Abstract
Context
The need for improved treatment options for patients with major depressive disorder (MDD) is critical. Faster-acting antidepressants and biomarkers that predict clinical response will facilitate treatment. Scopolamine produces rapid antidepressant effects and thus offers the opportunity to characterize potential biomarkers of treatment response within short periods.
Objective
To determine if baseline brain activity when processing emotional information can predict treatment response to scopolamine in MDD.
Design
A double-blind, placebo-controlled, crossover study together with repeated functional magnetic resonance imaging, acquired as participants performed face-identity and face-emotion working memory tasks.
Setting
National Institute of Mental Health Division of Intramural Research Programs.
Participants
Fifteen currently depressed outpatients meeting DSM-IV criteria for recurrent MDD and 21 healthy participants, between 18 and 55 years of age.
Main Outcome Measure
The magnitude of treatment response to scopolamine (percentage of change in the Montgomery-Asberg Depression Rating Scale score between study end and baseline) was correlated with blood oxygen level–dependent (BOLD) signal associated with each working memory component (encode, maintenance, and test) for both identity and emotion tasks. Treatment response also was correlated with change in BOLD response (scopolamine vs baseline). Baseline activity was compared between healthy and MDD groups.
Results
Baseline BOLD response in the bilateral middle occipital cortex, selectively during the stimulus-processing components of the emotion working memory task (no correlation during the identity task), correlated with treatment response magnitude. Change in BOLD response following scopolamine administration in overlapping areas in the middle occipital cortex while performing the same task conditions also correlated with clinical response. Healthy controls showed higher activity in the same visual regions than patients with MDD during baseline.
Conclusion
These results implicate cholinergic and visual processing dysfunction in the pathophysiology of MDD and suggest that neural response in the visual cortex, selectively to emotional stimuli, may provide a useful biomarker for identifying patients who will respond favorably to scopolamine.
Trial Registration
clinicaltrials.gov Identifier: NCT00055575
Traditional antidepressant treatments produce varied responses in patients with mood disorders, and often multiple trials with distinct antidepressant agents are necessary before an effective therapy is identified.1 Given that a sufficient trial requires several weeks of treatment for adequate assessment, patients often go for many weeks or longer before obtaining relief of their symptoms.2 Improving the current approach to treating patients with mood disorders is critical and will require both the identification of agents that have a more rapid clinical onset and the development of methods that predict treatment response prior to or early in treatment to attain a more rapid clinical response.
Efforts have been made to identify biomarkers that predict antidepressant treatment response. Measures associated with clinical symptoms or functional neuroimaging assessments have been considered as potential markers of response to conventional antidepressant treatments such as selective serotonin response inhibitors,3–6 cognitive behavior therapy,7 and transcranial magnetic stimulation,8 as well as more experimental approaches with a rapid onset of antidepressant action such as ketamine9,10 and scopolamine.11 The identification of biomarkers that accurately predict treatment response may prove particularly useful for guiding treatment decisions and hastening clinical response. Importantly, the use of antidepressant agents that produce a rapid clinical onset offers the potential for the assessment and identification of biomarkers that can predict treatment response prior to or early in treatment. In addition to predicting treatment response to specific or experimental agents, identified biomarkers carry the potential to be applicable to conventional treatments as well.
Interest in the role of the cholinergic system in mood disorders has resurfaced.12–16 Recently, we demonstrated12,13 that blocking cholinergic muscarinic receptors with scopolamine produces a rapid improvement (within 3 days) in depressive symptoms in a substantial proportion of currently depressed patients with major depressive disorder (MDD) or bipolar disorder, although not all patients showed a clinically significant response. Because conventional antidepressant agents require several weeks prior to the onset of antidepressant effects, the relatively rapid onset of antidepressant effects renders scopolamine an appealing candidate for studies designed to evaluate putative biomarkers of treatment response.
Working memory (WM) is a cognitive process that temporarily retains an active representation of information for further processing or recall17 and is critically linked to the cholinergic neurotransmitter system. Moreover, in healthy humans, muscarinic cholinergic receptor agonists enhance WM18 and antagonists impair WM,19 explicitly implicating the muscarinic receptor system in WM function. Results from functional brain imaging studies indicate that increasing cholinergic activity modulates neural response across brain regions that respond to WM20–23 but only visual processing areas consistently show increases in neural activity during cognitive tasks. The recruitment of classic WM prefrontal cortical regions is diminished significantly during cholinergic augmentation,21,24 and thus, improved WM performance is thought to be mediated at least partly via enhanced activity in visual cortices. These findings support the hypothesis that improvement in WM following cholinergic enhancement is mediated by influences on stimulus processing20–23,25 and may more generally suggest that cholinergic modulation influences cognitive functions through stimulus-processing mechanisms. Evidence also indicates that the relationship between cholinergic activity and cognitive performance is characterized by an inverted U function.26,27 These findings suggest that elevated cholinergic activity ultimately leads to impaired cognitive functioning, which also may occur through stimulus-processing mechanisms. Importantly, WM tasks have been shown to predict treatment response to the selective serotonin response inhibitor fluoxetine3 and to the N-methyl-D-aspartate receptor antagonist ketamine10 in patients with MDD. Thus, the evaluation of a WM task as a potential tool to identify biomarkers of treatment response offers a unique opportunity for use in conjunction with an antidepressant agent that targets cholinergic function and has a rapid onset of action.
Critically, the cholinergic system has extensive influence on neural activity, because the nucleus basalis sends widespread efferent cholinergic projections throughout the cortex,28 including to the posterior occipital cortices. While other neurotransmitters contribute to neural function in these regions,29,30 the cholinergic system in general and the cholinergic muscarinic receptors specifically play a key role in neural processing and stimulus-processing mechanisms in visual cortical regions.29
The most consistently reported cognitive feature observed in mood disorders is a mood-congruent processing bias, defined as a tendency or bias toward the processing of negative emotional information over positive or neutral information.31–34 Patients with MDD recall more negatively toned information over positively toned information in memory tasks,17 and depression-related negative words produce more interference than do happy or neutral words in attention tasks.10,33 This mood-congruent processing bias readily can be characterized within the framework of the cholinergic system and stimulus-processing mechanisms.25 Specifically, increased cholinergic muscarinic function in mood disorders may lead selectively to increased processing of stimuli-based emotional features and, in this way, contribute to the emotional processing bias observed in mood disorders. In healthy participants, the manipulation of the cholinergic system differentially modulates response to stimuli in visual processing areas of the brain based on the emotional content.35 The biased processing of negative or sad information in MDD is potentially consistent with a hyperstimulated muscarinic cholinergic system that results in the overrepresentation of specific types of emotional information.25 This framework would hypothesize that the presence of visual stimuli would engage the muscarinic cholinergic system, and the hyperresponsiveness of this system in MDD would bias stimulus processing preferentially based on specific emotional stimulus features. Thus, a WM task that uses emotional stimuli has the potential to be an effective tool to identify pre-treatment brain activity that reflects underlying muscarinic cholinergic dysfunction and that will predict anti-depressant response to a muscarinic cholinergic antagonist.
The goal of the current study was to evaluate the potential for neural activity during a WM task to predict antidepressant response to scopolamine administration. We expected that the putatively hyperresponsive muscarinic cholinergic system in MDD would be expressed in differential responses in stimulus-processing areas of the visual cortex, dependent on stimulus features (ie, emotional/nonemotional). Moreover, we predicted that the extent of cholinergic dysfunction would be reflected in baseline neural responses to stimuli and that the magnitude of dysfunction would predict treatment response to scopolamine.
METHODS
PARTICIPANTS
Twenty-one healthy participants (9 women, mean [SD] age=30.5 [8.5] years) and 15 participants with MDD, currently depressed without psychotic features (4 women, mean [SD] age=32.9 [7.8] years), were enrolled in this study; diagnosis was confirmed by the Structured Clinical Interview for DSM-IV Axis I Disorders36 and an unstructured interview conducted by a psychiatrist. The mean (SD) baseline Montgomery-Asberg Depression Rating Scale (MADRS) score was 30 (6.0) and 33% (5 of 15) had a current comorbid anxiety disorder (including combinations of simple phobia, social phobia, panic disorder, and posttraumatic stress disorder) that was secondary to the primary MDD diagnosis. All participants were evaluated at the National Institute of Mental Health outpatient clinic. Inclusion criteria for the depressed sample were the diagnosis of MDD, an MADRS score of at least 20, and a current major depressive episode of at least 4 weeks’ duration. Exclusion criteria included other Axis I disorders except anxiety disorders and a remote history of substance abuse (see later), exposure to psychotropic or other medications likely to affect central nervous system or cholinergic function within 3 weeks (8 weeks for fluoxetine), suicidal ideation suggestive of high suicide risk, current delusions or hallucinations, lifetime history of substance dependence or substance abuse within 1 year, medical or neurological disorders, abnormal electrocardiogram or blood pressure, narrow-angle glaucoma (based on ophthalmological examination of the anterior chambers for narrow angles), hypersensitivity to anticholinergic agents, hepatic dysfunction, electrolyte disturbance, human immunodeficiency virus or hepatitis viral infection, current nicotine use, weight more than 125 kg, or positive urine toxicology test results at screening. Patients were medication free and were not told to stop taking medication for the purpose of participating in this study. Pregnant or nursing women also were excluded. Healthy volunteers also were excluded if any first-degree relative had received a diagnosis for a major psychiatric disorder.
The study was approved by the Combined Neuroscience Institutional Review Board of the National Institutes of Health. All subjects provided written informed consent before entry into the study.
STUDY DESIGN
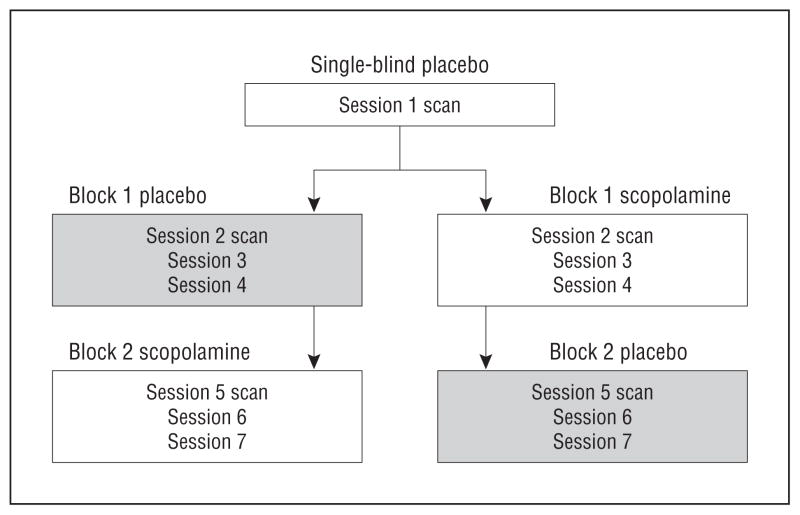
During each of 7 sessions, healthy subjects and subjects with MDD received a 15-minute intravenous infusion of either a placebo saline solution or 4.0 μg/kg of scopolamine. A single-blind lead-in session was used in which all subjects received a placebo infusion (to obtain baseline functional imaging data in all participants before administering scopolamine). Individuals subsequently were randomized into either a placebo/scopolamine or scopolamine/placebo double-blind, placebo-controlled, crossover design whereby placebo constituted a series of 3 placebo infusions and scopolamine comprised a series of 3 scopolamine infusions (Figure 1; for methodological details see Drevets and Furey,12 Furey and Drevets,13 and Furey et al37). Sessions were scheduled 3 to 5 days apart. Nonpregnancy was established prior to each session via laboratory testing. Randomization sequences were determined by the National Institutes of Health outpatient pharmacy and assigned by subject number at time of consent. To assess acute changes in mood, visual analog scale scores (components include happy, sad, drowsy, irritated, alert, anxious, and restless) were obtained at baseline and 20, 60, 120, and 150 minutes postinfusion, and the Profile of Mood States was administered at baseline and 20, 60, and 150 minutes postinfusion. Blood pressure and heart rate were obtained at baseline, at 15-minute intervals for 60 minutes postinfusion, and then at 30-minute intervals to the end of the study.
Figure 1.
Experimental design.
Immediately prior to each infusion, psychiatric interviews were conducted that included the MADRS.38 Follow-up interviews were obtained 3 to 5 days after the final infusion to provide the final clinical assessment and establish treatment response. Treatment response magnitude was calculated as a percentage of change at study end relative to the baseline MADRS score obtained in session 1 (because these measures were obtained just before baseline scanning). Patients also were characterized as achieving (1) full response (≥50% reduction in MADRS score from baseline) or (2) nonresponse (<50% reduction).39
FUNCTIONAL IMAGING
Functional magnetic resonance imaging scans were conducted during 3 sessions, including session 1 (following single-blind placebo to establish baseline) and during sessions 2 and 5 to ensure that data were acquired following scopolamine infusion. Following the infusion, a 45-minute waiting period followed (during which participants remained on the Day Hospital unit) to allow the peak cognitive effects to develop and the peak adverse effects (ie, drowsiness) to diminish.40 A 3-T General Electric scanner (GE Signa) and an 8-channel phased-array head coil were used to obtain an echo-planar imaging sequence for the blood oxygen level–dependent (BOLD) data (echo time=24 milliseconds; repetition time=2500 milliseconds; sagittal slices=35; voxel dimensions=3.75×3.75×3.5 mm; and 238 points per run) and a spoiled gradient echo sequence for the anatomical data (matrix=224×224; number of sagittal slices=128–140 [to obtain full brain coverage]; and slice thickness=1.2 mm). Four images were discarded from the beginning of each echo-planar imaging acquisition to allow for steady-state tissue magnetization.
WM TASK
On each of the 3 scanning days, participants performed a WM task (Figure 2). For each trial, participants were presented for 3 seconds with a picture of a face to encode, followed by a 9-second delay period, followed by the presentation for 3 seconds of a test face. Trials were separated by a 9-second inter-trial interval. Subjects were instructed in blocks of 8 trials to attend to either the identity or the emotional expression of the face during the encoding period and to indicate a match or non-match during the test period based only on the attended feature. For each block, each of 4 individual actors with 4 emotion expressions (happy, sad, fearful, and neutral) made up the 16-picture stimulus set.41 The same stimulus set was used within a scanner run for both the emotion and identity tasks (1 block of each task per run); unique stimulus sets were used in each of 6 scanner runs per session and over the 3 scanning sessions. The sequence of the attention condition was randomized based on subject number. Each run included 30-second baseline periods at the beginning and end of the run, as well as in between the 2 task conditions (ie, attend to emotion and attend to identity). Participants were instructed to focus on a cross-hair presented in the middle of the screen during baseline periods. Reaction time and accuracy data were collected.
Figure 2.

Working memory task. Participants were presented with a 3-second picture of a face to encode, followed by a 15-second delay/maintenance period, followed by a presentation of a 3-second picture of a test face. Trials were separated by a 15-second intertrial interval (ITI). Subjects were instructed to attend to either the identity or the emotional expression of the face during the encoding period and to indicate a match or nonmatch during the test period based only on the attended feature. Images from the Karolinska Directed Emotional Faces41 stimulus set were used to create the task; the models in the Figure include F06, F15, and F31.
IMAGING DATA ANALYSES
Echo-planar images were registered, smoothed, time-corrected, and normalized to the mean. AFNI42 was used to conduct multiple regression analysis to estimate BOLD response to each of the task components (encode, maintenance, and test) and for each of the 2 attention conditions (identity and emotion). Results of statistical analyses were spatially normalized to the stereotaxic array of Talairach and Tournoux.43
For the patient group, β estimates from each of the 6 task components as obtained during the baseline assessment in session 1 were correlated individually (ie, 6 separate correlations) with magnitude of treatment response (defined earlier) using AFNI software42 (3dRegAna), which applies a random-effects model. Voxelwise significance was defined as P<.005; significant brain regions were identified following whole-brain correction (WBC), requiring 16 or more contiguous voxels (P<.05) as defined by using AFNI software AlphaSim.
Task conditions that produced significant correlations between baseline BOLD measures and treatment response (percentage of change) were selected for further analysis. To evaluate the relation between baseline BOLD correlations, cholinergic mediation, and treatment response, a change in BOLD response (Δ) was calculated (BOLD response during scopolamine–BOLD response during single-blind placebo), and this calculated difference also was correlated with treatment response. Because the baseline correlations were observed in the occipital cortices (see the “Results” section), these analyses were restricted to the inferior, middle, and superior occipital cortical regions (using anatomically defined regions in AFNI). Voxel-level significance was defined as P<.05, and a small volume correction was applied, requiring 25 or more contiguous voxels (P<.05) as defined by using AFNI software AlphaSim.
A more exploratory post hoc analysis also was conducted to evaluate the specificity of correlations in the Δ BOLD response results to visual regions identified in the baseline BOLD response analysis. Thus, in this analysis, the results were not restricted to the occipital cortical regions. Voxel-level significance was defined at P<.05 and WBC was applied, requiring 93 or more contiguous voxels (P<.05) as defined by using AFNI software AlphaSim.
To compare baseline levels of activity in the MDD and control groups within regions found to correlate with treatment-responsive inpatients, baseline BOLD estimates from the brain regions and task conditions that produced correlations were compared. Region masks were created based on significant correlations (voxel-level P<.005; WBC P<.05) and baseline BOLD estimates were obtained for healthy and patient groups for task conditions that produced significant correlations. Group comparisons were performed using t tests to determine if differences in baseline levels of activity were present. Change in BOLD signal (BOLD response during scopolamine–BOLD response during placebo) also was assessed in healthy participants. The region masks (defined earlier) were used to obtain mean BOLD response during WM conditions under scopolamine administration and were compared with baseline placebo BOLD estimates using t tests to determine what scopolamine-induced changes in neural activity occurred in healthy participants.
PERFORMANCE DATA ANALYSIS
In the patient group, reaction time and performance accuracy were determined for each task condition (attend identity and attend emotion) and for placebo and drug conditions. Repeated-measures analysis of variance was used to evaluate task condition×drug effects (P<.05) and t tests were used to characterize significant interactions.
RESULTS
Overall, participants with MDD showed a significant reduction in symptoms of depression as indicated by a decrease in MADRS score from baseline to study end (t=7.9; P <.001). The magnitude of treatment response, reflected as the percentage of reduction from baseline, ranged from 10% to 99%, with a mean (SD) reduction of 63% (29). Of the 15 patients, 11 showed a clinical response following scopolamine administration (ie, a reduction in MADRS score of ≥50%) and 4 showed a non-response (a reduction in MADRS score of <50%). Of the 5 patients with comorbid anxiety disorder, 3 showed a clinical response. For those receiving scopolamine first, there was no worsening in depression severity between the end of the scopolamine block and the end of the study (P>.20). All patients successfully completed all 7 infusions and all 8 assessments. Depression severity did not differ for the 2 baseline assessments (P >.50) and the MADRS scores from the 2 baseline assessments correlated significantly (r=0.81; P=.001). One of the 15 patients was excluded poststudy when investigators learned of previously undisclosed current substance abuse; no data for this subject were included in the functional imaging or behavioral analyses. These clinical findings have been reported previously.12,37
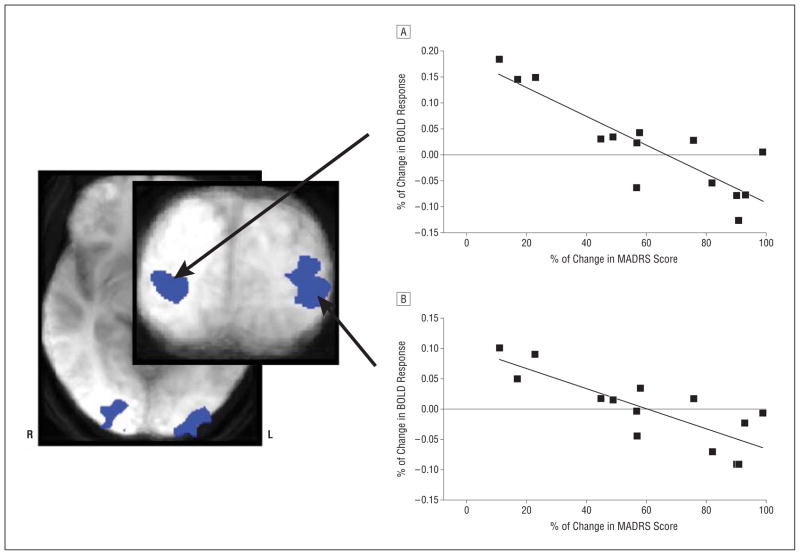
Treatment response magnitude correlated significantly with BOLD response in the bilateral middle occipital cortex during the encoding (right, r=−0.77; left, r = −0.85; Figure 3) and test (right, r = −0.81; left, r=−0.87; Figure 4) components of the WM task, selectively when attending to emotion. Coordinates of peak voxel effects are presented in the Table. These negative correlations show that larger reductions in MADRS scores are associated with lower neural activity in these brain regions during the processing of emotional information (encoding and test components), as measured at the pre-drug baseline assessment. The bilateral middle occipital cortical areas, identified separately in independent analyses based on the encoding and retrieval task components, showed a large extent of spatial overlap. No significant correlation between BOLD response and any component of the WM task was observed while attending to identity, nor during the maintenance component while attending to emotion (thus, no results for these analyses are presented). All participants (ie, excluding the patient identified with undisclosed substance abuse) were included in these analyses.
Figure 3.
Areas of the bilateral middle occipital cortex that show correlations (voxel P <.005; whole-brain correlation P <.05) between treatment response to scopolamine and baseline blood oxygen level–dependent (BOLD) response to the encoding component of a working memory task when attending to emotion are shown. The representative scatterplots and the best-fit lines for the right (A) and left (B) hemispheres are presented. MADRS indicates Montgomery-Asberg Depression Rating Scale.
Figure 4.
Areas of the bilateral middle occipital cortex that show correlations (voxel P<.005; whole-brain correlation P<.05) between treatment response to scopolamine and baseline blood oxygen level–dependent (BOLD) response to the test component of a working memory task when attending to emotion are shown. The representative scatterplots and best-fit lines for the right (A) and left (B) hemispheres are presented. MADRS indicates Montgomery-Asberg Depression Rating Scale.
Table 1.
Table Locations in the Middle Occipital Cortex That Show Peak Correlation Effects Between BOLD Response During a Working Memory Task and Antidepressant Response in Patients With Major Depressive Disorder
| Task Condition | Left Middle Occipital Cortex
|
Right Middle Occipital Cortex
|
||
|---|---|---|---|---|
| TA Coordinate (Voxel Counta) | t Value | TA Coordinate (Voxel Counta) | t Value | |
| Baseline | ||||
| Emotion encodingb | −23, −92, 3 (71)c | 4.1 | 26, −85, 3 (18)c | 3.7 |
| Emotion testb | −26, −95, 3 (49)c | 4.6 | 26, −92, −4 (21)c | 3.4 |
| Δ(Drug –placebo) | ||||
| Emotion encodingd | −23, −88, −1 (26) | 3.6 | NA | |
| Emotion testd | −29, −88, 4 (179)c | 3.7 | −24, −89, −3 (41) | 3.6 |
Abbreviations: BOLD, blood oxygen level dependent; NA, not applicable; TA, coordinates based on the Talairach and Tournoux Coplanar Stereotaxic Atlas of the Human Brain.43
The number of significant voxels is indicated in parenthesis.
Voxel-level significance P <.005.
Significant following whole-brain correction, P <.05.
Voxel-level significance P <.05; small volume correction P <.05.
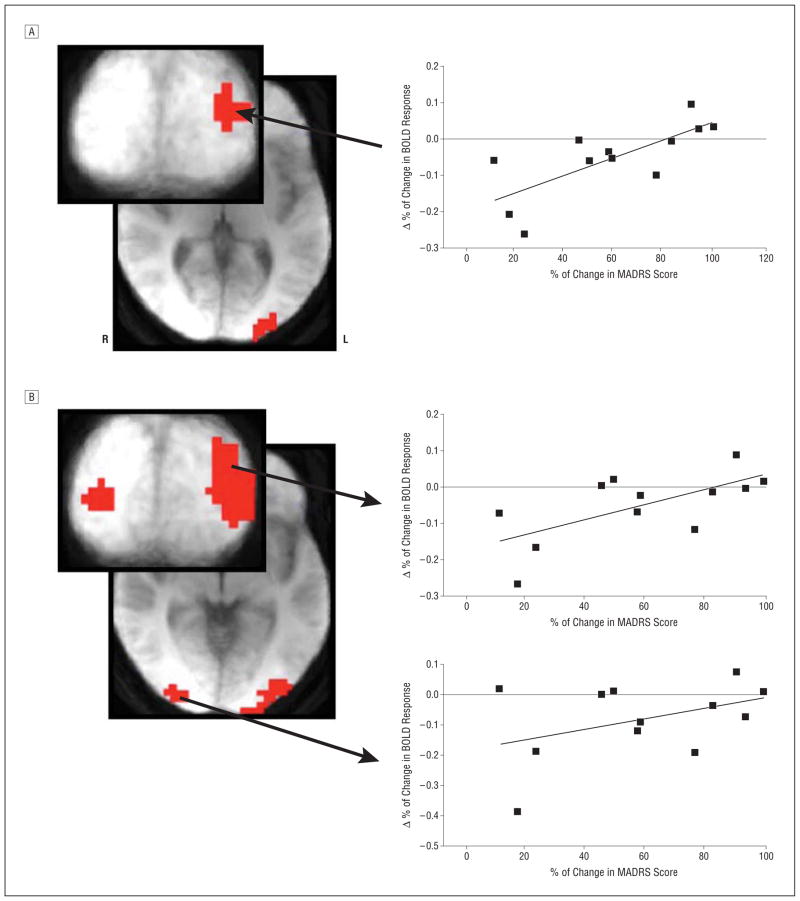
Because BOLD signal from the encoding and test components of the WM task when attending to emotion correlated with treatment response, the change in BOLD signal (drug –placebo) was calculated in patients for these 2 task conditions, and the result was correlated with treatment response magnitude. A positive correlation (small volume correction P <.05) between Δ BOLD response and treatment response was observed in the left middle occipital cortex for the encoding and test components of the emotion WM task (Figure 5) and in the right middle occipital cortex for the test component only. These correlations show that smaller reductions in MADRS score are associated with higher BOLD response in this area at baseline than during scopolamine administration. Conversely, larger reductions in MADRS score are associated with a larger BOLD response in this region during scopolamine administration than during baseline. No baseline correlation during the identify task was observed, and therefore, analyses assessing how changes in BOLD response following scopolamine administration correlate with treatment outcome are restricted to the emotion task condition. These analyses, examining correlations in the change in BOLD response, were restricted to 12 participants; the remaining 2 did not have useable echo-planar imaging data from the scopolamine session because of adverse effects (ie, sleepiness, as assessed by repeated nonresponse trials) and movement (as defined by movement that exceeded 1 voxel). (No region within the anterior cingulate cortex was identified in these analyses. See the eFigure [http://www.jamapsych.com] for post hoc region of interest analyses and results for the anterior cingulate cortex.)
Figure 5.
The area of the left middle occipital cortex where the change in blood oxygen level–dependent (BOLD) response following scopolamine administration (drug –placebo) correlates (voxel P <.05; small volume correction P <.05) with subsequent treatment response as measured during the encoding (A) and test (B) components of a working memory task while attending to emotion. The representative scatterplots and best-fit lines are shown. MADRS indicates Montgomery-Asberg Depression Rating Scale.
Post hoc whole-brain correlation between Δ BOLD response and treatment response identified areas of the middle occipital cortex that overlap with regions identified in the baseline correlations. In the encoding emotion condition, an overlapping area in the left middle occipital cortex was identified that did not reach the extent criteria for WBC (P > .05). Nonetheless, this was the largest region identified in this analysis, and the extent substantially overlapped spatially with the results of the independent analysis conducted with baseline data.
In the test emotion condition, an area in the left middle occipital cortex that overlapped substantially with the region identified in the baseline correlation was the only region to reach the extent criteria for WBC (P < .05), and thus, this independent whole-brain analysis highlighted the specificity of the Δ correlation to the same middle occipital brain region. An overlapping area also was identified in the right middle occipital cortex that did not reach the extent criteria for WBC. Coordinates of peak voxel effects are presented in the Table.
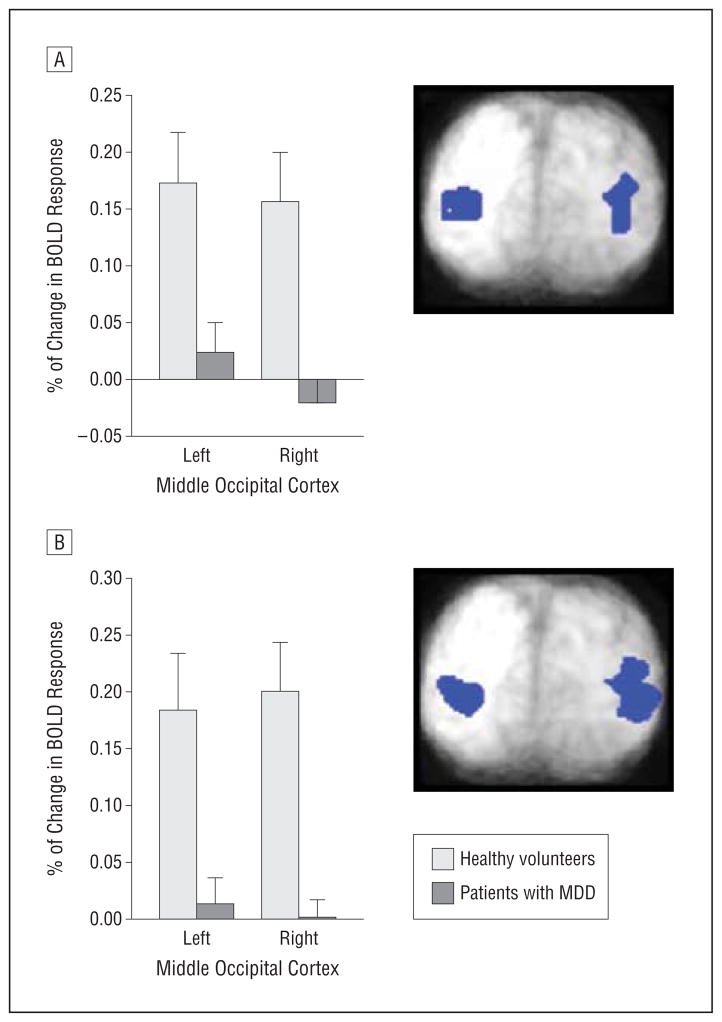
In the middle occipital cortical regions that showed correlations with treatment response in MDD, baseline BOLD activity was significantly higher in healthy participants than in patients with MDD during the encoding (left [t = 2.9; P < .01]) and right [t = 3.3; P < .01]) and test (left [t = 2.7; P < .01] and right [t = 3.7; P < .001]) components of the emotion WM condition (Figure 6). Similarly, the change in mean BOLD signal in healthy participants during the encode component of the emotion WM task following scopolamine administration (relative to placebo) decreased from baseline in the left (t = 1.9; P = .08) and right (t = 1.9; P = .08) middle occipital regions at trend level.
Figure 6.
Baseline blood oxygen level–dependent (BOLD) responses during the encoding (A) and test (B) components of a working memory task while attending to emotion are shown for healthy participants and patients with major depressive disorder (MDD) for the left and right middle occipital cortex. Regions were defined from correlation analyses. The error bars reflect standard error.
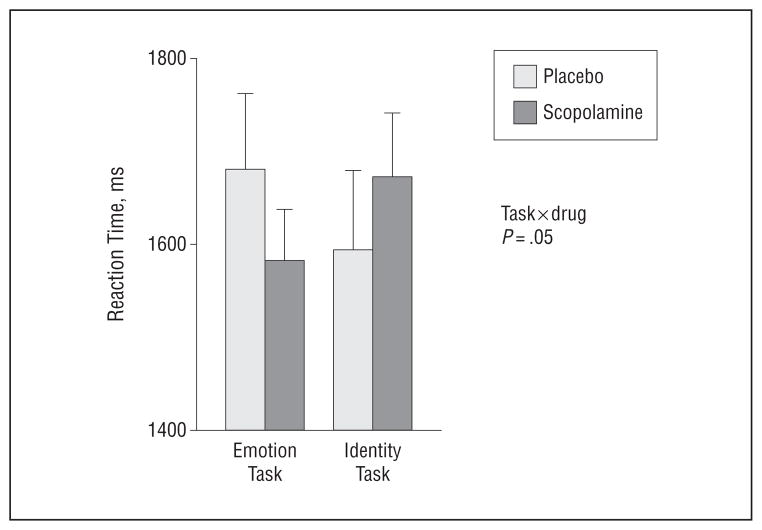
Performance data showed a task × drug interaction (Figure 7) (P = .05). At baseline, patients were relatively faster during the identity task relative to the emotion task, and this relative difference reversed during scopolamine. Thus, patients were relatively faster during the emotion task following scopolamine administration as compared with placebo.
Figure 7.
Mean reaction times when performing the emotion and identity working memory tasks are shown for patients as measured during placebo and scopolamine administration. The error bars reflect standard error of the mean.
COMMENT
These data demonstrate that levels of neural activity in visual processing areas that respond to specific stimulus features potentially provide a biomarker for antidepressant response to the antimuscarinic agent scopolamine. Levels of response in the bilateral middle occipital cortex during the processing of emotional information correlate with subsequent treatment response to scopolamine. Thus, neural activity in visual processing areas may reflect the potential for response to treatment with scopolamine. Importantly, this effect was specific to the WM condition when participants were attending to emotional features of the stimuli. When viewing the same stimuli but attending to face identity and ignoring emotional information, no such correlation occurred. Thus, the magnitude of neural response in the middle occipital cortex selectively to emotional features of face stimuli may provide a biomarker that predicts response to anti-depressant treatment with scopolamine.
The change in neural activity in the same region of the left middle occipital cortex during stimulus-processing components (encode and test) of the emotion WM task (as identified in the region of interest analysis) following short-term scopolamine administration (relative to baseline activity) also correlated with treatment response. Importantly, in the whole-brain analyses, the middle occipital cortex was identified independently and showed substantial overlap with the brain area showing predictive value at baseline. While the region identified in the encoding component did not reach the extent criteria for WBC, the area did overlie the region identified in the baseline condition. Critically, the area identified in the whole-brain analysis of the test component did reach WBC and overlapped substantially with the region identified in the baseline analysis. This finding demonstrates that the cortical areas in the occipital cortex that show baseline predictive value for treatment response also respond to cholinergic modulation, which argues that baseline differences are cholinergically mediated. Moreover, the region responds to cholinergic modulation to an extent that reflects treatment response magnitude, perhaps suggesting that at baseline this brain region retains a level of cholinergic dysfunction when processing emotional stimuli that reflects the potential for subsequent treatment response to scopolamine. Importantly, these effects are seen acutely following scopolamine administration and thus precede the onset of antidepressant effects.
These regions in the middle occipital cortex where BOLD activity shows correlations with treatment response in MDD also differ from healthy volunteers at baseline with respect to hemodynamic activity. Control participants had higher levels of BOLD activity bilaterally in the middle occipital cortex than patients with MDD during stimulus-processing components of the emotion WM task. Importantly, based on the correlation patterns, the data indicate that patients showing the lowest levels of activity in these areas, and who thus were the most dissimilar to healthy participants, also were the patients who subsequently showed the greatest treatment response (Figure 3 and Figure 4). The patients who did not respond to scopolamine (ie, <50% improvement in MADRS score) were the patients whose baseline activity levels appeared similar to/overlapped with activity levels observed in healthy participants. The level of activity in these brain regions at baseline conceivably may reflect the extent of cholinergic dysfunction, and the extent of underlying cholinergic dysfunction may in turn predict subsequent treatment response. If so, then perhaps the patients who show BOLD response of the same magnitude as healthy participants do not evince cholinergic dysfunction in these brain regions and subsequently show little or no response to scopolamine. Finally, patients with the lowest levels of activity at baseline and who subsequently showed a treatment response also expressed the largest increase in neural activity in this region following short-term scopolamine administration, and thus, following drug administration, the BOLD response in the middle occipital visual cortex when processing emotion information in these patients became more similar to healthy participants.
Similarly, healthy volunteers showed a trend toward a reduction in neural activity following scopolamine (vs placebo). Again, the patients who did not show a clinical response tended to show decreases in BOLD response following short-term scopolamine administration while those patients who did experience a clinical response tended to show increases in BOLD signal. Together with the baseline data described earlier, these findings again suggest that the patients who show similar patterns in BOLD response to healthy participants do not have evidence of cholinergic dysfunction in these brain regions and thus show little or no clinical response.
The behavioral data also showed that the effects of cholinergic modulation were dependent on attended stimulus features. Although patients viewed the same stimuli under 2 different attention conditions, the effects of scopolamine on reaction time differ when attending to identity and emotion, thus highlighting the stimulus specificity of the scopolamine effects. Importantly, patients tended to respond more quickly when attending to emotional features following scopolamine administration than following placebo administration, indicating that scopolamine was not impairing attention at the dose being used in this study.
Our findings also contribute to the understanding of emotional processing biases in mood disorders, which have been well characterized in the literature.31–34 Patients with mood disorders experience stimulus-processing biases that are driven by the emotional content of stimuli, as has been shown behaviorally32,33 and by functional brain imaging methods,32,34 and these biases reverse following treatment.34 Moreover, healthy individuals show differential levels of activity in visual processing areas (such as the middle occipital cortex) based on the emotional content of stimuli,35,44 and scopolamine has been shown to alter emotion recognition behaviorally.45,46 Visual cortical regions recently have been implicated in mood disorders,47–49 and the link provided herein between cholinergic dysfunction, stimulus-processing biases, and differential response to emotional stimuli in visual areas may provide insight into the underlying nature of altered visual cortical responses in mood disorders.
Some might expect that cholinergic interactions with WM would be reflected in prefrontal brain regions. Previous work evaluating the impact of cholinergic enhancement on the functional brain response to a face WM task identified stimulus-specific increases in neural response in visual cortical areas.21,22,50 In these studies, the dorso-lateral prefrontal cortex, a classic WM region, showed large decreases in activity during the task. Together these findings indicated that WM was modulated via the processing of stimuli in the visual cortex and suggested that improved visual percepts of task-related stimuli rendered the task easier and reduced the need for prefrontal cortical input.21,22,50 These earlier studies would predict that the role of cholinergic function in WM would be evident in visual cortical regions.
The impact of repeated scanning sessions on BOLD estimates while using the same WM task merits consideration. The primary analysis depends only on the data acquired in the first session, and thus, multiple-session practice effects or reproducibility concerns do not apply. Nonetheless, the possibility that practice effects influence BOLD measures obtained from the postscopolamine scan session does exist. The literature regarding the impact of repeated tasks over multiple functional magnetic resonance imaging sessions is complex, indicating that the BOLD response in some brain regions remains unchanged, while in other regions the BOLD activity either increases or decreases across sessions.51–53 Reproducibility of activation in the visual cortex specifically54,55 is reportedly relatively good, with between 75% and 90% overlap in significant voxels. Evidence does indicate that repeated presentations of the same stimuli result in the suppression of BOLD signal,56 and thus, we used unique stimuli in each scanning session. Importantly, in the current study, BOLD signal did not change in a manner that was systematic relative to repeated sessions; instead some patients showed BOLD signal increases and others showed BOLD signal decreases in later sessions relative to session 1, and these changes were related to treatment response.
A potential limitation to these findings is related to the fact that cholinergic activity has direct effects on the vasculature57; thus, the possibility exists that scopolamine may have a direct effect on vessels and an indirect effect on the BOLD signal. The effect of scopolamine on regional cerebral blood flow has been evaluated previously using the xenon Xe 133 inhalation technique.57,58 These studies demonstrated that the intravenous administration of scopolamine at a dose of 6.1 μg/kg produced cognitive effects, but no change in regional cerebral blood flow was observed. At a higher dose of 7.3 μg/kg, regional cerebral blood flow effects were observed but were primarily restricted to prefrontal cortical areas. Thus, we do not anticipate that the dose of scopolamine used in the current study (4 μg/kg) alters regional cerebral blood flow, especially in the posterior occipital regions where our effects are observed.
The need to improve current methods of treatment selection for individual patients is clear, and the identification of biomarkers of response has the potential to do so. Recently, functional brain imaging methods have been used in an effort to discriminate between treatment responders and nonresponders.3,10 Such studies have begun to characterize pretreatment, brain-based differences among patients that reflect subsequent treatment response. Moreover, the results of these studies would suggest that biological variables underlie the clinical variability associated with treatment response, further supporting the concept of a more personalized approach to treatment for patients with mood disorders. Our results identify selective baseline brain responses during the processing of emotional stimuli that reflect the subsequent potential to respond clinically to treatment with scopolamine. Further, given the lack of predictive power associated with the WM identity task, our results demonstrate that brain function per se is not sufficient to predict treatment response but rather task- and/or stimulus-specific neural activity is necessary to successfully predict treatment outcome. Whether this approach also will successfully predict outcome following other treatments remains an empirical question.
Continued exploration of the antidepressant effects of cholinergic modulators, like scopolamine, may ultimately lead to the development of new treatments for MDD. The results presented herein strongly implicate cholinergic and visual processing dysfunction in the pathophysiology of MDD and support the idea that neural response in visual cortical areas to emotional stimuli might be a useful biomarker for identifying a subgroup of patients who will respond favorably to scopolamine.
Supplementary Material
Acknowledgments
Funding/Support: This research was supported by the National Institutes of Health National Institute of Mental Health Division of Intramural Research Programs.
Footnotes
Conflict of Interest Disclosures: The National Institute of Mental Health has filed a use patent for the use of scopolamine in the treatment of depression, and Drs Furey and Drevets are identified as coinventors on this pending patent application in the United States and an existing patent in Europe. Dr Zarate is listed as a coinventor on a patent application for the use of ketamine and its metabolites in major depression. Dr Zarate has assigned his rights in the patent to the US government but will share a percentage of any royalties that may be received by the government.
Online-Only Material: The eFigure is available at http://www.jamapsych.com.
Additional Contributions: We thank Michele Drevets, RN, and Joan Williams, RN, MSW, for patient recruitment and evaluation, Paul Carlson, Alan Mallinger, and the 5SW Day Hospital nursing staff for medical support.
References
- 1.Simon GE, Perlis RH. Personalized medicine for depression: can we match patients with treatments? Am J Psychiatry. 2010;167(12):1445–1455. doi: 10.1176/appi.ajp.2010.09111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Insel TR, Wang PS. The STAR*D trial: revealing the need for better treatments. Psychiatr Serv. 2009;60(11):1466–1467. doi: 10.1176/ps.2009.60.11.1466. [DOI] [PubMed] [Google Scholar]
- 3.Korb AS, Hunter AM, Cook IA, Leuchter AF. Rostral anterior cingulate cortex theta current density and response to antidepressants and placebo in major depression. Clin Neurophysiol. 2009;120(7):1313–1319. doi: 10.1016/j.clinph.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee TW, Wu YT, Yu YW, Chen MC, Chen TJ. The implication of functional connectivity strength in predicting treatment response of major depressive disorder: a resting EEG study. Psychiatry Res. 2011;194(3):372–377. doi: 10.1016/j.pscychresns.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 5.MacQueen GM. Magnetic resonance imaging and prediction of outcome in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34(5):343–349. [PMC free article] [PubMed] [Google Scholar]
- 6.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36(1):183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry. 2006;163(4):735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- 8.Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am J Psychiatry. 2003;160(5):835–845. doi: 10.1176/appi.ajp.160.5.835. [DOI] [PubMed] [Google Scholar]
- 9.Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Jr, Manji HK. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65(4):289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, Holroyd T, DiazGranados N, Machado-Vieira R, Grillon C, Drevets WC, Zarate CA., Jr Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35(7):1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furey ML, Nugent AC, Speer AM, Luckenbaugh DA, Hoffman EM, Frankel E, Drevets WC, Zarate CA., Jr Baseline mood-state measures as predictors of antidepressant response to scopolamine. Psychiatry Res. 2012;196(1):62–67. doi: 10.1016/j.psychres.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67(5):432–438. doi: 10.1016/j.biopsych.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63(10):1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janowsky DS, el-Yousef MK, Davis JM, Hubbard B, Sekerke HJ. Cholinergic reversal of manic symptoms. Lancet. 1972;1(7762):1236–1237. doi: 10.1016/s0140-6736(72)90956-7. [DOI] [PubMed] [Google Scholar]
- 15.Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- 16.Janowsky DS, Overstreet DH, Nurnberger JI., Jr Is cholinergic sensitivity a genetic marker for the affective disorders? Am J Med Genet. 1994;54(4):335–344. doi: 10.1002/ajmg.1320540412. [DOI] [PubMed] [Google Scholar]
- 17.Bradley BP, Mogg K, Williams R. Implicit and explicit memory for emotion-congruent information in clinical depression and anxiety. Behav Res Ther. 1995;33(7):755–770. doi: 10.1016/0005-7967(95)00029-w. [DOI] [PubMed] [Google Scholar]
- 18.Ragozzino ME, Artis S, Singh A, Twose TM, Beck JE, Messer WS., Jr The selective M1 muscarinic cholinergic agonist CDD-0102A enhances working memory and cognitive flexibility. J Pharmacol Exp Ther. 2012;340(3):588–594. doi: 10.1124/jpet.111.187625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou X, Qi XL, Douglas K, Palaninathan K, Kang HS, Buccafusco JJ, Blake DT, Constantinidis C. Cholinergic modulation of working memory activity in primate prefrontal cortex. J Neurophysiol. 2011;106(5):2180–2188. doi: 10.1152/jn.00148.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furey ML, Pietrini P, Alexander GE, Schapiro MB, Horwitz B. Cholinergic enhancement improves performance on working memory by modulating the functional activity in distinct brain regions: a positron emission tomography regional cerebral blood flow study in healthy humans. Brain Res Bull. 2000;51(3):213–218. doi: 10.1016/s0361-9230(99)00219-1. [DOI] [PubMed] [Google Scholar]
- 21.Furey ML, Pietrini P, Haxby JV. Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science. 2000;290 (5500):2315–2319. doi: 10.1126/science.290.5500.2315. [DOI] [PubMed] [Google Scholar]
- 22.Furey ML, Ricciardi E, Schapiro MB, Rapoport SI, Pietrini P. Cholinergic enhancement eliminates modulation of neural activity by task difficulty in the prefrontal cortex during working memory. J Cogn Neurosci. 2008;20(7):1342–1353. doi: 10.1162/jocn.2008.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bentley P, Husain M, Dolan RJ. Effects of cholinergic enhancement on visual stimulation, spatial attention, and spatial working memory. Neuron. 2004;41(6):969–982. doi: 10.1016/s0896-6273(04)00145-x. [DOI] [PubMed] [Google Scholar]
- 24.Furey ML, Pietrini P, Haxby JV, Alexander GE, Lee HC, VanMeter J, Grady CL, Shetty U, Rapoport SI, Schapiro MB, Freo U. Cholinergic stimulation alters performance and task-specific regional cerebral blood flow during working memory. Proc Natl Acad Sci U S A. 1997;94(12):6512–6516. doi: 10.1073/pnas.94.12.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furey ML. The prominent role of stimulus processing: cholinergic function and dysfunction in cognition. Curr Opin Neurol. 2011;24(4):364–370. doi: 10.1097/WCO.0b013e328348bda5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braida D, Paladini E, Griffini P, Lamperti M, Maggi A, Sala M. An inverted U-shaped curve for heptylphysostigmine on radial maze performance in rats: comparison with other cholinesterase inhibitors. Eur J Pharmacol. 1996;302(1–3):13–20. doi: 10.1016/0014-2999(96)00072-6. [DOI] [PubMed] [Google Scholar]
- 27.Canal N, Imbimbo BP Eptastigmine Study Group. Relationship between pharmacodynamic activity and cognitive effects of eptastigmine in patients with Alzheimer’s disease. Clin Pharmacol Ther. 1996;60(2):218–228. doi: 10.1016/S0009-9236(96)90138-1. [DOI] [PubMed] [Google Scholar]
- 28.Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev. 2005;48(1):98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Origlia N, Kuczewski N, Pesavento E, Aztiria E, Domenici L. The role of cholinergic system in neuronal plasticity: focus on visual cortex and muscarinic receptors. Arch Ital Biol. 2008;146(3–4):165–188. [PubMed] [Google Scholar]
- 30.Sannita WG. Electrophysiology of the visual system: from neuroscience to human neuropharmacology. Neuropsychobiology. 1995;32(4):208–213. doi: 10.1159/000119237. [DOI] [PubMed] [Google Scholar]
- 31.Roiser JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology. 2012;37(1):117–136. doi: 10.1038/npp.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Neuroreport. 2000;11(8):1739–1744. doi: 10.1097/00001756-200006050-00028. [DOI] [PubMed] [Google Scholar]
- 33.Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29(6):1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- 34.Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry. 2010;67(11):1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bentley P, Vuilleumier P, Thiel CM, Driver J, Dolan RJ. Cholinergic enhancement modulates neural correlates of selective attention and emotional processing. Neuroimage. 2003;20(1):58–70. doi: 10.1016/s1053-8119(03)00302-1. [DOI] [PubMed] [Google Scholar]
- 36.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 37.Furey ML, Khanna A, Hoffman EM, Drevets WC. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479–2488. doi: 10.1038/npp.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan A, Khan SR, Shankles EB, Polissar NL. Relative sensitivity of the Montgomery-Asberg Depression Rating Scale, the Hamilton Depression Rating Scale and the Clinical Global Impressions Rating Scale in antidepressant clinical trials. Int Clin Psychopharmacol. 2002;17(6):281–285. doi: 10.1097/00004850-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Nierenberg AA, DeCecco LM. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):5–9. [PubMed] [Google Scholar]
- 40.Safer DJ, Allen RP. The central effects of scopolamine in man. Biol Psychiatry. 1971;3(4):347–355. [PubMed] [Google Scholar]
- 41.Lundqvist D, Flykt A, Öhman A. The Karolinska Directed Emotional Faces—KDEF, CD ROM. Stockholm, Sweden: Department of Clinical Neuroscience, Psychology Section, Karolinska Institutet; 1998. [Google Scholar]
- 42.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 43.Talairach J, Tournoux P. Coplanar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme Medical Publisher; 1998. [Google Scholar]
- 44.Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45(1):174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Kamboj SK, Curran HV. Scopolamine induces impairments in the recognition of human facial expressions of anger and disgust. Psychopharmacology (Berl) 2006;185(4):529–535. doi: 10.1007/s00213-006-0332-4. [DOI] [PubMed] [Google Scholar]
- 46.Kamboj SK, Curran HV. Neutral and emotional episodic memory: global impairment after lorazepam or scopolamine. Psychopharmacology (Berl) 2006;188 (4):482–488. doi: 10.1007/s00213-006-0552-7. [DOI] [PubMed] [Google Scholar]
- 47.Keedwell PA, Drapier D, Surguladze S, Giampietro V, Brammer M, Phillips M. Subgenual cingulate and visual cortex responses to sad faces predict clinical outcome during antidepressant treatment for depression. J Affect Disord. 2010;120(1–3):120–125. doi: 10.1016/j.jad.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 48.Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57(3):201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 49.Surguladze SA, El-Hage W, Dalgleish T, Radua J, Gohier B, Phillips ML. Depression is associated with increased sensitivity to signals of disgust: a functional magnetic resonance imaging study. J Psychiatr Res. 2010;44(14):894–902. doi: 10.1016/j.jpsychires.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ricciardi E, Pietrini P, Schapiro MB, Rapoport SI, Furey ML. Cholinergic modulation of visual working memory during aging: a parametric PET study. Brain Res Bull. 2009;79(5):322–332. doi: 10.1016/j.brainresbull.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin Y, Sohn MH, Anderson JR, Stenger VA, Fissell K, Goode A, Carter CS. Predicting the practice effects on the blood oxygenation level-dependent (BOLD) function of fMRI in a symbolic manipulation task. Proc Natl Acad Sci U S A. 2003;100(8):4951–4956. doi: 10.1073/pnas.0431053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tegeler C, Strother SC, Anderson JR, Kim SG. Reproducibility of BOLD-based functional MRI obtained at 4 T. Hum Brain Mapp. 1999;7(4):267–283. doi: 10.1002/(SICI)1097-0193(1999)7:4<267::AID-HBM5>3.0.CO;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirschen MP, Chen SH, Schraedley-Desmond P, Desmond JE. Load- and practice-dependent increases in cerebro-cerebellar activation in verbal working memory: an fMRI study. Neuroimage. 2005;24(2):462–472. doi: 10.1016/j.neuroimage.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 54.Miki A, Raz J, Englander SA, Butler NS, van Erp TG, Haselgrove JC, Liu GT. Reproducibility of visual activation in functional magnetic resonance imaging at very high field strength (4 Tesla) Jpn J Ophthalmol. 2001;45(1):1–4. doi: 10.1016/s0021-5155(00)00304-x. [DOI] [PubMed] [Google Scholar]
- 55.Rombouts SA, Barkhof F, Hoogenraad FG, Sprenger M, Scheltens P. Within-subject reproducibility of visual activation patterns with functional magnetic resonance imaging using multislice echo planar imaging. Magn Reson Imaging. 1998;16(2):105–113. doi: 10.1016/s0730-725x(97)00253-1. [DOI] [PubMed] [Google Scholar]
- 56.Weigelt S, Muckli L, Kohler A. Functional magnetic resonance adaptation in visual neuroscience. Rev Neurosci. 2008;19(4–5):363–380. doi: 10.1515/revneuro.2008.19.4-5.363. [DOI] [PubMed] [Google Scholar]
- 57.Hamel E. Cholinergic modulation of the cortical microvascular bed. Prog Brain Res. 2004;145:171–178. doi: 10.1016/S0079-6123(03)45012-7. [DOI] [PubMed] [Google Scholar]
- 58.Honer WG, Prohovnik I, Smith G, Lucas LR. Scopolamine reduces frontal cortex perfusion. J Cereb Blood Flow Metab. 1988;8(5):635–641. doi: 10.1038/jcbfm.1988.110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.