Abstract
There is evidence from EEG studies that unexpected perturbations to standing posture induce a differential modulation of cortical activity compared to self-initiated and/or predictable conditions. However, the neural correlates of whole body postural response to visually-induced perturbations on standing posture have not been examined. Here we employ a novel experimental paradigm via combined Virtual Reality (VR) and EEG measures to examine the effects of visually induced perturbations on the dynamics of postural responses. Twelve Penn State student-athletes without prior history of neurologic disorders and/or orthopaedic injuries participated in this study. There were no differences in response/reaction time measures between both spatially and temporally unpredictable and fully predictable conditions (p>.05). However, significantly stronger modulation of frontal-central EEG theta activity was present prior to onset of unpredictable postural perturbations (p<. 05). It is postulated that enhanced EEG theta in unpredictable conditions reflects increased effort to recruit additional brain resources to meet the demands of the postural tasks.
Keywords: Virtual Reality, Postural Responses, EEG
While many studies have used a continuously moving “virtual moving room” to analyze postural stability [4,5,7,10,13,14,16], the introduction of a VR-induced postural perturbation is a relatively novel approach to examining the neural substrates of preparatory postural responses in humans. Indeed, VR can serve as the means of inducing optic flow to subjects to provoke postural instability [4,5,10,16]. Moreover, the VR environment allows one to develop the task in which an individual can control and manipulate movement along with the sense of self-motion while retaining the head fixation requirement of the EEG environment [7,16]. In this study, we develop the VR-induced perturbation of dynamic postural task paradigm enabling: (a) the subject to experience the sense of presence [9]; (b) to examine the preparatory postural response to optic flow with various degrees of uncertainty; and (c) to track the modulation of brain activation patterns via EEG prior to onset of postural response initiation.
Twelve Penn State student-athletes (6 males and 6 female), without history of neurological and orthopaedic injuries, aged 20+/− 2.3 years old participated in this study. All subjects signed an informed consent form and the Institutional Review Board of the Pennsylvania State University approved this protocol. Subjects produced anterior-posterior (A–P) postural oscillation to follow optic flow and were required to respond via whole body medio-lateral (M–L) motion pivoted around the waist to: (a) predictable change in direction of “moving room” always to the right or to the left (13s after trial initiation); and (b) unpredictable (random) change in direction of “moving room.” In the unpredictable conditions, both direction (right or left) and timing of the perturbations (between 7–21s after trial initiation) was randomized. In both predictable and unpredictable conditions, the subjects were instructed to respond as fast and accurate as possible. Trial length was set at 30s and each subject performed 15 trials for each condition (predictable right, predictable left, and unpredictable). Total number of trials was 60 plus 3 trials per condition as practice trials. The subjects’ head motion was recorded via Intrasense, LLC motion tracking system.
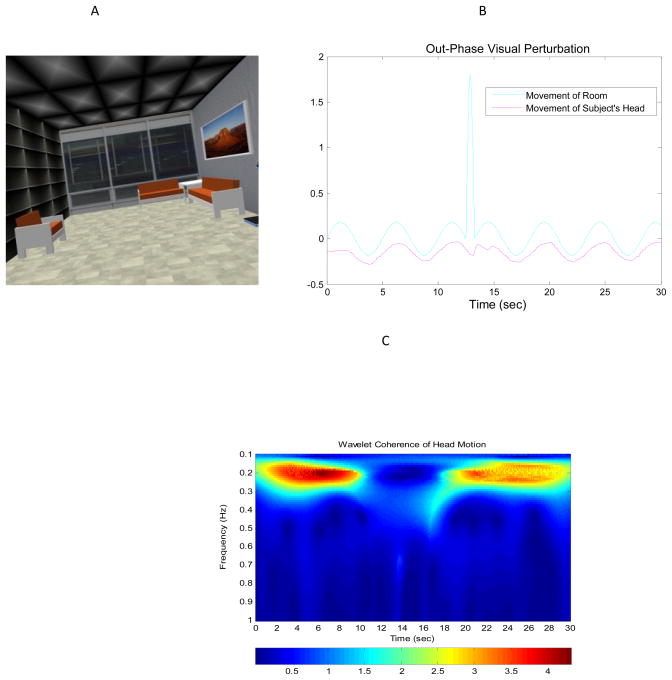
InnovativeVR, LLC Pulse Room hardware and software (www.HeadRehab.com) was used for this study. The subjects stood 2.5 feet away from a 4′ × 5′ screen that was positioned 60 degrees from the floor with the visual stimulus projected from a rear-projection system manufactured by Fakespace Systems. Surrounding the screen were two black curtains to each side. A IS900 device and wand was used to coordinate head tracking input, start the Pulse Room motion sequence and timer, and actively capture and record subject head motion data. The IS900 device was synchronized with an EGI 128-channel EEG system. The actual visual stimulus depicted a realistically looking room forming a “virtual room” with a height of 4′, width of 5′, depth of 6′ in a window size of 1400×1050 (see Fig. 1a.). CrystalEyes stereo glasses were worn by subjects and fit to head with a connective head strap if needed for fit. An adjustable harness was fitted to each subject and clipped to a sturdy ceiling beam as a safety precaution. Illustration of virtual room motion and visual perturbation as well as the subject’s responses is depicted in Fig 1b & c.
Figure 1.
(a) visual stimulus via realistically Virtual “moving room” enable to shift optic flow from Anterior-posterior to Medial-Lateral directions; (b) Time serious of moving room oscillation (.2 Hz) superimposed on a subject’s responses via head motion tracking; (c) wavelet transform depicting the dynamics of head motion/virtual room oscillation and response to visual perturbation. Where: X-axis is time of trial duration (30s); y-axis is the frequency of moving room and the subject’s head oscillation (.2 Hz); Color scale indicates the magnitude of moving room/head motion coherence within .2 Hz frequency cluster.
Recent technological advancements enable researchers to study electrocortical dynamics during whole body movement including head motion [1,6,8,15]. In this study, an EGI 128-channel system was used to collect EEG signals. Data were recorded during the predictable and unpredictable conditions after a task orientation was given to each participant. Raw signal processing was done through NetStation (Electrical Geodesics, Inc.). From the 128-channel array, 15 channels in the frontal-central region were selected for data analysis specifically focused on modulation of low-frequency EEG signal associated with postural responses under study. We focused primarily on frontal-central EEG theta power because previous research has documented that modulation of frontal-central theta power may relate to cortical control of balance [6,15,16], error monitoring during motor performance [1], and successful performance of perceptual-motor tasks [11]. The recording was filtered in the 4–30 Hz frequency range. The data were checked and corrected for artifacts and eye blinks were removed. The EEG recording was segmented into pieces that contained data from 500ms prior to the pulse perturbation to 100ms after the pulse. The segmented pieces were averaged together for all 15 trials in the predictable and unpredictable conditions. The processed files were subjected to EEG power analysis [see 15 for details].
All subjects under study experienced vection (e.g., sense/feeling of moving that may occur even in absence of ego-motion) in addition to ego-motion, as evidenced by subjective reports after the completion of each experimental condition. Specifically, the subjects reported vection strength was on average 8 on an 10 point scale with 0 representing “no vection” was perceived and 10 representing “vection so strong that the perceived self-motion could not be differentiated from real physical motion” [9].
Subjects’ behavioral responses are summarized as follows. No significant differences in reaction time to VR-induced postural perturbation were observed between predictable (455 +/− 45ms) and unpredictable conditions, (469 +/− 39 ms), p>.05). However, there was a trend to increase the “time-to-recovery” from perturbation, defined as reappearance of coherent head-VR oscillatory motion, for the unpredictable condition (p=.08). Success rate of the accuracy of postural responses was similar (98.8% for predictable and 97.9 % for unpredictable conditions). Overall, there was no performance difference between predictable and unpredictable responses to postural perturbation induced by VR moving room.
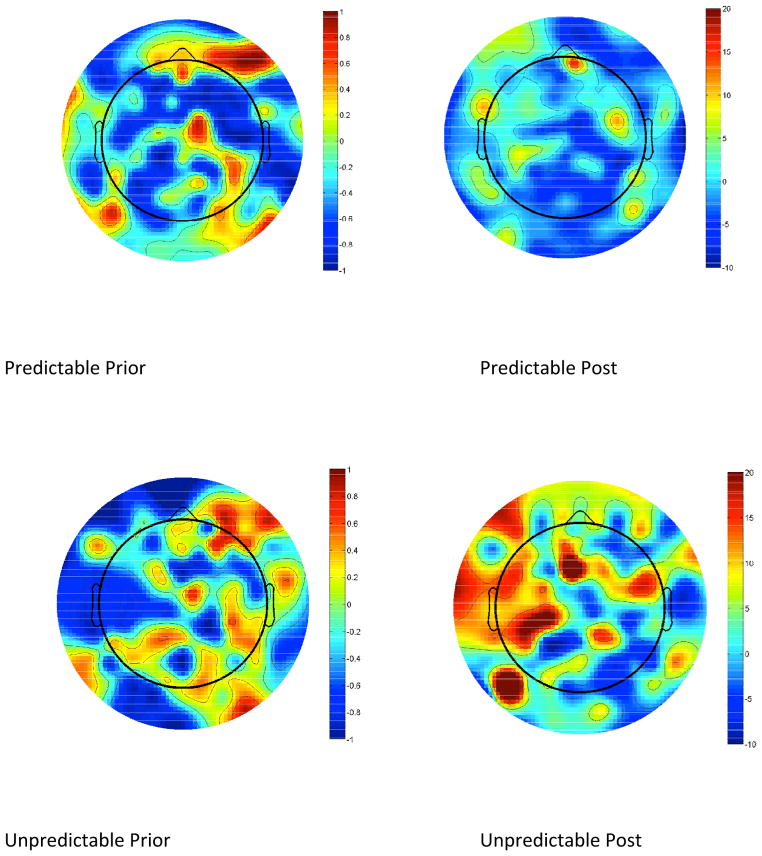
EEG theta power analysis revealed the following. First, there was a trend of decrease of theta power at the onset on initiation of postural responses in predictable (t=2.039, df=6, p=.058) and a significant decrease in unpredictable (t=2.895, df=6, p=.028) conditions. Second, and most importantly, theta power within 500s prior to onset of postural perturbation was significantly higher during unpredictable condition (t=2.783, df=6, p=.032). 2D plots of EEG power 500 ms prior to and 100 ms after onset of postural perturbations narrowed within theta band (4–7Hz) for both conditions are depicted in Fig. 2. No significant modulations of EEG signal in alpha (8–13 Hz) and beta (14–30) bands in frontal-central regions as a function of experimental conditions were observed.
Figure 2.
2D plots of EEG theta power prior to predictable and unpredictable perturbations. It should be noted that reduction of central theta power after the pulse in unpredictable condition was accompanied by significant increase of theta power in frontal and temporal-parietal ROIs. This interesting finding requires additional experimentation and beyond the scope of this report.
The major behavioral finding from this study indicates that increased demands for information processing associated with a more complex task is not necessarily related with increased reaction time. This unexpected finding may be explained by modulation of cortical activity within the frontal-central theta rhythm reflecting the utilization of additional brain resources to meet the behavioral task demands. A number of previous studies have documented that modulation of frontal-central theta power may relate to postural control [15], efficiency of performance of perceptual-motor tasks [11], and underlay the neural mechanism in charge of error monitoring during perceptual-motor tasks [1]. Specifically, we have reported significantly higher spectral power in low theta (4–5 Hz) and alpha (8–12 Hz) frequency bands from scalp electrodes located over the anterior cingulate during unstable balance when standing on one leg [15]. This finding is consistent with our previous EEG report suggesting increase of frontal-central theta power as a function of successful performance of perceptual-motor tasks [11]. Increase in theta band power over frontal-central areas, as seen in this study during unpredictable conditions, may presumably reflect the activation of anterior cingulate cluster [6] and appears to be related to: (a) reduction of the time of information processing for response initiation; and (b) speedy and error free whole body postural responses. This finding supports the idea that the primary role of the anterior cingulate cortex is to compare current sensory information with that expected from an internal forward-looking model, with sufficient mismatch triggering a postural response [2].
Unexpectedly, neither differences in postural response time nor accuracy of task performance (% error) were observed between predictable and unpredictable conditions in this study. A similar finding was reported in the Adkin et al., (2006) study [1]. They showed that balance responses evoked by both predictable and unpredictable perturbations were similar in terms of AP COP excursion and EMG responses after perturbations. However, unpredictable perturbations evoked multi-component cortical responses reflected in N1 potential with maximum at CZ and FCZ electrode sites. In contrast, predictable perturbations did not appear to elicit any changes in cortical potentials prior to the onset of perturbation [1]. Moreover, unpredictability of physically induced perturbation was shown to be associated with increased CNV amplitude at the CZ electrode site in both normal controls and PD patients [17]
Our most important finding is the increase in EEG theta power 500ms prior to the pulse in the unpredictable condition compared to the predictable condition. We have postulated that the magnitude of movement-related cortical potentials relates to the perceived effort of the motor task [12]. The enhanced cortical activity prior to postural perturbation may occur in association with setting of the CNS state in advanced of a forthcoming event that evoke balance reactions to meet the task demands [8]. Collectively, with observed behavioral data it is conceivable that enhanced electrocortical activity in general, and EEG theta in particular, in unpredictable condition reflects increased effort to recruit additional brain resources to meet the postural tasks demands. Therefore, exploration and utilization of these brain resources may be a promising tool for performance enhancement in a clinical setting.
Highlights.
Virtual Reality (VR) and EEG was used to investigate the effect of visual perturbation on standing posture
Whole body postural responses were not different between predictable and unpredictable conditions
Unpredictable timing and direction of perturbation
Acknowledgments
This research was supported by National Institutes of Health Grant RO1NS056226 0 01A2 “Identification of athletes at risk for traumatic brain injury”. We would like to thank Tracy Brewer for help with data collection, Kai Zhang for Matlab programming, and Elena Slobounov for VR programming.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adkin AL, Quant S, Maki BE, McIlroy WE. Cortical responses associated with predictable and unpredictable compensatory balance reactions. Experimental Brain Research. 2006;172:85–93. doi: 10.1007/s00221-005-0310-9. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed AA, Ashton-Miller JA. On use of a nominal internal model to detect a loss of balance in a maximal forward reach. Journal of Neurophysiology. 2007;97:2439–2447. doi: 10.1152/jn.00164.2006. [DOI] [PubMed] [Google Scholar]
- 3.Anguera JA, Seidler RD, Gehring WJ. Changes in performance monitoring during sensorimotor adaptation. Journal of Neurophysiology. 2009;102:1868–1879. doi: 10.1152/jn.00063.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Asten WNJC, Gielen CCAM, Denier van der Gon JJ. Postural adjustments induced by simulated motion of differently structures environments. Experimental Brain Research. 1998;73(2):371–383. doi: 10.1007/BF00248230. [DOI] [PubMed] [Google Scholar]
- 5.Dijkstra TM, Schoner G, Gielen CC. Temporal stability of the action-perception cycle for postural control in a moving visual environment. Experimental Brain Research. 1994;97(3):477–486. doi: 10.1007/BF00241542. [DOI] [PubMed] [Google Scholar]
- 6.Gramann K, Gwin JT, Ferris DP, Oie K, Jung TP, Lin CT, Liao LD, Makeig S. Cognition in action: imaging brain/body dynamics in mobile humans. Annual Review of Neuroscience. 2011;22:593–608. 448. doi: 10.1515/RNS.2011.047. [DOI] [PubMed] [Google Scholar]
- 7.Haibach P, Slobounov S, Newell K. The potential application of a virtual moving environment for assessing falls in the elderly adults. Gait and Posture. 2008;27(2):303–308. doi: 10.1016/j.gaitpost.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs JV, Horak FB. Cortical control of postural responses. Journal of Neural Transmission. 2007;114:1339–1348. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jancke L, Cheetham M, Baumgartner T. Virtual reality on the role of prefrontal cortex in adults and children. Frontiers Neuroscience. 2009;3(1):52–59. doi: 10.3389/neuro.01.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keshner E, Kenyon R. The influence of an Immersive Virtual Environment on the Segmental Organization of Postural Stabilizing Responses. Journal of Vestibular Research. 2000 Jul;:1–12. [PubMed] [Google Scholar]
- 11.Slobounov S, Fukada K, Simon R, Rearick M, Ray W. Neurophysiological and behavioral indices of time pressure effects on visuomotor task performance. Cognitive Brain Research. 2000;9(3):287–298. doi: 10.1016/s0926-6410(00)00009-4. [DOI] [PubMed] [Google Scholar]
- 12.Slobounov S, Hallett M, Newell K. Perceived effort in force production as reflected in motor-related cortical potentials. Clinical Neurophysiology. 2004;115(10):2391–2402. doi: 10.1016/j.clinph.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Slobounov S, Newell K, Slobounov E. Application of virtual reality graphics in assessment of concussion, Cyberpsychology. Behavior & Social Networking. 2006;9(2):188–191. doi: 10.1089/cpb.2006.9.188. [DOI] [PubMed] [Google Scholar]
- 14.Slobounov S, Hallett M, Wu T, Shibasaki H, Newell K. Neural underpinning of postural responses to visual field motion. Biological Psychology. 2006;72:188–197. doi: 10.1016/j.biopsycho.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Slobounov S, Cao C, Jaiswal N, Newell K. Predictor of postural instability relealed by VTC and EEG. Experimental Brain Research. 2006;191(1):1–16. doi: 10.1007/s00221-009-1956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slobounov S, Sebastianelli W, Newell K IEEE. Incorporating virtual reality graphics with brain imaging for assessment of sport-related concussions. Conference Proceedings IEEE Engineering and Medical Biology; 2011. pp. 1383–6. [DOI] [PubMed] [Google Scholar]
- 17.Smith B, Jacobs J, Horak F. Effects of magnitude and magnitude predictability of postural perturbations on preparatory cortical activity in older adults with and without Parkinson’s disease. Experimental Brain Research. 2012;222(4):455–70. doi: 10.1007/s00221-012-3232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]