Abstract
BACKGROUND
Δ9-Tetrahydrocannabinol (THC) is the most frequently observed illicit drug in investigations of accidents and driving under the influence of drugs. THC-glucuronide has been suggested as a marker of recent cannabis use, but there are no blood data following controlled THC administration to test this hypothesis. Furthermore, there are no studies directly examining whole-blood cannabinoid pharmacokinetics, although this matrix is often the only available specimen.
METHODS
Participants (9 men, 1 woman) resided on a closed research unit and smoked one 6.8% THC cannabis cigarette ad libitum. We quantified THC, 11-hydroxy-THC (11-OH-THC), 11-nor-9-carboxy-THC (THCCOOH), cannabidiol (CBD), cannabinol (CBN), THC-glucuronide and THCCOOH-glucuronide directly in whole blood and plasma by liquid chromatography/ tandem mass spectrometry within 24 h of collection to obviate stability issues.
RESULTS
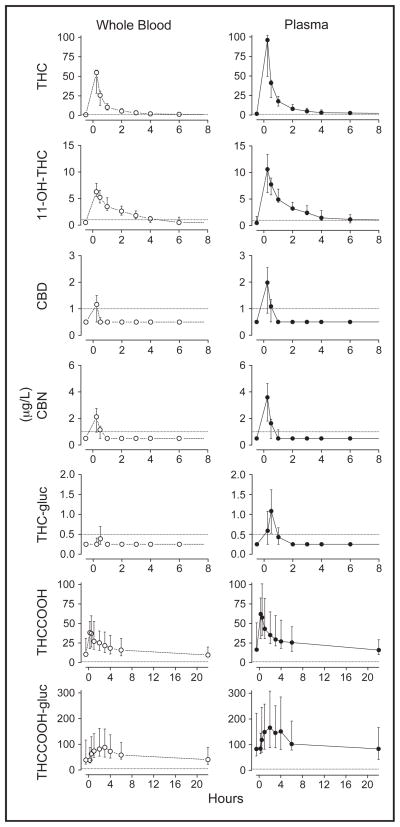
Median whole blood (plasma) observed maximum concentrations (Cmax) were 50 (76), 6.4 (10), 41 (67), 1.3 (2.0), 2.4 (3.6), 89 (190), and 0.7 (1.4) μg/L 0.25 h after starting smoking for THC, 11-OH-THC, THCCOOH, CBD, CBN, and THCCOOH-glucuronide, respectively, and 0.5 h for THC-glucuronide. At observed Cmax, whole-blood (plasma) detection rates were 60% (80%), 80% (90%), and 50% (80%) for CBD, CBN, and THC-glucuronide, respectively. CBD and CBN were not detectable after 1 h in either matrix (LOQ 1.0 μg/L).
CONCLUSIONS
Human whole-blood cannabinoid data following cannabis smoking will assist whole blood and plasma cannabinoid interpretation, while furthering identification of recent cannabis intake.
Cannabis intake substantially impacts public safety, since many individuals drive or operate complex equipment soon after self-administration. Indeed, cannabis is the most common illicit substance detected in blood and oral fluid of surveyed drivers (1). Drug presence does not necessarily imply impairment; however, cannabis detection windows in these matrices are relatively short for less-than-daily cannabis smokers (2–4), increasing impairment probability following a positive test.
The primary psychoactive chemical in cannabis, Δ9-tetrahydrocannabinol (THC),2 is metabolized via cytochrome P450 (CYP) 2C9 and 2C19 isoenzymes to several phase I metabolites, most prominently 11-hydroxy-THC (11-OH-THC) and 11-nor-9-carboxy-THC (THCCOOH) (5, 6). These analytes undergo phase II metabolism by uridine 5′-diphosphoglucuronosyltransferase (UGT) to produce cannabinoid glucuronides in vivo (7–9). Glucuronide formation facilitates excretion, but cannabinoid glucuronide pharmacological activity and detection windows following cannabis smoking are unknown, although these metabolites have been hypothesized to be markers of recent (10) or frequent (11) use. Characterization of major and minor cannabinoids in blood or plasma following cannabis smoking may enable researchers, physicians and law enforcement personnel to document recent cannabis intake, identify driving under the influence of drugs (DUID), or determine cannabinoid contributions to accident causation.
No studies, to our knowledge, have directly examined whole-blood cannabinoid pharmacokinetics in humans following cannabis smoking, although this matrix is often used for forensic and DUID work. Additionally, we know of no studies that have examined THC- or THCCOOH-glucuronide in whole blood or plasma following controlled, smoked cannabis administration. Plasma glucuronide/free ratios for THCCOOH following a single oral cannabis dose have been investigated (12 ), as have free and conjugated plasma THCCOOH concentrations following 5-mg intravenous THC (13 ). Other studies have examined glucuronides after self-reported administration (11, 14 ) or oral THC (dronabinol) dosing (15 ). Glucuronide concentrations typically have been determined indirectly by calculating the difference between total and free concentrations, with total analyte concentrations obtained after enzyme and/or alkaline hydrolysis.
In this study, we directly characterized, for the first time, free and glucuronidated THC and THCCOOH, 11-OH-THC, cannabidiol (CBD), and cannabinol (CBN) in cannabis smokers after a single smoked cannabis cigarette. Authentic whole-blood and plasma samples were simultaneously collected before and after smoking. These data provide an accurate and comprehensive cannabinoid metabolic profile in humans following cannabis smoking, yielding further insight into cannabinoid metabolism and documenting windows of drug detection for novel markers of recent cannabis smoking.
Materials and Methods
PARTICIPANTS
Participants provided written informed consent for this institutional review board–approved protocol. Inclusion criteria for participants were age 18–45 years, cannabis smoking at least twice monthly for 3 months before entry, positive urine cannabinoid test, normal cardiac function, and veins suitable for intravenous catheter placement. Clinically significant medical or psychiatric disease, physical dependence other than cannabis, pregnancy or nursing, history of cannabis-related psychosis or adverse reaction, drug treatment request, or blood donation in the previous 30 days were exclusionary. Participants were admitted to the secure research unit 15–20 h before dosing, with no cannabis use restrictions enforced before admission.
SMOKED CANNABIS ADMINISTRATION AND BLOOD COLLECTION
Cannabis cigarettes were obtained through the NIDA Chemistry and Physiological Systems Research Branch and independently assayed to contain 6.8% (0.2%) THC, 0.25% (0.08%) CBD, and 0.21% (0.02%) CBN (wt/wt). Mean cigarette weight was 0.79 (0.16) g, yielding total THC, CBD, and CBN content of 54, 2.0, and 1.7 mg per cigarette, respectively. Participants smoked a single cannabis cigarette ad libitum for 10 min. We collected venous blood samples via indwelling intravenous catheter into sodium heparin blood tubes on ice 0.5 h before and 0.25, 0.5, 1, 2, 3, 4, and 6 h after the start of smoking. Participants could optionally stay a second night with an additional blood collection at 22 h. Blood collected for plasma was centrifuged (1600g, 15 min) and plasma separated within 2 h. All samples were stored in polypropylene tubes at 4 °C until analysis within 24 h of collection.
CANNABINOID ANALYSIS
We quantified cannabinoids by a previously validated liquid chromatography–tandem mass spectrometry (LC-MS/MS) method (16 ). Briefly, we added 1.5 mL acetonitrile to 0.5 mL specimen (whole blood or plasma) to precipitate proteins. After mixing, the sample was centrifuged and the supernatant diluted and subjected to solid-phase extraction. The eluent was evaporated and reconstituted in mobile phase. Extracts were chromatographed via gradient elution at 400 μL/min, and detection was via electrospray ionization. Limits of quantification (LOQ) were 1 μg/L for THC, 11-OH-THC, THCCOOH, CBD, and CBN; 0.5 μg/L for THC-glucuronide; and 5 μg/L for THCCOOH-glucuronide.
CALCULATIONS AND STATISTICAL ANALYSIS
We performed noncompartmental pharmacokinetic analyses with WinNonlin Professional 5.2 for Windows (Pharsight Software). Highest observed concentrations (Cmax) and times to Cmax (Tmax) calculations included data only from participants with quantifiable analyte concentrations. One-way ANOVA with Tukey honestly significant difference (HSD) posthoc testing for molar glucuronide/free ratios used SPSS® 15.0 for Windows; all other descriptive statistical calculations were performed with GraphPad Prism 5.2 for Windows (GraphPad Software).
Results
SAMPLES
Participant demographics and self-reported cannabis use histories are detailed in Table 1. There was a wide range in age (18.5–45.7 years), body mass index (BMI) (18.1–32.0 kg/m2), and self-reported number of joints smoked in the previous 14 days (10–168 joints). Ten participants (9 male, 1 female) completed the protocol; 6 stayed for an additional night, yielding n = 6 for the 22-h time point. Samples (n = 85 each matrix) were collected without difficulty or appreciable hemolysis in all but participant I, in whom the 0.25-h specimen was not obtained due to catheter dislodgement.
Table 1.
Demographics and self-reported cannabis smoking characteristics for 10 adult cannabis smokers.
| Subject | Sex | Ethnicity | Age, years | BMI, kg/m2 | Days since last cannabis | Typical joints/day, n | Days of cannabis use in last 14 | Age at first cannabis use, years | 22-h sample? |
|---|---|---|---|---|---|---|---|---|---|
| A | M | White | 37.1 | 18.1 | a | 6 | 14 | 22 | Yes |
| B | M | African American | 26.5 | 30.6 | 3 | 2 | 10 | 16 | Yes |
| C | M | White | 45.7 | 32.0 | 1 | 5 | 14 | 12 | Yes |
| D | F | White | 27.7 | 22.3 | 1 | 6 | 8–9 | 15 | Yes |
| F | M | White | 34.1 | 23.0 | 2 | 1 | 10 | 16 | No |
| G | M | White | 22.2 | 22.9 | 1 | 12 | 14 | 15 | No |
| H | M | African American | 18.5 | 22.3 | 2 | 6 | 9 | 13 | Yes |
| I | M | African American | 41.5 | 25.5 | 3 | 6 | 12 | 19 | Yes |
| K | M | White | 22.5 | 20.9 | 4 | 2 | 13 | 16 | No |
| L | M | African American | 30.7 | 30.6 | 1 | 3 | 9 | 15 | No |
| Mean | 30.6 | 24.8 | 2.0 | 4.9 | 11.7 | 15.9 | |||
| SD | 8.9 | 4.7 | 1.1 | 3.2 | 2.2 | 2.8 |
Data not collected.
CANNABINOID DISPOSITION FOLLOWING CANNABIS SMOKING
Median (range) baseline whole-blood concentrations at 0.5 h before smoking were <LOQ (<LOQ–5.9) μ g/L for THC, <LOQ (<LOQ–2.0) μ g/L for 11-OH-THC, 10.5 (5.2–66) μ g/L for THCCOOH, and 39.0 (23–160) μ g/L for THCCOOH-glucuronide. Median (range) plasma concentrations at 0.5 h before smoking were 1.6 (<LOQ–7.3) μ g/L for THC, <LOQ (<LOQ–2.3) μ g/L for 11-OH-THC, 17 (8.2–100) μ g/L for THCCOOH and 83 (46–450) μ g/L for THCCOOH-glucuronide. THC-glucuronide, CBD, and CBN were <LOQ for all participants in whole-blood and plasma samples before smoking.
Whole-blood and plasma concentrations for all analytes were substantially higher than baseline 0.25 h after starting smoking (Fig. 1), although there was high interindividual variability for all analytes due to differences in ad libitum smoking topography and/or cannabinoid metabolism. Table 2 details the Cmax and Tmax values for each matrix. Minor cannabinoids (CBD, CBN, and THC-glucuronide) were not detected in all participants’ whole blood or plasma after cannabis smoking; when they were detected, however, maximum whole blood (plasma) concentrations were 2.1 (3.4), 2.9 (4.7), and 0.8 (2.3) μg/L, respectively.
Fig. 1. Median (interquartile range) whole-blood (○) and plasma (●) concentrations following smoking of a 6.8% THC cannabis cigarette.
Samples collected at −0.5, 0.25, 0.5, 1.0, 2.0, 3.0, 4.0, 6.0, and 22 h after starting smoking. Dotted lines indicate limits of quantification: 1 μg/L for THC, 11-OH-THC, THCCOOH, CBD, and CBN; 0.5 μg/L for THC-glucuronide (THC-gluc) and 5 μg/L for THCCOOH-glucuronide (THCCOOH-gluc).
Table 2.
Median (range) pharmacokinetic parameters following a single smoked 6.8% THC (wt/wt) cannabis cigarette in healthy adult cannabis users.
| Analyte | na | Tmax, h | Apparent Cmax (μ g/L) | C1 h, μg/Lb | C6 h, μ g/Lc | AUC0–6 h, h · μg/Ld |
|---|---|---|---|---|---|---|
| Whole blood | ||||||
| THC | 10 | 0.26 (0.25–0.50) | 50 (13–63) | 10 (3.3–25) | 1.4 (<LOQ–7.4)e | 60 (14–110) |
| 11-OH-THC | 10 | 0.26 (0.25–0.50) | 6.4 (3.2–8.8) | 3.5 (1.5–6.3) | <LOQ (<LOQ–2.0) | 11 (3.9–23) |
| THCCOOH | 10 | 0.40 (0.25–2.0) | 41 (19–80) | 27 (11–72) | 16 (6.4–39) | 140 (61–360) |
| CBD | 6 | 0.25 (0.25–0.50) | 1.3 (<LOQ–2.1) | <LOQ | <LOQ | NCf |
| CBN | 8 | 0.27 (0.25–0.50) | 2.4 (<LOQ–2.9) | <LOQ (<LOQ–1.4) | <LOQ | NC |
| THC-glucuronide | 5 | 0.50 (0.48–0.50) | 0.7 (<LOQ–0.8) | <LOQ | <LOQ | NC |
| THCCOOH-glucuronide | 10 | 2.0 (0.98–3.0) | 89 (46–220) | 74 (30–220) | 58 (21–140) | 440 (180–1100) |
| Plasma | ||||||
| THC | 10 | 0.26 (0.25–0.50) | 76 (18–110) | 18 (5.2–28) | 2.6 (1.0–9.2) | 110 (25–135) |
| 11-OH-THC | 10 | 0.26 (0.25–0.50) | 10 (4.0–16) | 4.9 (2.0–14) | 1.2 (<LOQ–3.0) | 19 (7.5–42) |
| THCCOOH | 10 | 0.50 (0.25–0.52) | 67 (27–110) | 43 (19–87) | 26 (9.6–61) | 210 (93–480) |
| CBD | 9 | 0.25 (0.25–0.50) | 2.0 (<LOQ–3.4) | <LOQ (<LOQ–1.1) | <LOQ | NC |
| CBN | 10 | 0.25 (0.25–0.50) | 3.6 (<LOQ–4.7) | 1.2 (<LOQ–1.7) | <LOQ | NC |
| THC-glucuronide | 8 | 0.50 (0.48–0.52) | 1.4 (<LOQ–2.3) | 0.6 (<LOQ–1.0) | <LOQ | NC |
| THCCOOH-glucuronide | 10 | 2.0 (0.28–4.0) | 190 (68–460) | 150 (64–420) | 100 (48–350) | 840 (350–2400) |
Number of samples included in calculation of Tmax and Cmax.
Concentration at t = 1 h.
Concentration at t = 6 h.
Area under the concentration–time curve from 0 to 6 h.
LOQ for THC, 11-OH-THC, THCCOOH, CBD, and CBN, 1 μg/L; for THC-glucuronide, 0.5 μg/L; for THCCOOH-glucuronide 5 μg/L.
NC, not calculable (too few points to determine).
After Tmax, whole blood and plasma concentrations decreased rapidly for all analytes except THC-COOH-glucuronide; concentrations for this analyte remained increased for approximately 3–4 h after smoking. From 6 h postsmoking, median whole-blood and plasma THCCOOH-glucuronide concentrations decreased gradually, returning to baseline by 22 h. Within 1 h, median whole-blood concentrations for CBD, CBN, and THC-glucuronide were <LOQ, and median plasma concentrations were <2 μg/L for these analytes.
Twenty-two hours after smoking, whole blood concentrations (n = 6) for THC, 11-OH-THC, CBD, CBN, and THC-glucuronide were <LOQ for all but participant I, who had residual THC and 11-OH-THC concentrations of 5.0 and 1.7 μg/L, respectively. Similar results were obtained in plasma, although a second participant also was positive for THC and 11-OH-THC at this time. THCCOOH and THCCOOH-glucuronide concentrations were variable in these extended samples, ranging from 4.4–43 μg/L to 15–190 μg/L in whole blood and 7.0–59 μg/L to 36–180 μg/L in plasma, respectively.
GLUCURONIDE/FREE CANNABINOID RATIOS
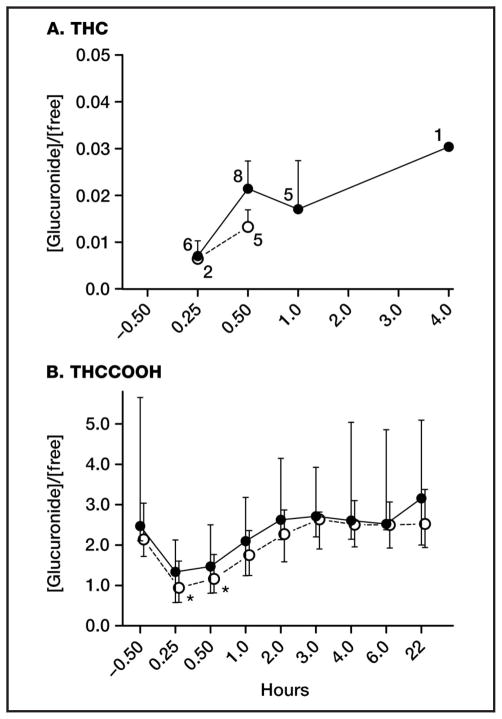
We determined molar glucuronide/free cannabinoid ratios for THC and THCCOOH in both matrices (Fig. 2, A and B). Throughout the study, THC (Fig. 2A) molar glucuronide/free ratios across participants ranged from 0.006 to 0.019 (median 0.013) in whole blood and 0.006 to 0.041 (median 0.019) in plasma, indicating minimal THC-glucuronide formation relative to free THC. THCCOOH (Fig. 2B) molar ratios were markedly different, ranging from 0.43 to 5.30 (median 2.05) in whole blood and 0.45 to 10.93 (median 2.39) in plasma across participants and time. Whole-blood median ratios were significantly lower immediately following smoking (0.25 and 0.5 h) compared with baseline (P = 0.009 and 0.037, respectively); similar nonsignificant differences were observed in plasma (P = 0.170 and 0.330). As free THCCOOH concentrations decreased and THCCOOH-glucuronide concentrations remained increased 0.5–2 h after smoking, glucuronide/free ratios returned to baseline concentrations in both matrices for the remainder of the session.
Fig. 2. Median (interquartile range) molar whole-blood (○) and plasma (●) [glucuronide]/[free] ratios for THC (A) and THCCOOH (B) following a 6.8% THC cannabis cigarette. n values indicate data points for THC; for THCCOOH, n = 9, 6, and 10 at 0.25 h, 22 h, and all other times, respectively.
THC-glucuronide was <LOQ (0.5 μg/L) after 1 h in whole blood and 4.0 h in plasma. *P < 0.05 compared to baseline, Tukey HSD.
CANNABINOID DETECTION RATES
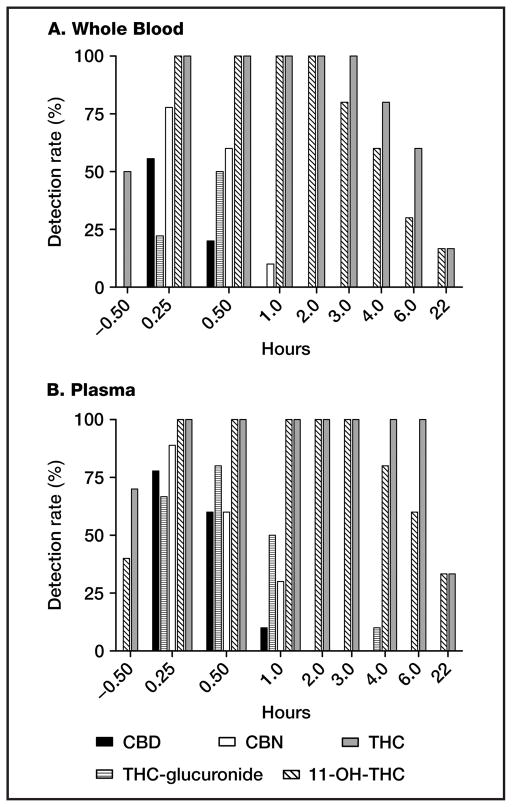
We investigated cannabinoid detection rates in whole blood and plasma following cannabis smoking (Fig. 3, A and B). THCCOOH and THCCOOH-glucuronide were quantified in all samples. THC and 11-OH-THC were quantified in all whole-blood and plasma samples for at least 2 h; after this time, detection rates decreased, with 10% detection in whole blood and 30% detection in plasma 22 h after smoking. In whole blood, CBD, CBN, and THC-glucuronide were detected 0.25 h after smoking in 60%, 80%, and 20% of samples, respectively (LOQs 1.0, 1.0, and 0.5 μg/L). By 1 h after smoking, however, CBN was the only minor cannabinoid detected in whole blood, and only for a single participant. In plasma, CBD, CBN, and THC-glucuronide were detected at 0.25 h in 80%, 90%, and 70% of samples, respectively, with detection of all 3 analytes for at least 1 participant at 1 h. Detection rates for THC-glucuronide were highest in both whole blood and plasma at 0.5 h, corresponding with the observed Tmax for this analyte, whereas other analyte detection rates were highest 0.25 h after smoking.
Fig. 3. Cannabinoid detection rates following a single smoked 6.8% THC cannabis cigarette in whole blood (A) and plasma (B). n = 9, 6, and 10 at 0.25 h, 22 h, and all other times, respectively.
All samples contained THCCOOH and THCCOOH-glucuronide. Limits of quantification: 1 μg/L for THC, 11-OH-THC, CBD, CBN, and THCCOOH; 0.5 μg/L for THC-glucuronide; 5.0 μg/L for THCCOOH-glucuronide.
Discussion
This study directly characterizes, for the first time, cannabinoid glucuronides in both whole blood and plasma following controlled smoked cannabis. The minor cannabinoids CBD and CBN were also included, yielding novel cannabinoid pharmacokinetic data following smoked cannabis administration. This study describes (1) directly determined whole-blood cannabinoid and cannabinoid glucuronide concentrations following cannabis smoking, providing a scientific database for interpretation of whole blood forensic and clinical investigations, and (2) whole-blood and plasma concentrations for minor cannabinoids THC-glucuronide, CBD, and CBN, which indicate that these minor cannabinoids warrant further investigation as markers of recent (<2 h) smoked cannabis intake.
All samples were collected on ice, processed, transferred to refrigerated storage within 2 h of collection, and analyzed by LC-MS/MS within 24 h to minimize concentration changes due to analyte instability. Many studies have investigated cannabinoid analyte instability in vitro, with results dependent on collection technique (17), storage container characteristics (18, 19), and storage time (20) and conditions (20–22). The ester-linked THCCOOH-glucuronide is susceptible to instability, especially at increased storage temperature and time (20). The present study circumvented these concerns by quantifying potentially unstable acyl glucuronides within 24 h of specimen collection, a period of <5% spontaneous hydrolysis (20).
Concentrations were variable between participants. Whole-blood THC observed Cmax values ranged from 13 to 63 μ g/L (median 50 μ g/L) 15 min after starting smoking. Similarly, plasma THC Cmax values ranged from 18 to 110 μ g/L (median 76 μ g/L). Plasma concentrations were similar to or slightly lower than those reported previously (2, 3, 23–25). Administration of 23.1% THC (69.4 mg) in cannabis/tobacco cigarettes via a paced smoking procedure over 22 min (24) yielded a mean plasma Cmax of 190 μ g/L (range 24–262 μ g/L). Huestis et al. (3) administered 3.55% THC (33.8 mg) in cannabis cigarettes via a paced procedure over 11.2 min; mean (range) peak plasma THC concentration for 6 participants was 162 (76–267) μ g/L.
Smoking topography (i.e., number of puffs, depth of inhalation, hold time, and time between puffs) and sidestream smoke production (26) during ad libitum smoking introduced variability in whole blood and plasma cannabinoid concentrations, similar to that observed in other well-controlled paced (3, 24, 27) and nonpaced (28) smoking studies. Ethical considerations currently prohibit paced smoking protocols, due to the possibility of forcing participants to consume more THC and at a faster rate than when self-administered. Ad libitum dosing, while more variable, is ideal, as it more closely represents authentic smoking behavior, providing more realistic concentrations while minimizing adverse events. Indeed, only 2 adverse events were reported in the present study, neither of which led to study withdrawal. Participants stopped smoking as they reached their desired “high,” limiting higher THC concentrations and untoward effects. The slight discrepancy in concentrations between this and prior studies is likely attributable to study design: both Huestis et al. (3) and Hunault et al. (24) collected plasma closer to the onset of smoking. Actual Cmax and Tmax likely occurred before the last puff (3), and data at these times were not captured in the present study.
Few prior studies have examined cannabinoid glucuronide concentrations in any matrix (10, 12–15, 29); none investigated whole-blood concentrations. Skopp and Potsch (14) measured THCCOOH-glucuronide directly (without hydrolysis) in serum 24–48 h after self-reported cannabis intake. Concentrations ranged from 9–1048 μg/L for “heavy” users (>1 joint per day), 35–368 μg/L for “moderate” users (>1 joint per week but <1 joint per day) and 10–204 μg/L for “light” users (<1 joint per week). In the present cohort, plasma THCCOOH-glucuronide concentrations were 35–460 μg/L over the entire study, with self-reported cannabis intake on more than half the days preceding study sessions. The highest observed THCCOOH-glucuronide concentrations at each blood collection were from the heaviest smoker (participant G, 12 joints/day on 14 of 14 days), whereas the lowest observed concentrations were often from participant K, who self-reported last smoking 4 days before dosing. Although lower THCCOOH-glucuronide concentrations generally were observed with decreasing self-reported intake, we could not differentiate between light, moderate, or heavy cannabis smokers solely on the basis of an individual blood specimen. This concurs with Skopp and Potsch (14), who observed variable cannabinoid concentrations, precluding accurate frequency of use determinations.
Median THCCOOH-glucuronide concentrations were substantially higher than free THCCOOH concentrations in whole blood and plasma in the present study, although the glucuronide/free ratio was variable within and between participants. This ratio was >5.0 in both matrices on several occasions, indicating extensive ester-linked glucuronidation. However, we observed significant and nonsignificant glucuronide/free ratio decreases in whole blood and plasma, respectively, 15–30 min after smoking as a result of rapid THCCOOH formation. UGT-catalyzed formation of THCCOOH-glucuronide from free THCCOOH is likely the rate-limiting step: THCCOOH concentrations increase more rapidly than THCCOOH-glucuronide concentrations immediately after smoking, decreasing the ratio. As time passes, remaining acid metabolite is glucuronidated, and the ratio returns to baseline within 1–2 h postsmoking in both matrices, remaining constant thereafter. This phenomenon was previously reported by Kelly and Jones (13), who also observed a brief decline in glucuronide/free ratios in plasma for up to 30 min following cannabis smoking. Further examination of this ratio may provide a marker of recent cannabis intake; however, a potential limitation with this approach is the frequent lengthy delay between cannabis smoking and sample collection during DUID and other investigations. Based on present and past data, samples obtained 2–3 h after smoking would not be useful because THCCOOH glucuronide/ free ratios may have already returned to baseline.
The ether glucuronide of THC has been proposed as a marker of recent cannabis intake (10, 12), yet few prior studies examined this metabolite in blood. Skopp et al. (11) observed low THC-glucuronide concentrations in urine from heavy and moderate cannabis users, but not occasional users. Schwilke et al. (15) observed significant increases of approximately 9.5% (P < 0.001) and 170% (P < 0.001) in plasma THC and THCCOOH concentrations, respectively, after Escherichia coli β-glucuronidase hydrolysis in samples collected during extended oral THC (dronabinol) administration. Our study reported here documented 2% median (0.6%–4.1%) glucuronide/free molar ratios for THC and 240% median (45%–1000%) glucuronide/free molar ratios for THCCOOH in plasma. The discrepancies in observed glucuronidation rates vs Schwilke et al. (15) are likely due to different routes of administration (smoked and oral) for THC and are likely methods-based for THCCOOH. Enteric glucuronidation after oral dronabinol potentially yielded higher THC glucuronidation rates, whereas inefficient enzymatic THCCOOH-glucuronide hydrolysis (30) may explain the discrepancy in observed THCCOOH glucuronidation rates.
THC-glucuronide concentrations were low in our study, with peak whole-blood and plasma concentrations of 0.8 and 2.3 μg/L, respectively. We are not aware of any in vitro or in vivo studies evaluating whether this metabolite possesses pharmacological activity similar to THC. Still, it is likely that observed concentrations would yield little or no effect on THC-induced impairment, as the glucuronide is present only immediately after smoking, when whole blood concentrations of THC are typically >10 μg/L. Additionally, this minor metabolite was not observed in all participants; maximum detection rates were 50% and 80% in whole blood and plasma, respectively. Concentrations decreased to <LOQ in all but 1 specimen by 2 h after smoking.
Observed detection rates render THC-glucuronide an inclusionary, but not exclusionary, marker for recent cannabis intake at a 0.5 μg/L LOQ. CBD and CBN had similar detection windows in whole blood and plasma, with CBN more prevalent than CBD between 0.25 and 1 h. These analytes were not detected beyond 2 h after smoking, rendering them possible candidates for markers of recent cannabis smoking. CBD and CBN are amenable to GC-MS analysis, are often readily extracted by current mixed-mode solid-phase extraction (SPE) procedures, and have commercially available deuterium-labeled internal standards, unlike cannabinoid glucuronides. However, concentrations of these analytes in cannabis vary depending on strain (31) and storage time and conditions (32), potentially altering detection rates. Additionally, these cannabinoids are present in cannabis smoke (33, 34) and, unlike THC-glucuronide, could possibly be detected following passive exposure. If detection limits improve for these minor cannabinoids, further study could suggest potential cutoffs and analytical approaches for confirming these analytes as markers of recent cannabis intake.
These data advance our understanding of THC metabolism following smoked cannabis. No data on 11-OH-THC-glucuronide, ether-linked THCCOOH-glucuronide, or di/bis-THCCOOH-glucuronide were collected, since standards for these analytes are not currently available; however, ester-linked THCCOOH-glucuronide is the predominant species (15, 35). THC, THCCOOH, and THCCOOH-glucuronide were observed in baseline samples owing to the relatively short (15–19 h) residential stay before dosing. The aim of the residential stay was to eliminate potential intoxication or impairment, not fully eliminate previously self-administered cannabinoids from biological samples. Because baseline concentrations of THC and 11-OH-THC were negligible compared with concentrations after dosing, error resulting from residual cannabinoids was minimal for these analytes. Candidates for markers of recent cannabis intake were <LOQ in all participants in the baseline specimen, enabling accurate detection window determination. Finally, varying cannabis composition could yield increased or decreased CBD or CBN content, leading to altered intake and detection windows. Cannabinoid pharmacotherapy such as Sativex® also complicates interpretation, as low CBD concentrations are likely following intake (36).
To our knowledge, these are the first directly measured authentic human whole-blood and plasma cannabinoid glucuronide data following cannabis smoking. Furthermore, we provide simultaneous THC, 11-OH-THC, THCCOOH, CBD, and CBN concentrations. Observed peak cannabinoid concentrations were variable, likely due to interindividual differences in smoking topography and prior cannabis smoking history. Direct glucuronide detection provided 3 primary benefits: (1) THCCOOH-glucuronide detection may improve interpretation of results, since high baseline THCCOOH-glucuronide concentrations may indicate chronic, daily cannabis smokers, precluding the need for recent cannabis-use predictive models (37); (2) detection of THC-glucuronide provides a potential new marker for recent cannabis intake, because concentrations rapidly fall below LOQ in whole blood and plasma; and (3) direct LC-MS/MS detection precludes time- and resource-intensive specimen hydrolysis and sample derivatization, yielding savings over typical GC-MS procedures and improving accuracy of measurement. Minor amounts of CBD and CBN were detected in some, but not all, participants’ samples for up to 2 h following smoking, rendering these cannabinoids additional potential markers of recent cannabis intake. These data provide valuable information for interpreting whole-blood cannabinoid concentrations in forensic DUID accident investigations and clinical monitoring scenarios, while furthering our understanding of potential markers of recent cannabis intake.
Acknowledgments
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
We acknowledge Karl Scheidweiler for technical assistance in manuscript preparation and the NIDA Intramural Research Program clinical research team, especially Rebecca Price, for clinical support. This research was supported by the Intramural Research Program, National Institute on Drug Abuse, NIH.
Footnotes
Nonstandard abbreviations: THC, Δ9-tetrahydrocannabinol; CYP, cytochrome P450; 11-OH-THC, 11-hydroxy-Δ9-tetrahydrocannabinol; THCCOOH, 11-nor-9-carboxy-Δ9-tetrahydrocannabinol; UGT, uridine 5′-diphosphoglucuronosyl-transferase; DUID, driving under the influence of drugs; CBD, cannabidiol; CBN, cannabinol; LC-MS/MS, liquid chromatography–tandem mass spectrometry; LOQ, limit of quantification; HSD, honestly significant difference; BMI, body mass index; Cmax, highest observed analyte concentration; Tmax, observed time to Cmax; SPE, solid-phase extraction.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Research Funding: D.M. Schwope, Intramural Research Program, National Institute on Drug Abuse, NIH; M.A. Huestis, Intramural Research Program, National Institute on Drug Abuse, NIH.
Expert Testimony: None declared.
References
- 1.Compton R, Berning A. Results of the 2007 national roadside survey of alcohol and drug use by drivers. Washington (DC): NHTSA; 2009. DOT HS 811 175. [Google Scholar]
- 2.Kauert GF, Ramaekers JG, Schneider E, Moeller MR, Toennes SW. Pharmacokinetic properties of Delta9-tetrahydrocannabinol in serum and oral fluid. J Anal Toxicol. 2007;31:288 –93. doi: 10.1093/jat/31.5.288. [DOI] [PubMed] [Google Scholar]
- 3.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16:276 –82. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- 4.Niedbala RS, Kardos KW, Fritch DF, Kardos S, Fries T, Waga J, et al. Detection of marijuana use by oral fluid and urine analysis following single-dose administration of smoked and oral marijuana. J Anal Toxicol. 2001;25:289 –303. doi: 10.1093/jat/25.5.289. [DOI] [PubMed] [Google Scholar]
- 5.Matsunaga T, Iwawaki Y, Watanabe K, Yamamoto I, Kageyama T, Yoshimura H. Metabolism of delta-9-tetrahydrocannabinol by cytochrome P450 isozymes purified from hepatic microsomes of monkeys. Life Sci. 1995;56:2089 –95. doi: 10.1016/0024-3205(95)00193-a. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe K, Yamaori S, Funahashi T, Kimura T, Yamamoto I. Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci. 2007;80:1415–9. doi: 10.1016/j.lfs.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Halldin MM, Carlsson S, Kanter SL, Widman M, Agurell S. Urinary metabolites of delta-1-tetrahydrocannabinol in man. Arzneim Forsch. 1982;32:764 – 8. [PubMed] [Google Scholar]
- 8.Williams PL, Moffat AC. Identification in human urine of Delta 9-tetrahydrocannabinol-11-oic acid glucuronide: a tetrahydrocannabinol metabolite. J Pharm Pharmacol. 1980;32:445– 8. doi: 10.1111/j.2042-7158.1980.tb12966.x. [DOI] [PubMed] [Google Scholar]
- 9.Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M. Metabolism, disposition, and kinetics of Delta-9-tetrahydrocannabinol in men and women. Clin Pharmacol Ther. 1983;34:352–63. doi: 10.1038/clpt.1983.179. [DOI] [PubMed] [Google Scholar]
- 10.Mareck U, Haenelt N, Geyer H, Guddat S, Kamber M, Brenneisen R, et al. Temporal indication of cannabis use by means of THC glucuronide determination. Drug Test Anal. 2009;1:505–10. doi: 10.1002/dta.106. [DOI] [PubMed] [Google Scholar]
- 11.Skopp G, Pötsch L, Ganßmann B, Mauden M, Richter B, Aderjan R, Mattern R. Freie und glucuronidierte Cannabinoide im Urin – Untersuchungen zur Einschätzung des Konsumverhaltens. Rechtsmedizin. 1999;10:21– 8. [Google Scholar]
- 12.Law B, Mason PA, Moffat AC, Gleadle RI, King LJ. Forensic aspects of the metabolism and excretion of cannabinoids following oral ingestion of cannabis resin. J Pharm Pharmacol. 1984;36:289 –94. doi: 10.1111/j.2042-7158.1984.tb04376.x. [DOI] [PubMed] [Google Scholar]
- 13.Kelly P, Jones RT. Metabolism of tetrahydrocannabinol in frequent and infrequent marijuana users. J Anal Toxicol. 1992;16:228 –35. doi: 10.1093/jat/16.4.228. [DOI] [PubMed] [Google Scholar]
- 14.Skopp G, Potsch L. Cannabinoid concentrations in spot serum samples 24 – 48 hours after discontinuation of cannabis smoking. J Anal Toxicol. 2008;32:160 – 4. doi: 10.1093/jat/32.2.160. [DOI] [PubMed] [Google Scholar]
- 15.Schwilke EW, Schwope DM, Karschner EL, Lowe RH, Darwin WD, Kelly DL, et al. Delta9-tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC plasma pharmacokinetics during and after continuous high-dose oral THC. Clin Chem. 2009;55:2180 –9. doi: 10.1373/clinchem.2008.122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwope DM, Scheidweiler KB, Huestis MA. Direct quantification of cannabinoids and cannabinoid glucuronides in whole blood by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401:1273– 83. doi: 10.1007/s00216-011-5197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCurdy HH, Callahan LS, Williams RD. Studies on the stability and detection of cocaine, benzoylecgonine, and 11-nor-Delta-9-tetrahydrocannabinol-9-carboxylic acid in whole blood using Abuscreen radioimmunoassay. J Forensic Sci. 1989;34:858–70. [PubMed] [Google Scholar]
- 18.Christophersen AS. Tetrahydrocannabinol stability in whole blood: plastic versus glass containers. J Anal Toxicol. 1986;10:129 –31. doi: 10.1093/jat/10.4.129. [DOI] [PubMed] [Google Scholar]
- 19.Stout PR, Horn CK, Lesser DR. Loss of THCCOOH from urine specimens stored in polypropylene and polyethylene containers at different temperatures. J Anal Toxicol. 2000;24:567–71. doi: 10.1093/jat/24.7.567. [DOI] [PubMed] [Google Scholar]
- 20.Skopp G, Potsch L. Stability of 11-nor-Delta(9)-carboxytetrahydrocannabinol glucuronide in plasma and urine assessed by liquid chromatography-tandem mass spectrometry. Clin Chem. 2002;48:301–6. [PubMed] [Google Scholar]
- 21.Wong AS, Orbanosky MW, Reeve VC, Beede JD. Stability of delta-9-tetrahydrocannabinol in stored blood and serum. In: Hawks RL, editor. The analysis of cannabinoids in biological fluids. Rockville (MD): National Institute on Drug Abuse; 1982. pp. 119–24. [PubMed] [Google Scholar]
- 22.Schwilke EW, Karschner EL, Lowe RH, Gordon AM, Cadet JL, Herning R, Huestis MA. Intra- and intersubject whole blood/plasma cannabinoid ratios determined by 2-dimensional, electron impact GC-MS with cryofocusing. Clin Chem. 2009;55:1188 –95. doi: 10.1373/clinchem.2008.114405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manno JE, Manno BR, Kemp PM, Alford DD, Abukhalaf IK, McWilliams ME, et al. Temporal indication of marijuana use can be estimated from plasma and urine concentrations of Delta-9-tetrahydrocannabinol, 11-hydroxy-Delta-9-tetrahydrocannabinol, and 11-nor-Delta-9-tetrahydrocannabinol-9-carboxylic acid. J Anal Toxicol. 2001;25:538 – 49. doi: 10.1093/jat/25.7.538. [DOI] [PubMed] [Google Scholar]
- 24.Hunault CC, Mensinga TT, de Vries I, Kelholt-Dijkman HH, Hoek J, Kruidenier M, et al. Delta-9-tetrahydrocannabinol (THC) serum concentrations and pharmacological effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg THC. Psychopharmacology (Berl) 2008;201:171– 81. doi: 10.1007/s00213-008-1260-2. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Reyes M, Di Guiseppi S, Davis KH, Schindler VH, Cook CE. Comparison of effects of marijuana cigarettes of three different potencies. Clin Pharmacol Ther. 1982;31:617–24. doi: 10.1038/clpt.1982.86. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Reyes M, Owens SM, Di Guiseppi S. The clinical pharmacology and dynamics of marijuana cigarette smoking. J Clin Pharmacol. 1981;21:201S–7S. doi: 10.1002/j.1552-4604.1981.tb02596.x. [DOI] [PubMed] [Google Scholar]
- 27.Ramaekers JG, Moeller MR, van Ruitenbeek P, Theunissen EL, Schneider E, Kauert G. Cognition and motor control as a function of Delta9-THC concentration in serum and oral fluid: limits of impairment. Drug Alcohol Depend. 2006;85:114 –22. doi: 10.1016/j.drugalcdep.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Lindgren JE, Ohlsson A, Agurell S, Hollister L, Gillespie H. Clinical effects and plasma levels of Delta9-tetrahydrocannabinol (Delta9-THC) in heavy and light users of cannabis. Psychopharmacology. 1981;74:208 –12. doi: 10.1007/BF00427095. [DOI] [PubMed] [Google Scholar]
- 29.Wall ME, Taylor HL. Conjugation of acidic metabolites of Delta-8 and Delta-9-THC in man. In: Harvey DJ, editor. Marihuana ’84: proceedings of the Oxford Symposium on Cannabis. Oxford: IRL Press; 1985. pp. 69–76. [Google Scholar]
- 30.Abraham TT, Lowe RH, Pirnay SO, Darwin WD, Huestis MA. Simultaneous GC-EI-MS determination of Delta9-tetrahydrocannabinol, 11-hydroxy-Delta9-tetrahydrocannabinol, and 11-nor-9-carboxy-Delta9-tetrahydrocannabinol in human urine following tandem enzyme-alkaline hydrolysis. J Anal Toxicol. 2007;31:477–85. doi: 10.1093/jat/31.8.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hemphill JK, Turner JC, Mahlberg PG. Cannabinoid content of individual plant organs from different geographical strains of Cannabis sativa L. J Nat Prod. 1980;43:112–22. [Google Scholar]
- 32.ElSohly MA. Chem constituents of cannabis. In: Grotenhermen F, Russo E, editors. Cannabis and cannabinoids: pharmacology, toxicology, and therapeutic potential. New York: Haworth Integrative Healing Press; 2002. pp. 27–36. [Google Scholar]
- 33.Pomahacova B, Van der Kooy F, Verpoorte R. Cannabis smoke condensate III: the cannabinoid content of vaporised Cannabis sativa. Inhal Toxicol. 2009;21:1108 –12. doi: 10.3109/08958370902748559. [DOI] [PubMed] [Google Scholar]
- 34.ElSohly HN, ElSohly MA. Marijuana smoke condensate: chemistry and pharmacology. In: El-Sohly, editor. Marijuana and the cannabinoids. Totowa (NJ): Humana Press; 2007. pp. 67–96. [Google Scholar]
- 35.Moran J, Radomińska-Pandya A, Miller G, Finel M, Gallus-Zawada A, Bratton S, et al. Characterization of human hepatic and extrahepatic UDP-glucuronosyltransferase enzymes involved in the metabolism of classic cannabinoids. Drug Metab Dispos. 2009;37:1496 –504. doi: 10.1124/dmd.109.026898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA. Plasma cannabinoid pharmacokinetics following controlled oral Delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem. 2011;57:66 –75. doi: 10.1373/clinchem.2010.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. II. Models for the prediction of time of marijuana exposure from plasma concentrations of Delta-9-tetrahydrocannabinol (THC) and 11-nor-9-carboxy-Delta-9-tetrahydrocannabinol (THCCOOH) J Anal Toxicol. 1992;16:283–90. doi: 10.1093/jat/16.5.283. [DOI] [PubMed] [Google Scholar]