Abstract
Effortful swallow and the Mendelsohn maneuver are two common strategies to improve disordered swallowing. We used high-resolution manometry (HRM) to quantify the effects of these maneuvers on pressure and timing characteristics. Fourteen normal subjects swallowed multiple, five ml water boluses using three techniques: normal swallow; effortful swallow; and Mendelsohn maneuver. Maximum pressure, rate, duration, area integral, and line integral were determined for the velopharynx and tongue base. Minimum pressure, duration of pressure-related change, duration of nadir pressure, maximum pre-opening and post-closure pressure, area integral, and line integral were recorded for the upper esophageal sphincter (UES). Area and line integrals of the velopharyngeal pressure curve significantly increased with the Mendelsohn maneuver; the line integral increased with the effortful swallow. Pre-opening UES pressure decreased significantly for the Mendelsohn, while post-closure pressure tended to increase insignificantly for both maneuvers. UES area and line integrals as well as nadir UES pressure duration increased with both maneuvers. Maneuver-dependent changes were observed primarily at the velopharynx and UES. These regions are critical to safe swallowing, as the velopharynx provides positive pressure at the bolus tail while the UES allows a bolus to enter the esophagus without risk of regurgitation. Integrals were more responsive than maximum pressure or duration and should be investigated further.
Keywords: pharyngeal pressure, swallowing maneuver, high-resolution manometry, deglutition, deglutition disorder
INTRODUCTION
The effortful swallow and Mendelsohn maneuver are two commonly employed techniques designed to improve swallowing function in patients with pharyngeal dysphagia. Effortful swallow requires patients to "swallow hard," which improves posterior tongue base movement and anterior movement of the pharyngeal wall (1, 2). The Mendelsohn maneuver requires patients to hold their tongue forcefully against the roof of their mouth while swallowing, which increases and prolongs laryngeal elevation (3–6), thereby increasing the duration and width of cricopharyngeal opening (2, 6, 7). The effect of these maneuvers on pharyngeal pressure has been evaluated using traditional manometry (1, 3, 8–10). Lazarus et al. found increased pressure at the tongue base for both the Mendelsohn maneuver and effortful swallow (1), while Bulow et al. found no difference in pharyngeal contraction pressures between maneuvers and normal swallows (3). The Mendelsohn maneuver has been shown to increase upper esophageal sphincter (UES) opening duration and pharyngeal peak contraction, as well as lower UES peak contraction pressure (8, 10). While these studies provided valuable information on how maneuvers affect swallowing physiology, results were not always in agreement (1, 3).
Traditional manometric catheters have utilized 1 – 3 pressure sensors typically placed at the level of the UES (1, 3, 4, 8, 11, 12). While these studies have contributed to our knowledge regarding normal and disordered swallowing physiology, technical limitations preclude a comprehensive analysis of all relevant pressure events. The pharynx is an asymmetric structure and includes highly mobile structures such as the UES which elevates 2 – 4 centimeters during swallowing (13). Accurate characterization of the rapidly changing pressures along the length of the pharynx demands greater spatial and temporal resolution than are allowed by traditional manometry.
High-resolution manometry (HRM) uses 36 circumferential pressure sensors spaced one centimeter apart and provides the resolution necessary to capture key pressure events along the entire length of the pharynx (14). When HRM is used in the pharynx, less than 15 of the 36 sensors are typically used due to the length of the pharynx. HRM has been used successfully to reveal subtle pressure differences due to head position (15, 16) and bolus size (17) not previously detected by traditional manometry.
Swallowing maneuvers are designed to improve swallowing in patients with pharyngeal dysphagia resultant from muscular dysfunction or decreased range of motion (18–21). The mechanism by which these maneuvers promote safe bolus passage may be elucidated by HRM. We used HRM to measure pressure events at the velopharynx, tongue base, and UES during normal swallowing, effortful swallowing, and the Mendelsohn maneuver in normal subjects. As the amount of data generated by HRM is significantly greater than traditional pressure catheters with only a few sensors, we are able to extract more information, including new variables such as area and line integrals of the pressure waveforms and pressure wave velocity. Area integrals, representative of total pressure generated within a region of interest, provide a more comprehensive picture of pharyngeal function than can be provided by isolated measurements of maximum pressure. Pressure wave velocity may be of particular interest in maneuvers designed to amplify normal bolus transit. Effortful swallow is purported to enhance tongue base retraction and pressure generation; therefore, we expected pressure wave velocity, velopharynx pressure, and tongue base pressure to increase, and swallow duration to decrease. Mendelsohn maneuver prolongs laryngeal elevation and might be predicted to cause increased duration of velopharynx pressure and UES opening, as well as decreased pre-opening UES pressure. Using HRM in healthy subjects may reveal how these maneuvers modulate swallowing physiology.
MATERIALS AND METHODS
Data collection
Equipment
A solid-state high-resolution manometer was used for all data collection (ManoScan360 High-Resolution Manometry System, Sierra Scientific Instruments, Los Angeles, CA). The manometric catheter has an outer diameter of 4 mm and 36 circumferential pressure sensors spaced 1 cm apart. Each sensor spans 2.5 mm and receives input from 12 circumferential sectors. These inputs are averaged and a mean pressure is recorded as the pressure detected by that individual sensor. The system is calibrated to record pressures between −20 and 600 mmHg with fidelity of 2 mmHg. Data were collected at a sampling rate of 50 Hz (ManoScan Data Acquisition, Sierra Scientific Instruments). Prior to calibration, the catheter was covered with a protective sheath to preserve sterility without the need to sterilize the catheter between uses (ManoShield, Sierra Scientific Instruments). The catheter was calibrated before each participant according to manufacturer specifications.
Participants
Seven males and seven females, aged 21.2 ± 2.0 years (range: 19 – 25), participated in this study with the approval of the Institutional Review Board of the University of Wisconsin-Madison. All subjects were without swallowing, neurological, or gastrointestinal disorders. Participants were instructed not to eat for four hours and not to drink liquids for two hours prior to testing to avoid any potential confounding effect of satiety.
Procedure
Topical 2% viscous lidocaine hydrochloride was applied to the nasal passages with a cotton swab and participants gargled a solution of 4% lidocaine (1 to 2 cc) for several seconds. The manometric catheter was lubricated with 2% viscous lidocaine to ease passage of the catheter through the pharynx. Once the catheter was positioned within the pharynx, participants rested for 5–10 minutes to adjust to the catheter prior to performing the experimental swallows.
Five 5 ml water boluses were swallowed using each technique: normal; effortful swallow; and the Mendelsohn maneuver. Task order was varied across subjects, so each possible order of the three tasks (of which there are six possibilities) was performed by 2–3 subjects. For the effortful swallow, subjects were instructed to swallow hard and contract their muscles forcefully. For the Mendelsohn maneuver, normal movement of the larynx during swallowing was discussed. Subjects were then instructed to maintain laryngeal elevation for 2–3 seconds after swallowing by squeezing their neck and throat muscles (18, 21). Unsuccessful trials were repeated and the erroneous trials were excluded from data analysis. Unsuccessful trials included instances where the subject did not understand how to perform the maneuver, swallowed the bolus normally (in the case of an effortful swallow or Mendelsohn maneuver), or was unable to maintain laryngeal elevation (for the Mendelsohn maneuver). All trials considered successful during data collection were included in data analysis. Each water bolus was delivered to the oral cavity via syringe. Fifteen swallows were analyzed for each participant.
Data analysis
Data extraction
Pressure and timing data were extracted using a customized MATLAB program (The MathWorks, Inc., Natick, MA) which locates areas of interest (maximum pressure attained in velopharynx, tongue base, and UES) and then calculates the requisite timing information. The basic workflow is automated, but in cases of anomalous data, the user may override program suggestions and manually select the correct manometric sensors corresponding to the area of interest. A sample three-dimensional spatiotemporal plot produced by HRM and the corresponding two-dimensional pressure waves produced by our MATLAB program are shown in figures 1A and 1B, respectively. Each pressure curve corresponds to one manometric sensor and the curves are oriented such that the nasopharynx is the first (top) curve and a sensor in the proximal esophagus is the last (bottom) curve. In a study of pharyngeal swallowing using HRM, Ghosh et al. employed an interpolation technique to increase resolution in the sensor axis (22). This method represents an interesting and potentially useful approach to HRM analysis. However, we have elected not to make use of such interpolation because we are unsure of the benefits it provides compared to the impact on data interpretation. As application of HRM to the pharyngeal swallow is still relatively new, it is not clear if the rapidly changing pressures in the pharynx may preclude accurate interpolation. Therefore, all pressure values presented in this study were obtained from the sensors of interest.
Figure 1.
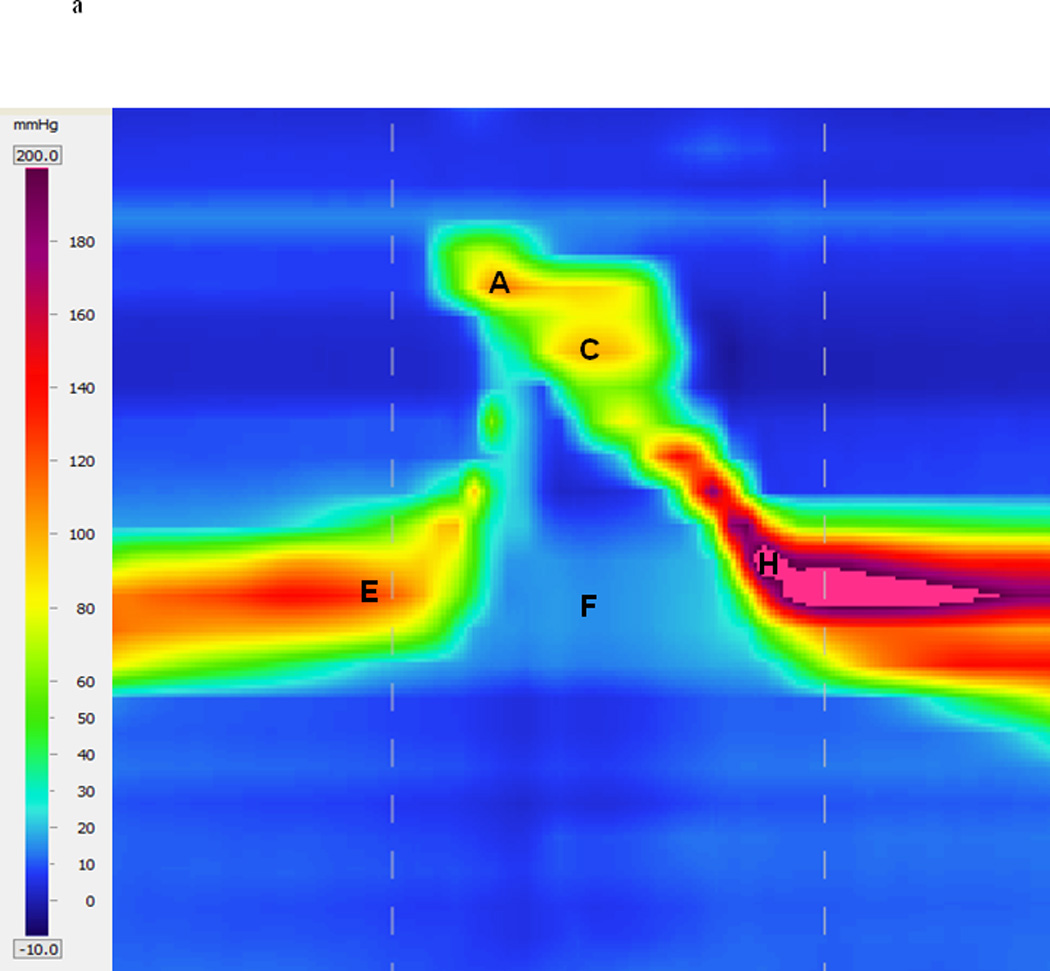
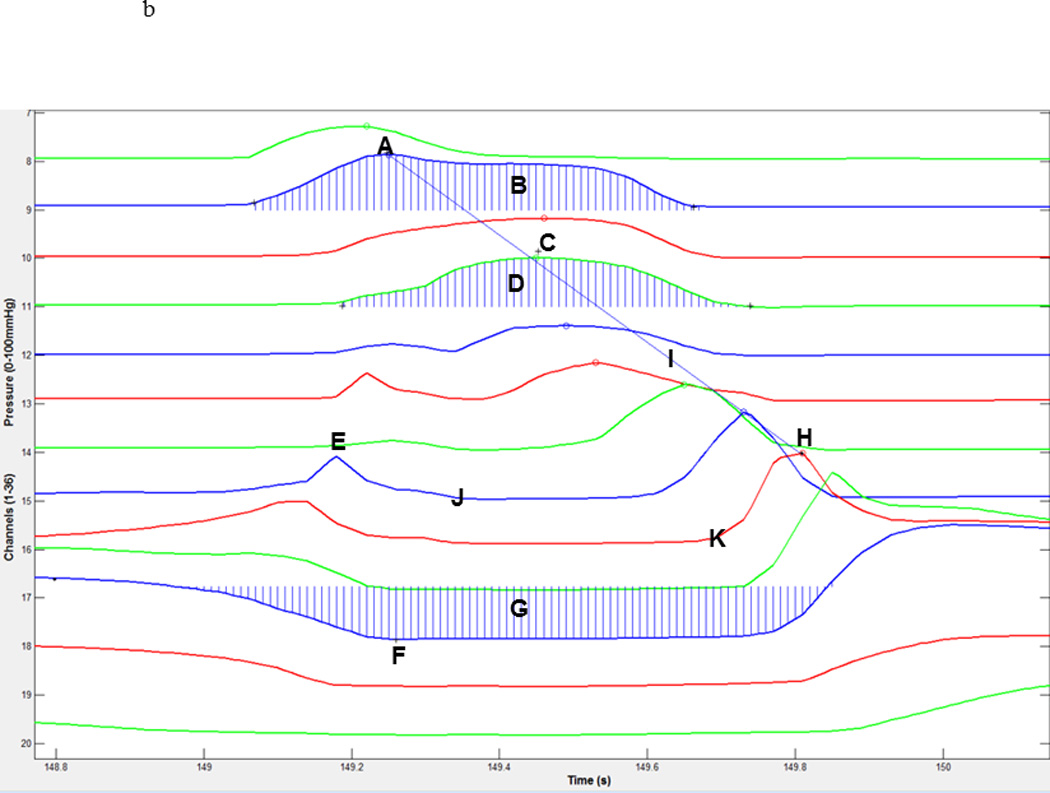
High-resolution manometry spatiotemporal plot of one normal swallow (A) and corresponding automated extraction of salient parameters (B). A = maximum velopharyngeal pressure; B = velopharyngeal pressure integral; C = maximum tongue base pressure; D = tongue base pressure integral; E = maximum pre-opening upper esophageal sphincter (UES) pressure; F = minimum UES pressure; G = UES pressure integral; H = maximum post-closure UES pressure; I = pressure wave velocity; J = onset of nadir UES pressure; K = offset of nadir UES pressure.
Regions of interest were defined manometrically as in McCulloch et al. (15). The velopharynx is the region of swallow-related pressure change just proximal to the area of continuous nasal cavity quiescence (baseline resting pressure of approximately zero in the sensors corresponding to the nasal cavity) and extending two centimeters distally. Maximum velopharyngeal pressure is detected by comparing the peak pressures of the most proximal (rostral) sensors. The peaks continually increase until the maximum velopharyngeal pressure is reached. The tongue base is the area of swallow related pressure change with a high pressure zone approximately midway between the nasopharynx and UES, with its epicenter at the high pressure point and extending two centimeters proximal and distal to that point. Maximum tongue base pressure is less obvious to detect in general, and occasionally requires intervention by the user. A scoring system considers several candidate sensors and their peak pressures, scoring them based on peak pressure, duration, and position relative to the velopharynx. The UES is the midpoint of stable high pressure just proximal (rostral) to the baseline low esophageal pressure zone, extending to a point of low esophageal pressure distally and low baseline pharyngeal pressure proximally. It is detected by computing the average resting pressures of each sensor, and selecting the sensor with the highest value. During swallowing, the UES is mobile along the catheter, moving rostrally as much as 4 cm. To account for this movement in our analysis, the algorithm scans the sensors both above and below the level of the resting UES when locating local maximums and minimums. The sensor corresponding to maximum pre-opening UES pressure, therefore, may be different than the sensor corresponding to maximum post-closure UES pressure. Once maximum pressure peaks are found, timing data can be extracted by marking the onset and offset of elevated pressure.
Mean and standard deviation values were recorded for maximum pressure, rate of pressure increase, duration of pressure above baseline, area integral, and line integral in the regions of the velopharynx and tongue base. Rate of pressure increase was calculated by subtracting baseline pressure from maximum pressure and dividing by the time lapse between these points. Duration of pressure above baseline within a region was defined as the time duration between the onset of pressure escalation and its return to or below baseline using the single senor where maximum pressure was recorded. Minimum pressure during UES opening as well as maximum pressures preceding and succeeding UES opening were also recorded. The time lapse between these pressure peaks is termed UES activity time. A measurement termed nadir UES pressure duration is determined by locating the region of lowest sustained UES pressure. That is, from the offset of the pre-opening UES pressure peak to the onset of the post-closure UES pressure peak. As the pre-opening and post-closure UES pressure peaks can occur at different sensors, the onset and offset of nadir UES pressure duration can also occur at different sensors; however, this is not a requirement of the measurement. Total swallow duration was defined as the time lapse between onset of velopharyngeal pressure rise and the post-closure UES pressure peak.
While maximum pressure can provide valuable information on swallowing physiology which can easily be compared to previous manometric investigations, it does not provide a complete picture of pharyngeal pressure events. Measuring the total pressure created in a specific region offers more information and, when combined with durative data, reveals more about the shape of the pressure curve. Integrals are calculated of the area beneath the velopharynx and tongue base pressure curves, as well as above the UES minimum with the UES resting pressure as an upper limit. Only one pressure trace (corresponding to one sensor) is used in this calculation. Area integrals are defined as the area under the curve, while line integrals are the distance along the curve. Area integrals have units of pressure*time, which in this case is mmHg*(s/50), as the sampling rate for the manometer is 50 Hz. Line integrals are unitless. Temporal bounds in all cases are the onset and offset of pressure elevation or depression determined previously.
The pharyngeal swallow can be thought of as a traveling pressure wave, with peak pressure traveling caudally and ending at the UES. We can calculate the velocity of this pressure wave by taking the distance from the velopharyngeal pressure peak to the maximum post-closure UES pressure peak and dividing by the time lapse between these two points.
Statistical analysis
SigmaPlot 11.0 software was employed for statistical analyses. Mean values recorded during effort swallow and Mendelsohn’s maneuver were compared to those recorded for normal swallows using two-tailed paired t-tests. Shapiro-Wilk and Levene’s tests were used to determine normality and equal variance, respectively. If data did not meet the statistical assumptions for parametric testing, a Wilcoxon-Mann-Whitney signed rank test was performed. A significance level of α = 0.05 was used for all tests.
RESULTS
Summary data are presented in table 1. Figure 2 provides sample spatiotemporal plots for the three tasks performed by a single subject. Figures 3, 4, and 5 present summary information on duration, pressure, and area integrals, respectively.
Table 1.
Summary data.
| Normal | Effortful | Mendelsohn | |||
|---|---|---|---|---|---|
| Parameter | Mean | Mean | P-value | Mean | P-value |
| VP max (mmHg) | 162 ± 93 | 183 ± 42 | 0.020* | 161 ± 45 | 0.635 |
| VP duration (s) | 0.87 ± 0.19 | 0.81 ± 0.15 | 0.303 | 1.66 ± 0.61 | <0.001* |
| VP rise rate (mmHg/s) | 869 ± 339 | 875 ± 309 | 0.920 | 680 ± 355 | 0.027* |
| VP integral (mmHg*(s/50)) | 6442 ± 2310 | 7896 ± 3297 | 0.124 | 14182 ± 7275 | <0.001* |
| VP line integral | 335 ± 71 | 383 ± 79 | <0.001* | 436 ± 151 | 0.008* |
| TB max (mmHg) | 245 ± 174 | 167 ± 77 | 0.326 | 161 ± 78 | 0.048* |
| TB duration (s) | 0.64 ± 0.13 | 0.58 ± 0.18 | 0.259 | 0.77 ± 0.21 | 0.086 |
| TB rise rate (mmHg/s) | 1150 ± 822 | 1030 ± 1141 | 0.676 | 805 ± 543 | 0.064 |
| TB integral (mmHg*(s/50)) | 4953 ± 1970 | 4619 ± 2060 | 0.464 | 5967 ± 2899 | 0.133 |
| TB line integral | 430 ± 340 | 408 ± 318 | 0.463 | 337 ± 178 | 0.273 |
| UES pre (mmHg) | 176 ± 122 | 145 ± 70 | 0.296 | 125 ± 72 | 0.005* |
| UES post (mmHg) | 249 ± 138 | 278 ± 192 | 0.358 | 294 ± 170 | 0.139 |
| UES min (mmHg) | −4 ± 6 | −1 ± 10 | 0.147 | −1 ± 10 | 0.174 |
| UES activity time (s) | 0.92 ± 0.13 | 0.86 ± 0.24 | 0.391 | 0.84 ± 0.16 | 0.135 |
| Nadir UES pressure duration (s) | 0.56 ± 0.08 | 0.65 ± 0.14 | 0.002* | 0.74 ± 0.16 | 0.001* |
| UES integral (mmHg*(s/50)) | 3605 ± 1942 | 4977 ± 2771 | 0.021* | 4729 ± 1948 | 0.157 |
| UES line integral | 163 ± 93 | 372 ± 283 | 0.004* | 358 ± 213 | 0.020* |
| Total swallow duration (s) | 0.93 ± 0.12 | 0.95 ± 0.22 | 0.673 | 0.92 ± 0.15 | 0.901 |
| Pressure velocity (cm/s) | 9.97 ± 1.68 | 10.47 ± 2.02 | 0.313 | 9.46 ± 1.46 | 0.317 |
VP = velopharynx; TB = tongue base; UES = upper esophageal sphincter.
Significant p-values are denoted by an asterisk.
Figure 2.
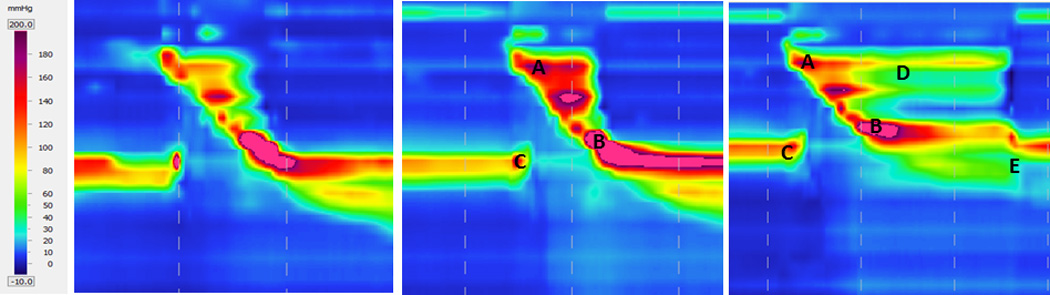
Spatiotemporal plots from one subject displaying normal swallow (left), effortful swallow (middle), and the Mendelsohn maneuver (right). Velopharyngeal pressure (A) and post-closure upper esophageal sphincter pressure (B) are elevated in both maneuvers, while pre-opening upper esophageal sphincter pressure is decreased (C). Duration of velopharyngeal pressure is prolonged in the Mendelsohn maneuver (D). Descent of the upper esophageal sphincter can be easily observed (E) at the conclusion of the Mendelsohn maneuver.
Figure 3.
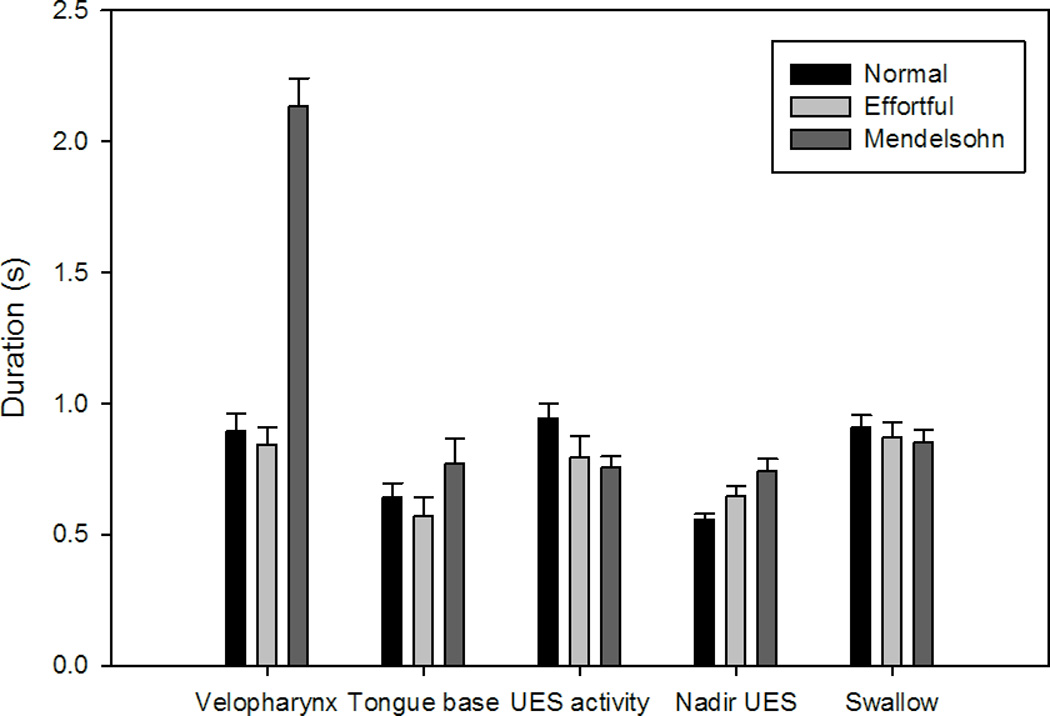
Bar charts displaying average duration for areas of interest and the total swallow. Error bars represent standard error of the mean. Duration of velopharyngeal pressure increased with the Mendelsohn maneuver. Duration of nadir upper esophageal sphincter (UES) pressure increased with both maneuvers.
Figure 4.
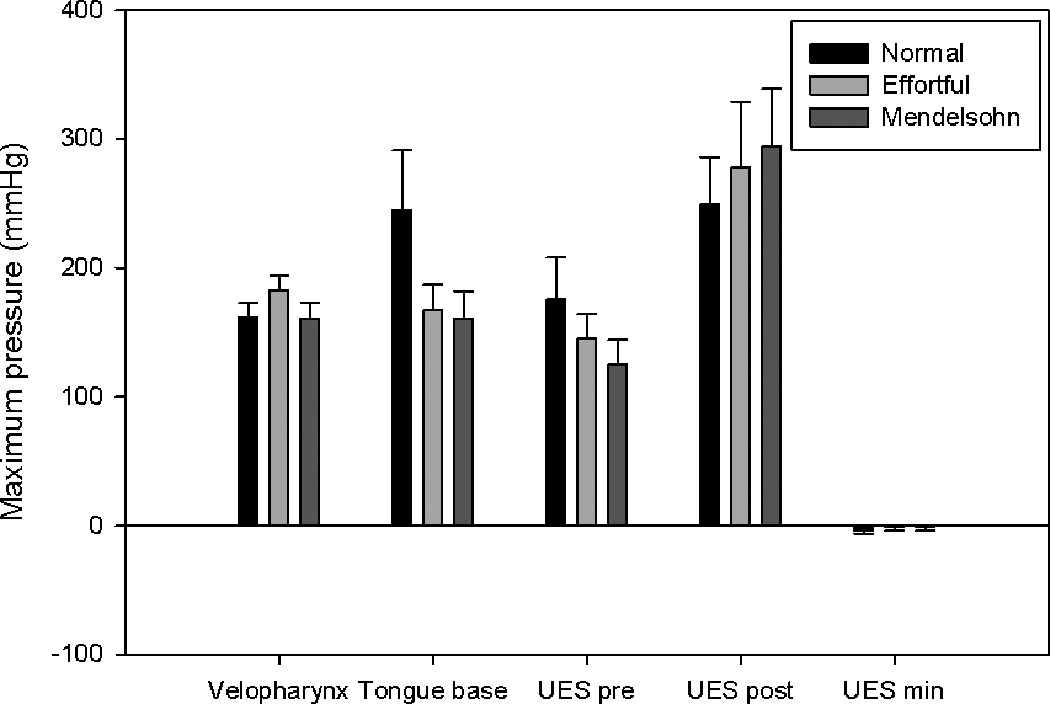
Bar charts displaying average maximum pressure in the areas of interest. Error bars represent standard error of the mean. Maximum tongue base pressure and pre-opening upper esophageal sphincter (UES) pressure were decreased by both maneuvers, while post-closure UES pressure and minimum UES pressure increased.
Figure 5.
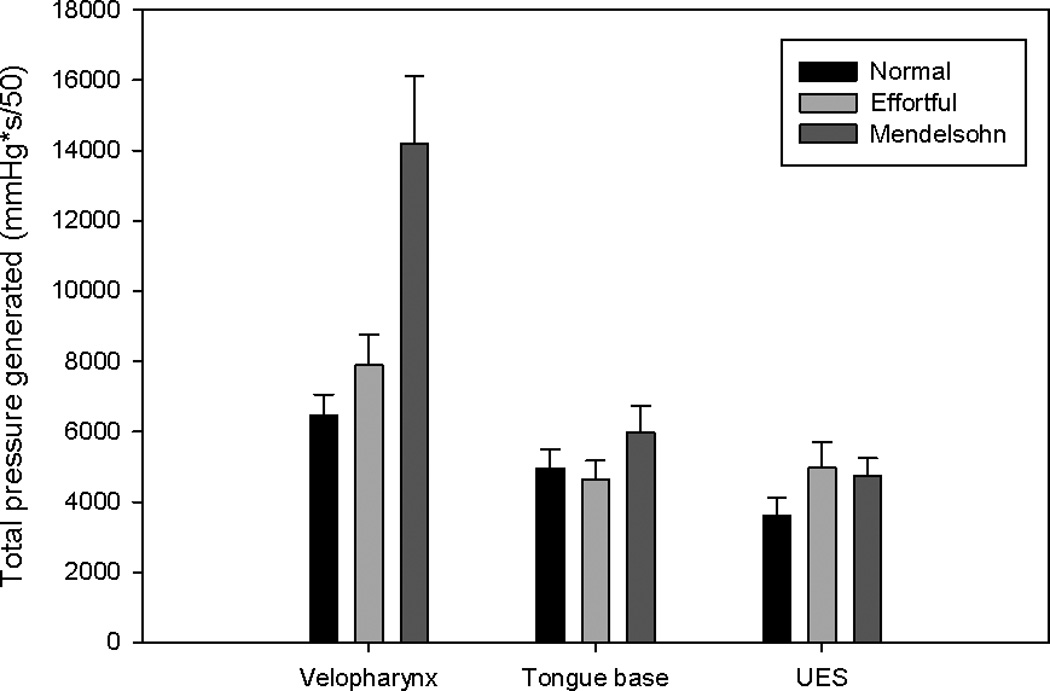
Bar charts displaying area integrals (total pressure generated) in the areas of interest. Error bars represent standard error of the mean. Total pressure generated in the velopharynx and upper esophageal sphincter (UES) increased for both maneuvers.
Velopharynx
Effortful swallow increased maximum velopharyngeal pressure (p=0.020). The Mendelsohn maneuver decreased velopharyngeal pressure rise rate (p=0.027). Duration of velopharyngeal pressure above baseline increased significantly (p<0.001) with the Mendelsohn maneuver (figure 3). Increases in the velopharyngeal pressure area and line integrals were observed for both effortful swallow (p=0.124; p<0.001) and the Mendelsohn maneuver (p<0.001; p=0.008), though the increase in the velopharyngeal area integral due to the effortful swallow was not significant (figure 5).
Tongue base
A significant decrease in maximum tongue base pressure was observed for the Mendelsohn maneuver (p=0.048) (figure 4). An increase in tongue base pressure duration (p= 0.086) occurred with the Mendelsohn maneuver, but this increase was not statistically significant. While an increase in the tongue base pressure area integral was observed for the Mendelsohn maneuver (p=0.133) (figure 5), this increase also did not reach statistical significance. No other notable trends were observed.
Upper esophageal sphincter
Maximum pre-opening UES pressure was decreased significantly by the Mendelsohn maneuver (p=0.005). Minimum UES pressure was increased but not significantly for both maneuvers (effortful swallow: p=0.147; Mendelsohn maneuver: p=0.174) (figure 4). Significant increases were observed for the area and line integrals with effortful swallow (p=0.021; p=0.004), while only a significant increase in the line integral was observed for the Mendelsohn maneuver (p=0.020) (figure 5). Nadir UES pressure duration was prolonged by both the Mendelsohn maneuver (p=0.001) and effortful swallow (p=0.002).
Total swallow duration was unaffected by either maneuver. The velocity of the pressure wave was not significantly affected by effortful swallow (p=0.313) or the Mendelsohn maneuver (p=0.318).
DISCUSSION
This is the first study using HRM to evaluate effortful swallow and the Mendelsohn maneuver. We also employed novel analysis techniques which take advantage of the multi-sensor array capabilities of HRM to better capture the complexity of pressure events, area integrals, line integrals, and pressure wave velocity. Both maneuvers elicited changes in pressure patterns in the pharynx, but these patterns were specific to the maneuver. The effects of effortful swallow were observed primarily at the velopharynx and UES, and not in the region of the tongue base. Effortful swallows displayed increased pressure wave velocity and area and line integrals. Widespread effects throughout the pharynx were observed for the Mendelsohn maneuver, including the tongue base. The Mendelsohn maneuver also yielded significantly longer velopharyngeal pressure duration and increased velopharyngeal area and line integrals.
Several effects previously reported using traditional manometry were also observed in this study. The Mendelsohn maneuver decreased UES pressure (8, 10) and increased duration of velopharyngeal pressure, likely attributable to prolonged laryngeal elevation (3–6). In contrast to previous reports (1), a decrease in tongue base pressure occurred with effortful swallow, though this decrease was not significant. This was accompanied by an increase in total pressure generated in the velopharynx. Decreased tongue base pressure could be attributed to increased hyoid movement occurring with the increased muscle contraction during effortful swallow, but we did not directly measure this. Theoretically, this would increase the volume of the oropharynx and consequently decrease pressure measured in the area of the tongue base. As structures of the pharynx are mobile during swallowing, previous investigations using single sensors may have recorded increased velopharynx pressure as an increase in tongue base pressure.
We also found changes in pressure patterns that have not been previously reported. Both maneuvers increased minimum UES pressure, though neither increase reached statistical significance. As laryngeal elevation creates a negative pressure vacuum in the UES, one could expect that the prolonged laryngeal elevation in the Mendelsohn maneuver may actually further decrease this negative pressure in normal swallows. However, increased pressure at the velopharynx created by either maneuver could require less negative pressure necessary at the bolus head to ensure safe bolus transit into the esophagus. Maximum pre-opening and post-closure UES pressures were also affected, with pre-opening pressure decreasing and post-closure pressure increasing with both maneuvers. These changes were more pronounced with the Mendelsohn maneuver, where significantly decreased pre-opening and increased post-closure pressure (not significant) were observed. Trends were not as evident for the effortful swallow. Each of these effects on UES pressure would facilitate safe bolus transit, as decreased pre-opening pressure would provide less resistance to bolus passage into the esophagus while elevated post-closure pressure would prevent reflux. As maneuvers tend to require significant effort and modification of the swallow pattern, multiple aspects of the pharyngeal swallow can be modulated (23).
The integral measurements used in this study demonstrated significant changes across tasks that were not revealed by traditional measurements of maximum pressure or duration of pressure above baseline. For example, though maximum velopharyngeal pressure was not affected by the Mendelsohn maneuver, obvious differences were observed for the area and line integrals. As integrals consider the entire shape of the pressure curve, they may be a more sensitive measurement than maximum pressure alone. Though only two-dimensional integrals were recorded in this study, considering the volume enclosed by three-dimensional pressure curves in regions of interest may provide an even more comprehensive characterization of the pharyngeal swallow.
Nadir UES pressure duration may be particularly valuable when evaluating swallowing maneuvers. As maneuvers are designed to improve bolus passage and passage can only occur through a patent UES, measuring the duration of UES patency, or duration of lowest measured UES pressure, may provide insight into why a maneuver is or is not successful. It is important to use this measurement in context, as a nadir pressure very near resting pressure, no matter how long in duration, will not contribute to bolus passage. Considering nadir UES pressure duration in the context of what that pressure is may be useful from a clinical and research standpoint.
There were three limitations to this study. First, while several significant differences could be observed between maneuvers, the sample size was relatively small. Findings that approached, but did not reach significance may do so with a larger sample size. Second, it is possible that the use of topical anesthesia affected our experimental measurements. In pilot experiments without topical anesthesia, subjects found it difficult not to gag and also displayed resting cricopharyngeal hypertonicity, both of which could confound data collection. While impairing afferent nerves in the pharynx could alter swallowing physiology, mechanoreceptors deep to the mucosa are largely responsible for modulating swallowing physiology (24) and these fibers were likely unaffected by the anesthetic. Also, the oral mucosa was not greatly affected and afferent information from this region is important in swallowing physiology. Though we feel the benefits of increased subject comfort outweigh the cost of short-term pain and temperature afferent alteration, conducting an experiment to determine the quantitative effect of anesthetic may be beneficial. Lastly, this study was conducted in healthy subjects. Evaluating the effect of these maneuvers in dysphagic patients using HRM will be the subject of future studies. Specifically, it would be interesting to determine if the relationships among velopharyngeal, tongue base, and UES pressure during effortful swallow are upheld in patients with tongue dysmotility. Further developing the novel parameters used in this study, such as area and line integrals, will also be investigated.
Similar to our previous study on head turn and chin tuck (15), effects were observed primarily at the velopharynx and UES. Proper muscular function in these two regions may be most critical to successful bolus passage and may also be easier to manipulate than the tongue base. Effortful swallow was introduced for patients with reduced tongue base retraction, but conflicting results on the biomechanical effects of this maneuver have been reported (25). Rather than normalizing tongue base function in patients with tongue dysmotility, effortful swallow may promote elevated velopharyngeal pressure which can overcome low tongue base pressure. This also leads to increased hyolaryngeal excursion, which enhances UES opening. Effortful swallow, then, is a floor of mouth event rather than a base of tongue event. This hypothesis was supported not only by increases in velopharyngeal pressure, but also by decreased tongue base pressure in our measurements. Decreased pre-opening UES pressure observed in this study would also promote bolus passage, as less resistance would be encountered at the UES. While success of the maneuver has previously been attributed to improved posterior tongue base motion (2), it may due to the elevated velopharyngeal and decreased UES pressures creating a favorable pressure gradient for bolus transit. The benefit of this gradient likely manifested in increased velocity of the traveling pressure wave that we observed.
HRM revealed several physiologic modifications that have not been previously reported. Our novel analysis techniques using area and line integrals as well as pressure wave velocity demonstrated prominent changes at the velopharynx and UES, with little effect at the tongue base. These findings increase our understanding of how effortful swallow and the Mendelsohn maneuver improve swallowing in dysphagic patients and may be used to enhance these maneuvers.
Acknowledgments
Grant Support: This research was supported by a grant from the University of Wisconsin School of Medicine and Public Health, Department of Surgery
REFERENCES
- 1.Lazarus C, Logemann JA, Song CW, Rademaker AW, Kahrilas PJ. Effects of voluntary maneuvers on tongue base function for swallowing. Folia Phoniatr Logop. 2002;54:171–176. doi: 10.1159/000063192. [DOI] [PubMed] [Google Scholar]
- 2.Logemann JA. Evaluation and Treatment of Swallowing Disorders. 2 ed. Austin: Pro-Ed; 1998. [Google Scholar]
- 3.Bulow M, Olsson R, Ekberg O. Videomanometric analysis of supraglottic swallow, effortful swallow, and chin tuck in healthy volunteers. Dysphagia. 1999;14:67–72. doi: 10.1007/PL00009589. [DOI] [PubMed] [Google Scholar]
- 4.Hind JA, Nicosia MA, Roecker EB, Carnes ML, Robbins J. Comparison of effortful and noneffortful swallows in healthy middle-aged and older adults. Arch Phys Med Rehabil. 2001;82:1661–1665. doi: 10.1053/apmr.2001.28006. [DOI] [PubMed] [Google Scholar]
- 5.Huckabee ML, Butler SG, Barclay M, Jit S. Submental surface electromyographic measurement and pharyngeal pressures during normal and effortful swallowing. Arch Phys Med Rehabil. 2005;86:2144–2149. doi: 10.1016/j.apmr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Logemann JA, Kahrilas PJ. Relearning to swallow after stroke--application of maneuvers and indirect biofeedback: a case study. Neurology. 1990;40:1136–1138. doi: 10.1212/wnl.40.7.1136. [DOI] [PubMed] [Google Scholar]
- 7.Ding R, Larson CR, Logemann JA, Rademaker AW. Surface electromyographic and electroglottographic studies in normal subjects under two swallow conditions: normal and during the Mendelsohn manuever. Dysphagia. 2002;17:1–12. doi: 10.1007/s00455-001-0095-3. [DOI] [PubMed] [Google Scholar]
- 8.Boden K, Hallgren A, Witt Hedstrom H. Effects of three different swallow maneuvers analyzed by videomanometry. Acta Radiol. 2006;47:628–633. doi: 10.1080/02841850600774043. [DOI] [PubMed] [Google Scholar]
- 9.Bulow M, Olsson R, Ekkberg O. Supraglottic swallow, effortful swallow, and chin tuck did not alter hypopharyngeal intrabolus pressure in patients with pharyngeal dysfunction. Dysphagia. 2002;17:197–201. doi: 10.1007/s00455-002-0050-y. [DOI] [PubMed] [Google Scholar]
- 10.Kahrilas PJ, Logemann JA, Krugler C, Flanagan E. Volitional augmentation of upper esophageal sphincter opening during swallowing. Am J Physiol. 1991;260:G450–G456. doi: 10.1152/ajpgi.1991.260.3.G450. [DOI] [PubMed] [Google Scholar]
- 11.Logemann JA, Kahrilas PJ, Kobara M, Vakil NB. The benefit of head rotation on pharyngoesophageal dysphagia. Arch Phys Med Rehabil. 1989;70:767–771. [PubMed] [Google Scholar]
- 12.Bulow M, Olsson R, Ekberg O. Supraglottic swallow, effortful swallow, and chin tuck did not alter hypopharyngeal intrabolus pressure in patients with pharyngeal dysfunction. Dysphagia. 2002;17:197–201. doi: 10.1007/s00455-002-0050-y. [DOI] [PubMed] [Google Scholar]
- 13.Kelly JH. Use of manometry in the evaluation of dysphagia. Otolaryngol Head Neck Surg. 1997;116:355–357. doi: 10.1016/S0194-59989770273-0. [DOI] [PubMed] [Google Scholar]
- 14.Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: moving from research into clinical practice. Gut. 2008;57:405–423. doi: 10.1136/gut.2007.127993. [DOI] [PubMed] [Google Scholar]
- 15.McCulloch T, Hoffman MR, Ciucci MR. High resolution manometry of pharyngeal swallow pressure events associated with head turn and chin tuck. Ann Otol Rhinol Laryngol. 2010;119(6):369–376. doi: 10.1177/000348941011900602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takasaki K, Umeki H, Kumagami H, Takahashi H. Influence of head rotation on upper esophageal sphincter pressure evaluated by high-resolution manometry system. Otolaryngol Head Neck Surg. 142:214–217. doi: 10.1016/j.otohns.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman MR, Ciucci MR, Mielens JD, Jiang JJ, McCulloch TM. Pharyngeal swallow adaptations to bolus volume measured with high resolution manometry. Laryngoscope. 2010;120(12):2367–2373. doi: 10.1002/lary.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheeler-Hegland KM, Rosenbek JC, Sapienza CM. Submental sEMG and hyoid movement during Mendelsohn maneuver, effortful swallow, and expiratory muscle strength training. J Speech Lang Hear Res. 2008;51:1072–1087. doi: 10.1044/1092-4388(2008/07-0016). [DOI] [PubMed] [Google Scholar]
- 19.Clark HM. Neuromuscular treatments for speech and swallowing: a tutorial. Am J Speech Lang Pathol. 2003;12:400–415. doi: 10.1044/1058-0360(2003/086). [DOI] [PubMed] [Google Scholar]
- 20.Langmore SE, Miller RM. Behavioral treatment for adults with oropharyngeal dysphagia. Arch Phys Med Rehabil. 1994;75:1154–1160. doi: 10.1016/0003-9993(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 21.Logemann JA. Behavioral management for oropharyngeal dysphagia. Folia Phoniatr Logop. 1999;51:199–212. doi: 10.1159/000021497. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Kahrilas PJ. Deglutitive upper esophageal sphincter relaxation: a study of 75 volunteer subjects using solid-state high-resolution manometry. Am J Physiol Gastrointest Liver Physiol. 2006;291(3):G525–G531. doi: 10.1152/ajpgi.00081.2006. [DOI] [PubMed] [Google Scholar]
- 23.Lazarus C, Logemann JA, Gibbons P. Effects of maneuvers on swallowing function in a dysphagic oral cancer patient. Head Neck. 1993;15:419–424. doi: 10.1002/hed.2880150509. [DOI] [PubMed] [Google Scholar]
- 24.Ali GN, Cook IJ, Laundl TM, Wallace KL, de Carle DJ. Influence of altered tongue contour and position on deglutitive pharyngeal and UES function. Am J Physiol. 1997;273:G1071–G1076. doi: 10.1152/ajpgi.1997.273.5.G1071. [DOI] [PubMed] [Google Scholar]
- 25.Witte U, Huckabee ML, Doeltgen SH, Gumbley F, Robb M. The effect of effortful swallow on pharyngeal manometric measurements during saliva and water swallowing in healthy participants. Arch Phys Med Rehabil. 2008;89:822–828. doi: 10.1016/j.apmr.2007.08.167. [DOI] [PubMed] [Google Scholar]


