Abstract
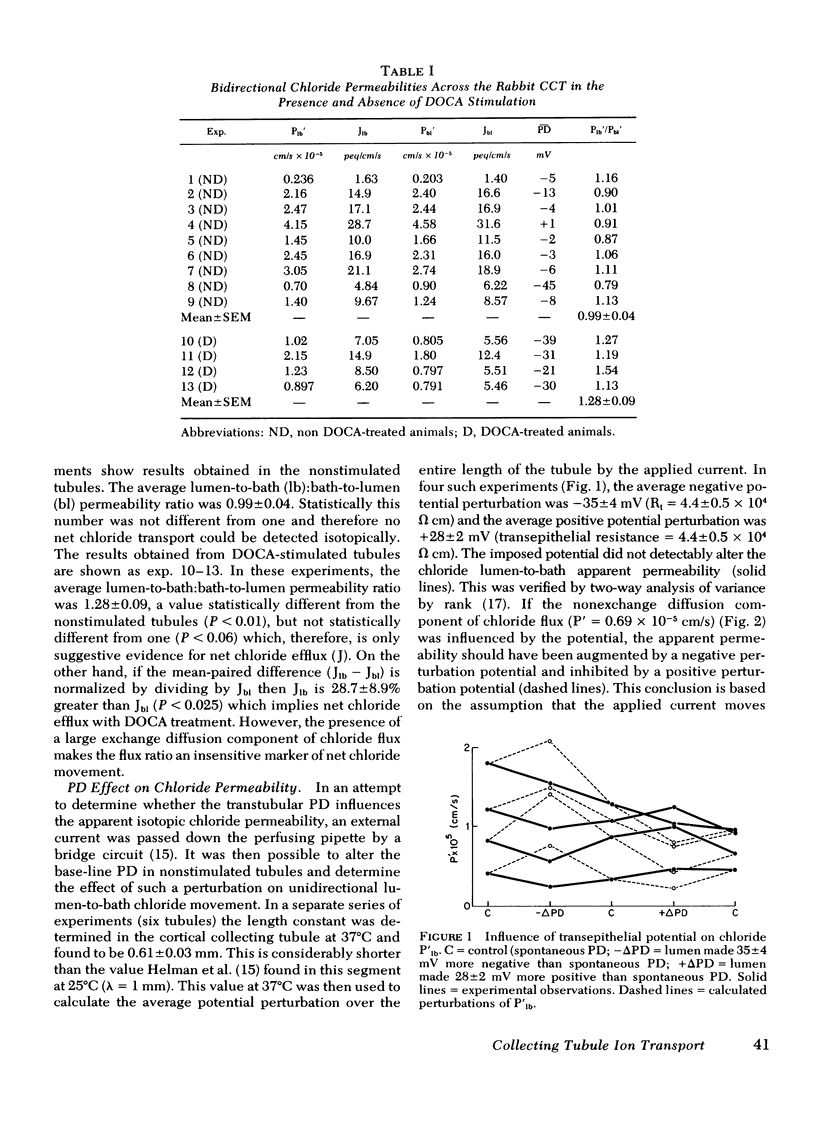
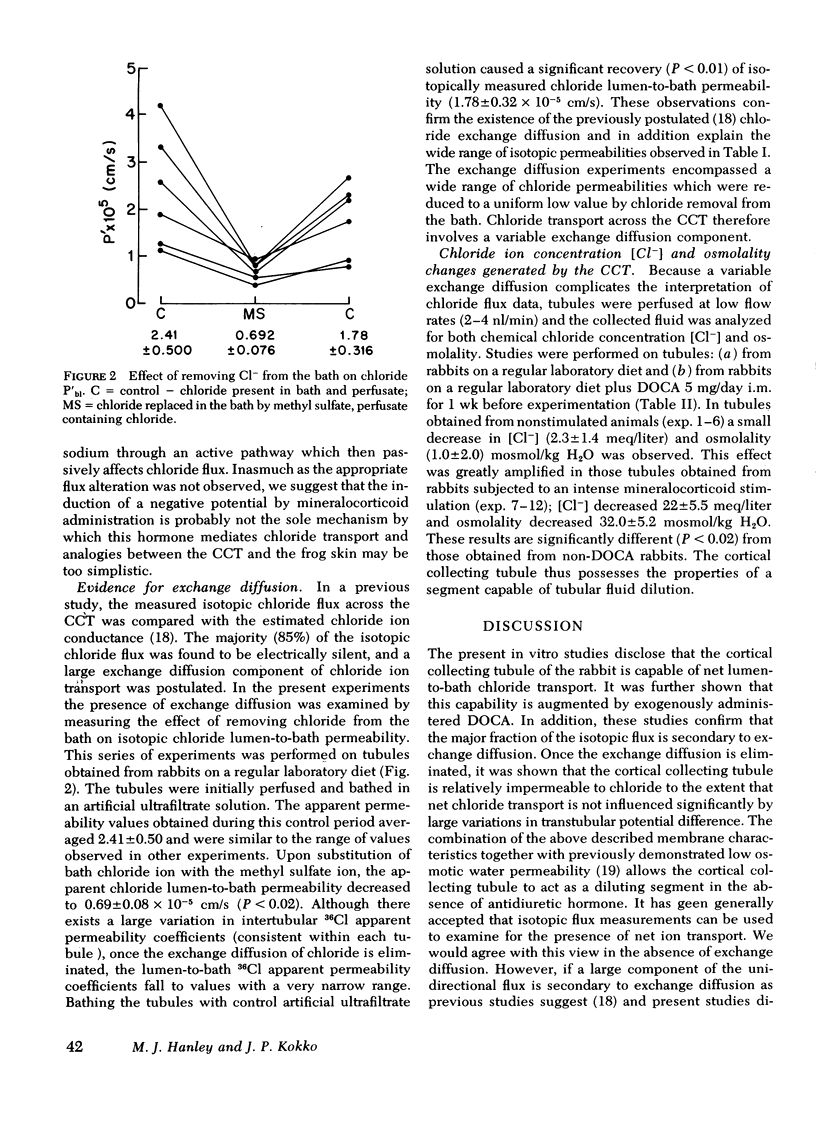
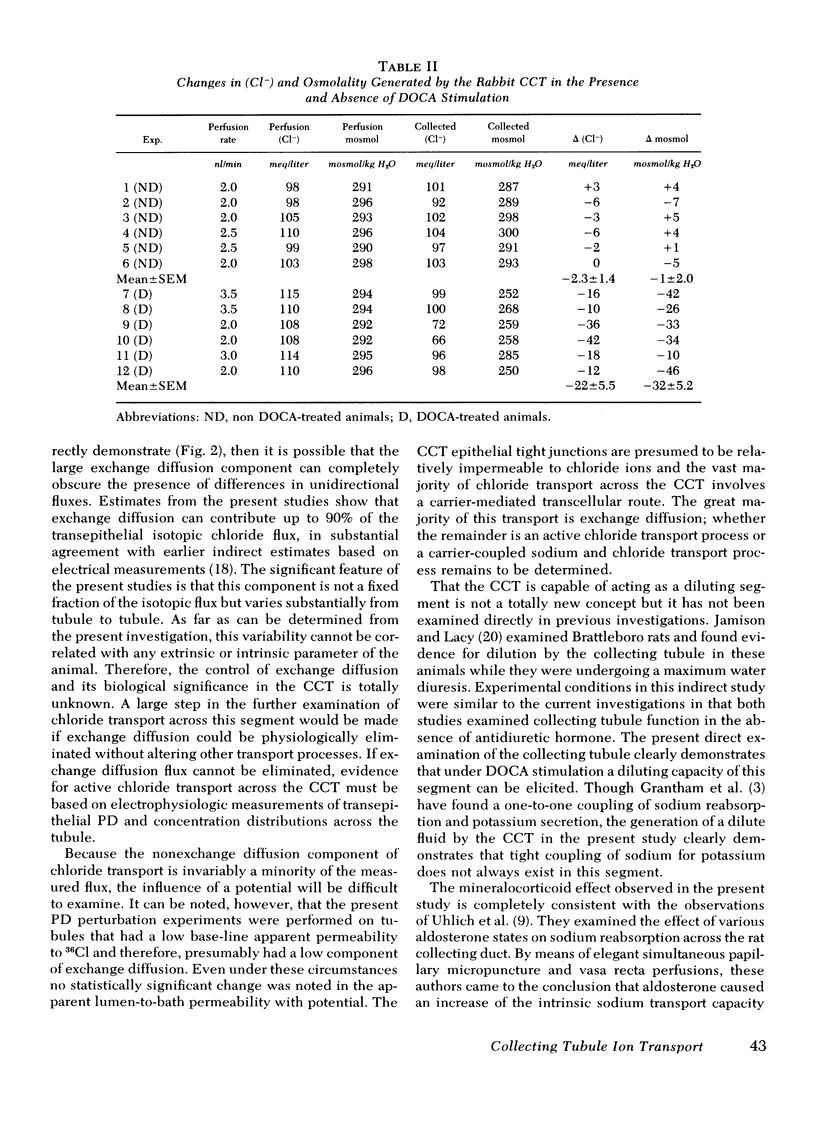
Recent micropuncture studies have suggested that the collecting tubule may be involved in the regulation of extracellular fluid volume. The present studies were designed to evaluate chloride transport across the in vitro-perfused rabbit cortical collecting tubule inasmuch as chloride ion would ultimately affect extracellular fluid volume. The tubules were perfused and bathed with artificial solutions simulating ultrafiltrate. Four groups of studies were conducted. In groups one and two, tubules from rabbits not receiving desoxycorticosterone (DOCA) were compared to tubules from rabbits which had received DOCA (5 mg/day) for 1 wk. In groups three and four, tubules were obtained only from rabbits not receiving DOCA. In group one, sequential bidirectional chloride fluxes were measured. The ratio of chloride efflux to influx was 0.99±0.04 in tubules obtained from rabbits not receiving DOCA whereas it was 1.28±0.09 in tubules obtained from rabbits receiving DOCA, suggesting stimulation of net chloride flux under these conditions. In group 2, chemical chloride concentration and osmolality of the collected fluid were measured. Neither the chemical chloride concentration nor the osmolality of the collected fluid decreased significantly below their respective perfusion fluid values in tubules from non-DOCA-treated rabbits but there was a significant decrease in the chemical chloride concentration (10-42 meq/liter) and osmolality (10-42 mosmol/kg H2O of the collected fluid in tubules from DOCA-treated rabbits. In group three, unidirectional chloride permeabilities from lumen-to-bath were determined during the passage of current down the perfusion pipette. The alterations of the average lumen potential, −35±4 and +28±2 mV, did not influence unidirectional chloride movement suggesting that the cortical collecting tubule is quite impermeable to chloride. In group four, unidirectional chloride permeability from lumen-to-bath was measured before and after substitution of NaCH3SO4 for sodium chloride in the bath. Replacement of chloride by CH3SO4 reversibly decreased the apparent chloride permeability from 2.41±0.50 to 0.69±0.08 (×10−5 cm/s) demonstrating that 36Cl permeability is dependent on the chemical concentration of chloride.
The current studies demonstrate that: (a) the cortical collecting tubule is able to reabsorb salt under the modulation of circulating mineralocorticoids and, thus, may participate in overall volume homeostasis; (b) the chloride permeability and the major portion of isotopic chloride flux across the cortical collecting tubule is via exchange diffusion; and (c) under certain circumstances the cortical collecting tubule may act as a diluting segment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado R. H., Dietz T. H., Mullen T. L. Chloride transport across isolated skin of Rana pipiens. Am J Physiol. 1975 Sep;229(3):869–876. doi: 10.1152/ajplegacy.1975.229.3.869. [DOI] [PubMed] [Google Scholar]
- Andreoli T. E., Watkins M. L. Chloride transport in porous lipid bilayer membranes. J Gen Physiol. 1973 Jun;61(6):809–830. doi: 10.1085/jgp.61.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diezi J., Michoud P., Aceves J., Giebisch G. Micropuncture study of electrolyte transport across papillary collecting duct of the rat. Am J Physiol. 1973 Mar;224(3):623–634. doi: 10.1152/ajplegacy.1973.224.3.623. [DOI] [PubMed] [Google Scholar]
- Finn A. L., Handler J. S., Orloff J. Active chloride transport in the isolated toad bladder. Am J Physiol. 1967 Jul;213(1):179–184. doi: 10.1152/ajplegacy.1967.213.1.179. [DOI] [PubMed] [Google Scholar]
- Forte J. G. Three components of Cl flux across isolated bullfrog gastric mucosa. Am J Physiol. 1969 Jan;216(1):167–174. doi: 10.1152/ajplegacy.1969.216.1.167. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Kurg M. B., Obloff J. The nature of transtubular Na and K transport in isolated rabbit renal collecting tubules. J Clin Invest. 1970 Oct;49(10):1815–1826. doi: 10.1172/JCI106399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. B., Imai M., Kokko J. P. A functional comparison of the cortical collecting tubule and the distal convoluted tubule. J Clin Invest. 1975 Jun;55(6):1284–1294. doi: 10.1172/JCI108048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman S. I., Grantham J. J., Burg M. B. Effect of vasopressin on electrical resistance of renal cortical collecting tubules. Am J Physiol. 1971 Jun;220(6):1825–1832. doi: 10.1152/ajplegacy.1971.220.6.1825. [DOI] [PubMed] [Google Scholar]
- Imai M., Kokko J. P. Sodium chloride, urea, and water transport in the thin ascending limb of Henle. Generation of osmotic gradients by passive diffusion of solutes. J Clin Invest. 1974 Feb;53(2):393–402. doi: 10.1172/JCI107572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison R. L., Lacy F. B. Evidence for urinary dilution by the collecting tubule. Am J Physiol. 1972 Oct;223(4):898–902. doi: 10.1152/ajplegacy.1972.223.4.898. [DOI] [PubMed] [Google Scholar]
- Kokko J. P. Proximal tubule potential difference. Dependence on glucose on glucose, HCO 3 , and amino acids. J Clin Invest. 1973 Jun;52(6):1362–1367. doi: 10.1172/JCI107308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko J. P. Sodium chloride and water transport in the descending limb of Henle. J Clin Invest. 1970 Oct;49(10):1838–1846. doi: 10.1172/JCI106401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko J. P. Urea transport in proximal tubule and the descending limb of Henle. J Clin Invest. 1972 Aug;51(8):1999–2008. doi: 10.1172/JCI107006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer J. A., Andreoli T. E. Cellular constraints to diffusion. The effect of antidiuretic hormone on water flows in isolated mammalian collecting tubules. J Clin Invest. 1972 May;51(5):1264–1278. doi: 10.1172/JCI106921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenblick E. H., Cannon P. J., Laragh J. H. THE NATURE OF THE ACTION OF INTRAVENOUS ALDOSTERONE: EVIDENCE FOR A ROLE OF THE HORMONE IN URINARY DILUTION. J Clin Invest. 1961 Jun;40(6):903–913. doi: 10.1172/JCI104329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. H., Reineck H. J. The role of the collecting duct in the regulation of excretion of sodium and other electrolytes. Kidney Int. 1974 Jul;6(1):1–9. doi: 10.1038/ki.1974.71. [DOI] [PubMed] [Google Scholar]
- Stoner L. C., Burg M. B., Orloff J. Ion transport in cortical collecting tubule; effect of amiloride. Am J Physiol. 1974 Aug;227(2):453–459. doi: 10.1152/ajplegacy.1974.227.2.453. [DOI] [PubMed] [Google Scholar]
- Uhlich E., Baldamus C. A., Ullrich K. J. Einfluss von Aldosteron auf den Natriumtransport in den Sammelrohren der Säugetierniere. Pflugers Arch. 1969;308(2):111–126. doi: 10.1007/BF00587019. [DOI] [PubMed] [Google Scholar]
- Uhlich E., Halbach R., Ullrich K. J. Einfluss von Aldosteron auf den Ausstrom markierten Natriums aus den Sammelrohren der Ratte. Pflugers Arch. 1970;320(3):261–264. doi: 10.1007/BF00587457. [DOI] [PubMed] [Google Scholar]


