Abstract
BACKGROUND
We measured Δ9-tetrahydrocannabinol (THC), 11-nor-9-carboxy-THC (THCCOOH), cannabidiol (CBD), and cannabinol (CBN) disposition in oral fluid (OF) following controlled cannabis smoking to evaluate whether monitoring multiple cannabinoids in OF improved OF test interpretation.
METHODS
Cannabis smokers provided written informed consent for this institutional review board–approved study. OF was collected with the Quantisal™ device following ad libitum smoking of one 6.8% THC cigarette. Cannabinoids were quantified by 2-dimensional GC-MS. We evaluated 8 alternative cutoffs based on different drug testing program needs.
RESULTS
10 participants provided 86 OF samples −0.5 h before and 0.25, 0.5, 1, 2, 3, 4, 6, and 22 h after initiation of smoking. Before smoking, OF samples of 4 and 9 participants were positive for THC and THCCOOH, respectively, but none were positive for CBD and CBN. Maximum THC, CBD, and CBN concentrations occurred within 0.5 h, with medians of 644, 30.4, and 49.0 μg/L, respectively. All samples were THC positive at 6 h (2.1–44.4 μg/L), and 4 of 6 were positive at 22 h. CBD and CBN were positive only up to 6 h in 3 (0.6–2.1 μg/L) and 4 (1.0–4.4 μg/L) participants, respectively. The median maximum THCCOOH OF concentration was 115 ng/L, with all samples positive to 6 h (14.8–263 ng/L) and 5 of 6 positive at 22 h.
CONCLUSIONS
By quantifying multiple cannabinoids and evaluating different analytical cutoffs after controlled cannabis smoking, we determined windows of drug detection, found suggested markers of recent smoking, and minimized the potential for passive contamination.
National and international drug monitoring surveys (1-3) document a high prevalence of cannabis intake. Cannabis was the most common illicit drug identified among drivers positive for potentially impairing drugs in a 2007 survey (4). Oral fluid (OF)2 drug testing in workplace, pain management, drug treatment, and driving under the influence of drugs (DUID) programs is increasing. Elucidating cannabinoid OF pharmacokinetics after controlled smoked cannabis is essential for determining drug detection windows, finding markers of recent smoking, and minimizing potential for passive environmental smoke contamination. The ideal drug detection window varies depending on the goals and design of drug-testing programs. For work-place, pain management, and drug treatment research follow-up visits, a long drug detection window is ideal because testing opportunities are widely separated. Drug testing during accident investigations or “for cause” testing, however, is focused on recent use and potential impairment. Additionally, drug treatment programs may test once, twice, or 3 times a week to evaluate abstinence and relapse, making an intermediate detection window ideal. A key characteristic would be the ability to differentiate new drug intake from residual drug excretion. A short detection window comparable to the period of cannabis intoxication and impairment is preferred for human performance testing. Controlled cannabis administration and sequestration of participants on closed research units to eliminate self-administered drugs provide data for rigorously determining windows of drug detection in OF and improving result interpretation.
Δ9-Tetrahydrocannabinolic acid in cannabis undergoes decarboxylation at high temperatures to produce Δ9-tetrahydrocannabinol (THC), the primary psychoactive constituent (5). THC is metabolized to multiple phase 1 metabolites, predominantly 11-hydroxy-THC (11-OH-THC) and 11-nor-9-carboxy-THC (THCCOOH), with further phase 2 conjugation with glucuronic acid and sulfate, facilitating elimination (6). Another cannabinoid, cannabidiol (CBD) is not psychoactive but has potential therapeutic applications (7, 8); some investigators suggest that CBD may attenuate THC-induced tachycardia, euphoria, and anxiety (9, 10). Cannabinol (CBN), a degradation product of THC oxidation, is approximately 10% as potent as THC and increases as cannabis ages (11, 12).
Defining the appearance, relative concentrations, and duration of multiple cannabinoids in OF will improve OF test interpretation. OF contamination was recently documented in nonsmoking controls sitting in close proximity to cannabis smokers for 3 h (13). THC and CBN were present, but THCCOOH and CBD were not. THCCOOH was identified in OF samples in ng/L concentrations (14-16). Milman et al. (17) later characterized the time course of THCCOOH OF concentrations after self-administered smoked cannabis, multiple 20-mg oral THC doses, and 22-h monitored abstinence in chronic daily cannabis smokers. Monitoring THCCOOH that is not present in cannabis smoke (18) may document intentional cannabis intake and minimize the potential for passive cannabis contamination.
We quantified THC, CBD, CBN, and THCCOOH OF concentrations before and after ad libitum smoking of a single cannabis cigarette to (a) determine OF cannabinoid disposition, (b) evaluate appropriate markers and cutoff concentrations for different drug testing programs, and (c) assess cannabinoid OF collection with the Immunalysis Quantisal™ device.
Materials and Methods
PARTICIPANTS
We recruited cannabis smokers (age 18–45 years) with a mean minimum cannabis intake of at least twice per month during the 3 months before study entry and a positive urine cannabinoid test. Additional inclusion criteria were peripheral veins suitable for venipuncture, blood pressure ≤ 140 mmHg systolic and 90 mmHg diastolic, heart rate ≤ 100 beats per minute, and electrocardiogram and 3-min rhythm strip without clinically relevant abnormalities. Exclusion criteria were history or presence of any clinically significant illness or adverse event associated with cannabis intoxication, donation of more than 450 mL blood within prior 30 days, interest or participation in drug abuse treatment within prior 60 days, and pregnancy or nursing.
SMOKED CANNABIS ADMINISTRATION
Participants resided on a closed clinical unit the night before (required) and after (optional) drug administration. Baseline measures for test parameters and biological samples were collected before drug administration. Mean (SD) cannabis cigarette weight was 0.79 (0.16) g and contained 6.8% (0.2%) THC, 0.25% (0.08%) CBD, and 0.21% (0.02%) CBN, yielding 54, 2.0, and 1.7 mg per cigarette, respectively. After ad libitum smoking (maximum 10 min), participants provided OF samples up to 22 h after smoking initiation. Participants were given breakfast 2 h before and lunch 2.5 h after dosing. Drinking water was prohibited 15 min before each OF collection. The study was approved by the National Institute on Drug Abuse Institutional Review Board, and participants gave voluntary written informed consent.
OF SAMPLE COLLECTION AND ANALYSIS
OF was collected with the Quantisal device (Immunalysis) at 0.5 h before and 0.25, 0.5, 1, 2, 3, 4, 6, and 22 h after the start of cannabis smoking. The device consists of an absorptive cellulose pad, a volume adequacy indicator that turns blue upon collection of 1.0 (0.1) mL OF, and a plastic tube containing 3 mL elution/stabilizing buffer, yielding a 1:4 OF dilution. Samples collected at all time points except 22 h were analyzed within 24 h after refrigeration at 4 °C; 22 h samples were stored at −20 °C until analysis 7 days later.
We quantified THC, CBD, CBN, 11-OH-THC, and THCCOOH in OF by use of a published method (15). Participants’ OF samples were diluted with drug-free OF-Quantisal buffer mixture if analyte concentrations exceeded the upper limit of linearity. Limits of quantification (LOQs) were 0.5 μg/L for THC, CBD, and 11-OH-THC; 1 μg/L for CBN; and 7.5 ng/L for THCCOOH. Intraassay imprecision was 2.2%–6.6%, and interassay imprecision was <5.2%. Analytical recovery was within 13.8% of target.
DATA ANALYSIS
We used IBM SPSS Statistics version 18.0 for Windows and Microsoft Excel for statistical evaluation. Cannabinoid concentrations were log-transformed due to outliers and consequent nonnormal distributions. We analyzed correlations by nonparametric Spearman correlation test; group medians and distributions were compared with nonparametric Fisher exact test and Mann–Whitney U-test. Values below LOQ were considered as one-tenth the LOQ. Results with P < 0.05 were considered significant.
Results
Ten cannabis smokers (9 men, 1 women; ages 18–45 years) spent the night before controlled administration at the secure research unit ensuring 15- to 20-h monitored abstinence. On completion of cannabis smoking the next day, participants provided 80 OF samples 0.5 h before and 0.25, 0.5, 1, 2, 3, 4, and 6 h after the start of smoking; 6 stayed for an additional night, providing 22 h samples. Participants reported median (range) cannabis smoking for 11.0 (8.5–14) of the last 14 days before screening. Median (range) age of first cannabis use was 15.5 (12-22) years old; lifetime duration of cannabis smoking was 9.0 (2-25) years. Participants’ body mass indices ranged from 18.1 to 32.0. Additional demographic data are presented in Table 1.
Table 1.
Demographics and self-reported cannabis history for the 10 participants.
| Participant | Sex | Racea | Age, years | BMI, kg/m2 | Cannabis smoking history | ||||
|---|---|---|---|---|---|---|---|---|---|
| Age first smoked, years | Last smoked, n days before | Days smoked in last 14, n | Joints smoked/day, n | Lifetime smoking duration, years | |||||
| A | M | W | 37 | 18.1 | 22 | 2 | 14 | 6 | 10 |
| B | M | AA | 26 | 30.6 | 16 | 3 | 10 | 2 | 8 |
| C | M | W | 45 | 32.0 | 12 | 1 | 14 | 5 | 25 |
| D | F | W | 27 | 22.3 | 15 | 1 | 8.5 | 6 | 6 |
| F | M | W | 34 | 23.0 | 16 | 2 | 10 | 1 | 10 |
| G | M | W | 22 | 22.9 | 15 | 1 | 14 | 12 | 5 |
| H | M | AA | 18 | 22.3 | 13 | 2 | 9 | 6 | 4 |
| I | M | AA | 41 | 25.5 | 19 | 3 | 12 | 6 | 22 |
| K | M | W | 22 | 20.9 | 16 | 4 | 13 | 2 | 2 |
| L | M | AA | 30 | 30.6 | 15 | 1 | 9 | 3 | 15 |
| Median | 28.5 | 23.0 | 15.5 | 2.0 | 11.0 | 5.5 | 9.0 | ||
| Mean | 30.2 | 24.8 | 15.9 | 2.0 | 11.4 | 4.9 | 10.7 | ||
| SD | 8.9 | 4.7 | 2.8 | 1.1 | 2.3 | 3.2 | 7.7 | ||
AA, African-American; W, white; BMI, body mass index.
At 0.5 h before smoking, 4 participants were positive for THC (range 2.0–13.6 μg/L) and 9 for THCCOOH (11.8–359 ng/L) owing to previously self-administered smoked cannabis. CBD and CBN were not detected. The Quantisal collection device indicator showed low-volume collections for all 0.25-h, 6 0.5-h, 5 1-h, and 1 2-h sample, owing to dry mouth after smoking. In the participant negative for THC and THCCOOH at baseline, positive results for both analytes were observed 0.25 h after the start of smoking despite a low-volume collection.
Maximum OF THC concentrations occurred at or before the first sample (0.25 h) after the start of smoking for all participants, except for 1 participant who had a 0.5-h peak. THC concentrations were highly increased within 2 h, with median (range) of 644 (68.0–10 284), 212 (40.0–6362), 287 (18.9–2440), and 94.1 (16.0–519) μg/L at 0.25, 0.5, 1, and 2 h, respectively. By 3 h postdose, all participants’ OF THC concentrations had decreased by more than 95% from the concentrations at 0.25 h. All participants’ samples were THC-positive 6 h postdose [9.4 (2.1–44.4) μg/L], with 4 of 6 still positive [2.1 (0.5–5.5) μg/L] at 22 h. The 2 participants (A and D) who were negative at baseline were positive 22 h after smoking a single 6.8% THC cigarette.
CBD and CBN had time courses similar to that of THC, with concentrations generally an order of magnitude lower. Maximum CBD and CBN concentrations occurred at or before the first sample (0.25 h) after the start of smoking for all participants except for the same participant with peak THC concentration at 0.5 h. At 0.25 h postdose, CBD and CBN concentrations were 30.4 (2.6–588) and 49.0 (4.8–1558) μg/L, respectively, decreasing by ≥95% 3 h after smoking to 0.9 (<LOQ–7.6) and 1.2 (<LOQ–17.3) μg/L, respectively. Three participants were positive for CBD and CBN at 6 h, with concentrations <5 μg/L; none were positive at 22 h.
Maximum THCCOOH concentrations generally occurred 1–2 h after the start of smoking, except in 2 participants with the highest concentrations at 0.25 h. OF THCCOOH concentrations decreased more slowly than those of THC, with 74.4 (9.6–647), 66.4 (<LOQ–567), 111 (12.1–665), and 83.6 (15.4–763) ng/L at 0.25, 0.5, 1, and 2 h, respectively. Median % change from 0.25 h at 3-h post dose was −50.6%, with 2 participants’ OF concentrations increasing; 39% of all samples demonstrated THCCOOH increases from a preceding collection. All participants were THCCOOH positive 6 h postdose [47.5 (14.8–263) ng/L], with 5 of 6 positive at 22 h [20.9 (9.7–103) ng/L]. 11-OH-THC was not present in any OF sample at the 0.5 μg/L LOQ. Median (interquartile range) THC, CBD, CBN, and THCCOOH OF concentrations over time are illustrated in Fig. 1, with individual data summarized in Table 2.
Fig. 1.
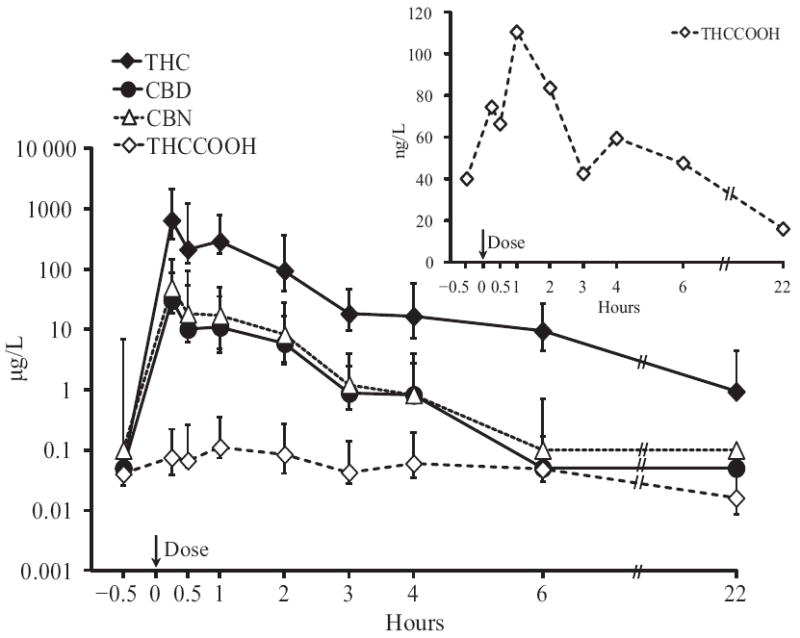
THC, CBD, CBN, and THCCOOH concentrations after smoking a single 6.8% THC cigarette (up to 6 h, n = 10; at 22 h, n = 6)
Error bars indicate interquartile ranges. Inset provides additional details for median THCCOOH concentrations over time.
Table 2.
THC, CBD, CBN, and THCCOOH OF concentrations in 10 cannabis smokers before and after smoking a single 6.8% THC cigarette.
| Participant | Smoking duration, mina | Final collection, h | THC, μg/L
|
THCCOOH, ng/L
|
CBD, μg/L
|
CBN, μg/L
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmaxa | Tmax, h | Clast | Tlast, h | Cmax | Tmax, h | Clast | Tlast, h | Cmax | Tmax, h | Clast | Tlast, h | Cmax | Tmax, h | Clast | Tlast, h | |||
| A | 10 | 22 | 700 | 0.25 | 0.5 | 22 | 75.1 | 1 | 18.7 | 6 | 47.8 | 0.25 | 0.9 | 4 | 36.5 | 0.25 | 1.0 | 3 |
|
| ||||||||||||||||||
| B | 5 | 22 | 588 | 0.25 | 3.0 | 6 | 52.2 | 0.25 | 20.9 | 22 | 29.6 | 0.25 | 0.6 | 4 | 50.0 | 0.25 | 6.5 | 2 |
|
| ||||||||||||||||||
| C | 7 | 22 | 1939 | 0.50 | 2.9 | 22 | 73.5 | 2 | 9.7 | 22 | 82.6 | 0.50 | 0.9 | 6 | 125 | 0.50 | 1.6 | 6 |
|
| ||||||||||||||||||
| D | 10 | 22 | 373 | 0.25 | 1.3 | 22 | 26.7 | 2 | 10.9 | 22 | 12.5 | 0.25 | 0.6 | 6 | 24.3 | 0.25 | 1.1 | 6 |
|
| ||||||||||||||||||
| F | 8 | 6 | 1162 | 0.25 | 7.6 | 6 | 265 | 2 | 125 | 6 | 31.2 | 0.25 | 0.9 | 3 | 82.5 | 0.25 | 1.5 | 3 |
|
| ||||||||||||||||||
| G | 10 | 6 | 478 | 0.25 | 11.0 | 6 | 156 | 2 | 118 | 6 | 19.8 | 0.25 | 0.8 | 4 | 48.0 | 0.25 | 1.0 | 6 |
|
| ||||||||||||||||||
| H | 8 | 22 | 215 | 0.25 | 5.6 | 6 | 320 | 1 | 103 | 22 | 10.8 | 0.25 | 2.2 | 2 | 11.6 | 0.25 | 3.0 | 2 |
|
| ||||||||||||||||||
| I | 10 | 22 | 10 284 | 0.25 | 5.5 | 22 | 212 | 1 | 38.4 | 22 | 588 | 0.25 | 2.1 | 6 | 1558 | 0.25 | 4.4 | 6 |
|
| ||||||||||||||||||
| K | 10 | 6 | 68.0 | 0.25 | 2.1 | 6 | 20.6 | 0.25 | 15.1 | 6 | 2.6 | 0.25 | 0.5 | 2 | 4.8 | 0.25 | 1.5 | 2 |
|
| ||||||||||||||||||
| L | 7 | 6 | 1524 | 0.25 | 11.9 | 6 | 763 | 2 | 263 | 6 | 67.0 | 0.25 | 1.2 | 4 | 104 | 0.25 | 1.8 | 4 |
Time participants took to finish smoking 1 cannabis cigarette (maximum time allowed = 10 min).
Tmax, time of Cmax; Clast, last sample concentration ≥LOQ (0.5 μg/L for THC and CBD, 7.5 ng/L for THCCOOH, 1 μg/L for CBN); Tlast, time of Clast.
THC concentrations were strongly correlated with CBD (ρ = 0.976; n = 86; P < 0.001) and CBN (ρ = 0.971; n = 86, P < 0.001) concentrations, with moderate correlation to THCCOOH (ρ = 0.405; n = 86; P < 0.001) concentrations. At each time point from 0.25 to 6 h (n = 10), correlation coefficients for THC to CBD or CBN were significant (P < 0.005) and ρ > 0.8, whereas correlation coefficients for THC and THCCOOH were ≤0.63 and nonsignificant (P ≥ 0.05) from 0.25 to 6 h. THC concentrations 0.25 h after smoking were significantly correlated with lifetime years of cannabis smoking (ρ = 0.948; P < 0.001) but not with body mass index, typical number of joints smoked per day, days since last smoked, or time taken to complete smoking (P > 0.05); THCCOOH concentrations were not significantly correlated with any of the above variables (all P > 0.05). Baseline THC concentrations were <1% (median 0.0%) of 0.25 h concentrations, whereas baseline THCCOOH concentrations ranged from 8% to 79% (49%) of 0.25 THCCOOH concentrations.
Participants with durations of lifetime cannabis smoking <10 years had significantly lower maximum concentrations (Cmax) (P = 0.008) for THC than participants with smoking durations >10 years (Fig. 2, A and C). The corresponding THCCOOH concentrations are shown in Fig. 2, B and D; there were no significant differences in THCCOOH concentrations between groups. Median (range) THC Cmax for the 5 participants smoking <10 years was 373 (68.0–588) μg/L; THCCOOH was 52.2 (20.6–320) ng/L. For the 5 participants who smoked ≥10 years, median THC and THCCOOH Cmax were 1524 (700–10 284) and 212 (73.5–763) ng/L, respectively. Large intersubject variability was also observed in other cannabinoid concentrations.
Fig. 2.
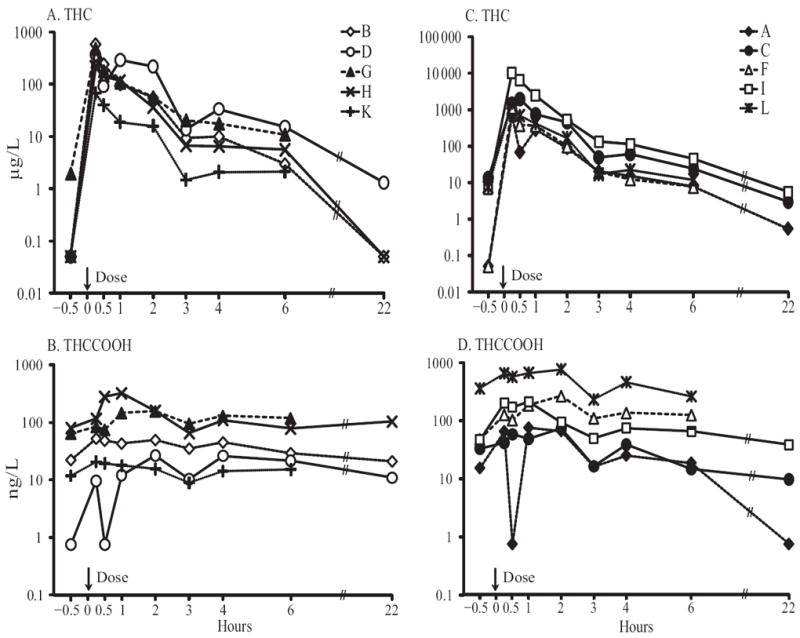
THC and THCCOOH oral fluid elimination profiles in 5 participants with lifetime cannabis intake of <10 years (A and B) and 5 participants with lifetime cannabis intake of ≥10 years (C and D).
At 6 h postsmoking, all 10 participants’ OF THC concentrations were greater than at baseline. By 22 h, however, 4 of 6 participants had THC <LOQ or lower than baseline concentrations. THCCOOH concentrations were more variable. Seven of 10 participants’ THCCOOH OF concentrations were higher at 6 h than at baseline. By 22 h, 4 of 6 THCCOOH concentrations were lower than those before smoking. THC and THCCOOH concentration differences from baseline at 6 and 22 h are shown in Fig. 3. At the Substance Abuse and Mental Health Services Administration (SAMHSA) proposed 2-μg/L THC cutoff, all participants were positive at 6 h and 2 of 6 at 22 h. At the 1-μg/L driving under the influence of drugs, alcohol, and medicines (DRUID) European Union program THC cutoff, all participants were positive at 6 h and 3 of 6 at 22 h. We explored other possible cutoffs (THC ≥1.5 μg/L, THCCOOH ≥20 ng/L, THC ≥1–2 μg/L + CBD ≥0.5 μg/L, THC ≥1–2 μg/L + CBN ≥1 μg/L, and THC ≥1–2 μg/L + THCCOOH ≥20, 30, 40, and 50 ng/L) and related detection windows to meet different drug-testing program goals (Fig. 4). Last detection times were dependent on CBD, CBN, or THCCOOH, not THC concentrations, within the narrow 1- to 2-μg/L cutoff range. Thus, when 2 cannabinoids were included in the combined analyte cutoffs, last detection times did not change whether THC ≥1, 1.5, or 2 μg/L was used.
Fig. 3.
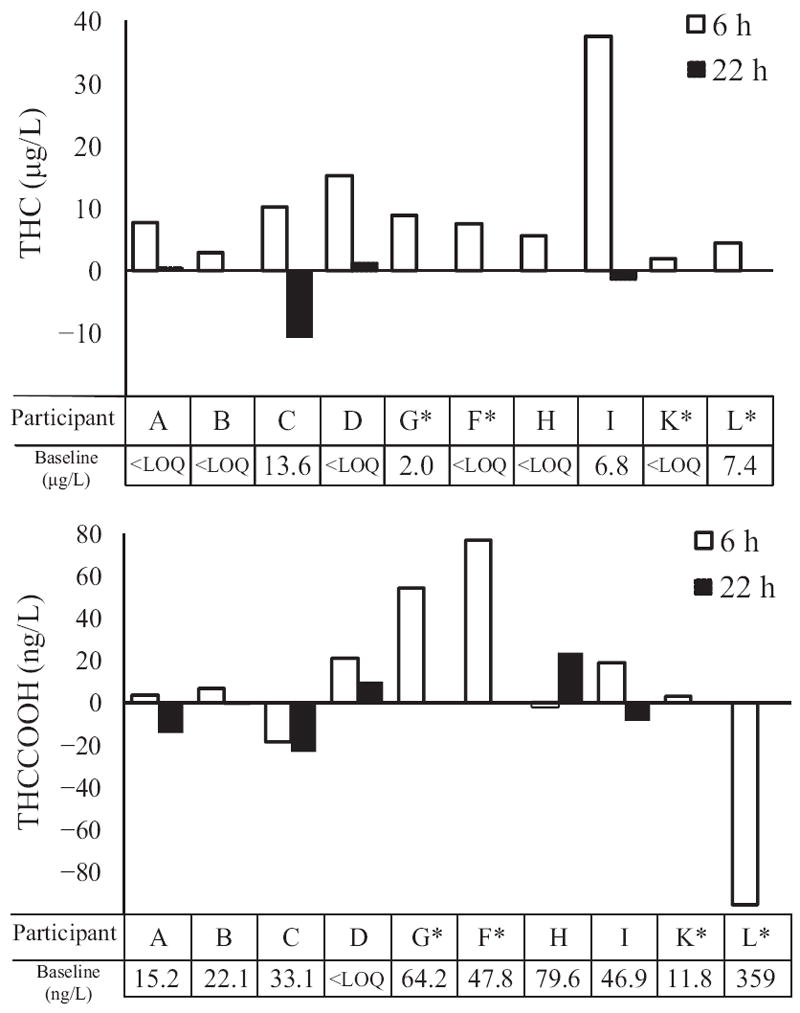
THC and THCCOOH concentration differences from baseline (−0.5 h) at 6 (n = 10) and 22 h (n = 6) after smoking
*Participants without 22-h samples.
Fig. 4.
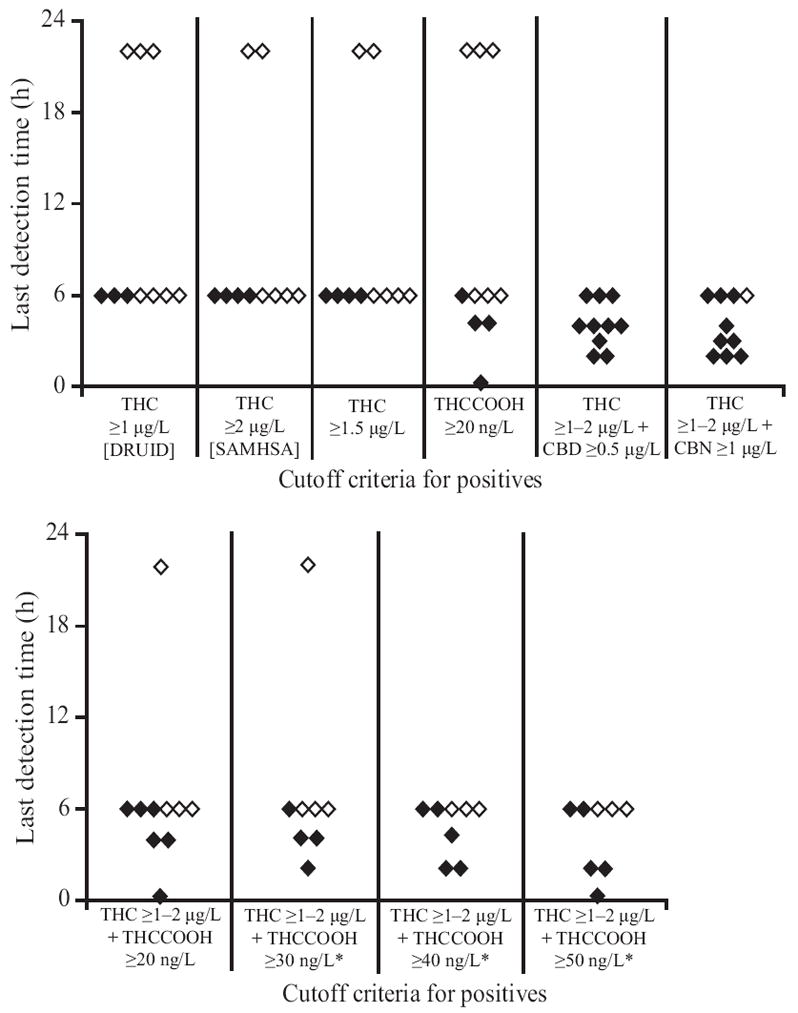
Cannabinoid last detection times for participants (n = 10) with cutoffs of THC ≥1 μg/L (DRUID), THC ≥2 μg/L (proposed SAMHSA), THC ≥1.5 μg/L, THCCOOH ≥20 ng/L, THC ≥1–2 μg/L + CBD ≥0.5 μg/L, THC ≥1–2 μg/L + CBN ≥1 μg/L, and THC 1–2 μg/L + THCCOOH ≥20, 30, 40, and 50 ng/L
◇, participants whose last positive sample = last collected sample; ◆, participants with defined last positive test (negative sample after last positive). *n = 8; 2 participants with no positives at the cutoff.
Discussion
The present study comprehensively characterized THC, CBD, CBN, and THCCOOH OF disposition after smoking of a single cannabis cigarette. Pharmacokinetic properties of cannabinoids in OF other than THC have not been adequately described. Also, for the first time, samples (all but 22-h samples) were refrigerated and analyzed within 24 h of collection to minimize concentration changes due to analyte instability. THC was stable under refrigerated conditions for 14 days in fortified OF samples (19), but long-term stability of other cannabinoid analytes and THC stability in authentic OF samples have not been evaluated.
Initial high OF THC, CBD, and CBN concentrations primarily reflected contamination of the oral mucosa directly with cannabinoids present in cannabis smoke. OF concentrations were influenced by cannabinoid concentrations in the cannabis plant, smoking topography (20), THC pyrolysis (21), and sidestream smoke losses. Participants can titrate the delivered drug dose, leading to high intra- and intersubject variability in blood THC concentrations even in studies where smoking topography was tightly controlled (22). Ad libitum smoking in this study introduced additional variability, but also provided more realistic concentrations by reflecting authentic smoking behavior while minimizing adverse events.
Our observed THC concentrations of 68–10 284 μg/L agree with those reported in other cannabinoid smoking studies. Niedbala et al. (23) reported OF THC 18–1080 μg/L immediately after smoking a cannabis cigarette with 5.4 or 10.4% THC. No significant difference in OF THC concentrations between low and high THC doses was found (23), further supporting smokers’ dose titration. Kauert et al. (24) documented peak OF THC concentration ranges of 245–2228 and 248–2544 μg/L 0.25 h after smoking cannabis cigarettes containing 18.2 and 36.5 mg THC, respectively. Moore et al. (25) reported OF THC of 15–93 μg/L in 3 repeated sessions in the same participant 0.5 h postdose, but after 5 days of abstinence, this individual’s peak OF THC exceeded 2000 μg/L 0.5 h postdose. Huestis and Cone (26) observed OF THC as high as 5800 μg/L 0.2 h after a cannabis cigarette containing 3.55% THC. In the present study, the high initial THC concentrations decreased to medians of 94.1 and 9.4 μg/L at 2 and 6 h, respectively. Other studies reported 4–54 and <LOQ to 17 μg/L at 2 and 6 h postdose, respectively (23, 25, 26). At 22 h, 4 of 6 participants were positive in our study, similar to reported THC detection at or beyond 24 h (25-28).
Median CBN concentrations 0.25–6 h postsmoking were 1%–50% higher than median CBD concentrations. Moore et al. (29) reported OF CBN, but not CBD, after cannabis smoking. The relatively high THC, CBD, and CBN concentrations detected in the present study could be due to higher cannabinoid content in the cigarette, analysis within 24 h (minimizing degradation), and/or participation by experienced chronic cannabis smokers whose smoking topography may have resulted in inhalation of higher cannabinoid amounts. Participant K, who had the lowest THC, CBD, and CBN concentrations throughout, self-reported smoking only 2 joints per day for 2 years. In contrast, participant I, who had the highest THC, CBD, and CBN concentrations, reported smoking 6 joints per day for 22 years. Toennes et al. (30) observed significantly higher maximum OF THC concentrations in chronic vs occasional smokers 5 min after 1 cannabis cigarette containing 500 μg THC/kg body weight. OF cannabinoid concentrations also can be affected by recovery of analytes from the collection device and extraction efficiency of the sample preparation assay.
THCCOOH is not present in cannabis smoke (18), and consequently OF THCCOOH concentrations likely reflect hepatic or oral mucosal metabolism following systemic THC ingestion. CYP2C expression was documented in human buccal (31) and tongue (32) cells; this enzyme is important to 11-OH-THC and THCCOOH formation from THC (33). In the present study, THCCOOH peak concentrations occurred 1–2 h after smoking, although maximum concentrations occurred at 0.25 h for participants D and K. Multiple factors may have contributed to these findings, including residual THCCOOH concentrations and low-volume collection at these time points. Clinical research on THCCOOH pharmacokinetic properties in OF is limited. One study reported OF THCCOOH concentrations of <134 ng/L 0.25–8 h after smoking in 1 participant (14). Even after 37 oral THC doses, no >1118 ng/L THCCOOH was observed (17). Likewise, even at its peak, OF THCCOOH was <764 ng/L in all participants. For participant L, THCCOOH concentrations did not fall below 230 ng/L, but clearly observable blood in the OF samples most likely contributed to these high concentrations. Whole blood THCCOOH concentrations in participant L were 38 400 ng/L at baseline and 79 900 ng/L at peak (34). His whole blood CBD and CBN were <LOQ within 0.5 h, with maximum concentrations ≤2.1 μg/L, much less than those detected in OF. Whole blood THC could have contributed; as the peak occurred at 0.25 h in OF and whole blood, OF THC concentration was 1524 μg/L whereas whole blood THC was 62.7 μg/L. OF samples collected with noticeable blood should be recollected to prevent inaccurate interpretation.
Sample volume is another important issue to be considered in OF collection. Twenty-two of 86 samples collected within 2 h after smoking cannabis had volumes <1 mL based on collection device indicator failures, but still had a high prevalence of positive tests (all positive for THC, CBD and CBN; 2 negative for THCCOOH). These data and the findings of others (35) suggest that low-volume samples should not be discarded, as many may be positive for stimulant or cannabinoid drugs. Current proposed SAMHSA guidelines suggest discarding low-volume samples and initiating a new collection. We suggest that low-volume OF collections should be analyzed, with a subsequent full 1-mL OF collection also tested to avoid false-negative results that could occur owing to greater dilution with elution buffer in low-volume samples.
We previously evaluated potential cannabinoid OF cutoffs in a population of chronic daily cannabis smokers during 4–33 days of abstinence (28). Including CBD, CBN, or THCCOOH with a THC cutoff could document recent cannabis exposure and rule out residual THC excretion. The present study evaluated multiple cutoffs following acute cannabis smoking. Cutoffs with THC and CBD or CBN limited the detection window to ≤6 h, equivalent to a cognitive and motor performance impairment window suggested by Ramaekers et al. (36). Others also reported impairment in psychomotor tasks from 15 min to 4 h after cannabis ingestion (37, 38). In DUID cases, it is important to identify recent cannabis exposure of <8–12 h, especially as cannabis was often the most prevalent drug identified in injured drivers (39). However, CBD and CBN content in the cigarette can vary due to selective breeding procedures, composition of plant material, and storage conditions (6), and tests for CBD and CBN do not protect against passive contamination from environmental cannabis smoke.
The current proposed THC confirmation cutoffs of ≥1 μg/L (DRUID) and ≥2 μg/L (SAMHSA) offer longer detection times but do not adequately protect against the possibility of passive cannabis smoke contamination. Cutoffs including THCCOOH with or without THC minimize the issue of passive exposure, although large intersubject variability in THCCOOH detection windows was observed. We noted in this study and in our previous work (28) that THCCOOH generally provides a longer detection time than THC. This longer detection time is a deterrent to drug intake. Incorporating additional cannabinoids or higher cutoff concentrations can shorten detection times for other testing programs, including DUID. Further research is needed to fully define OF cannabinoid detection windows, especially with the inclusion of additional occasional smokers.
These controlled smoked cannabis administration data provide valuable information for interpretation of OF cannabinoid concentrations, and suggest preliminary cutoffs and collection procedures for OF drug testing programs.
Acknowledgments
We acknowledge the contributions of the clinical staffs of the National Institute on Drug Abuse, Intramural Research Program, and Behavioral Pharmacology Research Unit. This research was funded by the Intramural Research Program, National Institute on Drug Abuse, NIH.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
Research Funding: The Intramural Research Program, National Institute on Drug Abuse, NIH.
Footnotes
Nonstandard abbreviations: OF, oral fluid; DUID, driving under the influence of drugs; THC, Δ9-tetrahydrocannabinol; 11-OH-THC, 11-hydroxy-THC; THCCOOH, 11-nor-9-carboxy THC; CBD, cannabidiol; CBN, cannabinol; LOQ, limit of quantification; SAMHSA, Substance Abuse and Mental Health Services Administration; DRUID, driving under the influence of drugs, alcohol, and medicines.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Previous presentation of the manuscript: 2011 Joint Meeting of the Society of Forensic Toxicologists and the International Association of Forensic Toxicologists, San Francisco, CA, September 2011.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Expert Testimony: None declared.
References
- 1.United Nations Office on Drugs and Crime. [September 2011];World drug report 2010. http://www.unodc.org/documents/wdr/WDR_2010/World_Drug_Report_2010_lo-res.pdf.
- 2.SAMHSA. Office of Applied Studies, NSDUH Series H-38A, HHS publ no SMA 10-4586 Findings. Rockville (MD): SAMHSA; 2010. Results from the 2009 National Survey on Drug Use and Health: national findings. [Google Scholar]
- 3.European Monitoring Centre for Drugs and Drug Addiction. [September 2011];Annual report 2010: the state of the drugs problem in Europe. http://www.emcdda.europa.eu/attachements.cfm/att_120104_EN_EMCDDA_AR2010_EN.pdf.
- 4.Lacey JH, Kelley-Baker T, Furr-Holden B, Voas RB, Romano E, Ramirez A, et al. Pacific Institute for Research and Evaluation, Calverton MD. 2007 National roadside survey of alcohol and drug use by drivers: drug results. Washington (DC): National Highway Traffic Safety Administration; 2009. p. 132. DOT HS 811 249. Contract no. DTNH22-06-C-00040. Available from: National Highway Traffic Safety Administration ( www.nhtsa.gov) [Google Scholar]
- 5.ElSohly MA, Jones AB. Drug testing in the workplace: could a positive test for one of the mandated drugs be for reasons other than illicit use of the drug? J Anal Toxicol. 1995;19:450–8. doi: 10.1093/jat/19.6.450. [DOI] [PubMed] [Google Scholar]
- 6.Huestis MA. Pharmacokinetics and metabolism of the plant cannabinoids, delta-9-tetrahydrocannabinol, cannabidiol and cannabinol. In: Pertwee RG, editor. Handbook of experimental pharmacology. New York: Springer; 2005. pp. 657–90. [DOI] [PubMed] [Google Scholar]
- 7.Ren Y, Whittard J, Higuera-Matas A, Morris CV, Hurd YL. Cannabidiol, a nonpsychotropic component of cannabis, inhibits cue-induced heroin-seeking and normalizes discrete mesolimbic neuronal disturbances. J Neurosci. 2009;29:14764–9. doi: 10.1523/JNEUROSCI.4291-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayakawa K, Mishima K, Abe K, Hasebe N, Takamatsu F, Yasuda H, et al. Cannabidiol prevents infarction via the non-CB1 cannabinoid receptor mechanism. Neuroreport. 2004;15:2381–5. doi: 10.1097/00001756-200410250-00016. [DOI] [PubMed] [Google Scholar]
- 9.Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta-9-THC in normal subjects. Psychopharmacology (Berl) 1982;76:245–50. doi: 10.1007/BF00432554. [DOI] [PubMed] [Google Scholar]
- 10.Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol interferes with the effects of delta 9-tetrahydrocannabinol in man. Eur J Pharmacol. 1974;28:172–7. doi: 10.1016/0014-2999(74)90129-0. [DOI] [PubMed] [Google Scholar]
- 11.ElSohly MA. Chemical constituents of cannabis. In: Grotenhermen F, Russo E, editors. Cannabis and cannabinoids: pharmacology, toxicology, and therapeutic potential. New York: Haworth Integrative Healing; 2002. pp. 27–36. [Google Scholar]
- 12.Turner CE, ElSohly MA, Boeren EG. Constituents of cannabis sativa L. XVII. A review of the natural constituents. J Nat Prod. 1980;43:169–234. doi: 10.1021/np50008a001. [DOI] [PubMed] [Google Scholar]
- 13.Moore C, Coulter C, Uges D, Tuyay J, van der Linde S, van Leeuwen A, et al. Cannabinoids in oral fluid following passive exposure to marijuana smoke. Forensic Sci Int. 2011;212:227–30. doi: 10.1016/j.forsciint.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Moore C, Coulter C, Rana S, Vincent M, Soares J. Analytical procedure for the determination of the marijuana metabolite 11-nor-Delta9-tetrahydrocannabinol-9-carboxylic acid in oral fluid specimens. J Anal Toxicol. 2006;30:409–12. doi: 10.1093/jat/30.7.409. [DOI] [PubMed] [Google Scholar]
- 15.Milman G, Barnes AJ, Lowe RH, Huestis MA. Simultaneous quantification of cannabinoids and metabolites in oral fluid by two-dimensional gas chromatography mass spectrometry. J Chromatogr A. 2010;1217:1513–21. doi: 10.1016/j.chroma.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day D, Kuntz DJ, Feldman M, Presley L. Detection of THCA in oral fluid by GC-MS-MS. J Anal Toxicol. 2006;30:645–50. doi: 10.1093/jat/30.9.645. [DOI] [PubMed] [Google Scholar]
- 17.Milman G, Barnes AJ, Schwope DM, Schwilke EW, Darwin WD, Goodwin RS, et al. Disposition of cannabinoids in oral fluid after controlled around-the-clock oral THC administration. Clin Chem. 2010;56:1261–9. doi: 10.1373/clinchem.2009.141853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sachs H, Dressler U. Detection of THCCOOH in hair by MSD-NCI after HPLC clean-up. Forensic Sci Int. 2000;107:239–47. doi: 10.1016/s0379-0738(99)00167-x. [DOI] [PubMed] [Google Scholar]
- 19.Moore C, Vincent M, Rana S, Coulter C, Agrawal A, Soares J. Stability of Delta(9)-tetrahydrocannabinol (THC) in oral fluid using the Quantisal collection device. Forensic Sci Int. 2006;164:126–30. doi: 10.1016/j.forsciint.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Reyes M. Marijuana smoking: factors that influence the bioavailability of tetrahydrocannabinol. In: Chiang CN, Hawks RN, editors. Research findings on smoking of abused substances. Rockville (MD): US Department of Health and Human Services, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute on Drug Abuse; 1990. pp. 42–62. NIDA research monograph; 99. [PubMed] [Google Scholar]
- 21.Davis KH, McDaniel IA, Cadwell IW, Moody PL. Some smoking characteristics of marijuana cigarettes. In: Agurell S, Dewey WL, Willette RE, editors. The cannabinoids: chemical, pharmacologic, and therapeutic aspects. Orlando (FL): Academic; 1984. pp. 97–109. [Google Scholar]
- 22.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16:276–82. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- 23.Niedbala RS, Kardos KW, Fritch DF, Kunsman KP, Blum KA, Newland GA, et al. Passive cannabis smoke exposure and oral fluid testing. II. Two studies of extreme cannabis smoke exposure in a motor vehicle. J Anal Toxicol. 2005;29:607–15. doi: 10.1093/jat/29.7.607. [DOI] [PubMed] [Google Scholar]
- 24.Kauert GF, Ramaekers JG, Schneider E, Moeller MR, Toennes SW. Pharmacokinetic properties of delta9-tetrahydrocannabinol in serum and oral fluid. J Anal Toxicol. 2007;31:288–93. doi: 10.1093/jat/31.5.288. [DOI] [PubMed] [Google Scholar]
- 25.Moore C, Rana S, Coulter C, Day D, Vincent M, Soares J. Detection of conjugated 11-nor-Delta9-tetrahydrocannabinol-9-carboxylic acid in oral fluid. J Anal Toxicol. 2007;31:187–94. doi: 10.1093/jat/31.4.187. [DOI] [PubMed] [Google Scholar]
- 26.Huestis MA, Cone EJ. Relationship of Delta 9-tetrahydrocannabinol concentrations in oral fluid and plasma after controlled administration of smoked cannabis. J Anal Toxicol. 2004;28:394–9. doi: 10.1093/jat/28.6.394. [DOI] [PubMed] [Google Scholar]
- 27.Niedbala RS, Kardos KW, Fritch DF, Kardos S, Fries T, Waga J, et al. Detection of marijuana use by oral fluid and urine analysis following single-dose administration of smoked and oral marijuana. J Anal Toxicol. 2001;25:289–303. doi: 10.1093/jat/25.5.289. [DOI] [PubMed] [Google Scholar]
- 28.Lee D, Milman G, Barnes AJ, Goodwin RS, Hirvonen J, Huestis MA. Oral fluid cannabinoids in chronic, daily cannabis smokers during sustained, monitored abstinence. Clin Chem. 2011;57:1127–36. doi: 10.1373/clinchem.2011.164822. [DOI] [PubMed] [Google Scholar]
- 29.Moore C, Rana S, Coulter C. Simultaneous identification of 2-carboxy-tetrahydrocannabinol, tetrahydrocannabinol, cannabinol and cannabidiol in oral fluid. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852:459–64. doi: 10.1016/j.jchromb.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Toennes SW, Ramaekers JG, Theunissen EL, Moeller MR, Kauert GF. Pharmacokinetic properties of Delta9-tetrahydrocannabinol in oral fluid of occasional and chronic users. J Anal Toxicol. 2010;34:216–21. doi: 10.1093/jat/34.4.216. [DOI] [PubMed] [Google Scholar]
- 31.Vondracek M, Xi Z, Larsson P, Baker V, Mace K, Pfeifer A, et al. Cytochrome P450 expression and related metabolism in human buccal mucosa. Carcinogenesis. 2001;22:481–8. doi: 10.1093/carcin/22.3.481. [DOI] [PubMed] [Google Scholar]
- 32.Yang SP, Raner GM. Cytochrome P450 expression and activities in human tongue cells and their modulation by green tea extract. Toxicol Appl Pharmacol. 2005;202:140–50. doi: 10.1016/j.taap.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Maurer HH, Sauer C, Theobald DS. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28:447–53. doi: 10.1097/01.ftd.0000211812.27558.6e. [DOI] [PubMed] [Google Scholar]
- 34.Schwope DM, Karschner EL, Gorelick DA, Huestis MA. Identification of recent cannabis use: whole-blood and plasma free and glucuronidated cannabinoid pharmacokinetics following controlled smoked cannabis administration. Clin Chem. 2011;57:1406–14. doi: 10.1373/clinchem.2011.171777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gjerde H, Normann PT, Christophersen A. The prevalence of alcohol and drugs in sampled oral fluid is related to sample volume. J Anal Toxicol. 2010;34:416–9. doi: 10.1093/jat/34.7.416. [DOI] [PubMed] [Google Scholar]
- 36.Ramaekers JG, Moeller MR, van Ruitenbeek P, Theunissen EL, Schneider E, Kauert G. Cognition and motor control as a function of Delta9-THC concentration in serum and oral fluid: limits of impairment. Drug Alcohol Depend. 2006;85:114–22. doi: 10.1016/j.drugalcdep.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Smiley A. Marijuana: on-road and driving simulator studies. Alcohol, Drugs Driving. 1986;2:121–34. [Google Scholar]
- 38.Berghaus G, Sheer N, Schmidt P. Effects of cannabis on psychomotor skills and driving performance - a metaanalysis of experimental studies. In: Kloeden CN, McLean AJ, editors. Alcohol, drugs and traffic safety Proceedings of the 13th International Conference on Alcohol, Drugs and Traffic Safety; August 13–18, 1995; Adelaide, Australia. Adelaide: NHMRC Road Accident Research Unit; pp. 403–9. Available from: http://casr.adelaide.edu.au/T95/paper/s16p2.html. [Google Scholar]
- 39.Walsh JM, de Gier JJ, Christopherson AS, Verstraete AG. Drugs and driving. Traffic Inj Prev. 2004;5:241–53. doi: 10.1080/15389580490465292. [DOI] [PubMed] [Google Scholar]


