Abstract
CD40L (CD154) expressed on activated CD4+ T cells has been shown to provide CD40+ dendritic cells (DCs), a critical signal for establishing CD8+ T-cell immunity. CD40L–CD40 interaction leads to DC maturation with IL-12 production and upregulation of various costimulatory molecules. In this study, we show that CD40 engagement provides a unique maturation signal for human monocyte-derived DCs to upregulate IL-7 production. Other inducers of DC maturation, such as TLR 4 and TLR 7/8 agonist, fail to induce IL-7 upregulation. Neutralization of IL-7 activity in human CD8+ T-cell cultures stimulated with CMV pp65-NLV peptide-pulsed mature DCs (mDCs) leads to a reduction in antigen-specific CD8+ T-cell yields suggesting a role for mDC-derived IL-7 during T-cell receptor (TCR) activation. Furthermore, IL-7 signaling requires a temporal coordination with TCR activation for maximal antigen-specific T-cell yields. These results show that CD40 signals regulate DC-derived IL-7 production that, in turn, may instruct CD8+ T cells at the time of TCR engagement for survival leading to an increased expansion of antigen-specific T cells.
Keywords: cytokine, dendritic cells, human, T cells, viral
Dendritic cells (DCs) are widely recognized as the most potent antigen-presenting cells, and development of adaptive immunity is determined, in part, by the maturational status of resident DC.1 The current paradigm holds that immature DCs (iDCs) capture and process antigen but are primarily non-stimulatory and, in certain situations, tolerogenic. In contrast, mature DC (mDC) migrates to T-cell rich areas within secondary lymphoid organs, efficiently present processed antigen to activate naive T cells and promote clonal expansion.2 A multitude of innate stimuli, in the form of pathogen products, can trigger DC maturation endowing these cells with the capacity to initiate adaptive immune responses.2 Furthermore, innate stimuli not only trigger DC maturation but also control and determine T-cell effector fate.3 In addition, CD40-delivered signals are required for DC to elicit optimal primary and recall T-cell immunity.4,5
In most instances, generation of CD8+ αβT-cell receptor (TCR) immunity requires the participation of CD4+ T cells, and more specifically, the interaction of activated CD4+ CD40L+ T cells with CD40+ DC.6–9 Under non-inflammatory conditions, CD40 signaling serves to license DC for priming naive or resting memory CD8+ T cells resulting in a rapid T-cell expansion. Experiments with genetically defined mice support the notion that CD40L–CD40 interactions are unique and non-redundant, as effective CD8 immunity does not develop in hosts deficient in either CD40 or CD40L10–12 Memory CD8+ T cell numbers are reduced approximately 50% in mice immunized with CD40-deficient DC suggesting an important role for CD40 signaling on APC during the development of CD8+ T-cell immunity.5,13 Moreover, soluble antigen and CD40 ligation are sufficient to induce robust primary and memory CD8+ T-cell immunity14
The exact mechanism by which CD40 engagement results in DC licensing for CD8 priming is not understood. CD40L-CD40 signaling, in the presence of IFN-γ, is a potent stimulus for IL-12 secretion by DC and IL-12 is required for the full acquisition of CD8 effector function.15–17 However, selected TLR agonists, primarily TLR3, 4 and 7/8, can also promote IL-12 production yet cannot fully substitute for CD40-CD40L interactions in the development of CD8+ T-cell memory4,9,18 Thus, CD40 engagement on DC results in additional signals that lead to effective cytotoxic T lymphocyte (CTL) generation. To begin to elucidate CD40 signals, we have examined cytokines produced upon maturation of human monocyte-derived DC by CD40L/ IFN-γ. Using the HLA-A*0201 -restricted CMV pp65 NLV peptide as antigen,19 and a cohort of healthy CMV-seropositive individuals, we investigate the consequences of CD40L/IFN-y-produced cytokines on CMV-specific CD8+ T-cell in vitro response.
RESULTS
Human monocyte-derived DC constitutively produces IL-7 and its production is upregulated by CD40 engagement
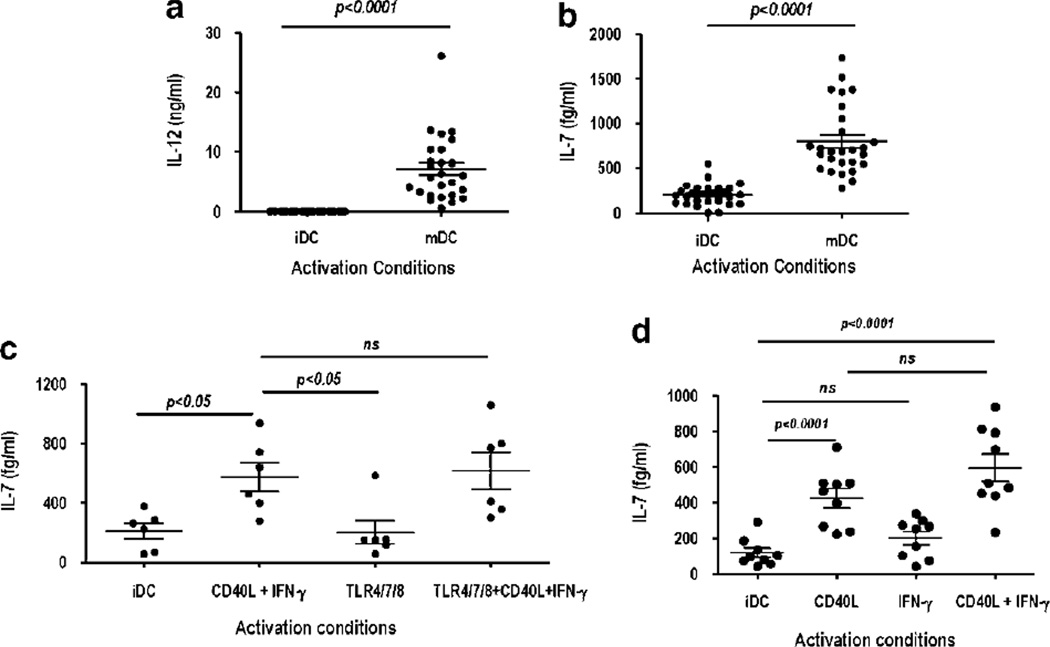
CD40L was used as a maturation signal in the presence of IFN-γ to evaluate the production of cytokines by human monocyte-derived DC. CD40L/IFN-γ-activated DC (referred throughout the paper as mDC) showed a mature phenotype with upregulation of major histocompatibility complex class II, CD83, CD86 and IL-15Rα expression (data not shown) compared with DC cultured in granulocyte macrophage colony-stimulating factor (GM-CSF)/IL-4 alone (referred as iDC). Cytokine multiplex analysis of cell culture supernatants obtained from iDC and mDC was performed as a screening assay to assess the differential expression of various cytokines. CD40L/IFN-γ-activated mDC de novo produced IL-12 and IL-23 as well as upregulated IL-lα, IL-6, IL-7 and TNF-α production (data not shown). To further evaluate IL-7 production, iDC and mDC were generated from healthy donors and culture supernatants, obtained 48 h after activation, were tested using a commercial high-sensitivity enzyme-linked immunosorbent assay (10 pg –156 fg ml−1) kit. As shown in Figure la, CD40L/IFN-γ activation induced IL-12 production in all donors (mean=7.1 ± 1 ng ml−1); in contrast, no IL-12 was detected in iDC supernatants (assay sensitivity, 15 pg ml−1). IL-7 could be detected in the supernatants of iDC with a mean value of 199.3±22 fg ml−1 (Figure lb). CD40L/IFN-γ activation leads to a ~four-fold increase with mean values of 800 ± 74 fg ml−1 (Figure lb). Overall, IL-7 levels range from undetectable (assay sensitivity, 156 fg ml−1) to 545 fg ml−1 in iDC cultures and 272 to 1735 fg ml−1 in mDC cultures. In all donors (n—27), an increase in IL-7 production was observed after CD40L/IFN-γ activation of DC. Upregulation of IL-7 in CD40L/IFN-γ-stimulated DC was confirmed by intracellular staining with anti-IL-7 antibody (Ab) (data not shown).
Figure 1.
IL-7 is constitutively produced by dendritic cell (DC) and uniquely upregulated upon CD40L/IFN-γ activation. DCs were generated and activated as described in ‘Dendritic cell generation and activation.’ Supernatants harvested from immature DC (iDC) and mature DC (mDC) 48h after activation were assayed by enzyme-linked immunosorbent assay (ELISA) for production of (a) IL-12 and (b) IL-7. P-values were obtained with the Student’s t-test; P-values <0.05 are significant (n=27). Horizontal bars represent mean values ± s.e.m. Activation of DC with CD40L-IFN-γ and/or TLR 4/7/8 agonists, (c) Supernatants harvested from iDC and CD40L-IFN-γ, TLR4 (ultrapure LPS, 500 ng ml−1) and/or TLR 7/8 (3M-007, 5 µg ml−1)-activated DC (48h) were assayed by ELISA for production of IL-7. (d) Supernatants harvested from iDC, CD40L, IFN-γ and CD40L-IFN-γ-activated DC (48h) were assayed by ELISA for production of IL-7. P-values were obtained with the repeated measure analysis of variance (ANOVA) followed by Tukey’s analysis; P-values <0.05 are significant (n=6). Horizontal bars represent mean values ± s.e.m.
Human monocyte-derived DC expressed a selected repertoire of TLRs (primarily TLR 2,3,4 and 8) and their ligation by viral/bacterial products can directly activate DC and induce maturation resulting in cytokine synthesis.20,21 Synergy between TLR agonists and CD40L/ IFN-γ has been reported for the induction of IL-12.22 Thus, we sought to determine whether TLR 4 and 7/8 agonist alone or in conjunction with CD40L/IFN-γ could induce IL-7 production. Upregulation of major histocompatibility complex class II, CD80, CD86 and CD83 was induced by TLR 4 and 7/8 and CD40L/IFN-γ activation, and in agreement with earlier reports, TLR agonist and CD40L/IFN-γ alone and in combination could induce IL-12 production (data not shown). In contrast, TLR 4/7/8 agonists did not enhance IL-7 production above levels detected in iDC supernatants nor did it synergize with CD40L/IFN-γ (Figure 1c). IFN-γ enhances IL-12 production induced by CD40L,15,16 and thus, we wished to determine whether IFN-γ alone or in combination with CD40L signals was necessary and/or sufficient for IL-7 production by DC. We find that IL-7 levels produced upon CD40L and CD40L/IFN-γ activation were nearly identical (Figure 1d). Altogether, this data indicates that CD40L–CD40 interactions provide distinct, and possibly unique, signals that result in the upregulation of IL-7 production by human monocyte-derived DCs.
Quantitation of CMV-specific human CD8+ T cells upon in vitro activation with NLV peptide-pulsed iDC and mDC
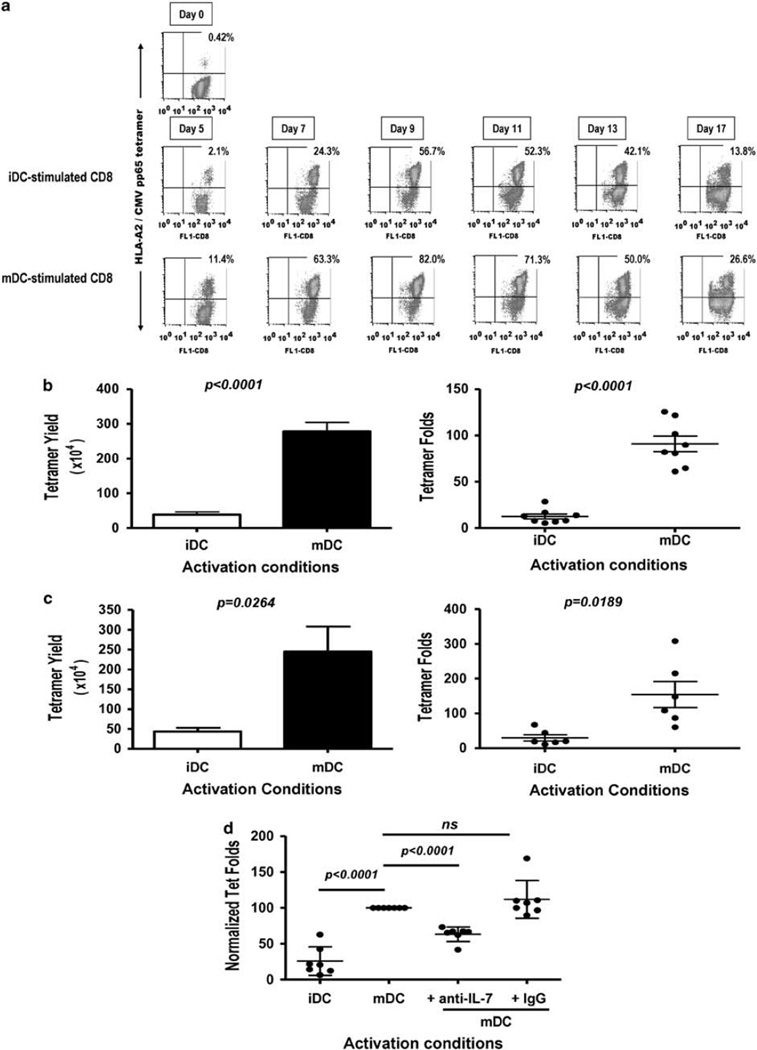
To evaluate the effect of CD40L/IFN-γ-mediated DC maturation on human CD8+ T cells, we chose to study CMV pp65 responses in healthy CMV-seropositive donors using the HLA-A*0201 -restricted NLV 495–503 peptide.19 The kinetics of response was assessed in vitro by stimulating purified HLA-A*0201+ CD8+ T cells with peptide-pulsed autologous iDC or mDC as described in Figure 2. T cells were harvested at the indicated time points after activation and stained using anti-CD8 Ab and HLA-A2/CMV pp65 NLV tetramers (Figure 2a). Owing to downregulation of TCR upon antigen stimulation,23 the percentages of tetramer-positive (tet+) CD8+ T cells could not be reliably determined at time points earlier than 5 days after stimulation (data not shown). As shown for a representative donor (Figure 2a) from day 5 onward, iDC- and mDC-activated cultures displayed similar kinetics with percentages of antigen-specific T cells increasing gradually in both cultures with a peak responses occurring 9–10 days after stimulation. However, activation of CD8+ T cells with NLV-pulsed mDC leads to higher percentages of antigen-specific T cells, (Figure 2a) which in turn results in higher yields of antigen-specific cells (Figure 2b). After day 11, a decrease in the percentage of antigen-specific cells is observed under both culture conditions. This decrease in antigen-specific CD8+ cells (Figure 2a) is reminiscent of the ‘contraction phase’ observed during the resolution of a primary viral infection.24,25 This phase of antigen-induced cell death (or contraction) is observed regardless of any replenishment of nutrients or cytokines (data not shown).
Figure 2.
IL-7 neutralization decreases the CMV pp65-specific CD8 T-cell peak response upon mature dendritic cell (mDC) stimulation, (a) Purified CD8+ T cells were in vitro stimulated with CMV pp65 495–503 NLV peptide-pulsed immature DC (iDC) or mDC. Human IL-2 (10 U well−1) was added 48h after stimulation and every 2–3 days thereafter. Cells were harvested on the indicated days, counted and stained with fluorescence isothiocyanate (FITC)-CD8 antibody (Ab) and phycoerythrin (PE)-HLA-A2/CMV pp65 peptide tetramer. Numbers in dot plots represent percentage of tetramer-positive (tet+) cells among lymphocyte/CD8-gated cells. A representative donor is shown (n=3). (b) Tetramer yield and fold increases in NLV peptide-specific CD8+ T cells upon peptide DC stimulation. Tetramer yield represents the percentage of tet+ T cells×total CD8+ T cells at day 9; tetramer fold represents tetramer yields/percentage of tet+ T cells×total CD8+ T cells at day 0. Each dot represents value obtained with an individual well/culture condition; horizontal bars represent mean values + s.e.m. A representative donor is shown (n=3). P-values were obtained with the Student’s t-test; P-values <0.05 are significant. Each individual well is harvested and counted separately and the mean ± s.e.m. is determined for both iDC and mDC groups. The coefficient of variation among wells (in both groups) is <15% for tetramer yields, (c) Tetramer yield and fold in NLV peptide-specific responses among individuals. Cultures were analyzed as described in (a and b). Tetramer yield and fold increases were calculated using a sample pooled from eight wells per culture condition per individual (represented by each dot). Six representative individuals are shown, (d) NLV peptide-pulsed mDCs were pre-incubated with anti-IL-7 Ab (1 µg ml−1), isotype control Ab (1 µg ml−1) or media for 1 h at 37°C before addition of T cells. Cells were harvested on day 9, stained with FITC-CD8 Ab and PE-HLA-A2/CMV pp65 tetramer, analyzed by fluorescence-activated cell sorting (FACS) and tetramer folds calculated using a sample pooled from eight wells per culture condition. For comparison among individuals, each individual’s tetramer fold values were normalized to those obtained in mDC-activated cultures. Each dot represents an individual and horizontal bars represent mean values ± s.d. P-values were obtained with the repeated measure analysis of variance (ANOVA) followed by Tukey’s analysis; P-values <0.05 are significant (n=8).
To compare antigen-specific T-cell increases among individuals, total cell counts were performed, tetramer percentages were assessed by flow cytometry and tetramer yields and tetramer fold increases calculated as described in Methods. Tetramer yields and folds obtained on day 9 from a representative individual (eight wells per condition, 2.5×105 CD8+ T cells per well) upon activation with NLV peptide-pulsed iDC or mDC are shown in Figure 2b. From the initial 2×l06 CD8+ T-cell starting population (1.53% tet+), tetramer yields on day 9 are 38.4×104 and 278.2×104 tet+ CD8+ T cells in iDC- and mDC-activated cultures, respectively. These values represent, respectively, 12.5 and 90.9 tetramer fold increases in NLV-specific CD8+ T-cell numbers (Figure 2b). NLV peptide-pulsed iDC and mDC activation of CD8+ T cells derived from six individual donors resulted in similar results. mDC-activated cultures resulted in higher tetramer yields and folds compared with iDC-activated cultures (Figure 2c). Among donors, the average tetramer increase is 29-fold in iDC-activated cultures and 154-fold in mDC-activated cultures. Thus, CD40L/IFN-γ mDC stimulation provides 5.3-fold increases in the peak antigen-specific response compared with iDC stimulation, showing that the activation status of DC influences the expansion of CMV pp65-specific CTL from a memory cell population.
Endogenous IL-7 neutralization results in decreased yields of antigen-specific CD8+ T cells after peptide stimulation
As CD40L/IFN-γ activation of DC leads to upregulation of IL-7 production (Figure 1), we wished to investigate the contribution of mDC-derived IL-7 to the magnitude of the antigen-specific CD8+ T-cell peak response. CD8+ T cells were activated with NLV peptide-pulsed mDC in the presence of neutralizing anti-IL-7 Abs, an isotype control or media, and the antigen-specific peak response was determined on day 9. The tetramer fold increase values were normalized to allow comparison among individuals (n=7). The addition of neutralizing anti-IL-7 Ab, at the initiation of each culture (time 0), results in a consistent decrease (~30%) in the peak response (tetramer fold increase) after mDC stimulation (Figure 2d), whereas the addition of a control Ab had no discernible effect. CD8+ T-cell cultures activated with peptide-pulsed iDC are shown for comparison and peak response in these cultures is inferior to mDC cultures treated with neutralizing IL-7 Ab. This finding suggests that IL-7 produced by mDC contributes to the antigen-driven expansion of CD8+ T cells, but it implies that additional cytokines (or costimulatory factors) contribute to the relative potency of CD40L/IFN-γ-stimulated DC.
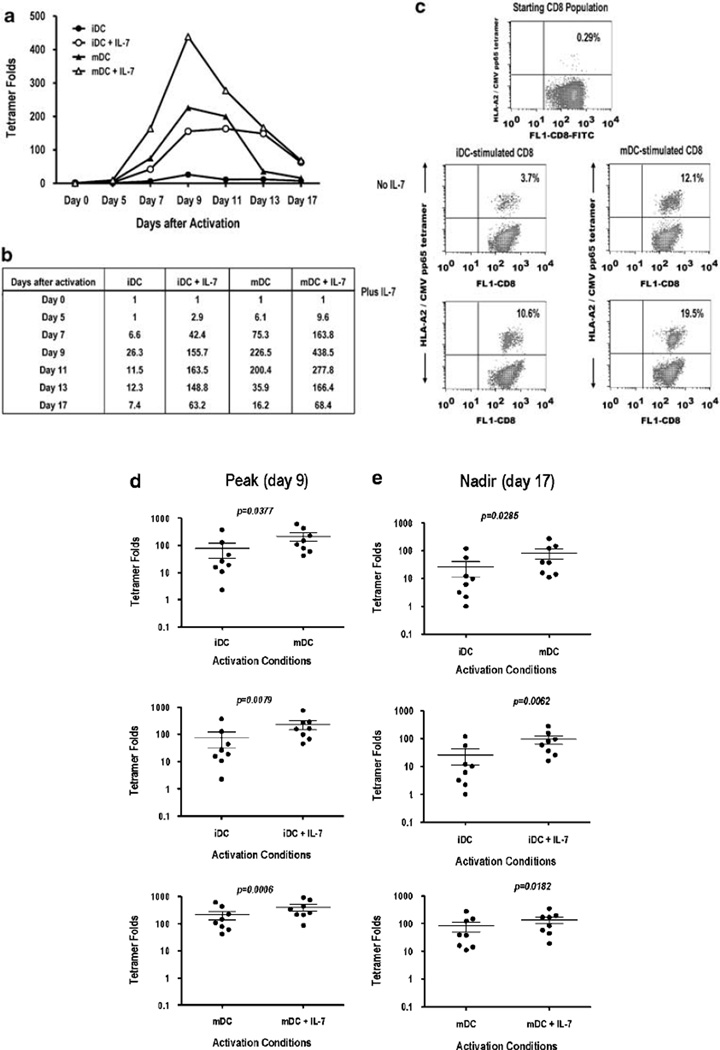
Exogenous IL-7 enhances the peak response, which results in an increased antigen-specific CD8+ T-cell survivor pool
Having shown an effect of mDC-derived IL-7 in antigen-specific T-cell numbers (Figure 2d), we wished to determine the effect of exogenous IL-7 on the size of the CD8+ antigen-specific survivor (memory) population at the end (nadir) of the culture period. Human CD8+ T cells were stimulated with NLV peptide-pulsed iDC or mDC in the presence or absence of rIL-7. Exogenous IL-7 (10 ng ml−1) was added only at the initiation of culture (time 0). The responding CD8+ T cells were harvested at various time points after activation, and a quantitative analysis was performed as described in Figure 2. The kinetics of NLV-specific tetramer fold changes after in vitro stimulation in a representative donor is shown in Figure 3a. Exogenous IL-7 had no effect on the kinetics of antigen-specific response. However, IL-7 affected the magnitudes at the peak (day 9) and the nadir (day 17) of the in vitro response (Figures 3a and b). Tetramer fold values at each time point are shown in Figure 3b. Exogenous IL-7 (provided only at time 0) increased the peak response of NLV-specific CD8+ T cells after either iDC or mDC stimulation (Figures 3b and c). For example, the percentage of tet+ CD8+ T cells from a representative donor (baseline, 0.29%) increased to 3.7% in iDC-stimulated cultures and increased further to 10.6% in iDC-stimulated cultures supplemented with IL-7 (Figure 3c). The effect of exogenous IL-7 is less pronounced in mDC cultures. The peak (day 9) response in mDC-stimulated cultures is 12.1% compared with the 19.5% in mDC-stimulated cultures supplemented with IL-7. After day 11, a reduction in the number of NLV-specific T cells is observed; however, the presence of IL-7 at the time of TCR activation leads to a less-pronounced ‘contraction’ resulting in greater NLV-specific T-cell numbers on day 17. Similar results were obtained when peak and nadir tetramer fold increases were assessed in eight healthy donors (Figures 3d and e). Significant differences in tetramer fold increases are observed between iDC- and mDC-activated cultures at the peak response (day 9; Figure 3d) and at the nadir (day 17; Figure 3e). Addition of exogenous IL-7 increases peak and nadir tetramer fold independently of the maturation status of the DC (Figures 3d and e). Thus, the presence of exogenous IL-7 at the time of TCR stimulation confers a survival/growth advantage leading to an increased yield of antigen-specific CD8+ T cells at the peak of the in vitro response. The increased magnitude of the peak response leads, after resolution of the in vitro response, to a larger antigen-specific survivor T-cell pool. Importantly in the presence of exogenous IL-7, the maturation status of the DC appears less critical in determining yields of antigen-specific CTL.
Figure 3.
Exogenous IL-7 increases the number of antigen-specific CD8+ T cells in immature dendritic cell (iDC)- and mature (mDC)-activated cultures. Purified CD8+ T cells were stimulated with NLV peptide-pulsed iDC or mDC in the presence or absence of IL-7 (10 ng ml−1, added exclusively at day 0). Cells were harvested at indicated days and stained with fluorescence isothiocyanate (FITC)-CD8 and phycoerythrin (PE)-HLA-A2/CMV pp65 tetramer. (a) Kinetics of tetramer fold and (b) tetramer fold values for a representative donor are shown (n=3 donors), (c) Dot plots of gated CD8+ T cells on day 9 (peak) after activation. Numbers represent percentage of NLV-specific T cells. Tetramer fold values at peak (d) and nadir (e) of the in vitro immune response. Each dot represents an individual (n=8) and horizontal bars represent mean values + s.e.m. P-values were obtained with the Student’s t-test; P-values <0.05 are significant.
IL-7 does not impact CTL effector function
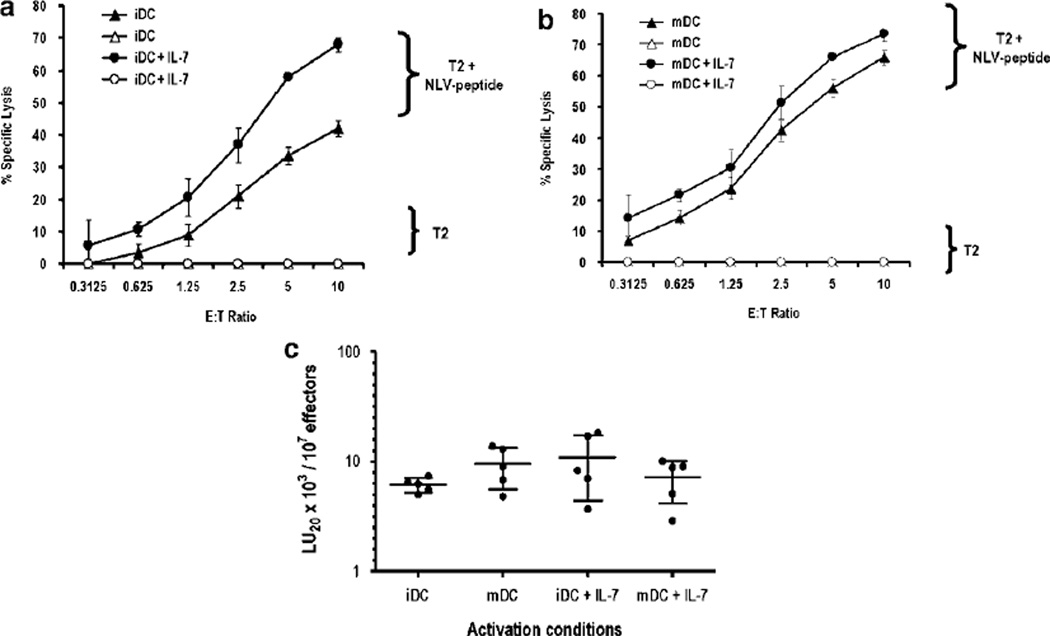
IL-7, IL-15 and IL-21 are all members of the γc (CD132) receptor cytokine family and both IL-15 and IL-21 have been reported to enhance the lytic function of human CD8+ T cells.26 To evaluate the effect of IL-7 on CTL effector function, CD8+ T cells were activated with NLV peptide-pulsed iDC or mDC in the presence or absence of exogenous IL-7, and then tested in a standard 4-h 51Cr-release assay 10 days later. As shown in Figure 4, apparent differences in lytic activity are observed among the various cultures. However, these differences can be attributed to the varying percentages of NLV tet+ CD8 cells in the different activation conditions. If the number of NLV-specific CD8+ T cells is corrected and normalized for each activation condition, all culture conditions yield similar lytic activity (Figure 4c). Thus, on a per cell basis, the lytic activity of NLV-specific CD8+ T cells is similar under the various activation conditions studied. This observation is consistent with IL-7 serving as a survival/growth factor and indicates that IL-7, in contrast to IL-15 and IL-21, does not influence the effector function of human CD8+ T cells.
Figure 4.
IL-7 has no effect on CD8+ effector function. Purified CD8+ T cells stimulated with (a) immature dendritic cell (iDC) or (b) mature DC (mDC) in the presence or absence of IL-7 were harvested on day 11 and tested in a standard 51Cr-release assay using NLV peptide-pulsed or non-peptide-pulsed T2 cells as targets. The percent specific lysis (mean of triplicates) ± 1 s.d. is shown for each E:T ratio. The spontaneous lysis is <10%. (c) A summary dot plot of lytic units (LU)/107 effectors as determined for five donors under each of the culture conditions is studied. The number of antigen-specific T cells for each donor per culture condition was determined before lytic assay by anti-CD8 antibody (Ab) and HLA-A2/CMV pp65 tetramer staining. The number of LU/107 effectors was calculated using the formula LU=107 exp{(Y*–Yp)/C}/(TXG).48 Y* is the mean of the logistically transformed specific lysis, p is the reference lysis percentage (taken to be 20%), Yp=ln(p/(100%–p) for each condition, T=104 target cells and XG is the geometric mean of the E:T ratio, where E is corrected by the number of tet+ cells present in the culture. On a per cell basis, there is no discernible effect of IL-7 on the lytic activity of CD8+ T cells.
IL-7 has a temporal effect on antigen-specific responses
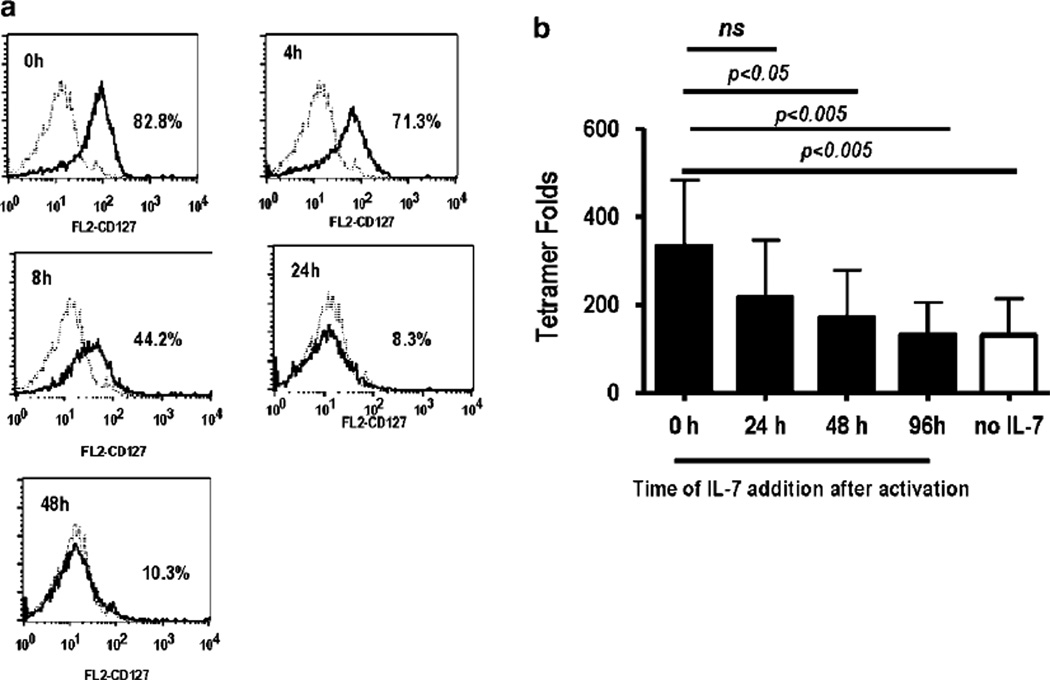
TCR activation has been reported to regulate the expression of IL-7Rα expression.27,28 We therefore examined the effect of TCR activation on IL-7Rα expression using purified CD8+ T cells and anti-CD3/CD28 beads to provide a polyclonal activation signal. CD8+ T cells were harvested at various points after activation and stained for IL-7Rα expression. Results shown in Figure 5a were obtained with a CD8+ T-cell donor expressing a large percentage of IL-7Rα-positive cells; percentages of CD8+/IL-7Rα+ expression among donors (n=7) varied from 50–80% (data not shown). A large percentage of IL-7Rα-positive cells are detected at the start of cultures (0h), and TCR activation leads to a time-dependent decrease in IL-7Rα expression. At 24–48 h after activation, < 10% of the cells expressed the IL-7Rα chain (Figure 5a). These findings would predict that upon antigen activation, T cells would lose their ability to respond to IL-7 in a temporal manner. To test this hypothesis, exogenous IL-7 was added to NLV peptide-pulsed iDC-stimulated cultures at various time points after TCR stimulation (0–96 h). Cells were harvested on day 9, stained with anti-CD8 Ab and HLA-A2/CMV pp65 tetramer and the tetramer fold increases were calculated. The timing of IL-7 addition dramatically affected the magnitude of the peak response on day 9. The largest tetramer fold increase was observed in cultures in which IL-7 was added at the time of TCR activation (IL-7 0h, Figure 5b). Addition of IL-7 at 24 h resulted in ~25% decrease in the peak tetramer fold recovery; however, this decrease was not statistically significant. Addition of IL-7 at later time points results in a statistically significant reduction of the peak response. No significant tetramer fold increase was observed in cultures given IL-7 at 96 h after activation compared with those cultures with no cytokine added. In addition, pre-incubation of T cells with IL-7 (30 min–3h and then washed out) before TCR activation had no significant effect on tetramer fold increases (data not shown). Taken together, these results suggest that TCR activation leads to loss of IL-7 responsiveness as a consequence of IL-7Rα downregulation and implies a temporal window in which IL-7 can promote antigen-specific CD8+ T-cell expansion during an ongoing antigen response. Our finding that addition of exogenous IL-7 at later time points (48–96 h) has no impact on peak antigen-specific response further supports the notion that IL-7 signaling occurs during TCR engagement, and is distinct from IL-7 homeostatic function to promote basal proliferation in the absence of cognate antigen.
Figure 5.
IL-7 during T-cell receptor (TCR) activation increases the antigen-specific response. Flow cytometry for CD127 expression upon TCR activation, (a) Purified CD8+ T cells were stimulated with anti-CD3/CD28 beads, harvested at the indicated times and stained with fluorescence isothiocyanate (FITC)-CD8 and phycoerythrin (PE)-CD127. Representative histogram plots depict IL-7Rα (CD127) expression on CD8+ T cells relative to isotype control at various time points after TCR activation (n=2). Numbers represent percentage of IL-7Rα+cells relative to isotype. (b) Purified CD8+ T cells were stimulated with NLV peptide-pulsed immature dendritic cell (iDC) as described in Figure 4a with IL-7 (10 ng ml−1) added at the indicated time after stimulation. Cells were harvested on day 9 and stained with FITC-CD8 antibody (Ab) and PE-HLA-A2/CMV pp65 tetramer and tetramer folds were calculated. Values represent mean ± s.d. obtained with four donors. P-values were obtained with the repeated measure analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test; P-values <0.05 are significant.
Human CD8+ T cells responsiveness to IL-7
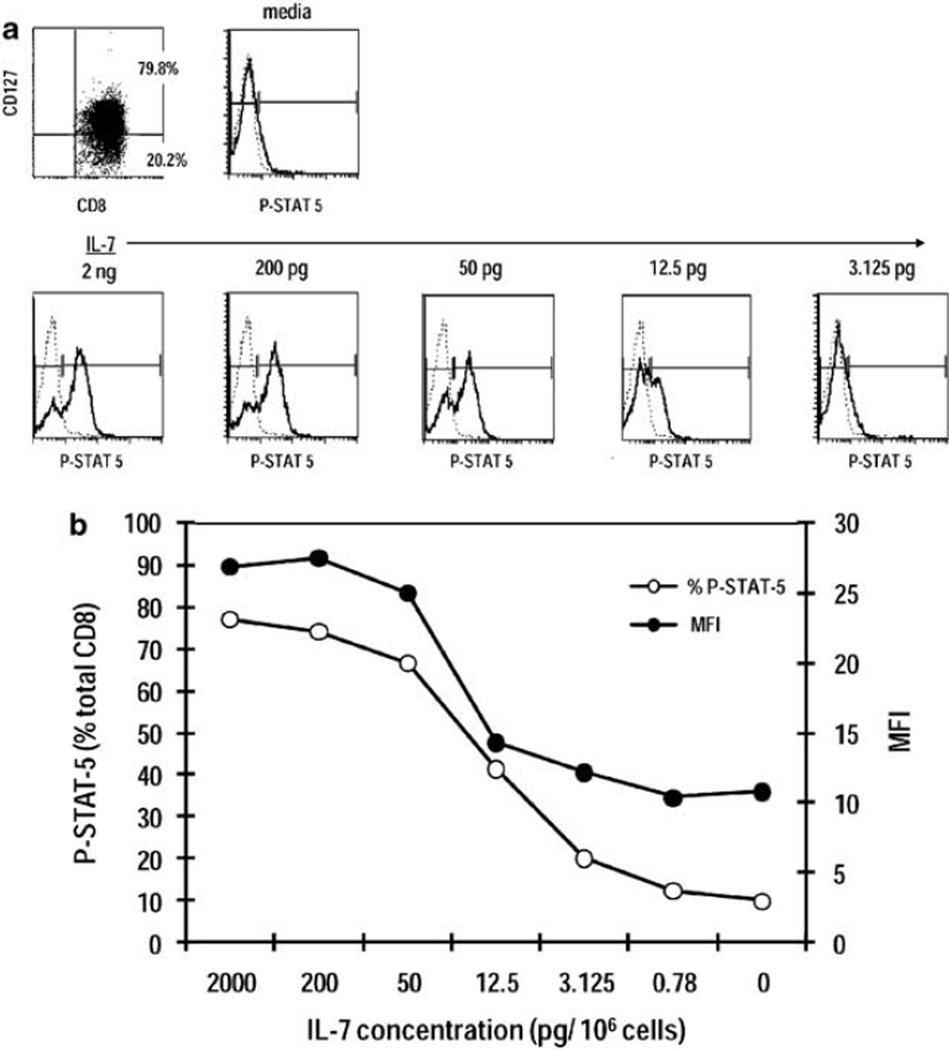
Having demonstrated IL-7 production by CD40L/IFN-γ-activated DC (Figure 1) and the ability of exogenous IL-7 to increase antigen-specific CD8+ T-cell responses independently of DC maturation status (Figure 3), we investigated IL-7 responsiveness in human CD8+ T cells as a function of STAT-5 phosphorylation. Purified CD8+ T cells were incubated in RPMI 1640 at 37 °C for 1 h, treated with increasing concentrations of IL-7 (range, 2 ng – 780 fg) for 30 min at 37 °C and stained intracellularly for STAT-5 using an Ab specific for the phosphorylated form (Y-694). As shown in Figures 6a and b, stimulation of cells with 2 ng–200 pg IL-7 leads to STAT-5 phosphorylation in ~80% of CD8+ T cells expressing IL-7Rα. STAT-5 phosphorylation falls with decreasing IL-7 concentrations and finally reaches background levels similar to those observed in inactivated cells (media only). A consistent correlation between the percentage of cells expressing IL-7Rα and STAT-5 phosphorylation is observed (data not shown), suggesting that receptor expression by CD8+ T cells dictates the extent of subsequent IL-7 signaling. Exogenous IL-7 concentrations in the 3–12 pg range are sufficient to activate a sizable percentage (~20–40%) of CD8+IL-7Rα+ cells.
Figure 6.
STAT-5 phosphorylation as a measurement of CD8+ T-cell responsiveness to IL-7. Purified CD8+ T cells were stained for IL-7Rα (CD127) expression or activated with the indicated concentrations of IL-7 for 30 min and stained for the phosphorylated form of STAT-5 (Y-649). (a) Representative histogram plots depict IL-7Rα expression on CD8+ T cells and P-STAT-5 staining relative to isotype control upon treatment with increasing concentrations of IL-7. (b) Quantitation of percentages of CD8+ T cells expressing P-STAT-5 and ΔMFI at each IL-7 concentration was tested. ΔMFI represents staining intensity with anti-P-STAT-5 minus isotype control antibody (Ab).
IL-7 signaling by CMV pp65-specific T cells correlates with IL-7Rα expression
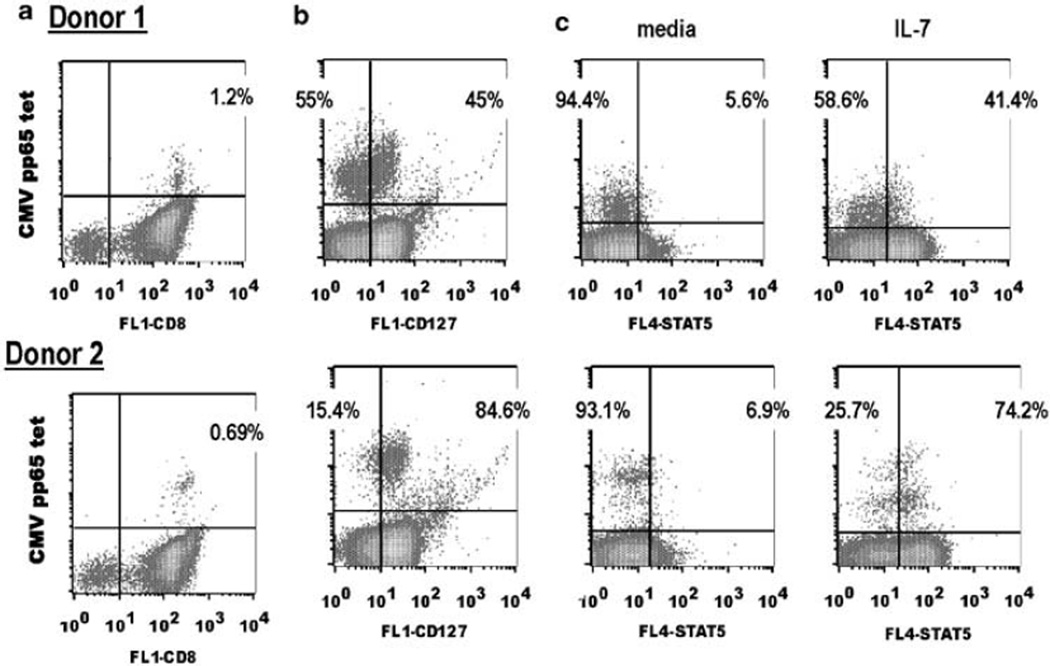
IL-7Rα expression is variable among NLV-specific CD8+ T cells,29 and thus it is hypothesized that the ability to respond to IL-7 will confer a growth advantage to IL-7Rα+ NLV-specific cells. We next evaluated IL-7Rα expression and IL-7 responsiveness of the NLV-specific T cells. To evaluate IL-7Rα expression, purified CD8+ T cells were incubated in RPMI 1640 at 37 °C for 1 h, and stained with HLA-A2/CMV pp65 tetramers and anti-CD127 for 30min at room temperature. To evaluate IL-7 signaling, cells were treated as described before in the absence of anti-CD127, incubated with media or IL-7 (10 ng ml−1) for 30min at 37 °C and stained intracellularly for STAT-5 using an Ab specific for the phosphorylated form (Y-694). Two representative donors with 0.7–1.2% NLV-specific cells among the CD8+ T-cell population are shown in Figure 7. In agreement with Sauce et al.,30 we observed that the larger the frequency of NLV-specific T cells, the lower the percentage of IL-7Rα+ cells within this population (Figures 7a and b). IL-7Rα expression (45–85%) within NLV-specific T cells is representative of that observed among our healthy CMV-seropositive donors (Figure 7b). Treatment with IL-7 results in a sizable percentage of the NLV-specific T cells showing STAT-5 activation, and these percentages correlate with numbers of cells expressing IL-7Rα (Figures 7b and c). Thus, these results suggest that IL-7Rα+NLV-specific memory CD8+ T cells may have a selective growth advantage relative to IL-7Rα–NLV-specific cells if antigen is re-encounter in the presence of IL-7.
Figure 7.
Within the CMV NLV-specific CD8+ T-cell pool, IL-7Rα expression correlates with STAT-5 phosphorylation upon IL-7 activation. Flow cytometry for CMV NLV-specific and IL-7Rα expression within CD8+ T cells. Purified CD8+ T cells were stained with (a) fluorescence isothiocyanate (FITQ-CD8 and phycoerythrin (PE)-HLA-A2/CMV pp65 tetramer or (b) FITC-CD127 and PE-HLA-A2/CMV pp65 tetramer. Overall, 2.5×l04 events were collected on the CD8/ lymphocyte gate to determine NLV-specific frequencies. Overall, 5×l03 events were collected on the tetramer/lymphocyte gate to evaluate CD127 expression. Representative dot plots are shown, and the percentages within the CD8 gate and tetramer gate are shown, (c) Cells were stained with HLA-A2/ CMV pp65 tetramers, incubated in media or IL-7 for 30min at 37°C and analyzed for STAT-5 phosphorylation. Overall, 5000 events were collected on the tetramer/lymphocyte gate. Representative dot plots are shown, and the percentages within the tet+ population are shown.
DISCUSSION
CD40L–CD40 interactions leading to DC maturation are crucial for priming naive CD8+ T cells, particularly under non-inflammatory conditions; however, the precise signals emanating from CD40-acti-vated DC are not fully defined.31 This study makes three distinct points. First, CD40 signals regulate DC-derived IL-7 production; CD40L activation of monocyte-derived human DC leads to ~ fourfold increases in IL-7 secretion. Second, neutralization of IL-7 in CD8+ T-cell cultures activated with mDC results in a diminished recovery (30% lower peak response) of antigen-specific T cells. Third, IL-7 signaling requires a temporal coordination with TCR activation for maximal antigen-specific T-cell expansion.
IL-7 is a member of the γc (CD132) receptor cytokine family and signals through the IL-7Rα (CD127)/CD132 receptor.32 IL-7 is essential for the survival of naive T cells and promotes homeostatic renewal and long-term maintenance of memory CD8+ T cells.33 An earlier report showed IL-7 mRNA transcripts in human peripheral blood DC.34 We extend this observation by providing direct evidence that human monocyte-derived DC not only produces IL-7 constitutively but can also upregulate IL-7 secretion upon CD40 signaling. These findings provide the first evidence of inducible/regulated IL-7 production by hematopoietic cells. Mounting evidence shows that monocyte-derived DC stimulation by microbial products through TLR engagement can result in distinct outcomes; for example, Mycobacterium tuberculosis induces IL-23 production by an engagement of TLR2 and NOD2, whereas LPS and R848 induces IL-12 production by an engagement of TLR4 and TLR8, respectively.22,35 These distinct cytokine patterns qualitatively shape the effector function of the adaptive immune response.3,36 As CD40 signals can synergize with TLR (TLR 4 and 8) and other cytokines (IFN-γ) to enhance IL-12 production,16,22 it has been proposed that CD40 signals amplify innate signals and enhance T-cell effector function. Our results provide evidence that CD40–CD40L interactions contribute unique survival and proliferative signals to antigen-responsive T cells by regulating the production of IL-7 by DCs. Thus, licensing of DC by activated CD4+CD40L+ T cells may induce IL-7 production by DC and consequent delivery of survival/proliferative signals to CD8+ T cells to maximize the clonal expansion.
In healthy adults, plasma levels of IL-7 are tightly regulated (range 2–8 pg ml−1); however, under conditions of CD4+ lymphopenia, such as bone marrow transplantation and HIV infection, levels can rise to 60 pg ml−1.37~40 On the basis of the inverse correlation between plasma IL-7 levels and CD4+ T-cell counts, it appears that circulating IL-7 levels are determined by the T-cell consumption rate.37,41 Fibroblastic reticular cells constitute the primary lymph node cellular source of IL-7 as cytokine transcripts are expressed uniquely by this, but not other, lymph node population.32,42 These cells are strategically localized through the T-cell zone, thus providing a local source of cytokine for naive T cells. Is IL-7 production by DC upon CD40 activation physiologically relevant? In this study, we find that IL-7 production by monocyte-derived DC can increase by ~ four-fold after CD40 engagement and can reach levels as high as 1–2 pg with an average of ~800 fg. In addition, we find that exogenous IL-7 concentrations in the 3-pg range can stimulate ~20% of CD8+ IL-7Rα+ T cells (Figure 6). Thus, these results suggest that mDC-derived IL-7 may provide a regulated and local source of cytokine which acts upon IL-7Rα+ T cells during antigen-specific stimulation. DC-derived IL-7 may synergize with IL-6 or additional cytokines to promote T-cell growth.43 Importantly, the strongest evidence for a role of DC-derived IL-7 is our finding that the treatment of mDC-stimulated T-cell cultures with anti-IL-7 Abs results in decreases in antigen-specific T-cell yields (Figure 2d). However, IL-7 levels produced by mDC are non-saturating as shown by the fact that addition of exogenous IL-7 to mDC-activated cultures can further increase the yield of antigen-specific T cells at both the peak and nadir after in vitro stimulation (Figure 3).
What effect does DC-produced IL-7 have on virus-specific naive and memory CD8+ T-cell populations? Although all naive cells express IL-7R, IL-7R expression by memory T cells depends on the nature and replication status of the virus. Within CMV-specific T cells, results by van Leeuwen et al.29 and Sauce et al.30 have shown that IL-7Rα+ memory T cells have a greater proliferative capacity to IL-7 or antigen than IL-7R cells. Indeed, we find a strong correlation between IL-7Rα expression and STAT-5 phosphorylation among CMV pp65-specific T cells (Figure 7). Thus at the time of DC/antigen encounter, virus-specific IL-7Rα+ T cells will have an advantage relative to IL-7Rα— cells; DC-produced IL-7 will preferentially promote an expansion of IL-7Rα+ cells, with minimal impact on effector function. Finally, our results suggest a dual role for IL-7 during antigen-specific responses, initially as a ‘costimulator’ cytokine during DC/antigen activation and later as a homeostatic cytokine to maintain memory T-cell pool. The temporal requirement for the ‘costimulatory effect of IL-7 after DC activation appears stringent (Figure 5). Exogenous IL-7 enhances the peak antigen-specific responses only if present within the first 24 h after TCR stimulation; addition of IL-7 up to 4 days after activation has a minimal impact on the recovery of antigen-specific CD8+ T cells (Figure 5). In agreement with this temporal requirement, we and others27,28,44,45 find that TCR/CD28 activation leads to downregulation of IL-7Rα expression. TCR engagement leads to a rapid and sustained decrease in IL-7Rα transcripts rendering cells unable to relay IL-7 signals.27,28 However, at later time points in culture, CMV pp65 (NLV)-stimulated T cells regain IL-7 responsiveness in culture and by day 14, approximately 75% of the CMV-specific cells are IL-7Rα+ (data not shown) and may be receptive to homeostatic IL-7 signals.
In summary, our data supports a model in which CD40L provides a unique signal to DC resulting in the upregulation of IL-7. Further studies in murine models, using IL-7-deficient DC, will be required to evaluate the role of DC-derived IL-7 during antigen stimulation in vivo. We conclude that IL-7 production is one of the many contributing features that accounts for the remarkable potency of CD40L–activated DCs in the induction of CTL responses.
METHODS
DC generation and maturation
DCs were prepared as described earlier.46 Briefly, peripheral blood mononuclear cells suspended in RPMI 1640 with 1% human-pooled plasma at 5×106 cells ml−1 were dispersed into T175 culture flasks. After a 2 h culture at 37 °C in 5% CO2, non-adherent cells were removed by two washes with phosphate-buffered saline. Adherent cells were then cultured in RPMI 1640 with 1% human-pooled plasma/10mmoll−1 HEPES/L-glutamine/penicillin/ streptomycin supplemented with GM-CSF (100 ng ml−1, Bayer HC Pharmaceuticals, Wayne, NJ, USA) and IL-4 (20ng ml−1, CellGenix, Antioch, IL, USA). Fresh medium containing GM-CSF and IL-4 was added every 2–3 days. Generally, an average yield of 5% DC was obtained from peripheral blood mononuclear cells. On day 6 of culture, DCs were harvested, washed twice in serum-free medium, and cultured (106 cells ml−1) for an additional 48 h in RPMI 1640/1% human plasma supplemented with GM-CSF/IL-4 (iDC) or with GM-CSF/IL-4 and CD40L–expressing J558 cells15 (a kind gift of Marco Colonna, Washington University) at a 5 DC:1 CD40L– J558 ratio and IFN-γ (100 U ml−1, Intermune, Brisbane, CA, USA) (mDC). For TLR activation, DCs were cultured in GM-CSF/IL-4 in the presence of ultrapure LPS (500 ng ml−1, InvivoGen, San Diego, CA, USA) and TLR7/8 (5 µgml−1, 3M-007, a kind gift of Richard Miller, 3M, St Paul, MN, USA) for 48 h. DCs were stained with fluorescence isothiocyanate-anti-HLA-DR, phycoerythrin- (PE)-anti-CD86, CD83, or biotinylated anti-IL-15R followed by strepavidin-PE (data not shown). Antibodies were obtained from BD Pharmingen (San Jose, CA, USA) and R&D Systems (Minneapolis, MN, USA). Cells were analyzed in a FACScan flow cytometer using CellQuest (BD Biosciences, San Jose, CA, USA).
Determination of cytokine production and STAT-5 phosphorylation
Cytokine production by DC was measured by enzyme-linked immunosorbent assay using either matched-paired antibodies for IL-12 p70 (Biosource, Carlsbad, CA, USA) or a high-sensitivity ELISA kit for IL-7 (R&D Systems). Both enzyme-linked immunosorbent assays were used according to the manufacturer’s instructions. To assess STAT-5 phosphorylation, T cells were allowed to equilibrate for 1 h at 37 °C in RPMI 1640, stained with fluorescence isothiocyanate-anti-CD8 (BD Pharmingen) for 15min at room temperature. Cells were then stimulated for 30 min with recombinant human IL-7 (Pepro-tech, Rocky Hill, NJ, USA) or media, then fixed in 2% paraformaldehyde, permeated in 90% methanol and stained for STAT-5 phosphorylation (anti-P-STAT-5 (Y-694), BD Pharmingen).
CD8+ T-cell DC cultures
CD8+ T cells were isolated from peripheral blood mononuclear cells using a CD8− selection kit (Miltenyi Biotech, Auburn, CA, USA). Purity of the selected cells was 85–90% in all experiments. Purified CD8+ T cells (5×l05 per ml) from HLA-A*0201/CMV-seropositive donors were cultured at a 20:1 ratio with irradiated (2500 Rads) autologous DC pulsed with CMV pp65 ND7 (495–503 aa sequence, NLVPMVATV) peptide (2 µg per 106 DCs ml−1, 2h, 37 °C) in 48-well trays (0.5mlwell−1) in Stemline media (Sigma, St Louis, MO, USA) containing 5% human-pooled sera. Human IL-7 (10ng ml−1, Peprotech) was added at the initiation of culture or as indicated; human IL-2 (10Uml−1, Chiron, Emeryville, CA, USA) was added to cultures every 2–3 days starting on day 2. Cell growth was monitored microscopically and if confluent, the cultures were transferred to 24-well trays. PE-HLA-A*0201/CMV pp65 tetramer (Beck-man Coulter, Fullerton, CA, USA) staining was performed on day 9 unless otherwise stated. For IL-7 neutralization, anti-IL-7 Ab (1µgml−1, goat polyclonal, R&D Systems) was incubated with peptide-pulsed DC for 1 h at 37 °C before addition of purified CD8+ T cells. HLA typing was performed by the Histocompatibility Laboratory (Barnes-Jewish Hospital). CMV-seropositive donors were identified from the Barnes-Jewish Hospital Blood Bank and confirmed by identification using PE-HLA-A*0201/CMV pp65 tetramer staining of peripheral blood CD8+ T cells. All individuals studied had greater than 0.01% tet+ peripheral blood CD8+ T cells.
Tetramer assay and analysis
CD8+ T cells (1×106) were harvested, washed with fluorescence-activated cell-sorting buffer (0.5% bovine serum albumin/phosphate-buffered saline w/o Ca+ and Mg+) and incubated with PE-HLA-A*0201/CMV pp65 tetramers for 30min at room temperature, followed by fluorescence isothiocyanate-anti-human CD8 Ab or APC-CD45RA, APC/Alexa750-CD27 and fluorescence isothiocyanate-CD127 (BD Pharmingen, San Diego, CA, USA) Abs for 15min at 4°C. After washing twice with fluorescence-activated cell-sorting buffer, cells were fixed by 2% paraformaldehyde in phosphate-buffered saline and analyzed in a modified FACscan flow cytometer with CellQuest (BD Biosciences). Percentages in dot plots indicate the frequency of tet+ CD8+ T cells; in all experiments, 2.5×104 events were collected in the lymphocyte/CD8+ gate to assess tetramer frequencies. For phenotypic characterization of tet+ cells, 5×103 events were collected in the tetramer gate. To compare experiments among individual CD8+ T-cell donors, tetramer yield and tetramer fold were calculated. Tetramer yield represents the percentage of tet+ CD8+ T cells×total CD8+ T-cell number at day 9 (or as indicated). The tetramer fold represents tetramer yield divided by the percentage of tet+ CD8+ T cells×total CD8+ T-cell number at day 0. Except for Figure 2b, which provides an example of intra-assay variability for individual wells (both tetramer yield and fold increases are shown), antigen-specific expansion is expressed as ‘tetramer fold’ values obtained using a sample pooled from eight replicate wells.
Cytolytic assay
CD8+ T cells were harvested on day 10–11 after stimulation and assayed for lytic activity in triplicates using a standard 4-h 51Cr-release assay. T2 (TAP1 deficient, A*0201+) cells were pulsed with NLV peptide (5 µg per 106 cells ml−1) and labeled with 50 µCi 51Cr for 1 h in RPMI+1% fetal calf sera, washed twice and used as targets in lysis assays in microtiter trays as described earlier.47 Lytic units (LU) were determined on the basis of the number of antigen-specific T cells (as assessed by tetramer staining) and calculated as described by Bryant et al.15 using the formula LU=107 exp{(Y*–Yp)/C}/(TXG) (see Figure 4 legend).
Statistical methods
Statistical differences between groups were examined by a Student’s f-test; significance was set at P-value of < 0.05 (Prism version 5 software, GraphPad software Inc., San Diego, CA, USA). For comparison among multiple activation conditions, repeated measure one-way analysis of variance was performed and Tukey’s test used post analysis of variance for pairwise comparison, differences were significant if P-values <0.05. Data represent the mean±s.e.m., unless otherwise indicated.
ACKNOWLEDGEMENTS
We thank Marco Colona for CD40L-J558 cell line, Marina Cella and Dan Link for critical reading of paper and Paul Thompson (Division of Biostatistics) for statistical advice. We acknowledge 3M Corporation for kindly providing the TLR agonists. We thank Bill Eades and the Siteman Cancer Center Cell Sorter Core for expert assistance (NIH P30 CA91842). This study was supported by NIH K23 CA80851 (GPL).
References
- 1.Steinman RM. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13:1155–1159. doi: 10.1038/nm1643. [DOI] [PubMed] [Google Scholar]
- 2.Macagno A, Napolitani G, Lanzavecchia A, Sallusto F. Duration, combination and timing: the signal integration model of dendritic cell activation. Trends Immunol. 2007;28:227–233. doi: 10.1016/j.it.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Diebold SS. Determination of T-cell fate by dendritic cells. Immunol Cell Biol. 2008;86:389–397. doi: 10.1038/icb.2008.26. [DOI] [PubMed] [Google Scholar]
- 4.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernandez MG, Shen L, Rock KL. CD40 on APCs is needed for optimal programming, maintenance, and recall of CD8+ T cell memory even i n the absence of CD4+T cell help. J Immunol. 2008;180:4382–4390. doi: 10.4049/jimmunol.180.7.4382. [DOI] [PubMed] [Google Scholar]
- 6.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 7.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labeur MS, Roters B, Pers B, Mehling A, Luger TA, Schwarz T, et al. Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J Immunol. 1999;162:168–175. [PubMed] [Google Scholar]
- 9.Kelleher M, Beverley PC. Lipopolysaccharide modulation of dendritic cells is insufficient to mature dendritic cells to generate CTLs from naive polyclonal CD8+ T cells in vitro, whereas CD40 ligation is essential. J Immunol. 2001;167:6247–6255. doi: 10.4049/jimmunol.167.11.6247. [DOI] [PubMed] [Google Scholar]
- 10.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 11.Borrow P, Tishon A, Lee S, Xu J, Grewal IS, Oldstone MB, et al. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response. J Exp Med. 1996;183:2129–2142. [Google Scholar]
- 12.Lee BO, Hartson L, Randall TD. CD40-deficient, influenza-specific CD8 memory T cells develop and function normally in a CD40-sufficient environment. J Exp Med. 2003;198:1759–1764. doi: 10.1084/jem.20031440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez MG, Shen L, Rock KL. CD40-CD40 ligand interaction between dendritic cells and CD8+ T cells is needed to stimulate maximal T cell responses in the absence of CD4+T cell help. J Immunol. 2007;178:2844–2852. doi: 10.4049/jimmunol.178.5.2844. [DOI] [PubMed] [Google Scholar]
- 14.Lefrancois L, Altman JD, Williams K, Olson S. Soluble antigen and CD40 triggering are sufficient to induce primary and memory cytotoxic T cells. J Immunol. 2000;164:725–732. doi: 10.4049/jimmunol.164.2.725. [DOI] [PubMed] [Google Scholar]
- 15.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;84:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snijders A, Kalinski P, Hilkens CM, Kapsenberg ML. High-level IL-12 production by human dendritic cells requires two signals. Int Immunol. 1998;10:1593–1598. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- 17.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 18.Toka FN, Gierynska M, Suvas S, Schoenberger SP, Rouse BT. Rescue of memory CD8+ T cell reactivity in peptide/TLR9 ligand immunization by codelivery of cytokines or CD40 ligation. Virology. 2005;331:151–158. doi: 10.1016/j.virol.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Wills MR, Carmichael AJ, Mynard K, Jin X, Weekes MP, Plachter B, et al. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;66:249–255. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 22.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drake DR, III, Ream RM, Lawrence CW, Braciale TJ. Transient loss of MHC class I tetramer binding after CD8+ T cell activation reflects altered T cell effector function. J Immunol. 2005;175:1507–1515. doi: 10.4049/jimmunol.175.3.1507. [DOI] [PubMed] [Google Scholar]
- 24.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 25.Butz E, Bevan MJ. Dynamics of theCD8+ T cell response during acute LCMV infection. Adv Exp Med Biol. 1998;452:111–122. doi: 10.1007/978-1-4615-5355-7_13. [DOI] [PubMed] [Google Scholar]
- 26.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alves NL, van Leeuwen EM, Derks IA, van Lier RA. Differential regulation of human IL-7 receptor a expression by IL-7 and TCR signaling. J Immunol. 2008;180:5201–5210. doi: 10.4049/jimmunol.180.8.5201. [DOI] [PubMed] [Google Scholar]
- 28.Swainson L, Verhoeyen E, Cosset FL, Taylor N. IL-7R alpha gene expression is inversely correlated with cell cycle progression in IL-7-stimulated T lymphocytes. J Immunol. 2006;176:6702–6708. doi: 10.4049/jimmunol.176.11.6702. [DOI] [PubMed] [Google Scholar]
- 29.van Leeuwen EM, de Bree GJ, Remmerswaal EB, Yong SL, Tesselaar K, ten Berge IJ, et al. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood. 2005;106:2091–2098. doi: 10.1182/blood-2005-02-0449. [DOI] [PubMed] [Google Scholar]
- 30.Sauce D, Larsen M, Leese AM, Millar D, Khan N, Hislop AD, et al. IL-7R alpha versus CCR7 and CD45 as markers of virus-specific CD8+ T cell differentiation: contrasting pictures in blood and tonsillar lymphoid tissue. J Infect Dis. 2007;195:268–278. doi: 10.1086/510248. [DOI] [PubMed] [Google Scholar]
- 31.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, et al. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Surh CD, Boyman 0, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 34.Sorg RV, McLellan AD, Hock BD, Fearnley DB, Hart DN. Human dendritic cells express functional interleukin-7. Immunobiology. 1998;198:514–526. doi: 10.1016/S0171-2985(98)80075-2. [DOI] [PubMed] [Google Scholar]
- 35.Gerosa F, Baldani-Guerra B, Lyakh LA, Batoni G, Esin S, Winkler-Pickett RT, et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med. 2008;205:1447–1461. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 37.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 38.Napolitano LA, Grant RM, Deeks SG, Schmidt D, De Rosa SC, Herzenberg LA, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–79. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 39.Bolotin E, Annett G, Parkman R, Weinberg K. Serum levels of IL-7 in bone marrow transplant recipients: relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant. 1999;23:783–788. doi: 10.1038/sj.bmt.1701655. [DOI] [PubMed] [Google Scholar]
- 40.Fry TJ, Connick E, Falloon J, Lederman MM, Liewehr DJ, Spritzler J, et al. A potential role for interleukin-7 in T-cell homeostasis. Blood. 2001;97:2983–2990. doi: 10.1182/blood.v97.10.2983. [DOI] [PubMed] [Google Scholar]
- 41.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 42.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 43.Gagnon J, Ramanathan S, Leblanc C, Cloutier A, McDonald PP, llangumaran S. IL-6, in synergy with IL-7 or IL-15, stimulates TCR-independent proliferation and functional differentiation of CD8(+) T lymphocytes. J Immunol. 2008;80:7958–7968. doi: 10.4049/jimmunol.180.12.7958. [DOI] [PubMed] [Google Scholar]
- 44.Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, et al. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 45.Park JH, Adoro S, Lucas PJ, Sarafova SD, Alag AS, Doan LL, et al. ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007;8:1049–1059. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- 46.Linette GP, Zhang D, Hodi FS, Jonasch EP, Longerich S, Stowell CP, et al. Immunization using autologous dendritic cells pulsed with the melanoma-associated antigen gp100-derived G280-9V peptide elicits CD8+ immunity. Clin Cancer Res. 2005;11:7692–7699. doi: 10.1158/1078-0432.CCR-05-1198. [DOI] [PubMed] [Google Scholar]
- 47.Carreno BM, Anderson RW, Coligan JE, Biddison WE. HLA-B37 and HLA-A2.1 molecules bind largely nonoverlapping sets of peptides. Proc Natl Acad Sci USA. 1990;87:3420–3424. doi: 10.1073/pnas.87.9.3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bryant J, Day R, Whiteside TL, Herberman RB. Calculation of lytic units for the expression of cell-mediated cytotoxicity. J Immunol Methods. 1992;146:91–103. doi: 10.1016/0022-1759(92)90052-u. [DOI] [PubMed] [Google Scholar]