cost of emergency care. Ann Emerg Med. 2005;45:491–2. doi: 10.1016/j.annemergmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Lougheed MD, Garvey N, Chapman KR, et al. The Ontario Asthma Regional Variation Study: emergency department visit rates and the relation to hospitalization rates. Chest. 2006;129:909–17. doi: 10.1378/chest.129.4.909. [DOI] [PubMed] [Google Scholar]
- 6.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138:273–87. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 7.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138:288–98. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- 8.Every NR, Hochman J, Becker R, Kopecky S, Cannon CP. Critical pathways : a review. Circulation. 2000;101:461–5. doi: 10.1161/01.cir.101.4.461. [DOI] [PubMed] [Google Scholar]
- 9.Fleischmann KE, Goldman L, Johnson PA, et al. Critical pathways for patients with acute chest pain at low risk. J Thrombos Thrombol. 2002;13:89–96. doi: 10.1023/a:1016246814235. [DOI] [PubMed] [Google Scholar]
- 10.Yealy DM, Auble TE, Stone RA, et al. Effect of increasing the intensity of implementing pneumonia guidelines: a randomized, controlled trial. Ann Intern Med. 2005;143:881–94. doi: 10.7326/0003-4819-143-12-200512200-00006. [DOI] [PubMed] [Google Scholar]
- 11.Lougheed MD, Garvey N, Chapman KR, et al. Variations and gaps in management of acute asthma in Ontario emergency departments. Chest. 2009;135:724–36. doi: 10.1378/chest.08-0371. [DOI] [PubMed] [Google Scholar]
- 12.The 2009 EM Model Review Task Force. The 2009 Model of the Clinical Practice of Emergency Medicine. doi: 10.1016/j.annemergmed.2010.11.015. Available at: http://www.acep.org/WorkArea/linkit.aspx?LinkIdentifier=id&ItemID=47823. Accessed Feb 7, 2011. [DOI] [PubMed]
- 13.Schull MJ, Vermeulen MJ, Stukel TA. The risk of missed diagnosis of acute myocardial infarction associated with emergency department volume. Ann Emerg Med. 2006;48:647–55. doi: 10.1016/j.annemergmed.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Mulley AG. Inconvenient truths about supplier induced demand and unwarranted variation in medical practice. BMJ. 2009;339:b4073. doi: 10.1136/bmj.b4073. [DOI] [PubMed] [Google Scholar]
- 15.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–50. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 16.Cucchiara B, Ross M. Transient ischemic attack: risk stratification and treatment. Ann Emerg Med. 2008;52:S27–39. doi: 10.1016/j.annemergmed.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 17.McCausland JB, Machi MS, Yealy DM. Emergency physicians: risk attitudes in acute decompensated heart failure patients. Acad Emerg Med. 2010;17:108–10. doi: 10.1111/j.1553-2712.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 18.Graff L, editor. Observation Medicine: The Healthcare System’s Tincture of Time. Irving, TX: The American College of Emergency Physicians; 2009. [Google Scholar]
- 19.Goodacre SW. Should we establish chest pain observation units in the UK? A systematic review and critical appraisal of the literature. J Accid Emerg Med. 2000;17:1–6. doi: 10.1136/emj.17.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zalenski RJ, McCarren M, Roberts R, et al. An evaluation of a chest pain diagnostic protocol to exclude acute cardiac ischemia in the emergency department. Arch Intern Med. 1997;157:1085–91. [PubMed] [Google Scholar]
- 21.Roberts RR, Zalenski RJ, Mensah EK, et al. Costs of an emergency department-based accelerated diagnostic protocol vs hospitalization in patients with chest pain: a randomized controlled trial. JAMA. 1997;278:1670–6. [PubMed] [Google Scholar]
- 22.Cunningham R, Walton MA, Weber JE, et al. One-year medical outcomes and emergency department recidivism after emergency department observation for cocaine-associated chest pain. Ann Emerg Med. 2009;53:310–20. doi: 10.1016/j.annemergmed.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Decker WW, Smars PA, Vaidyanathan L, et al. A prospective, randomized trial of an emergency department observation unit for acute onset atrial fibrillation. Ann Emerg Med. 2008;52:322–8. doi: 10.1016/j.annemergmed.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Ross MA, Compton S, Medado P, Fitzgerald M, Kilanowski P, O’Neil BJ. An emergency department diagnostic protocol for patients with transient ischemic attack: a randomized controlled trial. Ann Emerg Med. 2007;50:109–19. doi: 10.1016/j.annemergmed.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Peacock WF. Using the emergency department clinical decision unit for acute decompensated heart failure. Cardiol Clin. 2005;23:569–88. doi: 10.1016/j.ccl.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Weeks WB, Gottlieb DJ, Nyweide DJ, et al. Higher health care quality and bigger savings found at large multispecialty medical groups. Health Aff. 2010;29:991–7. doi: 10.1377/hlthaff.2009.0388. [DOI] [PubMed] [Google Scholar]
- 27.Shortell SM, Casalino LP, Fisher ES. How the Center For Medicare And Medicaid Innovation should test accountable care organizations. Health Aff. 2010;29:1293–8. doi: 10.1377/hlthaff.2010.0453. [DOI] [PubMed] [Google Scholar]
- 28.Kyriacou D, Handel D, Stein A, Nelson R. Brief report: factors affecting outpatient follow-up compliance of emergency department patients. J Gen Intern Med. 2005;20(10):938–42. doi: 10.1111/j.1525-1497.2005.0216_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asplin BR, Rhodes KV, Levy H, et al. Insurance status and access to urgent ambulatory care follow-up appointments. JAMA. 2005;294:1248–54. doi: 10.1001/jama.294.10.1248. [DOI] [PubMed] [Google Scholar]
- 30.Tang N, Stein J, Hsia RY, Maselli JH, Gonzales R. Trends and characteristics of US emergency department visits, 1997–2007. JAMA. 2010;304:664–70. doi: 10.1001/jama.2010.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodenheimer T, Grumbach K, Berenson RA. A lifeline for primary care. N Engl J Med. 2009;360:2693–6. doi: 10.1056/NEJMp0902909. [DOI] [PubMed] [Google Scholar]
- 32.Engel KG, Heisler M, Smith DM, Robinson CH, Forman JH, Ubel PA. Patient comprehension of emergency department care and instructions: are patients aware of when they do not understand? Ann Emerg Med. 2009;53:454–61. doi: 10.1016/j.annemergmed.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Niska RW, Bhuiya F, Xu J. National Hospital Ambulatory Medical Care Survey: 2007 emergency department summary. Natl Health Stat Rep. 2010;26:1–31. [PubMed] [Google Scholar]
- 34.Kline JA, Zeitouni RA, Hernandez-Nino J, Jones AE. Randomized trial of computerized quantitative pretest probability in low-risk chest pain patients: effect on safety and resource use. Ann Emerg Med. 2009;53:727–35. doi: 10.1016/j.annemergmed.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 35.Edwards A, Elwyn G. The potential benefits of decision aids in clinical medicine. JAMA. 1999;282:779–80. doi: 10.1001/jama.282.8.779. [DOI] [PubMed] [Google Scholar]
- 36.Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: The National Academies Press; 2009. [Google Scholar]
- 37.Press release from Foundation for Informed Medical Decision: “HR 3590 Enrolled Bill signed into Law”. Available at: http://www.informedmedicaldecisions.org/pdfs/Analysis_passed_legislation.pdf. Accessed March 1, 2011.
- 38.Charles C, Gafni A, Whelan T. Decision making in the physician-patient encounter: re-visiting the shared treatment decision model. Soc Sci Med. 1997;49:651–61. doi: 10.1016/s0277-9536(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 39.Krones T, Keller H, Sonnichsen A, et al. Absolute cardiovascular disease risk and shared decision making in primary care: a randomized controlled trial. Ann Fam Med. 2008;6:218–27. doi: 10.1370/afm.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Legare F, Labrecque M, Leblanc A, et al. Training family physicians in shared decision making for the use of antibiotics for acute respiratory infections: a pilot clustered randomized controlled trial. Health Expect. 2011 doi: 10.1111/j.1369-7625.2010.00616.x. in publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawthers AG, Localio AR, Laird NM, Lipsitz S, Hebert L, Brennan TA. Physicians’ perceptions of the risk of being sued. J Health Polit Policy Law. 1992;17:463–82. doi: 10.1215/03616878-17-3-463. [DOI] [PubMed] [Google Scholar]
- 42.Kessler DP, McClellan M. Do doctors practice defensive medicine? NBER Working Paper No. 5466. Q J Econ. 1996;111:356–90. [Google Scholar]
- 43.Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325:245–51. doi: 10.1056/NEJM199107253250405. [DOI] [PubMed] [Google Scholar]
- 44.Havighurst CC. Practice guidelines as legal standards governing physician liability. Law Contemp Probl. 1991;54:87–117. [PubMed] [Google Scholar]
- 45.Grimshaw JM, Eccles MP, Walker AE, Thomas RE. Changing physicians’ behavior: what works and thoughts on getting more things to work. J Contin Educ Health Prof. 2002;22:237–43. doi: 10.1002/chp.1340220408. [DOI] [PubMed] [Google Scholar]
- 46.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 47.Malenka DJ, O’Connor T. The Northern New England Cardiovascular Disease Study Group: a regional collaborative effort for continuous quality improvement in cardiovascular disease. Jt Comm J Qual Improv. 1998;24:594–600. doi: 10.1016/s1070-3241(16)30408-4. [DOI] [PubMed] [Google Scholar]
- 48.Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24:626–34. doi: 10.1200/JCO.2005.03.3365. [DOI] [PubMed] [Google Scholar]
- 49.Khuri SF, Daley J, Henderson WG. The comparative assessment and improvement of quality of surgical care in the Department of Veterans Affairs. Arch Surg. 2002;137:20–7. doi: 10.1001/archsurg.137.1.20. [DOI] [PubMed] [Google Scholar]
- 50.AHRQ. Prevention Quality Indicators Overview. Available at: http://www.qualityindicators.ahrq.gov/pqi_overview.htm. Accessed Feb 7, 2011.
- 51.Keenan PS, Normand ST, Lin Z, et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circulation. 2008;1:29–37. doi: 10.1161/CIRCOUTCOMES.108.802686. [DOI] [PubMed] [Google Scholar]
- 52.Society of Chest Pain Centers. Performance Measures for Chest Pain. Available at: http://www.scpcp.org/WebDocs/SCPC%20Chest%20Pain%20Metrics%20Specifications.pdf.Accessed Feb 7, 2011.
- 53.Krumholz HM, Keenan PS, Brush JE, Jr, et al. Standards for measures used for public reporting of efficiency in health care. A scientific statement from the American Heart Association Interdisciplinary Council on Quality of Care and Outcomes Research and the American College of Cardiology Foundation. Circulation. 2008;118:885–93. doi: 10.1161/CIRCULATIONAHA.108.190500. [DOI] [PubMed] [Google Scholar]
- 54.Martinez E, Reilly BM, Evans AT, Roberts RR. The observation unit: a new interface between inpatient and outpatient care. Am J Med. 2001;110:274–7. doi: 10.1016/s0002-9343(00)00710-5. [DOI] [PubMed] [Google Scholar]
- 55.Wynn BO. Medicare Payment for Hospital Outpatient Services: A Historical Review of Policy Options. Available at: http://www.rand.org/pubs/working_papers/WR267.html. Accessed Feb 7, 2011.
- 56.Moore E, Gilmer L. The recovery audit contractor program: an overview. Health Care Law Monthly. 2009:2–10. [PubMed] [Google Scholar]
- 57.Armstrong D. Medicare fraud plan brings surprise bills for elderly. Bloomberg Businessweek. 2010 Jul 12; Available at: http://www.businessweek.com/news/2010-07-12/medicare-fraud-plan-brings-surprise-bills-for-elderly.html. Accessed Feb 7, 2011.
- 58.Kharbanda AB, Taylor GA, Fishman SJ, Bachur RG. A clinical decision rule to identify children at low risk for appendicitis. Pediatrics. 2005;116:709–16. doi: 10.1542/peds.2005-0094. [DOI] [PubMed] [Google Scholar]
- 59.Samuel M. Pediatric appendicitis score. J Pediatr Surg. 2002;37:877–81. doi: 10.1053/jpsu.2002.32893. [DOI] [PubMed] [Google Scholar]
- 60.Owen TD, Williams H, Stiff G, Jenkinson LR, Rees BI. Evaluation of the Alvarado score in acute appendicitis. J R Soc Med. 1992;85:87–8. doi: 10.1177/014107689208500211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buckley RG, King KJ, Disney JD, Gorman JD, Klausen KH. History and physical examination to estimate the risk of ectopic pregnancy: validation of a clinical prediction model. Ann Emerg Med. 1999;34:589–94. doi: 10.1016/s0196-0644(99)70160-5. [DOI] [PubMed] [Google Scholar]
- 62.Graff L, Radford MJ, Werne C. Probability of appendicitis before and after observation. Ann Emerg Med. 1991;20:503–7. doi: 10.1016/s0196-0644(05)81603-8. [DOI] [PubMed] [Google Scholar]
- 63.Graff LG, 4th, Robinson D. Abdominal pain and emergency department evaluation. Emerg Med Clin North Am. 2001;19:123–36. doi: 10.1016/s0733-8627(05)70171-1. [DOI] [PubMed] [Google Scholar]
- 64.Pollack CV, Jr, Sites FD, Shofer FS, Sease KL, Hollander JE. Application of the TIMI risk score for unstable angina and non-ST elevation acute coronary syndrome to an unselected emergency department chest pain population. Acad Emerg Med. 2006;13:13–8. doi: 10.1197/j.aem.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 65.Lyon R, Morris AC, Caesar D, Gray S, Gray A. Chest pain presenting to the emergency department--to stratify risk with GRACE or TIMI? Resuscitation. 2007;74:90–3. doi: 10.1016/j.resuscitation.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 66.Christenson J, Innes G, McKnight D, et al. A clinical prediction rule for early discharge of patients with chest pain. Ann Emerg Med. 2006;47:1–10. doi: 10.1016/j.annemergmed.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 67.Mitchell AM, Garvey JL, Chandra A, Diercks D, Pollack CV, Kline JA. Prospective multicenter study of quantitative pretest probability assessment to exclude acute coronary syndrome for patients evaluated in emergency department chest pain units [Abstract] Ann Emerg Med. 2006;47:447. doi: 10.1016/j.annemergmed.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 68.Goldman L, Weinberg M, Weisberg M, et al. A computer-derived protocol to aid in the diagnosis of emergency room patients with acute chest pain. N Engl J Med. 1982;307:588–96. doi: 10.1056/NEJM198209023071004. [DOI] [PubMed] [Google Scholar]
- 69.Weber JE, Shofer FS, Larkin GL, Kalaria AS, Hollander JE. Validation of a brief observation period for patients with cocaine-associated chest pain. N Engl J Med. 2003;348:510–7. doi: 10.1056/NEJMoa022206. [DOI] [PubMed] [Google Scholar]
- 70.Chang AM, Shofer FS, Weiner MG, et al. Actual financial comparison of four strategies to evaluate patients with potential acute coronary syndromes. Acad Emerg Med. 2008;15:649–55. doi: 10.1111/j.1553-2712.2008.00159.x. [DOI] [PubMed] [Google Scholar]
- 71.Herren KR, Mackway-Jones K, Richards CR, Seneviratne CJ, France MW, Cotter L. Is it possible to exclude a diagnosis of myocardial damage within six hours of admission to an emergency department? Diagnostic cohort study. BMJ. 2001;323:372. doi: 10.1136/bmj.323.7309.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fesmire FM, Hughes AD, Fody EP, et al. The Erlanger chest pain evaluation protocol: a one-year experience with serial 12-lead ECG monitoring, two-hour delta serum marker measurements, and selective nuclear stress testing to identify and exclude acute coronary syndromes. Ann Emerg Med. 2002;40:584–94. doi: 10.1067/mem.2002.129506. [DOI] [PubMed] [Google Scholar]
- 73.Kirk JD, Diercks DB, Turnipseed SD, Amsterdam EA. Evaluation of chest pain suspicious for acute coronary syndrome: use of an accelerated diagnostic protocol in a chest pain evaluation unit. Am J Cardiol. 2000;85:40B–48B. doi: 10.1016/s0002-9149(00)00755-4. [DOI] [PubMed] [Google Scholar]
- 74.Udelson JE, Beshansky JR, Ballin DS, et al. Myocardial perfusion imaging for evaluation and triage of patients with suspected acute cardiac ischemia: a randomized controlled trial. JAMA. 2002;288:2693–700. doi: 10.1001/jama.288.21.2693. [DOI] [PubMed] [Google Scholar]
- 75.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 76.Roberts R. Management of patients with infectious diseases in an emergency department observation unit. Emerg Med Clin North Am. 2001;19:187–207. doi: 10.1016/s0733-8627(05)70175-9. [DOI] [PubMed] [Google Scholar]
- 77.Research Committee of the British Thoracic Society & Public Health Laboratory Service. Community-acquired pneumonia in adults in British hospitals in 1982–1983: a survey of aetiology, mortality, prognostic factors and outcome. QJM. 1987;62:195–220. [PubMed] [Google Scholar]
- 78.Ewig S, de Roux A, Bauer T, et al. Validation of predictive rules and indices of severity for community acquired pneumonia. Thorax. 2004;59:421–7. doi: 10.1136/thx.2003.008110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Flanders WD, Tucker G, Krishnadasan A, Martin D, Honig E, McClellan WM. Validation of the pneumonia severity index. Importance of study-specific recalibration. J Gen Intern Med. 1999;14:333–40. doi: 10.1046/j.1525-1497.1999.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med. 1990;113:664–70. doi: 10.7326/0003-4819-113-9-664. [DOI] [PubMed] [Google Scholar]
- 81.Dean NC, Bateman KA, Donnelly SM, Silver MP, Snow GL, Hale D. Improved clinical outcomes with utilization of a community-acquired pneumonia guideline. Chest. 2006;130:794–9. doi: 10.1378/chest.130.3.794. [DOI] [PubMed] [Google Scholar]
- 82.Gorelick M, Scribano PV, Stevens MW, Schultz T, Shults J. Predicting need for hospitalization in acute pediatric asthma. Pediatr Emerg Care. 2008;24:735–44. doi: 10.1097/PEC.0b013e31818c268f. [DOI] [PubMed] [Google Scholar]
- 83.Arnold DH, Gebretsadik T, Minton PA, Higgins S, Hartert TV. Assessment of severity measures for acute asthma outcomes: a first step in developing an asthma clinical prediction rule. Am J Emerg Med. 2008;26:473–9. doi: 10.1016/j.ajem.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rydman RJ, Isola ML, Roberts RR, et al. Emergency department observation unit versus hospital inpatient care for a chronic asthmatic population: a randomized trial of health status outcome and cost. Med Care. 1998;36:599–609. doi: 10.1097/00005650-199804000-00015. [DOI] [PubMed] [Google Scholar]
- 85.Roche N, Zureik M, Soussan D, Neukirch F, Perrotin D. Predictors of outcomes in COPD exacerbation cases presenting to the emergency department. Eur Respir J. 2008;32:953–61. doi: 10.1183/09031936.00129507. [DOI] [PubMed] [Google Scholar]
- 86.McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest. 2001;119:1190–209. doi: 10.1378/chest.119.4.1190. [DOI] [PubMed] [Google Scholar]
- 87.Monninkhof EM, van der Valk PD, van der Palen J, et al. Self-management education for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2003:CD002990. doi: 10.1002/14651858.CD002990. [DOI] [PubMed] [Google Scholar]
- 88.Auble TE, Hsieh M, Gardner W, et al. A prediction rule to identify low-risk patients with heart failure. Acad Emerg Med. 2005;12:514–21. doi: 10.1197/j.aem.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 89.Hsieh M, Auble TE, Yealy DM. Validation of the acute heart failure index. Ann Emerg Med. 2008;51:37–44. doi: 10.1016/j.annemergmed.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 90.Chin MH, Goldman L. Correlates of major complications or death in patients admitted to the hospital with congestive heart failure. Arch Intern Med. 1996;156:1814–20. [PubMed] [Google Scholar]
- 91.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin QJ, Adhere Scientific Advisory Committee, Study Group & Investigators Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–80. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 92.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–7. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 93.Peacock WF, Emerman CL. Emergency department management of patients with acute decompensated heart failure. Heart Fail Rev. 2004;9:187–93. doi: 10.1007/s10741-005-6128-5. [DOI] [PubMed] [Google Scholar]
- 94.Storrow AB, Collins SP, Lyons MS, Wagoner LE, Gibler WB, Lindsell CJ. Emergency department observation of heart failure: preliminary analysis of safety and cost. Congest Heart Fail. 2005;11:68–72. doi: 10.1111/j.1527-5299.2005.03844.x. [DOI] [PubMed] [Google Scholar]
- 95.Peacock WF, 4th, Young J, Collins S, Diercks D, Emerman C. Heart failure observation units: optimizing care. Ann Emerg Med. 2006;47:22–33. doi: 10.1016/j.annemergmed.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 96.Quinn J, McDermott D, Stiell I, Kohn M, Wells G. Prospective validation of the San Francisco Syncope Rule to predict patients with serious outcomes. Ann Emerg Med. 2006;47:448–54. doi: 10.1016/j.annemergmed.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 97.Colivicchi F, Ammirati F, Melina D, Guido V, Imperoli G, Santini M. Development and prospective validation of a risk stratification system for patients with syncope in the emergency department: the OESIL risk score. Eur Heart J. 2003;24:811–9. doi: 10.1016/s0195-668x(02)00827-8. [DOI] [PubMed] [Google Scholar]
- 98.Huff JS, Decker WW, Quinn JV, et al. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with syncope. J Emerg Nurs. 2007;33:e1–17. doi: 10.1016/j.jen.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 99.Shen WK, Decker WW, Smars PA, et al. Syncope evaluation in the emergency department Study (SEEDS): a multidisciplinary approach to syncope management. Circulation. 2004;110:3636–45. doi: 10.1161/01.CIR.0000149236.92822.07. [DOI] [PubMed] [Google Scholar]
- 100.Rodriguez-Entem F, Gonzalez-Enriquez S, Olalla-Antolin JJ, et al. Management of syncope in the emergency department without hospital admission: usefulness of an arrhythmia unit coordinated protocol [Spanish] Rev Esp Cardiol. 2008;61:22–8. [PubMed] [Google Scholar]
- 101.Sanders DS, Carter MJ, Goodchap RJ, Cross SS, Gleeson DC, Lobo AJ. Prospective validation of the Rockall risk scoring system for upper GI hemorrhage in subgroups of patients with varices and peptic ulcers. Am J Gastroenterol. 2002;97:630–5. doi: 10.1111/j.1572-0241.2002.05541.x. [DOI] [PubMed] [Google Scholar]
- 102.Masaoka T, Suzuki H, Hori S, Aikawa N, Hibi T. Blatchford scoring system is a useful scoring system for detecting patients with upper gastrointestinal bleeding who do not need endoscopic intervention. J Gastroenterol Hepatol. 2007;22:1404–8. doi: 10.1111/j.1440-1746.2006.04762.x. [DOI] [PubMed] [Google Scholar]
- 103.Stanley AJ, Ashley D, Dalton HR, et al. Outpatient management of patients with low-risk upper-gastrointestinal haemorrhage: multicentre validation and prospective evaluation. Lancet. 2009;373:42–7. doi: 10.1016/S0140-6736(08)61769-9. [DOI] [PubMed] [Google Scholar]
- 104.Hay JA, Maldonado L, Weingarten SR, Ellrodt AG. Prospective evaluation of a clinical guideline recommending hospital length of stay in upper gastrointestinal tract hemorrhage. JAMA. 1997;278:2151–6. [PubMed] [Google Scholar]
- 105.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 106.Hart RG, Pearce LA, McBride R, Rothbart RM, Asinger RW. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation : analysis of 2012 participants in the SPAF IñIII clinical trials. Stroke. 1999;30:1223–9. doi: 10.1161/01.str.30.6.1223. [DOI] [PubMed] [Google Scholar]
- 107.Wolf P, D’Agostino R, Belanger A, Kannel W. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–8. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 108.van Walraven C, Hart RG, Wells GA, et al. A clinical prediction rule to identify patients with atrial fibrillation and a low risk for stroke while taking aspirin. Arch Intern Med. 2003;163:936–43. doi: 10.1001/archinte.163.8.936. [DOI] [PubMed] [Google Scholar]
- 109.Ross MA, Davis B, Dresselhouse A. The role of an emergency department observation unit in a clinical pathway for atrial fibrillation. Crit Pathw Cardiol. 2004;3:8–12. doi: 10.1097/01.hpc.0000116582.64827.2f. [DOI] [PubMed] [Google Scholar]
- 110.Stiell IG, Clement CM, Perry JJ, et al. Association of the Ottawa aggressive protocol with rapid discharge of emergency department patients with recent-onset atrial fibrillation or flutter. CJEM. 2010;12:181–91. doi: 10.1017/s1481803500012227. [DOI] [PubMed] [Google Scholar]
- 111.Fothergill A, Christianson TJ, Brown RD, Jr, Rabinstein AA. Validation and refinement of the ABCD2 score: a population-based analysis. Stroke. 2009;40:2669–73. doi: 10.1161/STROKEAHA.109.553446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432–42. doi: 10.1016/S0140-6736(07)61448-2. [DOI] [PubMed] [Google Scholar]
- 113.Efstathiou SP, Pefanis AV, Tsioulos DI, et al. Acute pyelonephritis in adults: prediction of mortality and failure of treatment. Arch Intern Med. 2003;163:1206–12. doi: 10.1001/archinte.163.10.1206. [DOI] [PubMed] [Google Scholar]
- 114.Israel RS, Lowenstein SR, Marx JA, Koziol-McLain J, Svoboda L, Ranniger S. Management of acute pyelonephritis in an emergency department observation unit. Ann Emerg Med. 1991;20:253–7. doi: 10.1016/s0196-0644(05)80934-5. [DOI] [PubMed] [Google Scholar]
- 115.Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165–71. doi: 10.7326/0003-4819-144-3-200602070-00004. [DOI] [PubMed] [Google Scholar]
- 116.Miniati M, Pistolesi M. Assessing the clinical probability of pulmonary embolism. Q J Nucl Med. 2001;45:287–93. [PubMed] [Google Scholar]
- 117.Wolf SJ, McCubbin TR, Feldhaus KM, Faragher JP, Adcock DM. Prospective validation of Wells Criteria in the evaluation of patients with suspected pulmonary embolism. Ann Emerg Med. 2004;44:503–10. doi: 10.1016/j.annemergmed.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 118.Courtney DM, Sasser HC, Pincus CL, Kline JA. Pulseless electrical activity with witnessed arrest as a predictor of sudden death from massive pulmonary embolism in outpatients. Resuscitation. 2001;49:265–72. doi: 10.1016/s0300-9572(00)00374-9. [DOI] [PubMed] [Google Scholar]
- 119.Kline JA, Courtney DM, Kabrhel D, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6:772–80. doi: 10.1111/j.1538-7836.2008.02944.x. [DOI] [PubMed] [Google Scholar]
- 120.Donze J, Le Gal G, Fine MJ, et al. Prospective validation of the Pulmonary Embolism Severity Index. A clinical prognostic model for pulmonary embolism. Thromb Haemost. 2008;100:943–8. doi: 10.1160/th08-05-0285. [DOI] [PubMed] [Google Scholar]
- 121.American College of Emergency Physicians Clinical Policy Committee, Clinical Policies Committee Subcommittee on Suspected Pulmonary Embolism. Clinical policy: critical issues in the evaluation and management of adult patients presenting with suspected pulmonary embolism. Ann Emerg Med. 2003;41:257–70. doi: 10.1067/mem.2003.40. [DOI] [PubMed] [Google Scholar]
- 122.Snow V, Qaseem A, Barry P, et al. Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Fam Med. 2007;5:74–80. doi: 10.1370/afm.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kilroy DA, Ireland S, Reid P, Goodacre S, Morris F. Emergency department investigation of deep vein thrombosis. Emerg Med J. 2003;20:29–32. doi: 10.1136/emj.20.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Othieno R, Abu Affan M, Okpo E. Home versus in-patient treatment for deep vein thrombosis. Cochrane Datab Syst Rev. 2007;(3):CD003076. doi: 10.1002/14651858.CD003076.pub2. [DOI] [PubMed] [Google Scholar]
- 125.Agency for Healthcare Research and Quality. Diagnosis and treatment of deep venous thrombosis and pulmonary embolism. Rockville, MD: Agency for Healthcare Research and Quality; 2003. [Google Scholar]


