Abstract
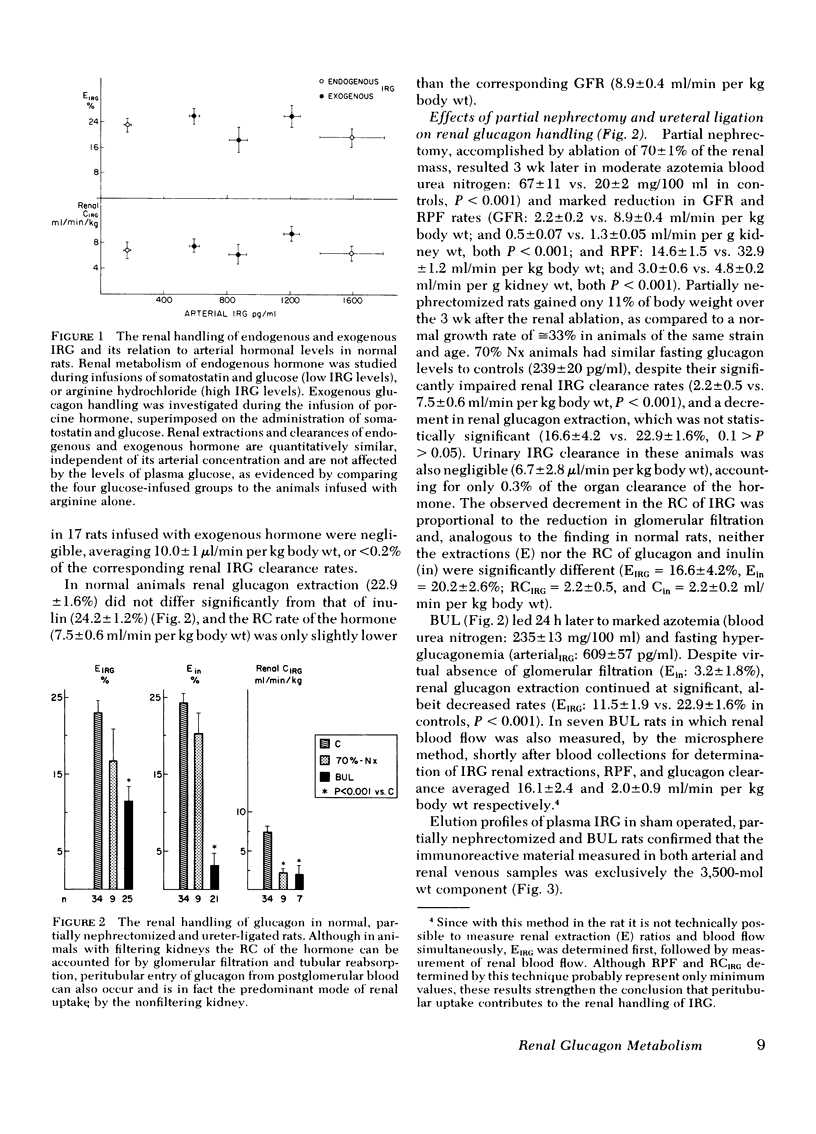
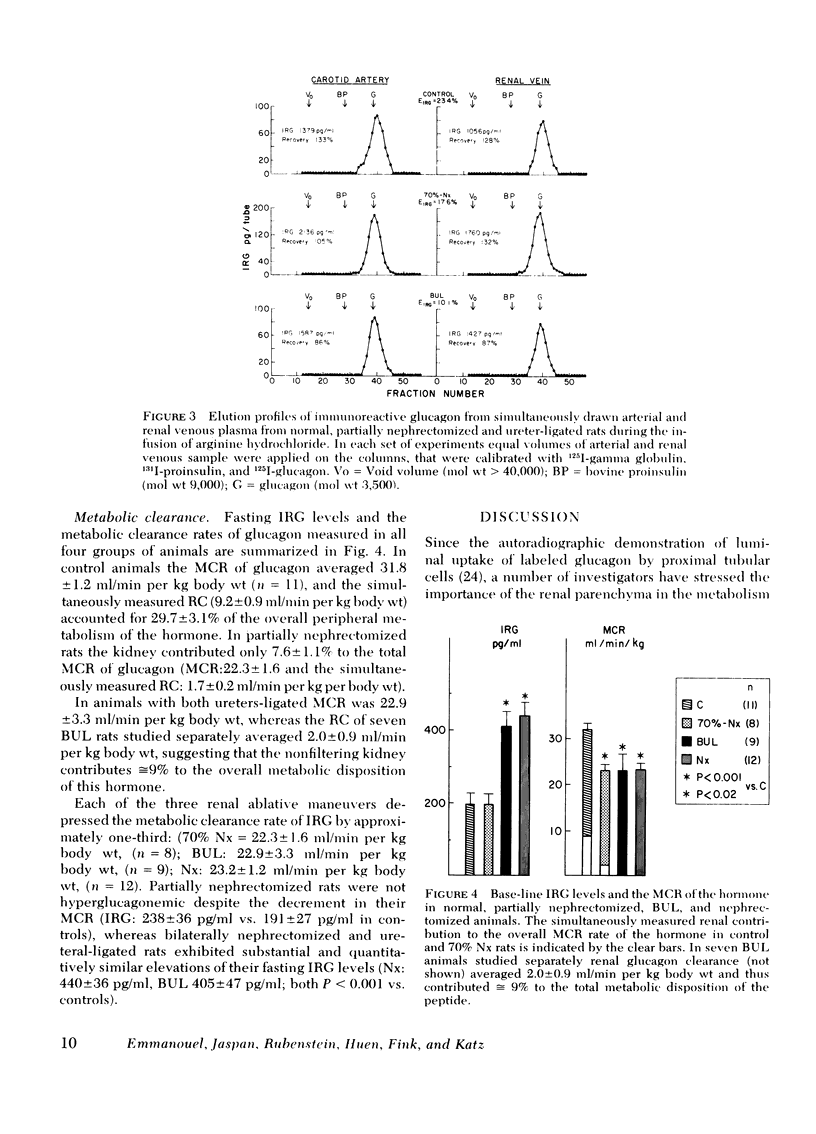
The renal handling of the biologically active glucagon component (the 3,500-mol wt fraction of immunoreactive glucagon [IRG]) and the contribution of the kidney to its overall peripheral metabolism were studied in normal and uremic rats. The metabolic clearance rate of glucagon was 31.8 ± 1.2 ml/min per kg in normal animals and was diminished by approximately one-third in each of three groups of rats with compromized renal function: 22.3±1.6 ml/min per kg in partially (70%) nephrectomized; 22.9±3.3 ml/min per kg in bilaterally ureteral ligated; and 23.2±1.2 ml/min per kg in bilaterally nephrectomized animals. In normal rats the kidney contributed 30% to the overall metabolic clearance of the hormone and the renal extraction of endogenous and exogenous glucagon was similar, averaging 22.9±1.6% and was independent of plasma IRG levels over a wide range of arterial concentrations. The remnant kidney of partially (70%) nephrectomized animals continued to extract substantial amounts (16.6±4.2%) of the hormone, but accounted for only 8% of the total peripheral catabolism of IRG. In the two groups of animals with filtering kidneys, renal glucagon uptake was linearly related to its filtered load and could be accounted for by glomerular filtration and tubular reabsorption. However, the kidneys of animals with both ureters ligated (renal extraction of inulin = 3.2±1.8%) and hence virtual absence of glomerular filtration, continued to extract 11.5±1.9% of the renal arterial glucagon, contributing by 9% to its overall metabolic clearance, indicating that IRG uptake occurs also from the post glomerular capillaries.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilbrey G. L., Faloona G. R., White M. G., Atkins C., Hull A. R., Knochel J. P. Hyperglucagonemia in uremia: reversal by renal transplantation. Ann Intern Med. 1975 Apr;82(4):525–528. doi: 10.7326/0003-4819-82-4-525. [DOI] [PubMed] [Google Scholar]
- Bilbrey G. L., Faloona G. R., White M. G., Knochel J. P. Hyperglucagonemia of renal failure. J Clin Invest. 1974 Mar;53(3):841–847. doi: 10.1172/JCI107624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M., Rivier J., Vale W. Biological activity of somatostatin and somatostatin analogs on inhibtion of arginine-induced insulin and glucagon release in the rat. Endocrinology. 1976 Feb;98(2):336–343. doi: 10.1210/endo-98-2-336. [DOI] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Chang R. L., Deen W. M., Robertson C. R., Brenner B. M. Permselectivity of the glomerular capillary wall: III. Restricted transport of polyanions. Kidney Int. 1975 Oct;8(4):212–218. doi: 10.1038/ki.1975.104. [DOI] [PubMed] [Google Scholar]
- Chideckel E. W., Palmer J., Koerker D. J., Ensinck J., Davidson M. B., Goodner C. J. Somatostatin blockade of acute and chronic stimuli of the endocrine pancreas and the consequences of this blockade on glucose homeostasis. J Clin Invest. 1975 Apr;55(4):754–762. doi: 10.1172/JCI107986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubresse J. C., Lerson G., Plamteux G., Rorive G., Luyckx A. S., Lefebvre P. J. Lipids and lipoproteins in chronic uraemia. A study of the influence of regular haemodialysis. Eur J Clin Invest. 1976 Mar 31;6(2):159–166. doi: 10.1111/j.1365-2362.1976.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Duckworth W. C. Insulin and glucagon degradation by the kidney. I. Subcellular distribution under different assay condition. Biochim Biophys Acta. 1976 Jul 21;437(2):518–530. doi: 10.1016/0304-4165(76)90020-9. [DOI] [PubMed] [Google Scholar]
- Duckworth W. C. Insulin and glucagon degradation by the kidney. II. Characterization of the mechanisms at neutral pH. Biochim Biophys Acta. 1976 Jul 21;437(2):531–542. doi: 10.1016/0304-4165(76)90021-0. [DOI] [PubMed] [Google Scholar]
- Emmanouel D. S., Jaspan J. B., Kuku S. F., Rubenstein A. H., Katz A. I., Huen A. H. Pathogenesis and characterization of hyperglucagonemia in the uremic rat. J Clin Invest. 1976 Nov;58(5):1266–1272. doi: 10.1172/JCI108581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman H. A., Singer I. Endocrinology and metabolism in uremia and dialysis: a clinical review. Medicine (Baltimore) 1975 Sep;54(5):345–376. doi: 10.1097/00005792-197509000-00001. [DOI] [PubMed] [Google Scholar]
- Fisher M., Sherwin R. S., Hendler R., Felig P. Kinetics of glucagon in man: effects of starvation. Proc Natl Acad Sci U S A. 1976 May;73(5):1735–1739. doi: 10.1073/pnas.73.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Jaspan J. B., Huen A. H., Morley C. G., Moossa A. R., Rubenstein A. H. The role of the liver in glucagon metabolism. J Clin Invest. 1977 Aug;60(2):421–428. doi: 10.1172/JCI108791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A. I., Rubenstein A. H. Metabolism of proinsulin, insulin, and C-peptide in the rat. J Clin Invest. 1973 May;52(5):1113–1121. doi: 10.1172/JCI107277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J. M., DiMeola H. J., Siegel N. J., Lytton B., Kashgarian M., Hayslett J. P. Compensatory adaptation of structure and function following progressive renal ablation. Kidney Int. 1974 Jul;6(1):10–17. doi: 10.1038/ki.1974.72. [DOI] [PubMed] [Google Scholar]
- Kuku S. F., Jaspan J. B., Emmanouel D. S., Zeidler A., Katz A. I., Rubenstein A. H. Heterogeneity of plasma glucagon. Circulating components in normal subjects and patients with chronic renal failure. J Clin Invest. 1976 Sep;58(3):742–750. doi: 10.1172/JCI108521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuku S. F., Zeidler A., Emmanouel D. S., Katz A. I., Rubenstein A. H. Heterogeneity of plasma glucagon: patterns in patients with chronic renal failure and diabetes. J Clin Endocrinol Metab. 1976 Jan;42(1):173–176. doi: 10.1210/jcem-42-1-173. [DOI] [PubMed] [Google Scholar]
- Lefebvre P. J., Luyckx A. S. Effect of acute kidney exclusion by ligation of renal arteries on peripheral plasma glucagon levels and pancreatic glucagon production in the anesthetized dog. Metabolism. 1975 Oct;24(10):1169–1176. doi: 10.1016/0026-0495(75)90153-5. [DOI] [PubMed] [Google Scholar]
- Lefebvre P. J., Luyckx A. S., Nizet A. H. Independance of glucagon and insulin handling by the isolated perfused dog kidney. Diabetologia. 1976 Aug;12(4):359–365. doi: 10.1007/BF00420980. [DOI] [PubMed] [Google Scholar]
- Lefebvre P. J., Luyckx A. S., Nizet H. Renal handling of endogenous glucagon in the dog: comparison with insulin. Metabolism. 1974 Aug;23(8):753–761. doi: 10.1016/0026-0495(74)90007-9. [DOI] [PubMed] [Google Scholar]
- Lefebvre P. J., Luyckx A. S. Plasma glucagon after kidney exclusion: experiments in somatostatin-infused and in eviscerated dogs. Metabolism. 1976 Jul;25(7):761–768. doi: 10.1016/0026-0495(76)90147-5. [DOI] [PubMed] [Google Scholar]
- Mortimer C. H., Tunbridge W. M., Carr D., Yeomans L., Lind T., Coy D. H., Bloom S. R., Kastin A., Mallinson C. N., Besser G. M. Effects of growth-hormone release-inhibiting hormone on circulating glucagon, insulin, and growth hormone in normal, diabetic, acromegalic, and hypopituitary patients. Lancet. 1974 Apr 20;1(7860):697–701. doi: 10.1016/s0140-6736(74)92903-1. [DOI] [PubMed] [Google Scholar]
- NARAHARA H. T., EVERETT N. B., SIMMONS B. S., WILLIAMS R. H. Metabolism of insulin-I 131 and glucagon-I 131 in the kidney of the rat. Am J Physiol. 1958 Feb;192(2):227–231. doi: 10.1152/ajplegacy.1958.192.2.227. [DOI] [PubMed] [Google Scholar]
- Rigopoulou D., Valverde I., Marco J., Faloona G., Unger R. H. Large glucagon immunoreactivity in extracts of pancreas. J Biol Chem. 1970 Feb 10;245(3):496–501. [PubMed] [Google Scholar]
- Sherwin R. S., Bastl C., Finkelstein F. O., Fisher M., Black H., Hendler R., Felig P. Influence of uremia and hemodialysis on the turnover and metabolic effects of glucagon. J Clin Invest. 1976 Mar;57(3):722–731. doi: 10.1172/JCI108330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAIT J. F. REVIEW: THE USE OF ISOTOPIC STEROIDS FOR THE MEASUREMENT OF PRODUCTION RATES IN VIVO. J Clin Endocrinol Metab. 1963 Dec;23:1285–1297. doi: 10.1210/jcem-23-12-1285. [DOI] [PubMed] [Google Scholar]


