Abstract
BACKGROUND
Cannabis is the illicit drug most frequently reported with impaired driving and motor vehicle accidents. Some “per se” laws make it illegal to drive with any amount of drug in the body, while others establish blood, saliva, or urine concentrations above which it is illegal to drive. The persistence of Δ9-tetrahydrocannabinol (THC) in chronic daily cannabis smokers’ blood is unknown.
METHODS
Thirty male chronic daily cannabis smokers resided on a secure research unit for up to 33 days, with daily blood collection. Samples were processed in an ice bath during sample preparation to minimize cannabinoid adsorption onto precipitant material. We quantified THC by 2-dimensional GC-MS.
RESULTS
Of the 30 participants, 27 were THC-positive on admission, with a median (range) concentration of 1.4 μg/L (0.3–6.3). THC decreased gradually; only 1 of 11 participants was negative at 26 days, 2 of 5 remained THC-positive (0.3 μg/L) for 30 days, and 5.0% of participants had THC ≥1.0 μg/L for 12 days. Median 11-hydroxy-THC concentrations were 1.1 μg/L on admission, with no results ≥1.0 μg/L 24 h later. 11-Nor-9-carboxy-THC (THCCOOH) detection rates were 96.7% on admission, decreasing slowly to 95.7% and 85.7% on days 8 and 22, respectively; 4 of 5 participants remained THCCOOH positive (0.6–2.7 μg/L) after 30 days, and 1 remained positive on discharge at 33 days.
CONCLUSIONS
Cannabinoids can be detected in blood of chronic daily cannabis smokers during a month of sustained abstinence. This is consistent with the time course of persisting neurocognitive impairment reported in recent studies.
Cannabis is the most widely used illicit drug worldwide (1). An estimated 17.4 million Americans age 12 years or older smoked cannabis in 2010, with about 6600 new initiates daily (2). Acutely intoxicated cannabis smokers show impairment on cognitive, perceptual, and psychomotor tasks, including those assessing short-term memory, sustained or divided attention, complex decision-making, and reaction time (3–5), and experience euphoria, relaxation, altered sensory perception, slowing of time, anxiety/paranoia, increased appetite, increased heart rate, and sometimes hallucinations or psychosis (6, 7). Acute impairment is well documented for hours after cannabis intake, whereas the persistence of chronic impairment is less clear. Some studies show neurocognitive impairment for 7–28 days or longer after last cannabis intake. Eldreth et al. (8) showed no impairment in heavy cannabis smokers in executive cognitive functioning 25 days after initiation of abstinence compared with controls. Pope et al. (9) found neurocognitive impairment for at least 7 days after initiation of abstinence but no significant differences 28 days later in chronic daily cannabis smokers vs less-than-daily smokers. Bolla et al. (10) found significant impairment after 28 days of monitored abstinence compared to light users.
Cannabis is second only to alcohol for causing impaired driving and motor vehicle accidents. In 2009, 12.8% of young adults (age 18–25 years) reported driving under the influence of illicit drugs (11). In the 2007 National Roadside Survey, more drivers tested positive for drugs (16.6%) than for alcohol (12.4%). Of weekend nighttime drivers who provided oral fluid and/or blood in a random traffic stop, 16.3% were drug-positive, with 8.6% positive for cannabinoids (12), whereas only 2.2% of drivers had a blood alcohol concentration of ≥0.08% (13). In 2003, 14% of fatally injured and 19% of non–fatally injured US drivers were positive for Δ9-tetrahydrocannabinol (THC),4 the primary psychoactive component of cannabis (14).
Cannabis smokers had a 10-fold increase in car crash injury compared with infrequent or nonusers after adjustment for blood alcohol concentration (15). THC blood concentrations >1 μg/L were associated with a 2.7-fold increase in driver responsibility for their road accident compared with drug-free drivers; culpability increased 6.6-fold when THC concentrations were ≥5 μg/L in driving fatalities (16, 17). In laboratory tests, THC serum concentrations of 2–5 μg/L were associated with perceptual/motor control impairment in 71% of drivers (18).
In light of this strong association between cannabis use and road accidents, legal “per se” limits have been established for blood THC concentrations while driving, analogous to those established for alcohol. Fifteen US states and 12 European countries established THC concentration limits in blood, and 4 European countries established limits in plasma or serum (19, 20). Blood concentrations above the per se limit are considered evidence of driving impairment.
The relationship between THC concentrations and pharmacodynamic effects is complex and nonlinear, in contrast to the comparable relationship for alcohol, for which per se driving laws are widespread. THC bioavailability is approximately 25% via the smoked route, with a plasma half-life of approximately 4 days (21). Concentrations initially decrease rapidly due to distribution into tissues, first-pass hepatic metabolism, and excretion into urine and feces (22). The liver enzyme CYP2C9 hydroxylates THC at the C11 position, producing the equipotent metabolite, 11-hydroxy-THC (11-OH-THC) (23). THC was present in brain of motor vehicle fatalities when no longer detectable in blood (24). Thus, blood concentrations may be low or not detected while brain concentrations might be sufficient to cause impairment. These pharmacokinetic characteristics make it difficult to identify a minimum blood THC concentration consistently associated with impairment (25).
In this study, we characterized cannabinoid elimination in blood from daily cannabis smokers during monitored sustained abstinence for up to 33 days. These new data complicate interpretation of cannabinoid blood concentrations in clinical and forensic cases, including impaired driving, and highlight the usefulness of monitoring multiple blood cannabinoids to improve the accuracy of result interpretation.
Materials and Methods
PARTICIPANTS
Participants with a history of chronic, daily cannabis smoking were recruited by print, radio, internet, and television advertisements. Subjects were required to be male, 18–65 years old, and physically and psychologically healthy on the basis of comprehensive medical and psychological evaluation. Women were excluded because this was part of a larger study of positron emission tomography (PET) imaging to evaluate brain cannabinoid CB1 receptors (26), and the female hormonal cycle may affect CB1 receptor density (27). Self-reported cannabis smoking of >1 year, typical smoking pattern of >5 days per week for the 6 months before admission, and a positive urine cannabinoid screen (Iscreen™ 50 ng/mL) were required for inclusion. Positive cannabinoid screens were not confirmed, since the purpose was only to verify self-report of cannabis smoking.
Exclusion criteria were clinically significant illness, schizophrenia, bipolar or other psychotic disorder diagnosis, participation in drug or alcohol abuse treatment within 90 days, or dependence on any substance other than cannabis, nicotine, or caffeine. Additional exclusion criteria due to the PET scanning component (26) included positive HIV test, metallic foreign body in the head, history of head trauma or seizures, fetal alcohol syndrome or other neurodevelopment disorder, and radiation exposure in the prior year.
All subjects provided written informed consent to participate in this Combined Neuroscience Institutional Review Board, NIH–approved study. Participants resided on a secure clinical research unit for up to 33 days with constant 24-h surveillance, preventing access to unauthorized illicit substances. There were no dietary or physical activity restrictions.
SAMPLE COLLECTION
Blood (3 mL) was collected each morning by indwelling peripheral intravenous catheters into sodium heparin BD Vacutainer® tubes (Becton Dickinson). Samples were stored at −20 °C in 4-mL polypropylene cryotubes until analysis.
BLOOD CANNABINOID ANALYSIS
Blood cannabinoid analysis measured free cannabinoids with minor temperature program modifications to a validated plasma cannabinoid method (28). We used modifications to the oven temperature program to separate chromatographic interferences not present in plasma. Samples were stored frozen at −20 °C until analysis; thus, samples were hemolysed. The method was validated with blank hemolysed whole blood and fortified hemolysed blood for calibrators and quality control samples. We processed samples in an ice bath during sample preparation to minimize cannabinoid adsorption onto precipitant material. Calibration curves were linear from 0.25 to 25 μg/L for THC, 0.50 to 50 μg/L for 11-OH-THC, and 0.25 to 50 μg/L for 11-nor-9-carboxy-THC (THCCOOH). Three QC concentrations were analyzed in each batch across the linear range of the assay. Intra- and interassay imprecision were <6.4%, and analytical bias was within 91.6%–111.5%.
DATA ANALYSIS
Body mass index (BMI), calculated as BMI = weight (kg)/height (m)2, classified participants as underweight (<18.5), normal weight (18.5–24.9), overweight (≥25.0), or obese (≥30.0). Cannabinoid blood detection rates were calculated at the limits of quantification (LOQ) of the method (0.25 μg/L for THC and THCCOOH and 0.50 μg/L for 11-OH-THC), 1 μg/L (the lower range of a per se THC limit for defining performance impairment above which drivers are at increased risk for motor vehicle accidents) (18), and 5 μg/L (a concentration shown to have a 6.6 odds ratio for fatal accident culpability and observed impairment in cognitive performance and motor tasks related to driving skills) (16).
We evaluated associations between time of last detectable cannabinoid concentration and participant demographics with Spearman rank correlation and differences in cannabinoid concentrations between days by Wilcoxon rank test. A Kaplan–Meier analysis was performed to evaluate the duration of cannabinoid detection in blood after admission, on the basis of last detection times in each participant. Participant data were censored if the participant left the study before achieving negative THC or negative 11-OH-THC results on 4 or 2 consecutive days, respectively (i.e., if participants failed to fulfill the THC or 11-OH-THC criteria they were not considered negative on discharge). The THC criterion required more consecutive negative days than the 11-OH-THC criterion because there were frequent negative THC samples interspersed between positive ones. Requiring a fewer number of negative days for THC would have biased the study toward classifying participants as falsely negative on discharge. THCCOOH was not included in the survival analysis because only 1 participant was negative for at least 2 consecutive days before discharge.
Investigation via boxplot and Kolmogorov–Smirnov normality test showed that data were not normally distributed; therefore, nonparametric tests were conducted with SPSS Statistics for Windows version 19.0. Values below LOQ were replaced with 0.5*LOQ for statistical comparisons. Statistical tests were considered significant if two-tailed P < 0.05.
Results
Thirty male chronic daily cannabis smokers participated [26 African American, 3 white, 1 mixed race; mean (SD) age 28.3 (7.9) years] (Table 1). Subjects’ BMIs indicated that 2 were underweight, 16 normal weight, 9 overweight, and 3 obese. According to self-report, subjects smoked a median 9 cannabis joints each day of the 14 days before study screening. Subjects began smoking cannabis at a median of 14 years old, with 10 years’ median duration of use. For example, subject A (age 33 years) reported first smoking cannabis at age 6, but smoked cannabis for a total of 13 of the intervening 27 years. All participants reported using alcohol, 2 illicit opioids, 1 amphetamine, 1 minor tranquilizers, and 80% tobacco. One participant reported cocaine consumption in the 2 weeks before admission, and 1 was administratively withdrawn because of cocaine use during transfer for a PET scan.
Table 1.
Demographic characteristics and self-reported cannabis use for 30 male participants.
| Participant | Age, years | Raceb | BMI, kg/m2 | Self-reported cannabis joints smoked per daya | Days smoked in prior 14a | Age at first use, years | Lifetime duration of cannabis smoking, years |
|---|---|---|---|---|---|---|---|
| A | 33 | AA | 20.4 | 6 | 14 | 6 | 13 |
| B | 25 | AA | 29.5 | 6 | 13 | 16 | 7 |
| C | 38 | AA | 25.6 | 18 | 14 | 21 | 15 |
| D | 19 | AA | 24.4 | 12 | 10 | 14 | 4 |
| E | 43 | AA | 25.1 | 4 | 12 | 13 | 28 |
| F | 29 | AA | 20.6 | 18 | 14 | 14 | 14 |
| G | 29 | AA | 21.0 | 12 | 13 | 14 | 10 |
| H | 27 | AA | 24.4 | 15 | 14 | 16 | 10 |
| I | 26 | C | 20.2 | 5 | 14 | 16 | 10 |
| J | 24 | AA | 19.7 | 18 | 14 | 18 | 5 |
| K | 22 | AA | 25.2 | 6 | 14 | 12 | 6 |
| L | 29 | AA | 23.7 | 9 | 14 | 14 | 15 |
| M | 36 | C | 16.4 | 1 | 13 | 22 | 10 |
| N | 30 | AA | 30.2 | 18 | 14 | 14 | 17 |
| O | 29 | AA | 29.3 | 9 | 14 | 11 | 17 |
| P | 25 | AA | 32.8 | 12 | 14 | 17 | 7 |
| Q | 24 | AA | 26.4 | 18 | 14 | 13 | 10 |
| R | 25 | AA | 27.7 | 6 | 14 | 15 | 10 |
| S | 21 | AA | 17.6 | 30 | 13 | 11 | 9 |
| T | 40 | AA | 25.4 | 6 | 12 | 18 | 22 |
| U | 25 | AA | 31.7 | 6 | 13 | 13 | 4 |
| V | 25 | AA | 20.5 | 12 | 13 | 17 | 5 |
| W | 52 | C | 24.9 | 3 | 14 | 14 | 38 |
| X | 38 | AA | 22.4 | 3 | 14 | 17 | 17 |
| Y | 20 | AA | 19.0 | 9 | 13 | 13 | 6 |
| Z | 31 | AA | 20.8 | 6 | 14 | 16 | 15 |
| 2A | 21 | AA | 20.9 | 6 | 12 | 14 | 5 |
| 2B | 21 | AA | 20.5 | 15 | 14 | 13 | 8 |
| 2C | 21 | AA | 21.1 | 9 | 14 | 12 | 6 |
| 2D | 21 | AA+C | 26.3 | 8 | 11 | 14 | 7 |
| Mean | 28.3 | 23.8 | 10.2 | 13.3 | 14.6 | 11.7 | |
| SD | 7.9 | 4.2 | 6.3 | 1.0 | 3.1 | 7.5 |
Before study screening.
AA, African American; C, Caucasian.
Participants (n = 30) provided a total of 570 blood samples: 326 were positive for THC (57.2%), with concentrations ranging from 0.25 to 6.3 μg/L; 531 (93.2%) were positive for THCCOOH; and 33 (5.8%) were positive for 11-OH-THC. 11-OH-THC concentrations were ≤4.1 μg/L. Participants resided on the secure unit for at least 1 (n = 24), 2 (n = 20), 3 (n = 14), or 4 (n = 11) weeks, with the longest residence 33 days. On days 26 and 27, subject O refused blood draws, but accepted on day 28. From day 27 forward, subject A was no longer enrolled in the study. Thus, there were 11, 10, and 11 participants on days 26, 27, and 28, respectively.
Table 2 and Fig. 1 present cannabinoid concentrations and detection rates (cannabinoids ≥LOQ) in blood during sustained abstinence. Twenty-seven of the 30 participants (90% at 0.25 μg/L) were THC positive on admission (day 0), 77.8% ≥1.0 μg/L, and 3 (11.1%) ≥5.0 μg/L. Two were THC negative at admission but later positive. One participant (M) was THC negative from admission through discharge on day 29.
Table 2.
Blood cannabinoid concentrations (μg/L) in chronic daily male cannabis smokers during sustained monitored abstinence.
| Day | n | THC
|
11-OH-THC
|
THCCOOH
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≥LOQ, % | Median | Maximum | ≥LOQ, % | Median | Maximum | ≥LOQ, % | Median | Maximum | ||
| Admission | 30 | 90.0 | 1.4 | 6.3 | 73.3 | 1.1 | 4.1 | 96.7 | 26.5 | 93.4 |
|
| ||||||||||
| 1 | 22 | 68.2 | 1.8 | 2.9 | 31.8 | 0.7 | 0.8 | 100.0 | 11.3 | 35.2 |
|
| ||||||||||
| 2 | 30 | 80.0 | 1.2 | 2.2 | 10.0 | 0.5 | 0.6 | 100.0 | 10.6 | 26.3 |
|
| ||||||||||
| 3 | 28 | 78.6 | 1.3 | 2.6 | 3.6 | 0.5 | 0.5 | 100.0 | 8.0 | 26.1 |
|
| ||||||||||
| 4 | 28 | 78.6 | 1.1 | 2.3 | 0.0 | NAa | <LOQ | 100.0 | 6.2 | 20.3 |
|
| ||||||||||
| 5 | 26 | 76.9 | 1.0 | 1.9 | 0.0 | NA | <LOQ | 100.0 | 5.2 | 19.4 |
|
| ||||||||||
| 6 | 25 | 72.0 | 1.0 | 2.2 | 0.0 | NA | <LOQ | 100.0 | 4.1 | 17.8 |
|
| ||||||||||
| 7 | 24 | 79.2 | 0.9 | 2.0 | 0.0 | NA | <LOQ | 100.0 | 3.1 | 14.4 |
|
| ||||||||||
| 8 | 23 | 65.2 | 0.8 | 2.4 | 0.0 | NA | <LOQ | 95.7 | 3.0 | 12.7 |
|
| ||||||||||
| 9 | 21 | 61.9 | 0.7 | 2.0 | 0.0 | NA | <LOQ | 95.2 | 2.3 | 7.6 |
|
| ||||||||||
| 10 | 21 | 61.9 | 0.5 | 1.8 | 0.0 | NA | <LOQ | 95.2 | 2.0 | 6.6 |
|
| ||||||||||
| 11 | 21 | 71.4 | 0.5 | 1.2 | 0.0 | NA | <LOQ | 95.2 | 2.0 | 6.9 |
|
| ||||||||||
| 12 | 20 | 65.0 | 0.5 | 1.0 | 0.0 | NA | <LOQ | 95.0 | 1.7 | 5.4 |
|
| ||||||||||
| 13 | 20 | 55.0 | 0.4 | 0.8 | 0.0 | NA | <LOQ | 90.0 | 1.8 | 4.3 |
|
| ||||||||||
| 14 | 20 | 60.0 | 0.4 | 1.0 | 0.0 | NA | <LOQ | 85.0 | 1.8 | 5.1 |
|
| ||||||||||
| 15 | 18 | 72.2 | 0.4 | 0.9 | 0.0 | NA | <LOQ | 94.4 | 1.5 | 5.0 |
|
| ||||||||||
| 16 | 18 | 44.4 | 0.3 | 0.8 | 0.0 | NA | <LOQ | 94.4 | 1.6 | 4.8 |
|
| ||||||||||
| 17 | 17 | 47.1 | 0.5 | 0.8 | 0.0 | NA | <LOQ | 94.1 | 1.3 | 5.2 |
|
| ||||||||||
| 18 | 16 | 43.8 | 0.4 | 0.7 | 0.0 | NA | <LOQ | 87.5 | 1.3 | 5.7 |
|
| ||||||||||
| 19 | 14 | 42.9 | 0.4 | 0.5 | 0.0 | NA | <LOQ | 85.7 | 1.1 | 4.7 |
|
| ||||||||||
| 20 | 15 | 40.0 | 0.3 | 0.7 | 0.0 | NA | <LOQ | 86.7 | 1.2 | 4.0 |
|
| ||||||||||
| 21 | 14 | 42.9 | 0.4 | 0.5 | 0.0 | NA | <LOQ | 85.7 | 1.2 | 3.4 |
|
| ||||||||||
| 22 | 14 | 21.4 | 0.4 | 0.5 | 0.0 | NA | <LOQ | 85.7 | 0.9 | 3.2 |
|
| ||||||||||
| 23 | 13 | 23.1 | 0.4 | 0.5 | 0.0 | NA | <LOQ | 84.6 | 0.8 | 3.3 |
|
| ||||||||||
| 24 | 12 | 25.0 | 0.4 | 0.7 | 0.0 | NA | <LOQ | 83.3 | 1.1 | 3.1 |
|
| ||||||||||
| 25 | 12 | 8.3 | 0.3 | 0.3 | 0.0 | NA | <LOQ | 91.7 | 0.7 | 2.5 |
|
| ||||||||||
| 26 | 11 | 9.1 | 0.4 | 0.4 | 0.0 | NA | <LOQ | 81.8 | 0.6 | 2.8 |
|
| ||||||||||
| 27 | 10 | 0.0 | NA | <LOQ | 0.0 | NA | <LOQ | 70.0 | 0.7 | 2.5 |
|
| ||||||||||
| 28 | 11 | 0.0 | NA | <LOQ | 0.0 | NA | <LOQ | 90.9 | 0.7 | 2.6 |
|
| ||||||||||
| 29 | 8 | 0.0 | NA | <LOQ | 0.0 | NA | <LOQ | 75.0 | 0.6 | 1.8 |
|
| ||||||||||
| 30 | 5 | 40.0 | 0.3 | 0.3 | 0.0 | NA | <LOQ | 80.0 | 1.1 | 2.7 |
|
| ||||||||||
| 31 | 1 | 0.0 | NA | <LOQ | 0.0 | NA | <LOQ | 0.0 | NA | <LOQ |
|
| ||||||||||
| 32 | 1 | 0.0 | NA | <LOQ | 0.0 | NA | <LOQ | 100.0 | 0.9 | 0.9 |
|
| ||||||||||
| 33 | 1 | 0.0 | NA | <LOQ | 0.0 | NA | <LOQ | 100.0 | 0.7 | 0.7 |
NA, not available.
Fig. 1.
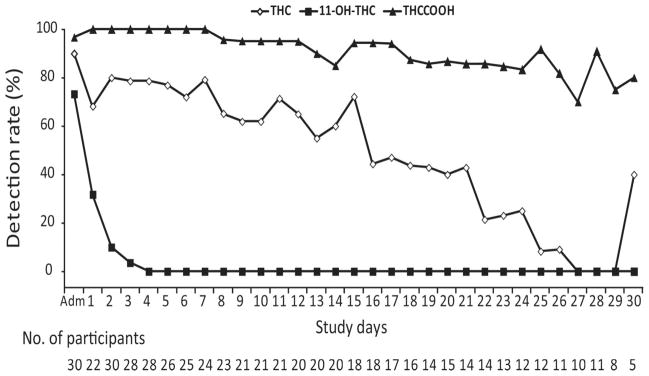
Cannabinoid detection rates in chronic daily cannabis smokers on the basis of the method’s limits of quantification, 0.25 μg/L for THC and THCCOOH and 0.5 μg/L for 11-OH-THC.
Twelve of 22 subjects (59.1%) had THC concentrations ≥1.0 <24 h after admission, but none ≥5.0 μg/L. On day 1, blood was unable to be collected from 8 subjects for a variety of reasons including inability to collect blood, refusal to have blood drawn, or nursing error. The highest THC concentration was 2.9 μg/L. All subjects’ THC concentrations were ≤1 μg/L within 7 days. THC median and maximum concentrations and percentage of subjects THC positive did not always decrease in a consistent manner. Fewer than 50% of blood samples from chronic daily cannabis smokers were THC positive after 16 days. The last THC-positive blood samples for 2 participants occurred on day 30 (0.3 μg/L for both), with interspersed negative and positive samples before this time. These subjects (C and O) smoked cannabis for 15–17 years and, during screening, reported smoking 9–18 cannabis joints/blunts per day every day, similar to other participants (Table 1). THC concentrations from 1 subject were >1.0 μg/L for 12 consecutive days after admission, the longest consecutive period at this threshold.
Twenty-two of 30 participants (73.3%) were 11-OH-THC positive on admission, 40.0% ≥1 μg/L. Less than 24 h later, median 11-OH-THC concentrations significantly decreased (P = 0.028) to 0.7 μg/L (maximum 0.8 μg/L). Only 1 of 28 participants’ blood was still 11-OH-THC positive <72 h after admission, at a concentration of 0.5 μg/L. Samples from 7 participants were never 11-OH-THC positive.
All but 1 participant (96.7%) were THCCOOH positive at admission, and all were positive 24 h later. THCCOOH concentrations were <10 μg/L by day 3 and <5 μg/L by day 6, even in these chronic daily cannabis smokers. All samples were THCCOOH positive through day 7 and 85% through day 22. One participant was still THCCOOH positive (0.7 μg/L) at discharge after 33 days of abstinence.
Negative samples were interspersed with positive samples for THC, 11-OH-THC, and THCCOOH in 12, 1, and 2 participants, respectively.
Eleven participants had exponential THC decreases. In 4 subjects, THC concentrations increased from admission to day 1; blood from 3 participants was positive only on admission. In contrast, 25 participants showed exponential THCCOOH declines, and THCCOOH increases from admission to day 1 were observed in blood from 3 individuals.
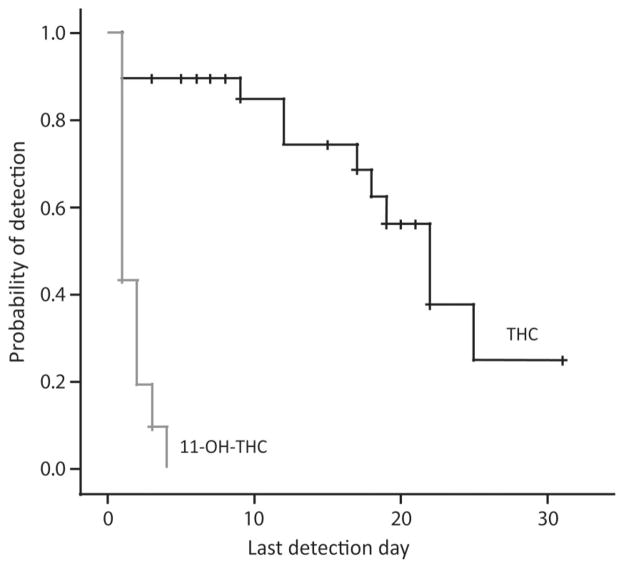
Last THC detection times were significantly, but weakly, correlated with self-reported quantity of cannabis smoking in the 2 weeks before screening (r = 0.372; P = 0.047). The median last detection time in blood after admission was 22 days (95% CI 17.8–26.2 days) for THC and 1 day (95% CI 0 days) for 11-OH-THC (Fig. 2).
Fig. 2. Kaplan–Meier plot for detection of THC and 11-OH-THC in blood during 33 days of sustained abstinence in chronic daily cannabis smokers.
THCCOOH was not included because it was positive in most individual’s blood samples until the time of discharge. Censored data represented by the small vertical ticks on the plotted lines are study participants who left the study before achieving negative THC or negative 11-OH-THC results on 4 or 2 consecutive days, respectively.
Discussion
To our knowledge, these are the first blood cannabinoid concentrations in chronic daily cannabis smokers during extended (up to 33 days) continuously monitored abstinence. These data are critical for understanding cannabinoid pharmacokinetics in this population, and for interpreting blood cannabinoid tests.
Both THC and its inactive metabolite THCCOOH were detected in blood up to 1 month after last smoking, 4 times longer than previously described (29). In contrast, the active THC metabolite, 11-OH-THC, had a maximum detection window of 72 h after admission, shorter than the 7 days reported in a previous study of cannabis smokers under monitored abstinence for 1 week (29). This difference may be due, in part, to sex difference, because in the prior study females had longer THC and 11-OH-THC detection windows than males.
Participants showed highly variable THC and THCCOOH concentrations over time, with positive samples occurring days to weeks after initiation of abstinence. The variable THC detection rate throughout the study, with positive samples interspersed with negatives ones (e.g., 2 participants THC positive on day 30), reflects large THC body burden (22). For instance, 2 participants with THC-positive samples on days 24 and 26, followed by negative samples, were THC-positive again on day 30 at 0.3 μg/L, a concentration close to the method’s LOQ. This increased the detection rate on day 30, as shown in Fig. 1 and Table 2. Although THC is mainly stored in adipose tissue (30, 31), we did not find a significant correlation between BMI and time of last detectable THC concentration. Persistence of THC impairment was shown in multiple studies for at least several weeks after initiation of abstinence (8–10, 32). Thus, our findings suggest an association between residual cannabinoid concentrations and impairment over the initial few weeks of abstinence, consistent with the approach of per se drugged driving laws. However, additional research is warranted on development of and dissipation of pharmacodynamic tolerance (33–35), acute cannabis withdrawal (which may also impair performance) (36, 37), and the relationship between concentrations in blood and brain (the site of action of impairment) (24).
THC serum concentrations of 2–5 μg/L were shown to impair driving (18), and concentrations of 7–10 μg/L produced impairment equivalent to a blood alcohol concentration of 0.05% (38). Sweden and Australia have zero tolerance for illegal drugs in drivers. If a 5-μg/L THC blood cutoff were adopted in Sweden, 90% of convicted impaired drivers would not have been prosecuted; 61% of prosecuted drivers would have been missed with a >1 μg/L cutoff (25).
Two states, Nevada and Ohio, set blood per se limits of ≥2 μg/L for THC or ≥5 μg/L for THCCOOH (19). In our study sample, 1 of 21 participants (4.8%) met this THC per se limit after 9 days of abstinence, and 1 of 16 participants (6.3%) met this THCCOOH per se limit after 18 days of abstinence, and thus would be prosecuted. Recommended blood cutoffs for forensic toxicology laboratories of 2, 2, and 5 μg/L for THC, 11-OH-THC, and THCCOOH, respectively (39), would result in 1 of 21 subjects (4.8%) prosecuted after 9 days of abstinence, no subjects 24 h after abstinence, and 1 of 16 subjects (6.3%) after 18 days of abstinence for THC, 11-OH-THC, and THCCOOH, respectively. Under the highest per se limits in Europe (20), 3 μg/L for THC in Portugal or 50 μg/L for THCCOOH in Poland, no participants would be prosecuted after 24 h of abstinence. The state of Colorado is currently considering a per se limit of 5.0 μg/L THC in blood. If applied to our study results, only 3 of 30 subjects (10%) would be prosecuted at admission, when subjects frequently self-reported recent smoking, and no participants after 24-h abstinence.
While existing laws focus on THC and THCCOOH per se concentrations, an appropriate cutoff might also be selected for 11-OH-THC due its shorter detection window. THC-glucuronide, cannabinol, and cannabidiol concentrations in blood may also indicate recent cannabis smoking (40).
This study has several limitations. Time of last cannabis smoking was based on participant self-report. However, our data report objective data from admission and document drug detection over days to weeks. If participants actually abstained from cannabis smoking immediately before admission, the length of detection could only be longer than times reported. Also, the majority (87%) of the study population was African American. Race/ethnicity may affect drug metabolism due to cytochrome P450 polymorphism and possibly excretion; additional research in other populations should be performed.
In conclusion, our results demonstrate, for the first time as far as we are aware, that cannabinoids can be detected in blood of chronic daily cannabis smokers during a month of sustained abstinence. This is consistent with the time course of persisting neurocognitive impairment reported in recent studies (9, 10, 26, 32). There is a strong public safety need to reduce morbidity and mortality from cannabis-impaired driving. Extended residual THC excretion in chronic daily cannabis smokers complicates prosecution. Establishment of per se THC legislation might achieve such a reduction in motor vehicle injuries and deaths. Per se alcohol legislation improved prosecution of drunk drivers and dramatically reduced alcohol-related deaths. By analogy, one way to protect the public from drugged drivers is to establish legislation making it illegal to smoke cannabis and drive.
Acknowledgments
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
The authors acknowledge the Chemistry and Drug Metabolism Section staff, especially David A. Gorelick for editing assistance and Allan Barnes for assistance with sample analysis.
Footnotes
Nonstandard abbreviations: THC, Δ9-tetrahydrocannabinol; 11-OH-THC, 11-hydroxy-THC; PET, positron emission tomography; THCCOOH, 11-nor-9-carboxy-THC; BMI, body mass index; LOQ, limit of quantification.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Research Funding: M.M. Bergamaschi, CAPES (Brazilian Federal Agency for the Support and Evaluation of Graduate Education); M.A. Huestis, Intramural Research Program, National Institute on Drug Abuse and National Institute of Mental Health, NIH.
Expert Testimony: None declared.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
References
- 1.United Nations Office on Drugs and Crime (UNODC) World Drug Report 2011. New York: 2011. p. 272. [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2010 National Survey on Drug Use and Health: summary of national findings. Rockville (MD): SAMHSA; 2011. NSDUH Series H-41, HHS publ. no. (SMA) 11-4658. [Google Scholar]
- 3.Elkashef A, Vocci F, Huestis M, Haney M, Budney A, Gruber A, el-Guebaly N. Marijuana neurobiology and treatment. Subst Abus. 2008;29:17–29. doi: 10.1080/08897070802218166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramaekers J, Kauert G, Theunissen E, Toennes S, Moeller M. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol. 2009;23:266–77. doi: 10.1177/0269881108092393. [DOI] [PubMed] [Google Scholar]
- 5.Lane SD, Cherek DR, Tcheremissine OV, Lieving LM, Pietras CJ. Acute marijuana effects on human risk taking. Neuropsychopharmacology. 2005;30:800–9. doi: 10.1038/sj.npp.1300620. [DOI] [PubMed] [Google Scholar]
- 6.Vandrey R, Haney M. Pharmacotherapy for cannabis dependence: how close are we? CNS Drugs. 2009;23:543–53. doi: 10.2165/00023210-200923070-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Frank RA. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–30. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- 8.Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. NeuroImage. 2004;23:914–20. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 9.Pope H, Gruber A, Hudson J, Huestis M, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58:909–15. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- 10.Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–43. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- 11.Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2009 National Survey on Drug Use and Health: volume I. Summary of national findings. Rockville (MD): SAMHSA; 2010. Office of Applied Studies, NSDUH Series H-38A, DHHS publ. no. (SMA) 10-4586. [Google Scholar]
- 12.Lacey JH, Kelley-Baker T, Furr-Holden D, Voas RB, Romano E, Ramirez A, et al. National Roadside Survey of Alcohol and Drug Use by Drivers: drug results. Pacific Institute for Research and Evaluation; Calverton, MD: Washington (DC): National Highway Traffic Safety Administration; 2007. 2009. Report No. DOT HS 811 249: Contract No. DTNH22-06-C-00040. [Google Scholar]
- 13.Compton R, Berning A. Results of the 2007 National Roadside Survey of Alcohol and Drug Use by Drivers. Washington (DC): National Highway Traffic Safety Administration; 2009. Traffic Safety Facts; DOT HS 811 175. [Google Scholar]
- 14.Jones RK, Shinar D, Walsh JM. State of knowledge of drug-impaired driving. Mid-America Research Institute; Winchester, MA: Washington (DC): National Highway Traffic Safety Administration; 2003. Report No. DOT HS 809 642; Contract or Grant No. DTNH22-98-D-25079. [Google Scholar]
- 15.Blows S, Ivers RQ, Connor J, Ameratunga S, Woodward M, Norton R. Marijuana use and car crash injury. Addiction. 2005;100:605–11. doi: 10.1111/j.1360-0443.2005.01100.x. [DOI] [PubMed] [Google Scholar]
- 16.Drummer OH, Gerostamoulos J, Batziris H, Chu M, Caplehorn J, Robertson MD, Swann P. The involvement of drugs in drivers of motor vehicles killed in Australian road traffic crashes. Accid Anal Prev. 2004;36:239– 48. doi: 10.1016/s0001-4575(02)00153-7. [DOI] [PubMed] [Google Scholar]
- 17.Laumon B, Gadegbeku B, Martin JL, Biecheler MB. Cannabis intoxication and fatal road crashes in France: population based case-control study. BMJ. 2005;331:1371. doi: 10.1136/bmj.38648.617986.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramaekers JG, Moeller MR, van Ruitenbeek P, Theunissen EL, Schneider E, Kauert G. Cognition and motor control as a function of Delta9-THC concentration in serum and oral fluid: limits of impairment. Drug Alcohol Depend. 2006;85:114–22. doi: 10.1016/j.drugalcdep.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Lacey J, Brainard K, Snitow S. Drug per se laws: a review of their use in states. Pacific Institute for Research and Evaluation; Calverton, MD: Washington (DC): National Highway Traffic Safety Administration; 2010. Report No. DOT HS 811 317; Contract or Grant No. DTNH22-02-D-95121 Task #13. [Google Scholar]
- 20.Verstraete A, Knoche A, Jantos R, Skopp G, Gjerde H, Vindenes V, et al. Per se limits - methods of defining cut-off values for zero tolerance. Deliverable 1.4.2. Driving under the Influence of Drugs, Alcohol and Medicines. 2011 6th Framework Programme. [Google Scholar]
- 21.Johansson E, Agurell S, Hollister LE, Halldin MM. Prolonged apparent half-life of Delta-1-tetrahydrocannabinol in plasma of chronic marijuana users. J Pharm Pharmacol. 1988;40:374–5. doi: 10.1111/j.2042-7158.1988.tb05272.x. [DOI] [PubMed] [Google Scholar]
- 22.Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4:1770– 804. doi: 10.1002/cbdv.200790152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bornheim LM, Lasker JM, Raucy JL. Human hepatic microsomal metabolism of Delta-1-tetrahydrocannabinol. Drug Metab Dispos. 1992;20:241–6. [PubMed] [Google Scholar]
- 24.Mura P, Kintz P, Dumestre V, Raul S, Hauet T. THC can be detected in brain while absent in blood. J Anal Toxicol. 2005;29:842–3. doi: 10.1093/jat/29.8.842. [DOI] [PubMed] [Google Scholar]
- 25.Jones AW, Holmgren A, Kugelberg FC. Driving under the influence of cannabis: a 10-year study of age and gender differences in the concentrations of tetrahydrocannabinol in blood. Addiction. 2008;103:452–61. doi: 10.1111/j.1360-0443.2007.02091.x. [DOI] [PubMed] [Google Scholar]
- 26.Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, et al. Reversible and regionally selective downregulation of brain cannabinoid CB(1) receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17:642–9. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fattore L, Fratta W. How important are sex differences in cannabinoid action? Br J Pharmacol. 2010;160:544– 8. doi: 10.1111/j.1476-5381.2010.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowe RH, Karschner EL, Schwilke EW, Barnes AJ, Huestis MA. Simultaneous quantification of Delta-9-tetrahydrocannabinol (THC), 11-hydroxy-Delta-9-tetrahydrocannabinol (11-OH-THC), and 11-nor-Delta-9-tetrahydrocannabinol-9-carboxylic acid (THCCOOH) in human plasma using two-dimensional gas chromatography, cryofocusing, and electron impact-mass spectrometry. J Chromatogr A. 2007;1163:318–27. doi: 10.1016/j.chroma.2007.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karschner E, Schwilke E, Lowe R, Darwin W, Pope H, Jr, Herning R, et al. Do Delta9-tetrahydrocannabinol concentrations indicate recent use in chronic cannabis users? Addiction. 2009;104:2041–8. doi: 10.1111/j.1360-0443.2009.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansson E, Noren K, Sjovall J, Halldin MM. Determination of Delta 1-tetrahydrocannabinol in human fat biopsies from marihuana users by gas chromatography-mass spectrometry. Biomed Chromatogr. 1989;3:35–8. doi: 10.1002/bmc.1130030109. [DOI] [PubMed] [Google Scholar]
- 31.Brunet B, Doucet C, Venisse N, Hauet T, Hebrard W, Papet Y, et al. Validation of large white pig as an animal model for the study of cannabinoids metabolism: application to the study of THC distribution in tissues. Forens Sci Int. 2006;161:169–74. doi: 10.1016/j.forsciint.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–31. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- 33.Ramaekers J, Theunissen E, de Brouwer M, Toennes S, Moeller M, Kauert G. Tolerance and cross-tolerance to neurocognitive effects of THC and alcohol in heavy cannabis users. Psychopharmacology (Berl) 2011;214:391–401. doi: 10.1007/s00213-010-2042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haney M, Comer SD, Ward AS, Foltin RW, Fischman MW. Factors influencing marijuana self-administration by humans. Behav Pharmacol. 1997;8:101–12. [PubMed] [Google Scholar]
- 35.Gorelick DA, Goodwin RS, Schwilke E, Schwope DM, Darwin WD, Kelly DL, et al. Antagonist-elicited cannabis withdrawal in humans. J Clin Psychopharmacol. 2011;31:603–12. doi: 10.1097/JCP.0b013e31822befc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez R. Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychol Rev. 2007;17:347–61. doi: 10.1007/s11065-007-9036-8. [DOI] [PubMed] [Google Scholar]
- 37.Pope HG, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. JAMA. 1996;275:521–7. [PubMed] [Google Scholar]
- 38.Grotenhermen F, Leson G, Berghaus G, Drummer OH, Kruger HP, Longo M, et al. Developing limits for driving under cannabis. Addiction. 2007;102:1910–7. doi: 10.1111/j.1360-0443.2007.02009.x. [DOI] [PubMed] [Google Scholar]
- 39.Farrell LJ, Kerrigan S, Logan BK. Recommendations for toxicological investigation of drug impaired driving. J Forensic Sci. 2007;52:1214– 8. doi: 10.1111/j.1556-4029.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- 40.Schwope DM, Karschner EL, Gorelick DA, Huestis MA. Identification of recent cannabis use: whole-blood and plasma free and glucuronidated cannabinoid pharmacokinetics following controlled smoked cannabis administration. Clin Chem. 2011;57:1406–14. doi: 10.1373/clinchem.2011.171777. [DOI] [PMC free article] [PubMed] [Google Scholar]