INTRODUCTION
More than 70 million patients undergo surgery every year in the United States, of which 80% experience acute postoperative pain, with 20% experiencing severe pain.1,2 Inadequate pain relief has both short- and long-term consequences that may delay surgical recovery, increase length of stay, increase readmissions, and decrease patient satisfaction, as well as increase overall health care costs.3 Patient-controlled analgesia (PCA) pumps have been shown to be more effective in treating pain than intermittent intramuscular or intravenous injections,3–6 providing higher patient satisfaction,7 increased perception of situational control,8 lower preoperative anxiety, lower postoperative depressive symptoms,9 and increased control over pain relief. Other advantages include not having to receive injections,10,11 not having to wait for pain relief, and not having to summon nurses.10–12 Despite these attributes, PCA has also been associated with negative experiences, including a lack of trust in the PCA pump,10–11fear of overdose or addiction,10–12 and adverse outcomes.13–18
Although some PCA devices have included limited feedback features, there are no PCA technologies in clinical use that provide robust feedback to the patient regarding the status of the PCA pump (eg, ready to deliver medicine, pump is in lockout) or clear and definitive feedback to the patient regarding whether they are receiving or being denied medication when they push the PCA button. Given that certain attributes of current PCA technology may contribute to suboptimal PCA use (eg, mistaking the call light for the PCA button, confusion related to lockout period), which may negatively impact patient outcomes, innovative strategies that seek to optimize the effectiveness of this important pain modality should be explored. Furthermore, if attributes related to the PCA patient interface impair patient outcomes, such characteristics should be identified and improved upon in order to optimize the impact of both current PCA as well as new and emerging PCA technologies (eg, iontophoresis and liposomal drug delivery).19,20 Designing the PCA patient interface to be more patient-centric may, therefore, remedy gaps identified with quality of acute pain management.21
We hypothesized that due to poor design of the PCA patient interface, patients using PCA may not know when they are receiving pain medication and that this ambiguity negatively affects satisfaction with pain control and self-reported ability to control pain. To pursue this hypothesis, we sought to (1) identify which attributes of the PCA patient interface contribute to negative experiences for patients, and (2) evaluate patient satisfaction with pain control, difficulties using PCA, lockout-period management, and evaluation of new PCA design features.
METHODS
Institutional Review Board approval (University of Michigan, Ann Arbor, MI) was obtained for this prospective survey study. Written informed consent was obtained from each participant at the time of enrollment. Adult patients (age ≥ 18 years) who received a PCA device post-operatively for a minimum of 24 hours at the University of Michigan were included. Exclusion criteria included patients whose primary language was not English, and patients who were deaf, blind, or quadriplegic. Enrolled patients with chronic pain were excluded from the present analysis. Patients with chronic pain were defined as those with daily use of opioid analgesics and non-opioid adjunctive medications (eg, gabapentinoids, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants) for greater than 30 days before their operation based on chart review.
Subjects were enrolled in the study within 24 hours after receiving intravenous pain medication via a PCA device for at least 24 hours. In order to identify whether or not actively using the PCA pump at the time of survey influenced how patients responded to the survey questions, block computer randomization was performed so that each sequential subject was then assigned to either complete the survey while using the PCA device or within 24 hours of the PCA device being discontinued. At the time of the survey, basic demographics (age, gender, and race) were collected, and the number of days using PCA was recorded. The research assistant recruited patients every 2 to 3 days. Therefore, patients that were randomized to complete the survey while currently using PCA had no more than 3 to 4 days of PCA use, whereas patients randomized to complete the survey after the PCA was discontinued allowed for the total length of use to be recorded.
Patients completed a 17-question survey about the PCA device, their satisfaction, overall experience, ability to control pain, difficulties using PCA and options for improving the PCA device (for full survey details, see Supplemental Digital Content 1, http://links.lww.com/AAP/A78). The survey was designed to assess perceived problems with the PCA interface and specific factors that may impact patient satisfaction and ability to control pain while using PCA. In order to assess for content validity, the survey was shown to several anesthesiologists with expertise in pain management before being finalized. For the purposes of phrasing questions in the correct tense, subjects who were using the PCA pump at the time of the interview were provided the “Current PCA Survey” and patients who were no longer using the PCA device were provided the “Post PCA Survey.” Other than differences in tense, questions were the same in both surveys.
For patients randomized to complete the survey after their PCA had been discontinued, a PCA pump was made available during the survey by the research assistant to demonstrate, as needed, the various PCA pump features and characteristics questioned in the survey. There was no formal demonstration of the device in either group, and the patients currently using the device were provided demonstrations in the same way, as needed. In order to clarify and understand the subject’s perspective, each closed-ended question requesting a “Yes” or “No” answer or a “0–10” Likert scale response was followed by an open-ended question.
Open-ended responses were analyzed by 2 of the authors (LSP and SD) for themes by first reading through each response and then creating response categories, labeling each comment with 1 or more categories, clarifying the content of the response and then identifying patterns and trends. The 2 authors identified similar categorical themes. Through a second review of patient quotes, both authors were easily able to agree on both the categorical theme and the organization of quotes without discrepancies.
Statistical Analysis
Data were analyzed using Statistical software IBM SPSS statistics version 19 (IBM Corp, Somers, New York). Before analysis, age was assessed for normality using the Kolmogorov-Smirnov statistic. The assumption of normality was deemed violated if the P value was < 0.05, and therefore nonparametric measures (eg, Mann-Whitney U test, Kruskal-Wallis test) were used. Per normal reporting standards, nonparametric data are presented as median and interquartile range (25th, 75th Interquartile Range) and parametric data are reported as mean ± standard deviation. Categorical data were analyzed using a 2-tailed Pearson chi-square test. A P value of < 0.05 was considered statistically significant.
Power Analysis
Sample size determination was based on standard survey methodology to provide a representative sample of the target population. The number of subjects needed to enroll in the study was determined based on an estimated target population of 1250 patients per year who met PCA inclusion criteria at our institution. Based on this assumption we determined that 475 patients would need to participate in order to provide a representative sample with a confidence level of 95% and a confidence interval (CI) of ± 4%. Furthermore, this sample size more than satisfies the rule of thumb of 10 participants per item in a questionnaire.22
RESULTS
A total of 512 patients were approached to participate in the study and, of these, 33 (6.4%) refused to participate, 129 (25.2%) had chronic pain and 350 (68.4%) met inclusion criteria and completed the survey. Subjects’ ages ranged from 18 to 93 years old with a mean age of 53 years. There were no significant differences between participants (n = 350) and patients who refused to participate (n = 33), by sex (participants 49.1% female and non-participants 63.6% female, P = 0.11, or race (participants non-Caucasian 13.4% and non-participants non-Caucasian 18.8%, P = 0.405). There was a significant difference in age (participants median age 55 [IQR 42, 64] and non-participants median age 47 years [40, 57], P = 0.035).
Forty-nine percent (n = 172) were female. The duration of PCA use for patients surveyed after the PCA was discontinued ranged from 1 to 12 days, with a median of 2 days. Self-reported satisfaction of pain control (Question 1) was not associated with age (P = 0.459) or race (P = 0.628), but there was an association with gender with male patients reporting lower satisfaction (male median 9 [7, 10] vs female 9 [8, 10]; P = 0.02). Self-reported ability to control pain (Question 5) was significantly lower in male patients (male median 8 [7, 10] vs female 9 [8, 10]; P = 0.001) and in Caucasian patients when compared to non-Caucasian patients (9 [7, 10] vs 10 [8, 10], respectively; P = 0.03). There was no association between age and the ability to control pain.
Responses to the individual survey questions were analyzed between the survey groups, (“Current PCA Use” [n = 182] and “Post PCA Use” [n = 168]) and no significant differences were associated with the time of survey administration. Furthermore, no significant differences were noted for age, , or race between the 2 time point administrations (data not shown). As such, all responses were grouped for subsequent analyses.
Factors Affecting PCA Use
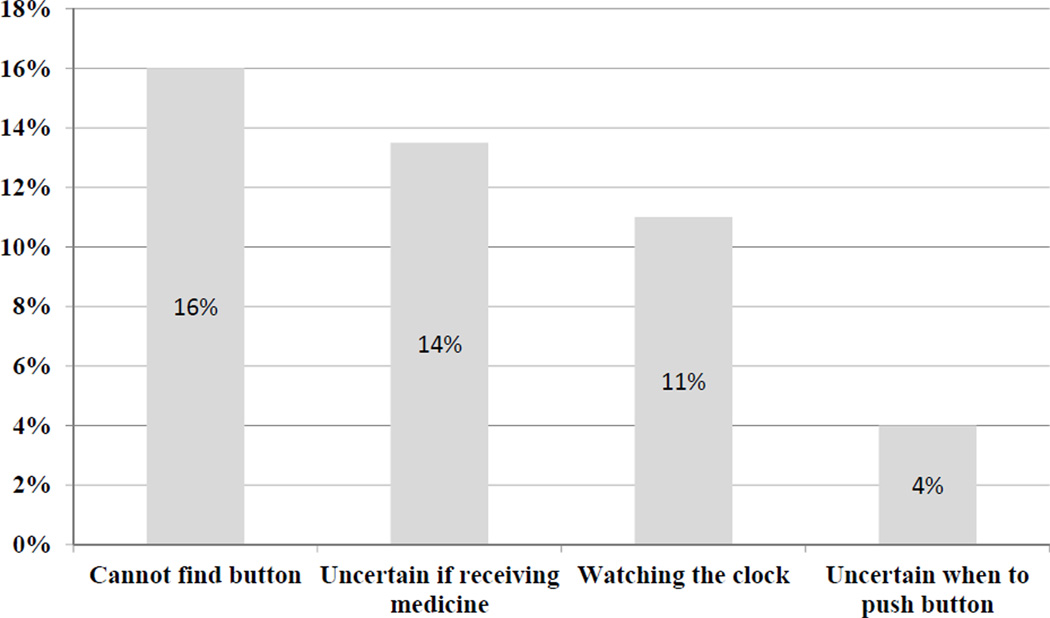
While the process of pushing a button in order to receive pain medicine is generally understood by most patients (n = 344, 98%), 92 patients (26%) still reported that they found the PCA difficult to use. From a list of possible reasons why the PCA was difficult to use (Figure 1), 56 patients (16%) reported times when they could not find the button to request a bolus, 48 (14%) reported they were uncertain if medicine was being delivered through the pump, 37 (11%) reported that having to watch the clock made it difficult, and 13 (4%) reported not understanding when they should push the button. With respect to the lockout period, 171 patients (49%) reported they did not know if they would receive medicine when they pushed the PCA button, of which 37 (22%) reported they believed that this uncertainty made their pain worse.
Figure 1.
Most common reported difficulties reported by patients while using PCA.
Factors Affecting Satisfaction and Ability to Control Pain
Out of the 350 patients surveyed, patient preferences were identified that negatively impacted patient satisfaction with pain control and ability to control pain (Questions 1 and 5, respectively, Supplemental Digital Content 1, http://links.lww.com/AAP/A78) using PCA (Tables 1 & 2). Significantly lower satisfaction scores (Question 1) were observed among patients that were unable to adequately control their pain (Question 2) and reported difficulty using PCA (Question 5; Data displayed in Table 1). There were no significant differences for questions regarding adequacy of PCA education, opinion regarding utility of a lighted button or cable, or the use of a button that would light up or vibrate to alert the patient when the PCA pump was allowed to deliver more medicine. Similar findings were noted when patients’ self-reported ability to control their pain (Question 5) was compared with the same Yes/No responses analyzed with the satisfaction question. With a trend toward significance (P = 0.06), however; patients who reported the inability to control their pain (Question 5) also preferred the PCA button to vibrate to alert them that the PCA pump was ready to deliver more medicine (Question 12; Data displayed in Table 2)
Table 1.
Patient Preferences Impacting Patient Satisfaction with PCA. The overall 335 satisfaction scores with the PCA device (Q1) were compared between groups responding 336 positively and negatively regarding specific Yes/No questions about their understanding, 337 use, and proposed innovations of the PCA device.
| Patient Preferences | YES Median Satisfaction Score [25th, 75th IQR] (n) |
NO Median Satisfaction Score [25th, 75th IQR] (n) |
p value |
|---|---|---|---|
| 1. Adequately able to control pain (Q2) | 9 [8,10] (293) | 5 [3,7] (57) | p < 0.001 |
| 2. Experienced difficulties using PCA (Q6) | 8 [5,10] (73) | 9 [8,10] (277) | p < 0.001 |
| 3. Provided adequate information about PCA (Q7) | 9 [7,10] (329) | 8 [7,10] (21) | p = 0.468 |
| 4. Finding the PCA pump difficult to use (Q9) | 8 [7,10] (92) | 9 [7,10] (258) | p = 0.133 |
| 5. A light on the button would have made it easier to find (Q10) | 9 [7,10] (199) | 9 [7,10] (151) | p = 0.983 |
| 6. A lit up cable would have made it easier to find (Q11) | 9 [7,10] (139) | 9 [7,10] (211) | p = 0.964 |
| 7. A vibrating button notifying when the PCA was ready to give more medicine (LockOut Period Over) would have made it easier to use (Q12) | 8 [7,10] (191) | 9 [8,10] (158) | p = 0.103 |
| 8. A light on the button that turned on notifying when the PCA was ready to give more medicine (LockOut Period Over) would have made it easier to use (Q13) | 9 [7,10] (246) | 9 [7,10] (104) | p = 0.135 |
| 9. Knowing when the PCA pump was ready so that medicine would be delivered if the button were pushed (LockOut Period Over) (Q14) | 8 [7,10] (179) | 9 [7,10] (171) | p = 0.123 |
Question number (Q) noted corresponds to patient survey. See Supplemental Digital Content 1, http://links.lww.com/AAP/A78, for further details. N = number of patients. α = 0.05.
Table 2.
Patient Preferences Impacting Patient Ability to Control Pain Using PCA. The overall ability to control pain scores with the PCA device (Q5) were compared between groups responding positively and negatively regarding specific Yes/No questions about their understanding, use, and proposed innovations of the PCA device.
| Patient Preferences | YES Median Ability to Control Score [25th, 75th IQR] (n) |
NO Median Ability to Control Score (25th, 75th IQR) (n) |
p value |
|---|---|---|---|
| 1. Satisfied with ability to control pain (Q1) | 9 [8,10] (293) | 5 [3,7] (57) | p < 0.001 |
| 2. Experienced difficulties using PCA (Q6) | 8 [5,10] (73) | 9 [8,10] (277) | p < 0.002 |
| 3. Provided adequate information about PCA (Q7) | 9 [7,10] (329) | 9 [5,10] (21) | p = 0.501 |
| 4. Finding the PCA pump difficult to use (Q9) | 8 [6,10] (92) | 9 [7,10] (258) | p = 0.259 |
| 5. A light on the button would have made it easier to find (Q10) | 9 [8,10] (199) | 9 [7,10] (151) | p = 0.360 |
| 6. A lit up cable would have made it easier to find (Q11) | 9 [8,10] (139) | 9 [7,10] (211) | p = 0.868 |
| 7. A vibrating button notifying when the PCA was ready to give more medicine (Lockout Period Over) would have made it easier to use (Q12) | 9 [7,10] (191) | 9 [8,10] (158) | p = 0.060 |
| 8. A light on the button that turned on notifying when the PCA was ready to give more medicine (Lockout Period Over) would have made it easier to use (Q13) | 9 [7,10] (246) | 10 [7,10] (104) | p = 0.640 |
| 9. Knowing when the PCA pump was ready so that medicine would be delivered if the button were pushed (Lockout Period Over) (Q14) | 9 [7,10] (179) | 9 [8,10] (171) | p = 0.116 |
Question number (Q) noted corresponds to patient survey. See Supplemental Digital Content 1, http://links.lww.com/AAP/A78, for further details. N = number of patients. α = 0.05.
Patient Impressions of Proposed PCA Innovations
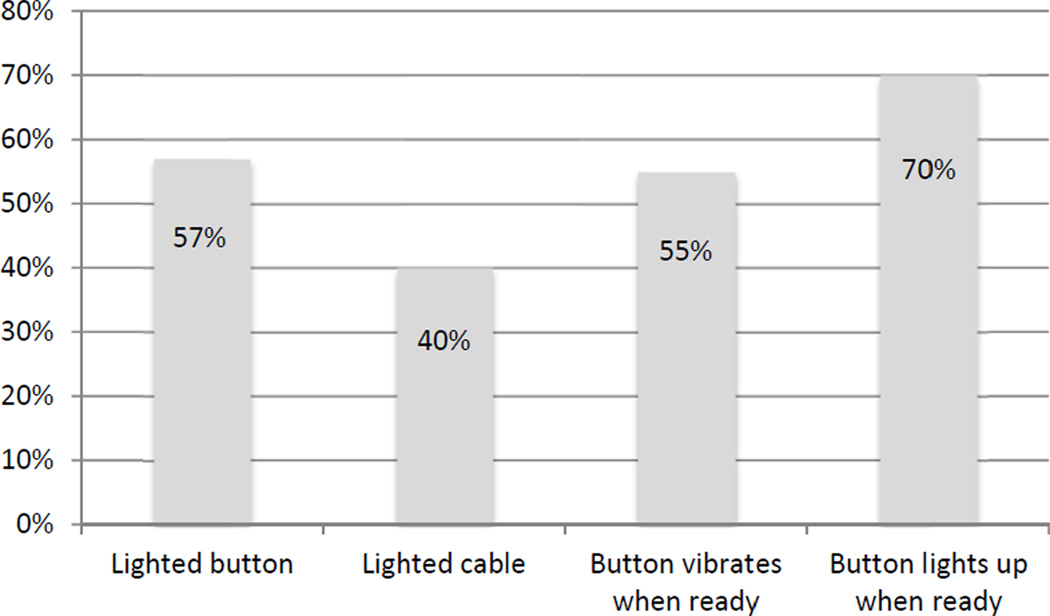
From a list of possible solutions presented to patients that would make it be easier to use PCA and manage the lockout period (Figure 2), 199 patients (57%) reported that it would be easier if the PCA button had a light that made it easier to find, 139 (40%) reported that it would be easier if the cable were lit, 191 (55%) reported that it would easier if the PCA button would vibrate when the pump was available to deliver more medicine, and 246 (70%) reported that it would be easier if the PCA button lit up when the pump was available to deliver more medicine.
Figure 2.
Patient responses to proposed solutions for improving ease of use and improving how patients manage the lockout period.
Open-ended responses to questions were reviewed for overall themes and reported in Table 3. Open-ended questions addressed the patient’s perceptions of difficulties with PCA, patient education with PCA, possible solutions to make PCA easier to use, management of the lockout period, and how PCA could be improved.
Table 3.
Summary of patient responses to open-ended questions.
| Open-ended Question | Themes extracted from patient responses |
|---|---|
| A. Difficulties with PCA |
|
| B. PCA patient education and information |
|
| C. Lit up button help locate the button |
|
| D. Lit up cable to help locate the button |
|
| E. Vibrating button to know when lock-out period is over |
|
| F. Button lighting up to know when lock-out period is over |
|
| G. Knowing when pump was ready to deliver more medicine |
|
| H. Best signal for alert when the lock-out period is over |
|
| I. Anything else that would help improve PCA |
|
DISCUSSION
To our knowledge, the present study represents one of the largest patient surveys of PCA and provides a unique, in-depth perspective of patient characteristics and preferences with PCA, the PCA patient interface, and how these factors affect both patient ability to control pain and patient satisfaction using PCA. Despite having a general understanding how to push the PCA button, some still experienced difficulties using PCA, due, in part, to deficiencies in the patient-interface design. Aligned with previous studies,23,24 patient satisfaction and ability to control pain were not affected by preoperative teaching or adequate information being provided to the patient on how to use PCA. The current patient interface is, however, still lacking what appears to be a simple feedback mechanism that would inform the patient about the lockout status of the pump. Of the possible patient interface design changes proposed, the one feature that may improve patients’ ability to control pain is having the button vibrate to notify the patient that the lockout period has ended, alerting the patient that the pump will deliver medicine when the button is pushed (Table 2). Indeed, despite a higher than expected satisfaction rate, the majority of patients preferred the device modifications proposed in the survey (Figure 2).
Impact of human factors on the PCA patient interface and patient’s perceptions of pain control and satisfaction using PCA
Prior research correlating both patient satisfaction with lower pain intensity and perceived control with good pain relief,8,9 suggests that efforts to improve aspects of PCA that increase patient satisfaction and control are worthwhile. Inadequate pain control for patients enduring acute or chronic pain may be due, in part, to poor engineering of the PCA patient interface and its communication with the patient. Although the PCA device allows patients to push a button when medication is desired, there remains some confusion about how the PCA works, which can be problematic. For example, because patients may not know when the pump is locked out, they may request medication that is unavailable at the time, , thus, do not know whether they are receiving pain medicine. In the open-ended response portion of the survey, patients commented on this ambiguity with the following comments: “anxious on whether or not to hit the button,” “left in the dark,” “making the pain worse,” and “always in pain because I never knew if I was actually getting any medicine.” The ambiguity around pain medication delivery and the denied attempts during the lockout period may contribute to patients’ perceptions that the PCA is an unreliable pain treatment modality.
More than 70% of patients preferred at least 1 of the 4 proposed changes to improve the PCA patient interface, the most popular being a “button that lights up as a notification that medicine is ready to be delivered,” and the most significant for improving patients’ ability to control pain being a “button that vibrates as a notification that medicine is ready to be delivered” (Figure 2). Many patients suggested having the option of both, with the light notification for nighttime and vibration notification during the day. In open-ended responses, patients reported that these 2 added features, by providing more information about the PCA pump status with respect to the lock out period, would (1) improve patient’s understanding about the lockout period, (2) improve how patients manage the lockout period, (3) improve confidence that the PCA pump was functioning properly, and () would help the patient find the button at night when the room was dark. Although PCA notification of pain medication availability might lead to increased usage and thereby increased adverse events and complications, this has not been studied, and the lockout function as well as the patient being awake enough to push the PCA button may be sufficient to prevent over-usage.
Proposing a new PCA patient interface
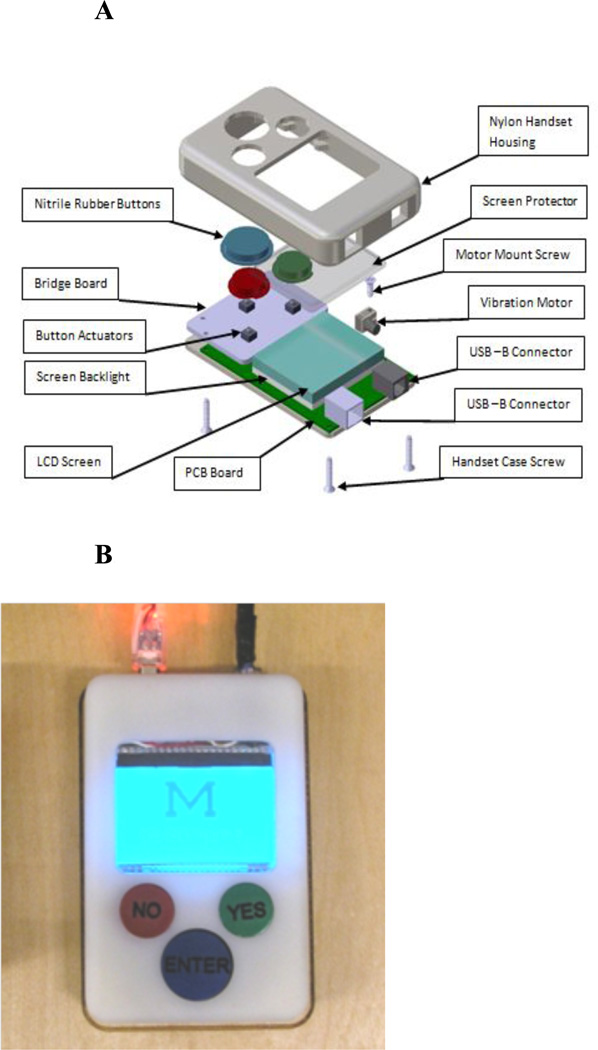
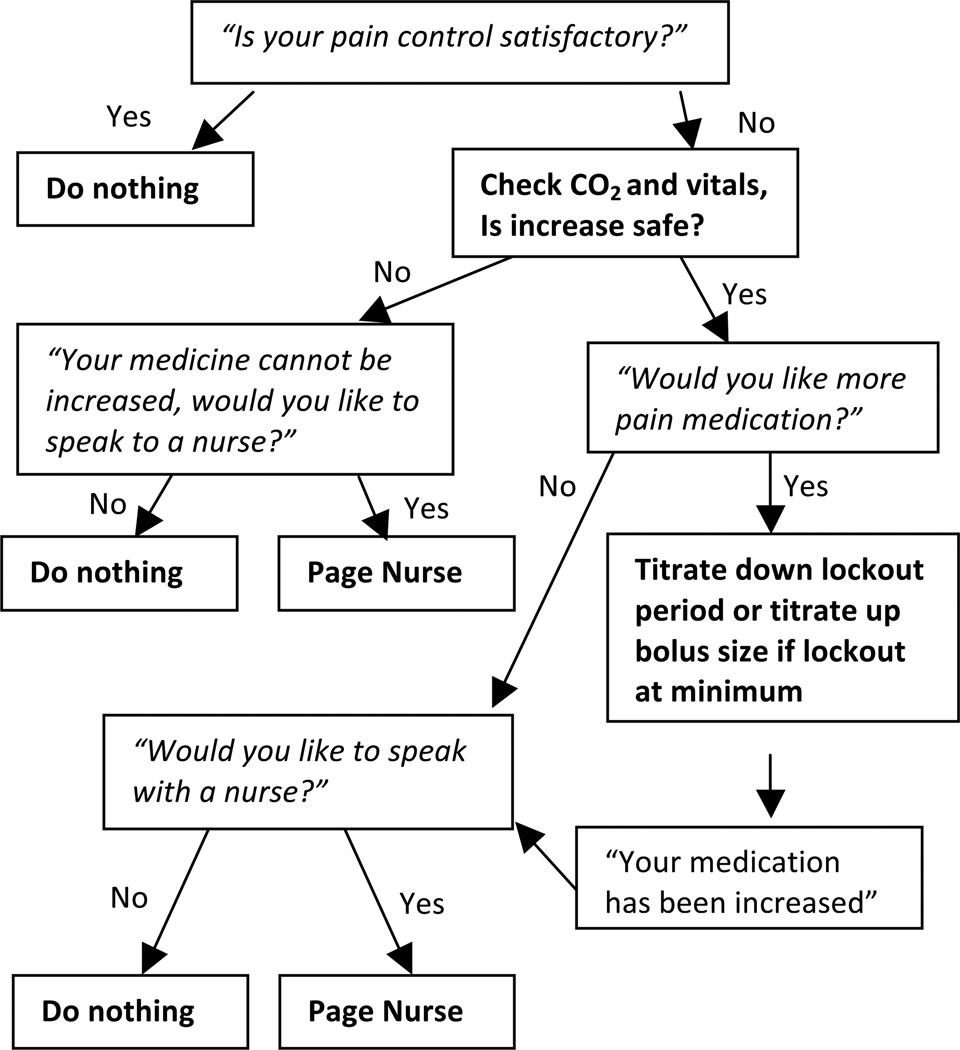
An overarching goal of this study was to identify features of the current PCA patient interface that negatively affect patient satisfaction and the ability to control pain with a goal of developing strategies to improve the usability and functionality of PCA. The University of Michigan used patient feedback from this study along with innovative strategies proposed by authors within the research team and guided a team of engineers through the design for a new PCA patient interface. The University of Michigan prototype handset engineered by a team of 4 engineering students (Figures 3A & 3B) incorporates an LCD screen to effectively communicate pump status (eg, in lockout, medication delivery readiness, real-time feedback of medication delivery) and offers pain score and satisfaction assessment questions to the patient, which enable auto-titration of pain medication along with PCA use data, patient reported data and strategic alerts that can be routed to provider portable devices. Improving the patient interface alone will not resolve the problems of under-dosing; however, this concern must be balanced against potential risks of increasing opioids. The pain assessment decision tree (Figure 4) allows for auto-titration based on binary satisfaction with pain control, which may include assessment of experienced side effects and the desire for more or less pain medication, rather than simply using pain scales as the basis for titration.25
Figure 3.
A. Exploded view of prototype PCA patient handset.
B. PCA prototype handset with liquid crystal display display (LCD) screen.
Figure 4.
Pain assessment decision tree used to solicit patient feedback and guide auto-titration of PCA dosing. This proposed algorithm uses patient feedback in conjunction with patient physiologic feedback (e.g. oxygen saturation, carbon dioxide level) and PCA use data. The basic assumption is that PCA use patterns may indicate the need for pain assessments and/or PCA dosing adjustments and so prompts can be generated to engage the patient and verify if indeed an assessment is warranted. If nurses are notified during such times, a more patient-centric approach to monitoring patients using PCA can be achieved. Although assessments are routinely performed on patients using PCA, there may be more appropriate times to assess the patient that fall outside of scheduled routine visits. At the same time, while PCA orders often include PCA titration, it may be better to build these allowable titrations into the PCA algorithm which can be incorporated into a more advanced patient interface. Rather than using pain scores to assess the need for more or less pain medication (e.g., increase or decrease bolus doses), this algorithm assumes the patient knows whether or not they need more pain medication and simply asks the binary question, “Would you like more pain medicine?” Consequently, if it is safe to do so and it is within the prescribed order set and programmed into the PCA, the PCA would titrate accordingly, otherwise the system would page the nurse and systematically establish a patient-nurse encounter that would be timelier than a routine assessment scheduled by the provider.
Limitations
Two limitations in this study were not including pain scores or PCA medication usage within the data collection and data analysis. Since the objective was to determine if patients knew when they were receiving pain medication and if any ambiguity related to medication delivery negatively impacted their satisfaction with pain control and self-reported ability to control pain, individual patient’s pain score and PCA medication usage was not collected. Although, pain scores could have provided additional information regarding patient satisfaction; given that prior research has already shown lower pain scores correlate with increased satisfaction,8,9 the present study was designed to assess the patient’s satisfaction with their experience of pain and ability to control pain. Furthermore, while a practitioner may strive to treat pain scores that scale up and down, what any practitioner is really addressing is the binary question, “Is the patient satisfied with their pain?” or “Does the patient need more pain medication?” Any pain score, while useful in documenting trends and monitoring immediate response to pain treatments, is usually followed by qualifying questions that seek to determine if the patient needs more or less pain medication. In addition to the above limitations, despite creating the survey with the assistance of pain physicians and researchers with experience in survey research (ART and CMB), the survey was not validated, which may impact the results. Whereas the removal of patients with chronic pain that completed the survey leaves a study population that is smaller than that indicated by the a priori power analysis, the included cohort was still sufficiently large to be representative of the institution’s target population. In addition, the general rule of thumb of at least 10 patients per question in survey research was still met (17 questions, 350 patients included).
Conclusions
Based on surveys from 350 patients in a tertiary care medical center, we conclude that the ambiguity of receiving pain medication when pain medication is requested leads to difficulties using PCA and may negatively impact patient satisfaction and patients’ perceived ability to control pain. Future study is needed to determine whether modifications in the patient interface can improve outcomes and satisfaction and the impact such features might have on patients with chronic pain who may have more anxiety about their ability to control pain during periods of acute post-operative pain. Future directions for product development and research can investigate the feasibility of safely allowing patients to increase their opioid dosing in the setting of uncontrolled pain, with appropriate positive- and negative-feedback loops in place along with using a more informative patient-centric PCA patient interface.
Supplementary Material
Acknowledgements
For the assistance in the development and production of the prototype, we thank the engineering team of Kevin Connolly, Joseph Dear, James Powers, Bryan Skulsky and Dr. Kathleen Sienko (School of Engineering, University of Michigan, Ann Arbor, MI)
Funding:
Department of Anesthesiology, University of Michigan
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentations:
America Society of Regional Anesthesia and Pain Medicine 36th Annual Regional Anesthesia Spring Meeting and Workshops, Las Vegas, NV, May 5–8, 2011
Disclosure:
Drs. Lance Patak, Chad Brummett, and Sunavo Dasgupta are co-authors of a pending patent and have assigned rights to this patent application to the University of Michigan Technology Transfer Office. The patent application details specifications for modifying a PCA device.
References
- 1.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: Results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–540. doi: 10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- 2.Warfield CA, Kahn CH. Acute pain management programs in U.S. hospitals and experiences and attitudes among U.S. adults. Anesthesiology. 1995;83:1090–1094. doi: 10.1097/00000542-199511000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Dolin SJ, Cashman JN, Bland JM. Effectiveness of acute postoperative pain management: I. evidence from published data. Br J Anaesth. 2002;89:409–423. [PubMed] [Google Scholar]
- 4.Boldt J, Thaler E, Lehmann A, Papsdorf M, Isgro F. Pain management in cardiac surgery patients: Comparison between standard therapy and patient-controlled analgesia regimen. J Cardiothorac Vasc Anesth. 1998;12:654–658. doi: 10.1016/s1053-0770(98)90237-3. [DOI] [PubMed] [Google Scholar]
- 5.Gust R, Pecher S, Gust A, Hoffmann V, Bohrer H, Martin E. Effect of patient-controlled analgesia on pulmonary complications after coronary artery bypass grafting. Crit Care Med. 1999;27:2218–2223. doi: 10.1097/00003246-199910000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Thomas V, Heath M, Rose D, Flory P. Psychological characteristics and the effectiveness of patient-controlled analgesia. Br J Anaesth. 1995;74:271–276. doi: 10.1093/bja/74.3.271. [DOI] [PubMed] [Google Scholar]
- 7.Ballantyne JC, Carr DB, Chalmers TC, Dear KB, Angelillo IF, Mosteller F. Postoperative patient-controlled analgesia: Meta-analyses of initial randomized control trials. J Clin Anesth. 1993;5:182–193. doi: 10.1016/0952-8180(93)90013-5. [DOI] [PubMed] [Google Scholar]
- 8.Pellino TA, Ward SE. Perceived control mediates the relationship between pain severity and patient satisfaction. J Pain Symptom Manage. 1998;15:110–116. [PubMed] [Google Scholar]
- 9.Jamison RN, Taft K, O'Hara JP, Ferrante FM. Psychosocial and pharmacologic predictors of satisfaction with intravenous patient-controlled analgesia. Anesth Analg. 1993;77:121–125. [PubMed] [Google Scholar]
- 10.Chumbley GM, Hall GM, Salmon P. Patient-controlled analgesia: An assessment by 200 patients. Anaesthesia. 1998;53:216–221. doi: 10.1046/j.1365-2044.1998.00314.x. [DOI] [PubMed] [Google Scholar]
- 11.Kluger MT, Owen H. Patients’ expectations of patient-controlled analgesia. Anesthesia. 1990;45:1072–1074. doi: 10.1111/j.1365-2044.1990.tb14893.x. [DOI] [PubMed] [Google Scholar]
- 12.Taylor NM, Hall GM, Salmon P. Patients’ experiences of patient-controlled analgesia. Anesthesia. 1996;51:525–528. doi: 10.1111/j.1365-2044.1996.tb12556.x. [DOI] [PubMed] [Google Scholar]
- 13.Ashburn MA, Love G, Pace NL. Respiratory-related critical events with intravenous patient-controlled analgesia. Clin J Pain. 1994;10:52–56. doi: 10.1097/00002508-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Fleming BM, Coombs DW. A survey of complications documented in a quality-control analysis of patient-controlled analgesia in the postoperative patient. J Pain Symptom Manage. 1992;7:463–469. doi: 10.1016/0885-3924(92)90132-2. [DOI] [PubMed] [Google Scholar]
- 15.Schug SA, Torrie JJ. Safety assessment of postoperative pain management by an acute pain service. Pain. 1993;55(3):387–391. doi: 10.1016/0304-3959(93)90016-I. [DOI] [PubMed] [Google Scholar]
- 16.Sidebotham D, Dijkhuizen MR, Schug SA. The safety and utilization of patient-controlled analgesia. J Pain Symptom Manage. 1997;14:202–209. doi: 10.1016/s0885-3924(97)00182-6. [DOI] [PubMed] [Google Scholar]
- 17.Wheatley RG, Madej TH, Jackson IJ, Hunter D. The first year’s experience of an acute pain service. Br J Anesth. 1991;67:353–359. doi: 10.1093/bja/67.3.353. [DOI] [PubMed] [Google Scholar]
- 18.Simes D, Power L, Priestley G. Respiratory arrest with patient-controlled analgesia. Anaesth Intensive Care. 1995;23:119–120. [PubMed] [Google Scholar]
- 19.Viscusi ER. Emerging treatment modalities: Balancing efficacy and safety. Am J Health Syst Pharm. 2007;64(6 Suppl 4):S6–S11. doi: 10.2146/ajhp060680. [DOI] [PubMed] [Google Scholar]
- 20.Viscusi ER. Patient-controlled drug delivery for acute postoperative pain management: A review of current and emerging technologies. Reg Anesth Pain Med. 2008;33:146–158. doi: 10.1016/j.rapm.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Carr DB, Reines HD, Schaffer J, Polomano RCm, Lande S. The impact of technology on the analgesic gap and quality of acute pain management. Reg Anesth Pain Med. 2005;30:286–291. doi: 10.1016/j.rapm.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Arrindell W, van der Ende J. An empiricial test of the utility of the observations-to-variables ratio in factor and component analysis. Appl Psychol Measure. 1985;9:165–178. [Google Scholar]
- 23.Chumbley GM, Hall GM, Salmon P. Patient-controlled analgesia: What information does the patient want? J Adv Nurs. 2002;39:459–471. doi: 10.1046/j.1365-2648.2002.02311.x. [DOI] [PubMed] [Google Scholar]
- 24.Chumbley GM, Ward L, Hall GM, Salmon P. Pre-operative information and patient-controlled analgesia: Much ado about nothing. Anaesthesia. 2004;59:354–358. doi: 10.1111/j.1365-2044.2004.03661.x. [DOI] [PubMed] [Google Scholar]
- 25.Gordon DB, Rees SM, McCausland MP, et al. Improving reassessment and documentation of pain management. Jt Comm J Qual Patient Saf. 2008;34:509–517. doi: 10.1016/s1553-7250(08)34065-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.