Abstract
Since 2004, when the World Anti-Doping Agency assumed the responsi-bility for establishing and maintaining the list of prohibited substances and methods in sport (i.e. the Prohibited List), cannabinoids have been prohibited in all sports during competition. The basis for this prohibition can be found in the World Anti-Doping Code, which defines the three criteria used to consider banning a substance. In this context, we discuss the potential of can-nabis to enhance sports performance, the risk it poses to the athlete’s health and its violation of the spirit of sport. Although these compounds are prohibited in-competition only, we explain why the pharmacokinetics of their main psychoactive compound, Δ9-tetrahydrocannabinol, may complicate the results management of adverse analytical findings. Passive inhalation does not appear to be a plausible explanation for a positive test. Although the prohibition of cannabinoids in sports is one of the most controversial issues in anti-doping, in this review we stress the reasons behind this prohibition, with strong emphasis on the evolving knowledge of cannabinoid pharmacology.
1. Introduction
Since the World Anti-Doping Agency (WADA) was conceived in 1999 by the sport movement and governments of the world to fight against doping in sport in all its forms, the prohibition of cannabis in sport has been one of the controversial issues debated by the scientific and political anti-doping authorities. Prior to 2004 and the establishment of the World Anti-Doping Code (Code) by WADA, cannabinoids were prohibited only in certain sports.[1] The decision was left to the governing international sport federation as to whether can-nabinoids were prohibited in their discipline(s) and whether anti-doping tests were conducted. Consequently, a limited population of athletes was tested and sanctioned for cannabis anti-doping rule violations.
In 2004, WADA assumed responsibility for establishing the list of prohibited substances and methods in sport (the Prohibited List). In the first Prohibited List, published under the auspices of WADA that same year, prohibition of cannabi-noids was extended to all sports in-competition.[2] When the Code and its related international standard publications: the Prohibited List, Testing, Laboratories and Therapeutic Use Exemptions were presented for discussion and adoption by the sport movement and world governments at the second World Conference on Doping in Sport, held in Copenhagen in March 2003, extension of the cannabinoids ban to all sports was one of the most controversial issues. Some delegates strongly argued that cannabinoids should not be included in sport regulations because consumption of can-nabis is not performance enhancing in sports and therefore it should remain a social issue; conversely, others claimed that cannabis is performance enhancing and, because it is an illegal substance in most countries and because athletes are role models in modern society, cannabinoids should be prohibited at all times, in- and out-of-competition. Facing such polarized opinions, the final decision to only prohibit cannabinoids in-competition in all sports appeared to be an acceptable compromise.
The criteria for inclusion of a substance, a class of substances, or a method in the Prohibited List are defined in section 4 of the Code.[3] The criteria are (i) potential to enhance performance; (ii) risk for the athletes’ health; and (iii) violation of the spirit of sport. Although heavily debated during drafting of the Code, the 2003 Code[4] and its revised 2009[3] version clearly confirmed the equal weight of the three criteria as an essential principle of the Prohibited List. After thorough consideration by the WADA scientific committees, prohibition of cannabinoids across all sports in-competition was established and this remained in force in subsequent versions of the Prohibited List. The vast majority of sport and governmental representatives agree on the status of cannabis; however, the subject continues to be frequently debated.
The objective of this review is to provide the scientific data to support the status of cannabis vis-à-vis the Prohibited List, and to further explore cannabinoid pharmacokinetics and pharmacology after acute and chronic exposure in the context of the fight against doping in sport.
2. Literature Search Methodology
The PubMed database was initially searched for scientific articles with no time restriction and with the following keywords: ‘cannabis’, ‘marijuana’, ‘anti-doping/antidoping’ and ‘drug testing’. Additional articles originated from references in selected manuscripts. The article describes WADA’s position on cannabis in sport, but acknowledges different points of view on this controversial issue. Therefore, there were no attempts to target and exclude information contradicting WADA’s views. Data from legal cases were obtained from the Court of Arbitration for Sport. Finally, original data collected in cannabis research conducted by one of the authors, Prof. Marilyn A. Huestis, were also included to inform the review.
3. Pharmacodynamic Effects of Cannabis
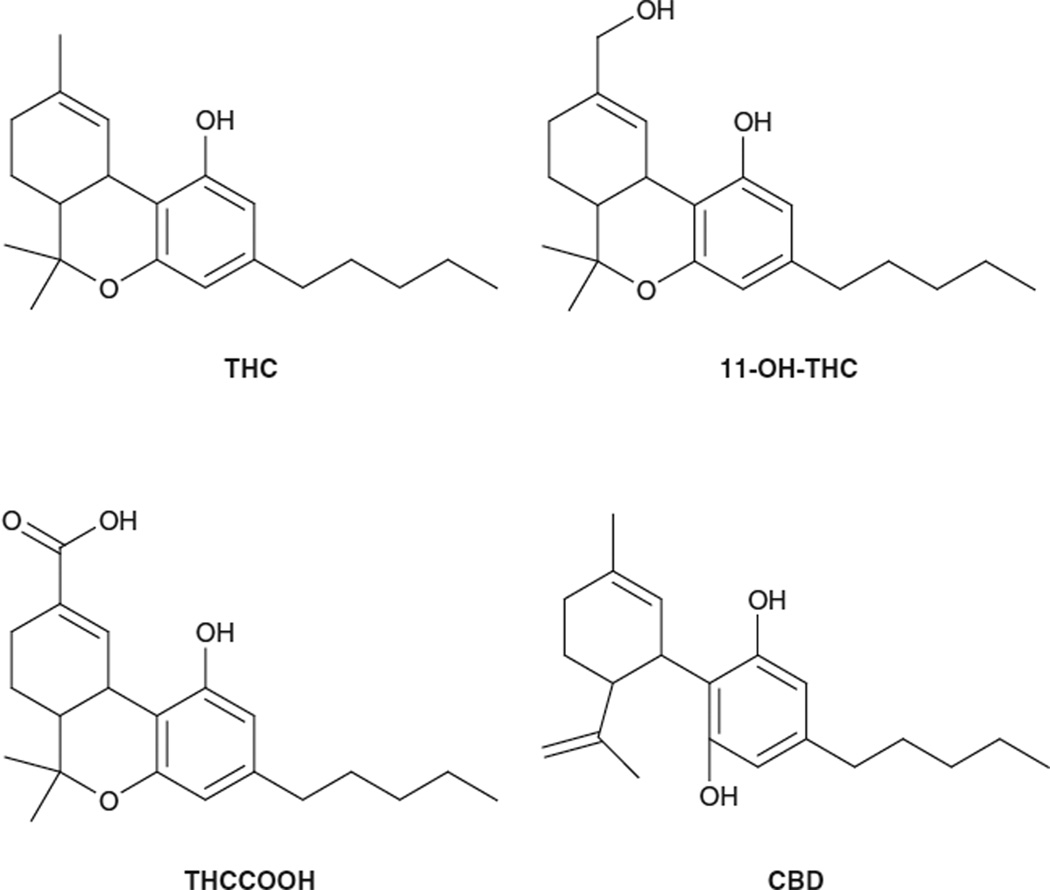
Cannabis contains over 400 different chemical compounds, including at least 61 cannabinoids.[5] During smoking, more than 2000 compounds may be produced by pyrolysis. Eighteen different classes of chemicals, including nitrogenous compounds, amino acids, hydrocarbons, sugars, ter-penes, and simple and fatty acids, contribute to the known pharmacological and toxicological properties of cannabis. The main psychoactive drug in cannabis is Δ9-tetrahydrocannabinol (THC) [figure 1], but other cannabinoids also contribute to its pharmacological effects. Cannabidiol (CBD) [figure 1] lacks psychoactivity but possesses an-xiolytic,[6–8] antipsychotic[9] and alerting[10] properties, and it is the basis of pharmacotherapies with multiple indications. CBD content in can-nabis is believed to modulate the effects of THC. Other cannabinoids include cannabinol, which is approximately 10% as psychoactive as THC, and cannabigerol, cannabichromene and multiple minor cannabinoid components.
Fig. 1.
Chemical structure of different cannabinoids. CBD = cannabidiol; THC = Δ9-tetrahydrocannabinol; 11-OH-THC = 11-hydroxy-Δ9-tetrahydrocannabinol; THCCOOH = 11-nor-9-carboxy-Δ9-tetrahydrocannabinol.
Over the last 20 years, our knowledge of canna-binoid pharmacology has increased tremendously. Discoveries include (i) identification of CB1 and CB2 cannabinoid receptors; (ii) multiple endogenous neurotransmitters (e.g. N-arachidonoyl ethanolamine [anandamide], 2-arachidonoylgly-cerol, 2-arachidonyl glyceryl ether, N-arachidonoyl-dopamine and virodhamine); (iii) synthetic pathways; (iv) enzymes for neurotransmitter inactivation (fatty acid amide hydrolase and monoacylglycerol lipase); and (v) transport across cell membranes.
CB1 receptors are primarily located in the CNS, in high density in the cerebral cortex, hippocampus, amygdala, striatum and cerebellum and functional areas associated with the most prominent behavioural effects of cannabinoids. The most common neurocognitive deficit observed during acute intoxication is short-term memory impairment.[11] Deficits in motor inhibition, decision making and inhibitory control are also prominent.[12] Less consistent results are available for risk taking after cannabis use.[13] After chronic cannabis exposure, it appears that cannabinoid receptors are desensitized and internalized.[14] The distinct CB2 cannabinoid receptor, primarily located in the periphery, has a critical role in immunomodulation.[15]
The endogenous cannabinoid system is highly conserved throughout evolution; it modulates important functions including locomotion, emotional behaviour, cognition, cardiovascular response, pain, feeding behaviour and drug dependence. The stimulating effects of THC on the brain reward system are characteristic of drugs with abuse liability and are similar to other drugs of abuse such as heroin, cocaine, methamphetamine and nicotine.[16] Cannabis produces substantial changes in human behaviour as well as physiological and biochemical changes.
The behavioural and subjective effects of can-nabis are highly dose-dependent and include euphoria, enhancement of sensory perception, sedation, relaxation, altered perceptions of time, lack of concentration, impairment of learning/ memory, mood changes, panic reaction, paranoia and impaired psychomotor activity.[17–21] Well described physiological effects include tachycardia, conjunctival injection, dry mouth and throat, increased appetite, vasodilation, bronchodila-tion, increased sleep and analgesia. This spectrum of behavioural effects is unique, preventing classification of the drug as a stimulant, sedative, tranquilizer or hallucinogen. Subjective and physiological effects of cannabis appear after the first puff of a THC-containing product.[22,23] Although cannabis smoking produces rapid changes in heart rate, pronounced hypotension and dizziness is observed in approximately 25% of individuals approximately 10 minutes after smoking.[24]
The Drug Abuse Warning Network[25] of the US National Institute on Drug Abuse reported 290 563 cannabis-related hospital emergency room visits in 2006, 9.1% of the total number of drug-related visits, while the number of cannabis-related hospital admissions has steadily increased in Australia between 1999 and 2005.[19] Over the last two decades, the potency of cannabis has increased in the US as well as in some European countries,[18] leading to higher demand for can-nabis rehabilitation treatment. Following inadvertent ingestion of cannabis by children, serious CNS depression may be observed.[26,27] However, with supportive care, these cases have resolved successfully with few residual health effects. Cannabis does not produce death directly, as there are few cannabinoid receptors in the brain stem, other than the vomiting centre, limiting cannabis’ effects on respiratory function; however, the drug is a major contributing factor to motor vehicle and other accidents.[18,19,28]
Additional research is needed on the development of tolerance after long-term, frequent exposure. Around-the-clock, high-dose THC is needed to produce tolerance to the physiological and behavioural effects of cannabinoids.[29] Controversy exists as to whether long-term exposure produces irreversible changes in brain function. Short-term (24-hour) neurocognitive impairment of verbal memory, language function and processing speed compared with controls has been reported, as well as impairment of memory, executive function, inhibitory control and psy-chomotor speed after a 28-day abstinence in chronic daily cannabis users.[30] Pope et al.[31] also demonstrated neurocognitive impairment at baseline and after 7 days of abstinence in current daily cannabis users; however, results were not significantly different from former heavy canna-bis users and controls who smoked fewer than 50 times in their lives after 28 days of abstinence. Furthermore, the observed impaired performance was correlated with concentrations in urine of the inactive metabolite of THC, 11-nor-9-carboxy-THC (THCCOOH) [figure 1], on admission. This suggests that after sustained cannabis abstinence neuropsychological performance can return to baseline. Recently, THC was quantified in the blood and plasma of chronic cannabis users for at least 7 days,[32,33] and in their urine for up to 28 days[34] after continuously monitored abstinence, suggesting that residual THC in the brain could be a mechanism for sustained impairment. Abstinence may permit elimination of cannabinoids from the brain and a return to baseline performance.
There are conflicting reports in the literature on the chronic toxic effects of cannabis. Impaired health including lung damage, behavioural changes and reproductive, cardiovascular and immuno-logical effects are associated with cannabis use.[35] Cannabis smoke condensate contains potential carcinogens.[36] In a comparison of toxic components in cannabis and tobacco smoke, ammonia was found at levels up to 20-fold greater and hydrogen cyanide, nitrous oxide and some aromatic amines at concentrations three to five times higher in cannabis than tobacco smoke.[37] Cannabinoids may readily cross placental membranes and expose the developing fetus.[38] Cannabinoids affect embryo implantation, female fertility[39] and child development[40] and may increase vulnerability to substance abuse problems later in life.[41] Cannabinoids alter immune function and decrease host resistance to microbial infections in experimental animal models and in vitro.[42,43]
Synthetic cannabinoid agonists and antagonists at CB1 and CB2 cannabinoid receptors are also approved or under development as pharmacotherapies. Cannabinoid agonists are approved for the treatment of nausea and vomiting from cancer chemotherapy and as an appetite stimulant in patients with HIV-AIDS wasting disease. Nabilone is being evaluated for posttraumatic stress disorder, IP-751 or ajulemic acid for analgesia and inflammation, and HU-210 (6aR)-trans-3-(1,1-Dimethylheptyl)-6a,7,10,10a–tetrahydro-1-hydroxy-6,6-dimethyl-6H–dibenzo [b,d]pyran-9-methanol, as an antipyretic, antiinflammatory, analgesic, antiemetic and antipsychotic agent.[44,45] Extracts from the cannabis plant also have potential as effective treatments. Sativex® (GW Pharmaceuticals, Inc., Wiltshire, UK), a cannabinoid medicine derived from cannabis plant extracts, contains approximately equal quantities of THC and CBD and has been approved for the indication of neuropathic pain and as an adjunct analgesic for cancer pain in multiple countries. Different combinations of these two cannabinoids are being investigated for the treatment of migraine, to reduce spasticity and incontinence, and for a wide variety of other illnesses. As we discover more about endogenous cannabinoid synthesis and metabolism, new targets are presented that offer the potential to develop pharmacotherapies without the psychoactive effects of traditional cannabinoids.
4. Criteria Under the World Anti-Doping Code
Section 4 of the Code defines the three criteria used to consider if a drug, class of drugs or method should be included in the Prohibited List; at least two of three criteria must be fulfilled. Experts of the WADA scientific committees endeavour to utilize all published scientific data to achieve the most objective judgement on the potential of substances or methods to fulfil the Code’s criteria. The WADA scientific committees are organized hierarchically. The Health, Medical and Research Committee considers recommendations of the four subcommittees (the List Expert Group, the Laboratory Expert Group, the Therapeutic Use Exemption Expert Group and the Gene Doping Expert Group), all comprised a diversity of experts in the field of sport medicine, pharmacology, toxicology, doping, analytical chemistry, endocrinology and haematology.
Other sources of information such as testimonies from athletes who formerly doped, and substances or paraphernalia seized during police operations, are employed to aid experts in forging the most objective opinion on the status of drugs or methods in sports. Data on cannabis from all sources have been scrutinized in light of the potential benefits and dangers for athletes in the context of abuse of cannabinoids for the purpose of doping in sport.
5. Potential Health Risk
One criterion to be considered when deciding if a drug, class of drugs or method should be included in the Prohibited List is the potential health risk. Anti-doping authorities are always concerned about the impact of doping on athletes’ health. Before WADA, doping was monitored and controlled by the International Olympic committee (IOC) Medical Commission, also overseeing the well-being and health of athletes. When the Code was drafted and adopted, the consideration of athlete health was included in section 4.3.1.2, taking into account all “Medical or other scientific evidence, pharmacological effect or experience that the use of the substance or method represents an actual or potential risk for the athlete.”[3] Concern about the detrimental effects of doping on health gained substantial governmental support because of the strong perception that doping is a public health issue, not limited to elite athletes but affecting the general public at large.
Cannabis can alter the perception of risk, potentially leading to poor decision making and/or risk for the athlete and their entourage. With negative influences on coordination, movement and time estimation, cannabis can impair essential technical skills that may also increase the probability of accidents and injuries, particularly when handling equipment or when high velocities are involved. CB1 receptor density in the cerebellum (involved in motor control and movement) and prefrontal cortex (involved in decision making and executive function) is high.[46] In this regard, there is an increased risk for motor vehicle and aeroplane accidents associated with the use of cannabis.[47,48] Although impairment is generally believed to last approximately 8 hours, there are some reports on adverse effects for 24 hours.[49] Furthermore, new preparations of herbal products laced with multiple highly potent cannabinoid analogues including, but not limited to, JWH-018JWH-073, JWH-250, CP-47497 and HU-210, have demonstrated prolonged intoxication and may have greater health risks.[50] These products are primarily sold over the Internet and in drug paraphernalia shops under many ‘street’ names such as Spice, K2 and fake marijuana. Athletes who smoke cannabis or Spice in-competition potentially endanger themselves and others because of increased risk taking, slower reaction times and poor executive function or decision making.
Acute effects of cannabis include increased heart rate, followed in many individuals by hypotension, dizziness and disorientation,[23] increased subjective feelings of euphoria or being ‘high’ and a state of intoxication or being ‘stoned’, and sometimes psychosis, panic reactions and para-noia.[51] Additional effects that could harm the athlete during competition are loss of vigilance,[52] increased reaction times[53] and short-term memory loss.
A different spectrum of effects occurs with chronic daily cannabis use. Multiple studies report decreased cognitive performance after long-term cannabis exposure.[30,31,54–57] Other chronic effects include pulmonary toxicity following smoking and cannabis smoke may induce bronchial irritation, chronic cough and wheeze.[58] Cardiovascular dam-age,[59] liver steatosis[60] and negative reproductive effects[61] are all associated with chronic cannabis exposure. The exacerbation of symptoms of schizophrenia and the early initiation of the disease has been noted by several investigators.[62,63]
Chronic cannabis use may induce tolerance if high amounts are consumed around the clock. There are increased reports of cannabis dependence and requests for drug treatment related to cannabis use.[64] The cannabis withdrawal syndrome is characterized by psychological rather than physical symptoms, including restlessness, anxiety, insomnia, muscle tremor and increased aggression.[21,65–69] THC increases dopamine release in the nucleus accumbens and prefrontal cortex similar to other reinforcing drugs of abuse such as cocaine and amphetamines.[70–73]
Based on objective preclinical and clinical research and consequences of the effects of acute and chronic cannabis exposure, cannabis fulfils the criterion of potential for health risks.
6. Potential to Enhance Performance
Judgement of the performance-enhancing effects of a substance is based on article 4.3.1.1 of the Code. This article stipulates that a substance shall be considered to be performance enhancing when “Medical or other scientific evidence, pharmacological effect or experience that the substance or method, alone or in combination with other substances or methods, has the potential to enhance or enhances sport performance.”[3]
Cannabis is often portrayed as a substance that has detrimental effects on performance. Cannabis decreases coordination, distorts spatial perception and alters perception and awareness of the passage of time.[74–77] Steadward and Singh[78] found that cannabis smoking did not increase vital capacity or grip strength, and Renaud and Cormier[79] found maximal exercise performance in 12 cyclists reduced from 16 to 15 minutes at 10 minutes after smoking a THC 1.7% cigarette. However, in this study vasodilation and bronchodilation were increased, suggesting that cannabis could also improve oxygenation to the tissues. Furthermore, hotlines developed in support of doped athletes report performance-enhancing capabilities (WADA, unpublished observations). Cannabis is presented as a drug that has significant positive effects in sports, such as improvement of vision for goalkeepers and muscle relaxation.
Smoked cannabis can decrease anxiety, fear, depression and tension.[75] THC is anxiolytic at low doses,[80] the doses reportedly consumed by athletes.[81] Animal studies also addressed can-nabinoid effects on aversive responses. Interfering with the hydrolysis or uptake of endocannabinoids reduced anxiety-like behaviours without motor impairment in rodents,[82–84] and CB1 knockout mice exhibited increased anxiety-like behaviour.[85] In human volunteers, THC and cannabis also increased impulsive responses leading to more risk-taking behaviour but without affecting decision making.[86,87] In this regard, and from a sports perspective, Martinez[88] suggested that cannabis smoking reduces anxiety, allowing athletes to better perform under pressure and to alleviate stress experienced before and during competition.[88] Furthermore, cannabinoids play a major role in the extinction of fear memories by interfering with learned aversive behaviours.[89–91] Athletes who experienced traumatic events in their sports career could benefit from such an effect. For these reasons, Wagner[92] described cannabis as ergogenic. The endocannabinoid system is also involved in the modulation of mood. Animal studies demonstrate antidepressant-like effects in models based on inescapable or chronic stress.[83,93,94] In adolescents and young adults, cannabis also helps in coping with negative mood and emotional distress.[95–97]
Catlin and Murray[98] indicated that cannabis could be performance enhancing in sports that require greater concentration. Iven[99] noted that athletes use cannabis for relief of anxiety and stress, and perhaps to reduce muscle spasm. Saugy et al.[81] suggested that athletes were mainly motivated to use cannabis due to its effects on relaxation and well-being, promoting better sleep.
In France, in 2002, 25% of IOC positive tests were for cannabis, prompting Lorente et al.[100] to conduct a survey in France of 1152 sport university students on their use of cannabis. Based on the students’ survey responses, the relaxing properties of cannabis were frequently used to enhance sports performance. Surprisingly, the higher the students’ level of competition, the more cannabis was employed to enhance performance. The percentage was higher in males than in females and, interestingly, it was more prevalent in sliding sports. A similar trend was observed in adolescents, where cannabis consumption was found to be highest among athletes seeking the high risk and excitement of competing in extreme sports.[101] In this regard, skateboarders Bob Burnquist and Jen O’Brien admitted that cannabis helps them relieve the pressure associated with their sport.[102]
Anecdotal evidence from blogs and drug hotlines also indicates that athletes abuse cannabi-noids to enhance sport performance. Athletes under the influence of cannabis indicate that their thoughts flow more easily and their decision making and creativity is enhanced; others claim that cannabis improves their concentration or reduces pain. Health professionals have encountered athletes including gymnasts, divers, football players and basketball players who claim smoking cannabis before play helps them to focus better.[102]
Much additional research is needed to determine the effects of cannabis on athletic performance. The endocannabinoid system was discovered in the 1980s, and each year since this discovery we learn more about cannabinoid pharmacology. Clearly, cannabis induces euphoria, improves self-confidence, induces relaxation and steadiness and relieves the stress of competition. Cannabis improves sleep and recovery after an event, reduces anxiety and fear and aids the forgetting of negative events such as bad falls and so forth. Cannabis increases risk taking and this perhaps improves training and performance, yielding a competitive edge. Cannabis increases appetite, yielding increased caloric intake and body mass. Cannabis enhances sensory perception, decreases respiratory rate and increases heart rate; increased bronchodilation may improve oxygenation of the tissues. Finally, cannabis is an analgesic that could permit athletes to work through injuries and pain induced by training fatigue.
In conclusion, although much more scientific information is needed, based on current animal and human studies as well as on interviews with athletes and information from the field, cannabis can be performance enhancing for some athletes and sports disciplines.
7. The Spirit of Sport
Of the three criteria to consider a substance or a method as prohibited in sport, the most difficult to define is probably the spirit of sport. Contrary to health risk and performance enhancement, the spirit of sport criterion does not rely on established scientific facts; rather, it relies more on ethical and societal considerations encompassing a wider view of sport beyond physical achievements and health. Therefore, the fundamental rationale for this aspect of the Code does not include a strict definition of the spirit of sport, but instead provides a collection of essential values to be shared in sport. The values included are ethics, fair play and honesty, health, excellence in performance, character and education, fun and joy, teamwork, dedication and commitment, respect for rules and laws, respect for self and other participants, courage, community and solidarity. These values are in essence contrary to doping. Such essential principles guide WADA scientists and ethicists when determining whether a substance or a method violates values embedded in the spirit of sport.
Cannabis is classified as an illegal substance in most of the world, with penalties ranging from no action to long-term incarceration. The consumption of cannabis and other illegal drugs contradicts fundamental aspects of the spirit of sport criterion. The international anti-doping community believes that the role model of athletes in modern society is intrinsically incompatible with use or abuse of cannabis. Although some anti-doping officials proposed also banning can-nabis for out-of-competition testing, this appeared beyond the anti-doping mandate and it was believed to violate athletes’ privacy. For these reasons, cannabis use is prohibited only in-competition. Use of illicit drugs that are harmful to health and that may have performance-enhancing properties is not consistent with the athlete as a role model for young people around the world. For example, a recent case involving a high-profile athlete smoking cannabis out-of-competition triggered negative reactions by the public, sponsors and the media, even if no anti-doping rule violation was committed. The national sport federation imposed a suspension on the athlete under its code of conduct and sponsorship support was lost.[103,104]
Banning a substance only in-competition creates challenges in differentiating new cannabis smoking during competition from evidence of prior out-of-competition cannabis use.
8. Identification of Cannabis Use In-Competition
In urine, the presence of THCCOOH equal to or greater than the threshold value of 15 ng/mL is reported by WADA-accredited laboratories as an adverse analytical finding (AAF).[105] For athletes, clinicians, coaches and sport federations to understand what this means in terms of detection of THCCOOH, it is necessary to turn to the scientific literature on cannabinoid pharmaco-kinetics.
9. Cannabinoid Pharmacokinetics
9.1 Absorption and Metabolism
THC, the primary psychoactive component of cannabis, is rapidly absorbed into the bloodstream following inhalation and is extensively metabolized in the liver into multiple metabolites. The equipotent metabolite 11-hydroxy-THC (11-OH-THC) of THC is further oxidized to THCCOOH and THCCOOH-glucuronide and sulphate.[106,107] THC is extensively metabolized to multiple other alcohols and acids, but THCCOOH was selected as the analyte monitored in urine for virtually all drug-testing programmes, including workplace, military, criminal justice and drug treatment programmes. After alkaline hydrolysis of urine to free THCCOOH from its conjugates, THCCOOH is the most abundant urinary marker of cannabis use.
9.2 Distribution and Excretion in Urine
THC is distributed initially to the highly perfused organs including the brain, heart, liver and kidneys, with secondary distribution into adipose tissue, because of its high lipophilicity. With chronic daily THC exposure, the THC body burden in fat is large; the rate-limiting step in THC elimination is the slow release of stored drug from the tissues.[29,108]
Cannabinoid concentrations in body fluids depend upon the cannabis potency, smoking topography, frequency of cannabis use and time since last use. In plasma, for example, THC was detected for 6–27 hours after smoking a single cannabis joint containing approximately THC 34mg (gas chromatography/mass spectrometry [GCMS] limit of quantification [LOQ] 0.5 ng/mL) in individuals who smoked less frequently than daily.[22,23] Mean (range) peak plasma THC concentrations were 162 (76–267) ng/mL. The mean detection time for THCCOOH in plasma was longer, from 3 to 7 days at the same LOQ. Recently, nondaily cannabis users smoked THC 69.4mg and achieved similar mean peak serum THC concentrations of 190 ± 106 ng/mL, despite twice the available dose.[109] This is consistent with other reports of cannabis smokers who titrate their dose to the desired level of intoxication and tolerable cardiovascular response.
Mean peak urinary THCCOOH concentrations were 89.8 ± 31.9 ng/mL and 153 ± 49 ng/mL approximately 8 and 14 hours after smoking cigarettes containing THC 16mg and 34mg, respectively.[107–110] All urine specimens were collected and individually analysed. THCCOOH was detected in urine at a concentration ≥15 ng/mL for 33.7 ± 9.2 hours (range 8–68.5 hours) and 88.6 ± 9.5 hours (range 57.0–122 hours) after these doses.[111] Similar values were reported by Niedbala et al.[112] when subjects smoked a cannabis cigarette containing THC 20–25mg; mean detection time for the last positive urine specimen was 58 ± 6 hours (range 16–72 hours). Thus, in cannabis users smoking less frequently than daily, a positive urine cannabinoid test would likely be positive for <4 days. However, when an individual smokes cannabis daily for an extended period, it is possible to have a positive urine specimen for at least 4 weeks.
When cannabis is smoked daily, the body burden of cannabinoids is high. Recently, Huestis and collaborators.[113] reported cannabinoid excretion data based on creatinine-normalized urine concentrations from 60 cannabis smokers who resided on a closed research unit under 24-hour monitoring for up to 30 days. All urine specimens were collected and individually analysed for THCCOOH. When urinary cannabinoid excretion data are normalized to urinary creatinine, the individual’s state of hydration is taken into account and the excretion curve is smoothed. Cannabinoid excretion data were divided into three groups based on the initial cannabinoid to creatinine ratio in ng/mg. The three groups were ≤50 ng/mg, 51–150 ng/mg and >150 ng/mg. In the ≤50 ng/mg group, normalized cannabinoid/creatinine concentrations on admission ranged from 0 to 47.3 ng/mg. These individuals reported smoking cannabis from 2 to 30 days per month for up to 25 years. The first negative urine specimen (≤50 ng/mL by immunoassay screen) in this group occurred from 0 to 2.2 days after admission, and the last positive specimen (immunoassay screen ≥50 ng/mL and THCCOOH GCMS ≥2.5 ng/mL) occurred up to 8.6 days after admission, except for one individual who was positive 21.8 days later. The latter individual reported smoking daily for 5 years. In the >150 ng/mg group, normalized cannabinoid/ creatinine concentrations on admission ranged from 155 to 1165 ng/mg. These individuals reported cannabis use from 12 to 30 days per month for up to 28 years. There were statistically significant correlations between groups and number of days until first negative and last positive urine specimens; mean number of days were 0.6 and 4.3, 3.2 and 9.7, and 4.7 and 15.4 days, respectively, for the three groups (table I). In individuals generally smoking cannabis on a daily basis, creatinine-normalized urine specimens would on average be negative after 15.4 days; however, the urine specimens of many cannabis smokers remained positive for up to 30 days after long-term use. Thus, for athletes who are occasional cannabis smokers, abstinence for 1 week prior to competition should result in negative cannabinoid urine tests and no AAFs, while chronic cannabis use would require abstinence of 1 month or longer. Based on the documented cognitive impairment and toxicity that occurs following chronic use, it is not expected that a significant number of elite athletes would be chronic daily cannabis smokers.
Table I.
Cannabinoid excretion data based on creatinine-normalized urine concentrationsa
| Variable | Groups according to cannabinoid to creatinine ratio (ng/mg) |
||
|---|---|---|---|
| ≤50 | 51–150 | >150 | |
| Smoking frequency (d/mo) | 2–30 | 6–30 | 12–30 |
| History of cannabis smoking (y) | ≤25 | ≤22 | ≤24 |
| Mean time until first negative specimen (d) | 0.6 | 3.2 | 4.7 |
| Mean time until last positive specimen (d) | 4.3 | 9.7 | 15.4 |
Sixty cannabis smokers resided on a closed research unit under 24-h monitoring for up to 30 d. All urine specimens were collected and individually analysed for 11-nor-9-carboxy-Δ9-tetrahydrocannabinol.
9.3 Differentiating New Cannabis Use from Residual Cannabinoid Excretion
When chronic cannabis users test positive, it is not possible to differentiate new cannabis use from residual cannabinoid excretion from a single urine specimen. Models were developed to predict whether new cannabis use has occurred between two urine specimens collected up to 21 days apart from cannabis users smoking less frequently than daily.[114] These models require the creatinine-normalized cannabinoid concentrations and the time between specimen collections. Minimum, median and maximum ratios between the later urine specimen normalized concentration to that of the first specimen are provided to guide interpretation of whether or not new can-nabis use has occurred between the two urine collection dates. For the first time, models were recently published for chronic daily cannabis smokers taking into account the creatinine-normalized concentrations and the time between the two specimen collections.[115]
Others suggested that measurement of the psychoactive components of cannabis, THC and/ or 11-OH-THC, in urine could also indicate recent cannabis use, even in chronic cannabis smokers.[116,117] Kemp et al.[116] indicated that following Escherichia coli β-glucuronidase hydrolysis of urine, THC and 11-OH-THC could be found for up to 8 hours after cannabis smoking. We evaluated cannabinoid excretion in blood, plasma and urine from the heaviest chronic daily cannabis users encountered in more than 15 years of research. Cannabis smokers resided in a closed research unit for up to 30 days of continuously monitored abstinence. Surprisingly, THC was quantified in blood[32] and plasma[33] from some chronic users for at least 7 days and in the urine for up to 24 days[34] after initiation of abstinence with LOQs of 0.25, 0.25 and 2.5 ng/mL, respectively. Interestingly, 11-OH-THC was present in urine for as long as THCCOOH was throughout the 30-day monitoring period. Thus, neither THC nor 11-OH-THC in urine identify recent cannabis smoking.
Considerable effort has been expended in identifying a better marker of recent cannabis smoking. Δ9-Tetrahydrocannabivarin (THCV) was suggested as a marker of recent cannabis smoking; the 11-nor-9-carboxy metabolite of THCV was shown to be present in the urine of a cannabis smoker.[118] However, recently Levin et al.[119] showed that because of the variability of THCV content in different cannabis sources, 50% of urine specimens from cannabis smokers did not contain detectable THCV. Furthermore, the excretion pattern and window of drug detection in urine for THCV is not yet known.
Δ9-Tetrahydrocannabinolic acid-A (THCA-A) is a precursor to THC in the cannabis plant. Upon heating, much of the THCA-A is decar-boxylated to form THC. Jung et al.[120] demonstrated that THCA-A could be found in human urine and serum. THCA-A has also been suggested as a better urinary marker of recent cannabis smoking, but human pharmacokinetic data after controlled cannabis administration have not yet been collected. Interestingly, rats were dosed orally with THCA-A 15mg/kg and urinary metabolites were identified.[121] THCA-A underwent a similar metabolism as THC in humans, producing 11-OH-THCA-A and 11-nor-9-car-boxy-THCA-A. Together, these data suggest that these metabolites may be useful markers of recent smoked cannabis, but much additional research is needed to determine if these markers solely reflect recent use or whether they also have a long window of detection after chronic cannabis smoking. The window of THCA-A detection in human urine is unknown.
Another approach to identifying recent can-nabis smoking is to monitor drug biomarkers in an alternative matrix other than urine. Oral fluid (saliva) testing offers a simple, fully observed specimen collection method, thereby reducing the potential for adulteration, and it does not require clinical personnel or same-sex doping control officers. THC appears immediately in oral fluids in high concentrations after cannabis smoking, because of the exposure of the oral mucosa to the drug in the cannabis smoke. Niedbala et al.[112] found that approximately 30–45 minutes after the end of smoking, THC concentrations in oral fluid decreased considerably, correlating temporally with blood concentrations. In 18 subjects administered smoked cannabis 20–25 mg, oral fluid specimens were positive on average 31 hours (range 2–72 hours), while the mean time to the last positive urine specimen from the same participants was 42 hours. Therefore, oral fluid testing would not unequivocally resolve the problem of in- and out-of-competition cannabis use. To date, there are no data on the window of THC detection in oral fluid after chronic cannabis smoking, and oral fluid is not an approved matrix for anti-doping testing. The WADA International Standard for Laboratories (ISL) specifically states that results obtained from another biological material such as oral fluid cannot counter an AAF from urine.[122] If future research supports the value of oral fluid testing, consideration should be given to revise the relevant ISL provisions.
10. Can Positive 11-Nor-9-Carboxy-Δ9-Tetrahydrocannabinol Urine Specimens be Produced from Passive Inhalation of Cannabis Smoke?
It has been argued that passive inhalation of cannabinoids can yield urinary THCCOOH concentrations ≥15 ng/mL. Under realistic exposure conditions, passive inhalation of cannabis or hashish smoke does not produce detectable levels of urinary cannabinoids;[123] however, under extreme conditions in laboratory settings with high cannabis smoke concentrations, measurable THCCOOH is possible.[124–130] Thus, passive inhalation is used as a line of defence following positive drug tests in the workplace.[131] Similarly, this argument could be used to refute an AAF of urinary cannabinoids following an anti-doping test, despite the principle of strict liability that does not question how the substance entered the body. Scientific data document that THCCOOH concentrations following passive THC inhalation under less than extreme experimental conditions do not exceed the threshold value of THCCOOH 15 ng/mL by GCMS.
A pioneer study by Perez-Reyes et al.[130] showed the presence of cannabinoids in urinary samples of two individuals passively exposed to the smoke of four cannabis cigarettes for 1 hour in small confined environments (approximately 3500 L and 15 500 L). However, in 80 specimens collected over a 24-hour period, only one quantified at THCCOOH 3.9 ng/mL was far below the 15 ng/mL threshold. In another study, four individuals passively inhaled the smoke of six cannabis cigarettes for 3 hours in a small office (27 900 L). Urinary samples collected for 6 hours contained cannabinoid concentrations of <7 ng/mL as determined by radioimmunoassay (RIA).[128] In addition, three subjects passively inhaling the smoke of four simultaneously burning cannabis cigarettes in a room mimicking “realistic … conditions” for 1 hour had <6 ng/mL urinary concentrations of cannabinoids as assayed by RIA.[123]
A series of studies by Cone and collaborators[125–127] determined urinary concentrations of cannabinoids following passive inhalation of different air concentrations of THC and different lengths of exposure. Five to seven subjects were exposed to the smoke of up to 16 cannabis cigarettes for 1 hour during 6 consecutive days in a small, unventilated room (2.1m×2.5m×2.4m, approximately 12 500 L). Urinary concentrations after a single exposure to four cigarettes, which generated moderate smoke in the room, yielded GCMS values well below the threshold of 15 ng/mL, ranging from THCCOOH 0 to 6 ng/mL, the maximum being 12 ng/mL after multiple exposures. When 16 cannabis cigarettes were simultaneously burned, the smoke was so dense that goggles were required.[127] Under these extreme experimental conditions, only one of seven individuals produced urine specimens with THCCOOH concentrations >15 ng/mL by GCMS after a single exposure, while other subjects required multiple exposures to these high THC room air concentrations to surpass this threshold.[127] Furthermore, following the second 1-hour exposure to the smoke of 16 cigarettes, positive psychoactive effects were experienced by the passive inhalers, effects that were absent during passive inhalation of smoke produced by four cannabis cigarettes.[125]
More recently, another experiment proved the low probability of detecting THCCOOH in the urine of subjects exposed to passive inhalation. Ten active and two passive smokers with no history of cannabis consumption were kept in an unventilated room (6m×6m×3.5m), with a window opened occasionally to relieve the accumulated smoke. Although each active smoker had a cannabis cigarette containing THC 20–25mg, none of the passive smokers produced a positive urinary sample (THCCOOH ≥15 ng/mL by GCMS) up to 72 hours following exposure.[112]
The scientific evidence on passive inhalation was recently reviewed by Westin and Slørdal,[124] who noted that only when the air volume was extremely low and the THC smoke amount unrealistically high, bordering on intolerable discomfort, was it possible to detect the presence of cannabinoids in the urine of participants smoking passively for more than 24 hours. Based on their review of all published articles on the topic, they concluded that ignorant passive cannabis smoking can be excluded with high certitude as a cause of positive samples.
Most studies on passive inhalation of cannabis smoke were conducted more than 20 years ago. THC cannabis content increased from 2.8% to as high as 40% in some preparations sold in Dutch coffee shops.[132] It could, therefore, be argued that since THC content is higher, there is a greater chance of testing positive if passively exposed to high-content cannabis. However, the average THC content in most street cannabis is 6–7%[132] and the passive exposure conditions required to produce positive urine tests are unrealistic. In addition, under these conditions participants would most likely feel effects, as was observed in previous controlled studies.[125] Furthermore, the cannabis included in a study by Law et al.[128] contained 9.8% THC, with no subjects’ urine exceeding THCCOOH 15 ng/mL. Since an AAF is reported if a concentration is THCCOOH >15 ng/mL in an athlete’s urine in-competition, such a threshold appears to be fair in an anti-doping context based upon the available literature. Similar rules apply for workplace and military drug testing programmes. Based on the studies described, this limit was established to distinguish between active cannabis smokers and athletes who may have been passively exposed to canna-bis smoke. In four cases adjudicated by the Court of Arbitration for Sport in the last 8 years, four athletes admitted active consumption of cannabis. Their urinary THCCOOH concentrations ranged from 35 to 318 ng/mL (WADA, unpublished observations).
11. Oral Δ9-Tetrahydrocannabinol or Cannabis Administration
Although most cannabis users smoke the drug, rapidly delivering drug to the brain and producing the typical pharmacodynamic responses of euphoria and tachycardia, cannabis can also be taken orally. When ingested, peak concentrations are much lower and peak later than after smoking. Less euphoria is experienced and exposure to the more toxic ingredients produced from burning cannabis is avoided. There are multiple therapeutic uses for oral synthetic THC, including as an appetite stimulant in patients with HIV-AIDS wasting disease and as an adjunct analgesic with opioids during cancer chemotherapy. Sativex® is approved in Canada for neuropathic pain and in Britain for spasticity associated with multiple sclerosis. The effects of THC on memory, loss of vigilance, fear reduction and so forth can be induced through any route of cannabis administration (oral, rectal, sublingual, transdermal), albeit based on dose, frequency of dosing and duration of treatment.
12. Cannabis Use in Sports
Cannabinoids accounted for 12.5–13.9% of all AAFs reported by the IOC between 1998 and 2003 (table II). Since the enforcement of the 2004 WADA Code, when cannabinoids were prohibited in all sports, a significant decrease in cannabinoid-related AAFs from 15.7% to 7.7% was observed (table I). These percentages place cannabinoids as the third most reported prohibited substance in 4 of the last 7 years of compiled WADA laboratory statistics (2003–9) and the second most reported in 2008–9 following anabolic agents. In a social context, cannabis is the most prevalent illicit drug abused in many countries.[133,134] The use of cannabinoids peaks during the late teens to early twenties, decreasing thereafter to half peak prevalence by age 30.[135] Based on these statistics, cannabis in sport would appear to reflect levels of recreational use reported in many countries, as elite athletes are in general young adults.
Table II.
Adverse analytical findings (AAFs) for cannabinoids from 1998 to 2009 from the International Olympic Committee (1998–2002) and the World Anti-Doping Agency (2003–9)
| Year | Specimens (n) | Total AAFs (n) | AAFs for cannabinoids (n) | AAFs for cannabinoids (%) |
|---|---|---|---|---|
| 2009 | 277 771 | 4567 | 352 | 7.7 |
| 2008 | 274 615 | 5523 | 496 | 9.0 |
| 2007 | 223 888 | 4850 | 576 | 11.9 |
| 2006 | 198 143 | 4332 | 553 | 12.8 |
| 2005 | 183 337 | 4298 | 503 | 11.7 |
| 2004 | 169 187 | 3305 | 518 | 15.7 |
| 2003 | 151 210 | 2716 | 378 | 13.9 |
| 2002 | 131 373 | 2371 | 347 | 14.6 |
| 2001 | 125 701 | 2075 | 298 | 14.3 |
| 2000 | 117 314 | 2228 | 295 | 13.2 |
| 1999 | 118 259 | 2341 | 312 | 13.3 |
| 1998 | 105 250 | 1926 | 233 | 12.1 |
Sanctions for a first positive result for canna-binoids range from a warning to a 2-year ban, with positive results on repeated occasions leading to a potential lifetime ban. In recent positive cannabinoid cases, there were serious consequences for the athlete’s image and sponsorship endorsement, even if the elite athlete stated the use was out-of-competition.
Based on WADA statistics, some sports report higher percentages of cannabis use than others, but there does not appear to be a pattern of abuse related to the degree of risk inherent in the sport, the abilities required for each type of sport or the psychological pressure related to exposure to the public. A specific culture of a sport and/or the athlete’s personal decision appears to be the primary motivation for using cannabis in sport. The significant decrease in AAFs for cannabinoids in-competition also stresses the importance of implementing such a tight anti-doping regulation in sport.
13. Conclusion
Recent advances in understanding the endogenous cannabinoid system demonstrate its important role in many critical functions that could positively affect sports performance. This fact, together with the detrimental health effects of cannabis and its violation of the spirit of sport, supports the prohibition of cannabis and its analogues.
Acknowledgements
We would like to thank Dr Patrick Schamasch (IOC) and Mr Thierry Boghosian (WADA) for providing statistics on AAFs on cannabinoids. We would also like to thank Ms Violet Maziar for her editing assistance. No funding was used to assist in the preparation of this review. The authors have no conflicts of interest to declare that are directly relevant to the content of this review.
References
- 1.International Olympic Committee. Prohibited classes of substances and prohibited methods. 2003 Olympic Movement Anti-Doping Code: appendix A. Lausanne: International Olympic Committee. [Google Scholar]
- 2.World Anti-Doping Agency (WADA) The 2004 prohibited list. Montreal (QC): WADA. 2004 [Google Scholar]
- 3.World Anti-Doping Agency (WADA) World anti-doping code. Montreal (QC): WADA. 2009;29 [Google Scholar]
- 4.World Anti-Doping Agency (WADA) World anti-doping code. Montreal (QC): WADA. 2003;14 [Google Scholar]
- 5.Turner CE, Elsohly MA, Boeren EG. Constituents of Cannabis sativa L: XVII A review of the natural constituents. J Nat Prod. 1980;43:169–234. doi: 10.1021/np50008a001. [DOI] [PubMed] [Google Scholar]
- 6.Musty RE, Conti LH, Mechoulam R. Anxiolytic properties of cannabidiol. In: Harvey DJ, editor. Marihuana ‘84: proceedings of the Oxford Symposium on Cannabis; 1994. pp. 713–719. [Google Scholar]
- 7.Onaivi ES, Green MR, Martin BR. Pharmacological characterization of cannabinoids in the elevated plus maze. J Pharmacol Exp Ther. 1990;253:1002–1009. [PubMed] [Google Scholar]
- 8.Zuardi AW, Crippa JAS, Hallak JEC, et al. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz J Med Biol Res. 2006;39:421–429. doi: 10.1590/s0100-879x2006000400001. [DOI] [PubMed] [Google Scholar]
- 9.Gardner DM, Baldessarini RJ, Waraich P. Modern anti-psychotic drugs: a critical overview. CMAJ. 2005;172:1703–1711. doi: 10.1503/cmaj.1041064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wade DT, Makela P, Robson P, et al. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004;10(4):434–441. doi: 10.1191/1352458504ms1082oa. [DOI] [PubMed] [Google Scholar]
- 11.Riedel G, Davies SN. Cannabinoid function in learning, memory and plasticity. In: Pertwee RG, editor. Handbook of experimental pharmacology. Vol. 168. New York (NY): Springer; 2005. pp. 446–470. [DOI] [PubMed] [Google Scholar]
- 12.Ramaekers JG, Berghaus G, van Laar M, et al. Dose-related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73:109–119. doi: 10.1016/j.drugalcdep.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Verdejo-Garcia A, Benbrook A, Funderburk F, et al. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend. 2007;90(1):2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blair RE, Deshpande LS, Sombati S, et al. Prolonged exposure to WIN55,212-2 causes downregulation of the CB1 receptor and the development of tolerance to its anticon-vulsant effects in the hippocampal neuronal culture model of acquired epilepsy. Neuropharmacology. 2009;57:208–218. doi: 10.1016/j.neuropharm.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabral GA, Griffin-Thomas L. Emerging role of the can-nabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med. 2009 Jan 20;11:e3. doi: 10.1017/S1462399409000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fattore L, Fadda P, Spano MS, et al. Neurobiological mechanisms of cannabinoid addiction. Mol Cell En-docrinol. 2008;286(1–2 Suppl. 1):S97–S107. doi: 10.1016/j.mce.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Huestis MA, Smith ML. Human cannabinoid pharmaco-kinetics and interpretation of cannabinoid concentrations in biological fluids and tissues. In: ElSohly MA, editor. Marijuana and the cannabinoids. Totowa (NJ): Humana Press; 2006. pp. 205–236. [Google Scholar]
- 18.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) A cannabis reader: global issues and local experiences. Vol. 2. Lisbon: EMCDDA; 2008. Monograph, series 8. [Google Scholar]
- 19.National Drug Strategy. Cannabis in Australia: use, supply and responses. Monograph Series no. 57. Canberra (ACT): Australian Government, Department of Health and Ageing; p. 2006. [Google Scholar]
- 20.National Drug Strategy. Cannabis and mental health: put into context. Monograph Series no. 68. Canberra (ACT): Australian Government, Department of Health and Ageing; 2008. [Google Scholar]
- 21.Moreira FA, Lutz B. The endocannabinoid system: emotion, learning and addiction. Addict Biol. 2008;13:196–212. doi: 10.1111/j.1369-1600.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 22.Huestis MA, Henningfield JE, Cone EJ. Blood cannabi-noids: I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16:276–282. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- 23.Huestis MA, Sampson A, Holicky B, et al. Characterization of the absorption phase of marijuana smoking. Clin Pharmacol Ther. 1992;52:31–41. doi: 10.1038/clpt.1992.100. [DOI] [PubMed] [Google Scholar]
- 24.Gorelick DA, Heishman SJ, Preston KL, et al. The can-nabinoid CB1 receptor antagonist rimonabant attenuates the hypotensive effect of smoked marijuana in male smokers. Am Heart J. 2006;151(754):e1–e5. doi: 10.1016/j.ahj.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Substance Abuse and Mental Health Services Administration, Office of Applied Studies. DAWN Series D-30. Rockville (MD: US Department of Health and Human Services; 2008. Drug abuse warning network, 2006: national estimates of drug-related emergency department visits. DHHS publication no.: (SMA) 08-4339. [Google Scholar]
- 26.Appelboam A, Oades PJ. Coma due to cannabis toxicity in an infant. Eur J Emerg Med. 2006;13:177–179. doi: 10.1097/01.mej.0000194405.38206.f2. [DOI] [PubMed] [Google Scholar]
- 27.Robinson K. Beyond resinable doubt? J Clin Forensic Med. 2005;12:164–166. doi: 10.1016/j.jcfm.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Richer I, Bergeron J. Driving under the influenceofcannabis: links with dangerous driving, psychological predictors, and accident involvement. Accid Anal Prev. 2009;41:299–307. doi: 10.1016/j.aap.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Hunt CA, Jones RT. Tolerance and disposition of tetra-hydrocannabinol in man. J Pharmacol Exp Ther. 1980;215:35–44. [PubMed] [Google Scholar]
- 30.Bolla KI, Brown K, Eldreth D, et al. Dose-related neuro-cognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- 31.Pope HG, Jr, Gruber AJ, Hudson JI, et al. Neuropsycho-logical performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- 32.Karschner EL, Schwilke EW, Lowe RH, et al. Do delta9-tetrahydrocannabinol concentrations indicate recent use in chronic cannabis users? Addiction. 2009;104:2041–2048. doi: 10.1111/j.1360-0443.2009.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karschner EL, Schwilke EW, Lowe RH, et al. Implications of plasma delta9-tetrahydrocannabinol, 11-hydroxy-THC, and 11-nor-9-carboxy-THC concentrations in chronic cannabis smokers. J Anal Toxicol. 2009;33:469–477. doi: 10.1093/jat/33.8.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowe RH, Abraham TT, Darwin WD, et al. Extended urinary delta9-tetrahydrocannabinol excretion in chronic cannabis users precludes use as a biomarker of new drug exposure. Drug Alcohol Depend. 2009;105:24–32. doi: 10.1016/j.drugalcdep.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374:1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- 36.Singh R, Sandhu J, Kaur B, et al. Evaluation of the DNA damaging potential of cannabis cigarette smoke by the determination of acetaldehyde derived N2-ethyl-2’-deox-yguanosine adducts. Chem Res Toxicol. 2009;22:1181–1188. doi: 10.1021/tx900106y. [DOI] [PubMed] [Google Scholar]
- 37.Moir D, Rickert WS, Levasseur G, et al. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol. 2008;21:494–502. doi: 10.1021/tx700275p. [DOI] [PubMed] [Google Scholar]
- 38.Bailey JR, Cunny HC, Paule MG, et al. Fetal disposition of delta 9-tetrahydrocannabinol (THC) during late pregnancy in the rhesus monkey. Toxicol Appl Pharmacol. 1987;90:315–321. doi: 10.1016/0041-008x(87)90338-3. [DOI] [PubMed] [Google Scholar]
- 39.Battista N, Bari M, Rapino C, et al. Regulation of female fertility by the endocannabinoid system. Hum Fertil (Camb) 2007;10:207–216. doi: 10.1080/14647270701429879. [DOI] [PubMed] [Google Scholar]
- 40.Campolongo P, Trezza V, Palmery M, et al. Developmental exposure to cannabinoids causes subtle and enduring neurofunctional alterations. Int Rev Neurobiol. 2009;85:117–133. doi: 10.1016/S0074-7742(09)85009-5. [DOI] [PubMed] [Google Scholar]
- 41.Porath AJ, Fried PA. Effects of prenatal cigarette and marijuana exposure on drug use among offspring. Neu-rotoxicol Teratol. 2005;27:267–277. doi: 10.1016/j.ntt.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Cabral GA. Drugs of abuse, immune modulation, and AIDS. J Neuroimmune Pharmacol. 2006;1:280–295. doi: 10.1007/s11481-006-9023-5. [DOI] [PubMed] [Google Scholar]
- 43.Croxford JL, Yamamura T. Cannabinoids and the immune system: potential for the treatment of inflammatory diseases? J Neuroimmunol. 2005;166:3–18. doi: 10.1016/j.jneuroim.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 44.Ottani A, Giuliani D. HU-210: a potent tool for investigations of the cannabinoid system. CNS Drug Rev. 2001;7:131–145. doi: 10.1111/j.1527-3458.2001.tb00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pertwee RG. Emerging strategies for exploiting cannabi-noid receptor agonists as medicines. Br J Pharmacol. 2009;156:397–411. doi: 10.1111/j.1476-5381.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77(2):299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 47.Drummer OH, Gerostamoulos J, Batziris H, et al. The incidence of drugs in drivers killed in Australian road traffic crashes. Forensic Sci Int. 2003;134:154–162. doi: 10.1016/s0379-0738(03)00134-8. [DOI] [PubMed] [Google Scholar]
- 48.Janowsky DS, Meacham MP, Blaine JD, et al. Simulated flying performance after marihuana intoxication. Aviat Space Environ Med. 1976;47:124–128. [PubMed] [Google Scholar]
- 49.Yesavage JA, Leirer VO, Denari M, et al. Carry-over effects of marijuana intoxication on aircraft pilot performance: a preliminary report. Am J Psychiatry. 1985;142:1325–1329. doi: 10.1176/ajp.142.11.1325. [DOI] [PubMed] [Google Scholar]
- 50.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) [Accessed 2011 Aug 31];Synthetic cannabinoids and ‘Spice’. 2009 [online]. Available from URL: http://www.emcdda.europa.eu/publications/drug-profiles/synthetic-cannabinoids.
- 51.Skinner R, Conlon L, Gibbons D, et al. Cannabis use and non-clinical dimensions of psychosis in university students presenting to primary care. Acta Psychiatr Scand. 2011;123:21–27. doi: 10.1111/j.1600-0447.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- 52.Scholes KE, Martin-Iverson MT. Alterations to pre-pulse inhibition (PPI) in chronic cannabis users are secondary to sustained attention deficits. Psychopharmacology (Berl) 2009;207:469–484. doi: 10.1007/s00213-009-1679-0. [DOI] [PubMed] [Google Scholar]
- 53.Hunault CC, Mensinga TT, Böcker KB, et al. Cognitive and psychomotor effects in males after smoking a combination of tobacco and cannabis containing up to 69mg delta-9-tetrahydrocannabinol (THC) Psychopharmacol-ogy (Berl) 2009;204:85–94. doi: 10.1007/s00213-008-1440-0. [DOI] [PubMed] [Google Scholar]
- 54.Solowij N. Do cognitive impairments recover following cessation of cannabis use? Life Sci. 1995;56:2119–2126. doi: 10.1016/0024-3205(95)00197-e. [DOI] [PubMed] [Google Scholar]
- 55.Pope HG, Jr, Gruber AJ, Hudson JI, et al. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 56.McHale S, Hunt N. Executive function deficits in short-term abstinent cannabis users. Hum Psychopharmacol. 2008;23:409–415. doi: 10.1002/hup.941. [DOI] [PubMed] [Google Scholar]
- 57.Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: a review. Addiction. 2000;95:1621–1630. doi: 10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- 58.Tashkin DP. Smoked marijuana as a cause of lung injury. Monaldi Arch Chest Dis. 2005;63:93–100. doi: 10.4081/monaldi.2005.645. [DOI] [PubMed] [Google Scholar]
- 59.Reece AS. Chronic toxicology of cannabis. Clin Toxicol (Phila) 2009;47:517–524. doi: 10.1080/15563650903074507. [DOI] [PubMed] [Google Scholar]
- 60.Purohit V, Rapaka R, Shurtleff D. Role of cannabinoids in the development of fatty liver (steatosis) AAPS J. 2010;12:233–237. doi: 10.1208/s12248-010-9178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whan LB, West MC, McClure N, et al. Effects of delta-9-tetrahydrocannabinol, the primary psychoactive can-nabinoid in marijuana, on human sperm function in vitro. Fertil Steril. 2006;85:653–660. doi: 10.1016/j.fertnstert.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 62.Welch KA, McIntosh AM, Job DE, et al. The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr Bull. Epub. 2010 Mar 11; doi: 10.1093/schbul/sbq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mazzoncini R, Donoghue K, Hart J, et al. Illicit substance use and its correlates in first episode psychosis. Acta Psychiatr Scand. Epub. 2009 Oct 13; doi: 10.1111/j.1600-0447.2009.01483.x. [DOI] [PubMed] [Google Scholar]
- 64.Vandrey R, Haney M. Pharmacotherapy for cannabis dependence: how close are we? CNS Drugs. 2009;23(7):543–553. doi: 10.2165/00023210-200923070-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Curr Opin Psychiatry. 2006;19:233–238. doi: 10.1097/01.yco.0000218592.00689.e5. [DOI] [PubMed] [Google Scholar]
- 66.Vandrey R, Budney AJ, Kamon JL, et al. Cannabis withdrawal in adolescent treatment seekers. Drug Alcohol Depend. 2005;78:205–210. doi: 10.1016/j.drugalcdep.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Copersino ML, Boyd SJ, Tashkin DP, et al. Cannabis withdrawal among non-treatment-seeking adult cannabis users. Am J Addict. 2006;15:8–14. doi: 10.1080/10550490500418997. [DOI] [PubMed] [Google Scholar]
- 68.Hasin DS, Keyes KM, Alderson D, et al. Cannabis withdrawal in the United States: results from NESARC. J Clin Psychiatry. 2008;69:1354–1363. doi: 10.4088/jcp.v69n0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cornelius JR, Chung T, Martin C, et al. Cannabis withdrawal is common among treatment-seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addict Behav. 2008;33:1500–1505. doi: 10.1016/j.addbeh.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lupica CR, Riegel AC, Hoffman AF. Marijuana and can-nabinoid regulation of brain reward circuits. Br J Pharmacol. 2004;143:227–342. doi: 10.1038/sj.bjp.0705931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 72.Zangen A, Solinas M, Ikemoto S, et al. Two brain sites for cannabinoid reward. J Neurosci. 2006;26:4901–4907. doi: 10.1523/JNEUROSCI.3554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Solinas M, Yasar S, Goldberg SR. Endocannabinoid system involvement in brain reward processes related to drug abuse. Pharmacol Res. 2007;56:393–405. doi: 10.1016/j.phrs.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matthew RJ, Wilson WH, Turkington TG, et al. Cerebellar activity and disturbed time sense after THC. Brain Res. 1998;797:183–189. doi: 10.1016/s0006-8993(98)00375-8. [DOI] [PubMed] [Google Scholar]
- 75.Ashton CH. Pharmacology and effects of cannabis: a brief review. Br J Psychiatry. 2001;178:101–106. doi: 10.1192/bjp.178.2.101. [DOI] [PubMed] [Google Scholar]
- 76.Wachtel SR, ElSohly MA, Ross SA, et al. Comparison of the subjective effects of delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 2002;161:331–339. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]
- 77.Grotenhermen F. The toxicology of cannabis and cannabis prohibition. Chem Biodivers. 2007;4:1744–1769. doi: 10.1002/cbdv.200790151. [DOI] [PubMed] [Google Scholar]
- 78.Steadward RD, Singh M. The effectsof smoking marihuana on physical performance. Med Sci Sports. 1975;7:309–311. [PubMed] [Google Scholar]
- 79.Renaud AM, Cormier Y. Acute effects of marihuana smoking on maximal exercise performance. Med Sci Sports Exerc. 1986;18:685–689. [PubMed] [Google Scholar]
- 80.Berrendero F, Maldonado R. Involvement of the opioid system in the anxiolytic-like effects induced by delta(9)-tetrahydrocannabinol. Psychopharmacology (Berl) 2002;163:111–117. doi: 10.1007/s00213-002-1144-9. [DOI] [PubMed] [Google Scholar]
- 81.Saugy M, Avois L, Saudan C, et al. Cannabis and sport. Br J Sports Med. 2006;40(Suppl. 1):13–15. doi: 10.1136/bjsm.2006.027607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bortolato M, Campolongo P, Mangieri RA, et al. Anxio-lytic-like properties of the anadamide transport inhibitor AM404. Neuropsychopharmacology. 2006;31:2652–2659. doi: 10.1038/sj.npp.1301061. [DOI] [PubMed] [Google Scholar]
- 83.Naidu PS, Varel SA, Ahn K, et al. Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology (Berl) 2007;192:61–70. doi: 10.1007/s00213-006-0689-4. [DOI] [PubMed] [Google Scholar]
- 84.Moreira FA, Aguiar DC, Guimaraes FS. Anxiolytic-like effect of cannabidiol in the rat Vogel conflict test. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1466–1471. doi: 10.1016/j.pnpbp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 85.Haller J, Varga B, Ledent C, et al. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur J Neurosci. 2004;19:1906–1912. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- 86.McDonald J, Schleifer L, Richards JB, et al. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- 87.Lane SD, Cherek DR, Tcheremissine OV, et al. Acute marijuana effects on human risk taking. Neuropsycho-pharmacology. 2005;30:800–809. doi: 10.1038/sj.npp.1300620. [DOI] [PubMed] [Google Scholar]
- 88. [Accessed 2011 Aug 31];Martinez D. Le dopage au cannabis releve surtout de la question des droits et des devoirs du sportif. 20 Minutes France, 2007 Jan 31 [online]. Available from URL: http://www.20minutes.fr/article/136277/Sport-Le-dopage-au-cannabis-releve-surtout-de-la-question-des-droits-et-des-devoirs-du-sportif.php.
- 89.Marsicano G, Wotjak CT, Azad SC, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 90.Chhatwal JP, Davies M, Maguschak KA, et al. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- 91.Lutz B. The endocannabinoid system and extinction learning. Mol Neurobiol. 2007;36:92–101. doi: 10.1007/s12035-007-8004-x. [DOI] [PubMed] [Google Scholar]
- 92.Wagner JC. Abuse of drugs used to enhance athletic performance. Am J Hosp Pharm. 1989;46:2059–2067. [PubMed] [Google Scholar]
- 93.Bambico FR, Katz N, Debonnel G, et al. Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J Neurosci. 2007;27:11700–11711. doi: 10.1523/JNEUROSCI.1636-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bortolato M, Mangieri RA, Fu J, et al. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62:1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 95.Chabrol H, Ducongé E, Casas C, et al. Relations between cannabis use and dependence, motives for cannabis use and anxious, depressive and borderline symptomatology. Addict Behav. 2005;30:829–840. doi: 10.1016/j.addbeh.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 96.Simons JS, Gaher RM, Correia CJ, et al. An affective-motivational model of marijuana and alcohol problems among college students. Psychol Addict Behav. 2005;19:326–334. doi: 10.1037/0893-164X.19.3.326. [DOI] [PubMed] [Google Scholar]
- 97.Bonn-Miller MO, Zvolensky MJ, Bernstein A. Marijuana use motives: concurrent relations to frequency of past 30-day use and anxiety sensitivity among young adult marijuana smokers. Addict Behav. 2007;32:49–62. doi: 10.1016/j.addbeh.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 98.Catlin DH, Murray TH. Performance-enhancing drugs, fair competition, and Olympic sport. JAMA. 1996;276:231–237. [PubMed] [Google Scholar]
- 99.Iven VG. Recreational drugs. Clin Sports Med. 1998;17(2):245–259. doi: 10.1016/s0278-5919(05)70079-x. [DOI] [PubMed] [Google Scholar]
- 100.Lorente FO, Peretti-Watel P, Grelot L. Cannabis use to enhance sportive and non-sportive performances among French sport students. Addict Behav. 2005;30:1382–1391. doi: 10.1016/j.addbeh.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 101.Pillard F, Cances-Lauwers V, Godeau E, et al. Sport practice and cannabis consumption in a representative sample of French high school adolescents [in French] Ann Med Interne (Paris) 2001;152(Suppl. 7):28–36. [PubMed] [Google Scholar]
- 102.Red C, Quinn TJ, O’Keeffe M. [Accessed 2011 Sep 7];Reefer madness: why more athletes are turning to marijuana. New York Daily News 2003 May 4 [online]. Available from URL: http://articles.nydailynews.com/2003-05-04/sports/18221489_1_wada-testing-marijuana.
- 103.USA Swimming. [Accessed 2011 Aug 31];Updated statement from USA Swimming regarding Michael Phelps. 2009 Feb 5; [online]. Available from URL: http://www.usaswimming.org/USASWeb/ViewNewsArticle.aspx?TabId=0&Alias=Rainbow&Lang=en&ItemId=2354&mid=2943.
- 104.Van Valkenburg K. Michael Phelps suspended 3 months. [Accessed 2011 Aug 31];Baltimore Sun. 2009 Feb 6; [online]. Available from URL: http://www.baltimoresun.com/sports/olympics/bal-sp.phelps06feb06,0,1898488.story.
- 105.World Anti-Doping Agency (WADA) WADA technical document: Decision limits for the confirmatory quantification TD2011DL. The world anti-doping code. [Accessed 2011 Sep 5];International Standards for Laboratories [online] Available from URL: http://www.wada-ama.org/Documents/World_Anti-Doping_Program/WADP-IS-Laboratories/WADA_TD2010DLv1.0_Decision%20Limits%20for%20the%20Confirmatory%20Quantification%20of%20Threshold%20Substances_May%2008%202010_EN.doc.pdf.
- 106.Goodwin RS, Gustafson RA, Barnes A, et al. Delta(9)-tetra-hydrocannabinol, 11-hydroxy-delta(9)-tetrahydrocannabinol and 11-nor-9-carboxy-delta(9)-tetrahydrocannabinol in human plasma after controlled oral administration of cannabinoids. Ther Drug Monit. 2006;28:545–551. doi: 10.1097/00007691-200608000-00010. [DOI] [PubMed] [Google Scholar]
- 107.Huestis MA, Mitchell JM, Cone EJ. Urinary excretion profiles of 11-nor-9-carboxy-delta 9-tetrahydrocannabinol in humans after single smoked doses of marijuana. J Anal Toxicol. 1996;20:441–452. doi: 10.1093/jat/20.6.441. [DOI] [PubMed] [Google Scholar]
- 108.McGilveray IJ. Pharmacokinetics of cannabinoids. Pain Res Manag. 2005;10(Suppl. A):15A–22A. doi: 10.1155/2005/242516. [DOI] [PubMed] [Google Scholar]
- 109.Hunault CC, Mensinga TT, de Vries I, et al. Delta-9-tetra-hydrocannabinol (THC) serum concentrations and pharmacological effects in males after smoking a combination of tobacco and cannabis containing up to 69mg THC. Psychopharmacology (Berl) 2008;201:171–181. doi: 10.1007/s00213-008-1260-2. [DOI] [PubMed] [Google Scholar]
- 110.Huestis MA, Cone EJ. Urinary excretion half-life of 11-Nor-9-carboxy-delta9-tetrahydrocannabinol in humans. Ther Drug Monit. 1998;20:570–576. doi: 10.1097/00007691-199810000-00021. [DOI] [PubMed] [Google Scholar]
- 111.Huestis MA, Mitchell JM, Cone EJ. Detection times of marijuana metabolites in urine by immunoassay and GC-MS. J Anal Toxicol. 1995;19:443–449. doi: 10.1093/jat/19.6.443. [DOI] [PubMed] [Google Scholar]
- 112.Niedbala RS, Kardos KW, Fritch DF, et al. Detection of marijuana use by oral fluid and urine analysis following single-dose administration of smoked and oral marijuana. J Anal Toxicol. 2001;25:289–303. doi: 10.1093/jat/25.5.289. [DOI] [PubMed] [Google Scholar]
- 113.Goodwin RS, Darwin WD, Chiang CN, et al. Urinary elimination of 11-nor-9-carboxy-delta9-tetrahydrocannnabinol in cannabis users during continuously monitored abstinence. J Anal Toxicol. 2008;32:562–569. doi: 10.1093/jat/32.8.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith ML, Barnes AJ, Huestis MA. Identifying new can-nabis use with urine creatinine-normalized THCCOOH concentrations and time intervals between specimen collections. J Anal Toxicol. 2009;33:185–189. doi: 10.1093/jat/33.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schwilke EW, Gullberg RG, Darwin WD, et al. Differentiating new cannabis use from residual urinary cannabinoid excretion in chronic, daily cannabis users. Addiction. 2011;106:499–506. doi: 10.1111/j.1360-0443.2010.03228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kemp PM, Abukhalaf IK, Manno JE, et al. Cannabinoids in humans: II. The influence of three methods of hydrolysis on the concentration of THC and two metabolites in urine. J Anal Toxicol. 1995;19:292–298. doi: 10.1093/jat/19.5.292. [DOI] [PubMed] [Google Scholar]
- 117.Manno JE, Manno BR, Kemp PM, et al. Temporal indication of marijuana use can be estimated from plasma and urine concentrations of delta9-tetrahydrocannabinol, 11-hydroxy-delta9-tetrahydrocannab-inol, and 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid. J Anal Toxicol. 2001;25:538–549. doi: 10.1093/jat/25.7.538. [DOI] [PubMed] [Google Scholar]
- 118.ElSohly MA, deWit H, Wachtel SR, et al. Delta9-tetra-hydrocannabivarin as a marker for the ingestion of marijuana versus Marinol: results of a clinical study. J Anal Toxicol. 2001;25:565–571. doi: 10.1093/jat/25.7.565. [DOI] [PubMed] [Google Scholar]
- 119.Levin FR, Mariani JJ, Brooks DJ, et al. Delta9-tetra-hydrocannabivarin testing may not have the sensitivity to detect marijuana use among individuals ingesting drona-binol. Drug Alcohol Depend. 2010;106:65–68. doi: 10.1016/j.drugalcdep.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jung J, Kempf J, Mahler H, et al. Detection of delta9-tet-rahydrocannabinolic acid A in human urine and blood serum by LC-MS/MS. J Mass Spectrom. 2007;42:354–360. doi: 10.1002/jms.1167. [DOI] [PubMed] [Google Scholar]
- 121.Jung J, Meyer MR, Maurer HH, et al. Studies on the metabolism of the delta9-tetrahydrocannabinol precursor delta9-tetrahydrocannabinolic acid A (delta9-THCA-A) in rat using LC-MS/MS, LC-QTOF MS and GC-MS techniques. J Mass Spectrom. 2009;44:1423–1433. doi: 10.1002/jms.1624. [DOI] [PubMed] [Google Scholar]
- 122.World Anti-Doping Agency. [Accessed 2011 Sep 7];International Standard for Laboratories. 2009 [online]. Available from URL: http://www.wada-ama.org/en/Science-Medicine/Anti-Doping-Laboratories/International-Standard-for-Laboratories/
- 123.Mulé SJ, Lomax P, Gross SJ. Active and realistic passive marijuana exposure tested by three imunoassays and GC/MS in urine. J Anal Toxicol. 1988;12:113–116. doi: 10.1093/jat/12.3.113. [DOI] [PubMed] [Google Scholar]
- 124.Westin AA, Slørdal L. Passive inhalation of cannabis smoke: is it detectable [in Norwegian]? Tidsskr Nor Lae-geforen. 2009;129:109–113. doi: 10.4045/tidsskr.09.33889. [DOI] [PubMed] [Google Scholar]
- 125.Cone EJ. Marijuana effects and urinalysis after passive inhalation and oral ingestion. NIDA Res Monogr. 1990;99:88–96. [PubMed] [Google Scholar]
- 126.Cone EJ, Johnson RE. Contact highs and urinary canna-binoid excretion after passive exposure to marijuana smoke. Clin Pharmacol Ther. 1986;40:247–256. doi: 10.1038/clpt.1986.171. [DOI] [PubMed] [Google Scholar]
- 127.Cone EJ, Johnson RE, Darwin WD, et al. Passive inhalation of marijuana smoke: urinalysis and room air levels of delta-9-tetrahydrocannabinol. J Anal Toxicol. 1987;11:89–96. doi: 10.1093/jat/11.3.89. [DOI] [PubMed] [Google Scholar]
- 128.Law B, Mason PA, Moffat AC, et al. Passive inhalation of cannabis smoke. J Pharm Pharmacol. 1984;36:578–581. doi: 10.1111/j.2042-7158.1984.tb04901.x. [DOI] [PubMed] [Google Scholar]
- 129.Morland J, Bugge A, Skuterud B, et al. Cannabinoids in blood and urine after passive inhalation of cannabis smoke. J Forensic Sci. 1985;30:997–1002. [PubMed] [Google Scholar]
- 130.Perez-Reyes M, Di Guiseppi S, Mason AP, et al. Passive inhalation of marijuana smoke and urinary excretion of cannabinoids. Clin Pharmacol Ther. 1983;34:36–41. doi: 10.1038/clpt.1983.125. [DOI] [PubMed] [Google Scholar]
- 131.ElSohly MA. Practical challenges to positive drug tests for marijuana. Clin Chem. 2003;49:1037–1038. doi: 10.1373/49.7.1037. [DOI] [PubMed] [Google Scholar]
- 132.Pijlman FT, Rigter SM, Hoek J, et al. Strong increase in total delta-THC in cannabis preparations sold in Dutch coffee shops. Addict Biol. 2005;10:171–180. doi: 10.1080/13556210500123217. [DOI] [PubMed] [Google Scholar]
- 133.Office of National Drug Control Policy. [Accessed 2011 Sep 7];National Household Survey on Drug Abuse (NHSDA) [online] 2001 Available from URL: http://oas.samhsa.gov/nhsda/2k1nhsda/PDF/cover.pdf.
- 134.European School Survey Project on Alcohol and Other Drugs (ESPAD) [Accessed 2011 Sep 5];Alcohol and drug use among European 17–18 year old students: data from the ESPAD Project. 2007 [online]. Available from URL: http://www.espad.org/documents/Espad/ESPAD_reports/ESPAD_17-18_Year_Old_2003.pdf.
- 135.US Department of Health and Human Services. [Accessed 2011 Aug 31];Substance Abuse and Mental Health Services Administration (SAMHSA): 2006 National Survey on Drug Use and Health [online] Available from URL: http://oas.samhsa.gov/nsduh/2k6nsduh/2k6results.cfm.