Abstract
The niche in which stem cells reside and differentiate is a complex physico-chemical microenvironment that regulates cell function. The role played by three-dimensional physical contours was studied on cell progeny derived from mouse embryonic stem cells using microtopographies created on PDMS membranes. While markers of differentiation were not affected, the proliferation of heterogeneous mouse embryonic stem cell-derived progeny was attenuated by 15 μm-, but not 5 μm-high microprojections. This reduction was reversed by Rho kinase and myosin light chain kinase inhibition, which diminishes the tension generating ability of stress fibers. Purified cardiomyocytes derived from embryonic stem cells also showed significant blunting of proliferation and increased beating rates compared to cells grown on flat substrates. Thus, proliferation of stem cell-derived progeny appears to be regulated by microtopography through tension-generation of contractility in the third-dimension. These results emphasize the importance of topographic cues in the modulation of stem cell progeny behavior.
Introduction
The microenvironment of a developing embryo has a three-dimensional surface topography and an abundance of extracellular matrix proteins (Timpl 1996) that alter the phenotype and function of developing cells (Scadden 2006). Cells, for example, require internal contractility rather than adhesivity to sort according to tension, with lower for endoderm and higher for mesoderm (Krieg et al. 2008). In general, physical effects of the local niche microenvironment are less well understood than the effects of soluble, molecular factors on cell growth, differentiation and proliferation. Improved understanding of the complex physico-chemical niche thus is needed to harness the potential of stem cells and their derivatives for regenerative medicine (Watt and Hogan 2000, Forouhar et al. 2006).
Physical links by cells from the extracellular matrix and neighboring cells coordinate cell growth, differentiation and apoptosis and involve the intracellular mechanics of the cytoskeleton (Ingber 2006, Engler et al. 2009). The rigidity of the substrate alone influences proliferation and migration of epithelial cells (Saez et al. 2007) and the fates of multipotent stem cells (Engler et al. 2006). Cells sense the environment through force transmission via transmembrane integrins in the focal adhesions attached to the substrate that trigger a plethora of intracellular signaling pathways remodeling of the interior cytoskeleton (Chen et al. 2004). Thus, tensional forces within cells are potent regulators of contractile stress cable assembly in many cells and specialized myofibrils in muscle (Samarel 2005).
Topography aligns or guides a variety of cell types, including endothelial cells, epithelial cells, fibroblasts, oligodendrocytes and astrocytes (Bettinger et al. 2006, Cheng and LeDuc 2006). Surface microtopography has significant effects on behavior of neonatal and adult cells (Motlagh et al. 2003a, Boateng et al. 2003, Thakar et al. 2008). Physical constraints created by microwells control stem cell growth and homogeneity (Karp et al. 2007). Even topographies in the nanometer scale affect cell behavior such as decreased proliferation of smooth muscle cells (Yim et al. 2005) and contact guidance of human embryonic stem (ES) cells altering cell shape (Gerecht et al. 2007).
ES cells from mouse and human have an almost unlimited capacity to proliferate in vitro and can give rise to many cell types (Wobus and Boheler 2005). In the undifferentiated state, ES cells do not seem to be subject to physical cues, as these cells are not contact inhibited in vitro (Gammill and Bronner-Fraser 2002). Loss of self-renewal, activation of differentiation, and lineage commitment are however associated with adhesivity, a decrease in pluripotency, up-regulation of differentiation markers, and important physiological changes that include an increased potential for cell death and checkpoint-apoptosis coupling (Yamanaka et al. 2008a). Since the niche environment can affect differentiation, we hypothesize that ES cells differentiating in vitro sense physical cues that have the potential to alter their physiological status.
The work presented here investigates the effect of microprojections selected to be in the micron size range found in the cells and tissues of the developing embryo. The data demonstrate that the local physical microenvironment regulates proliferation and cell function of mouse ES cell progeny and one of its lineages, the cardiomyocyte, through the role played by cell contractility.
RESULTS
Response of heterogeneous mES cell progeny near microprojections
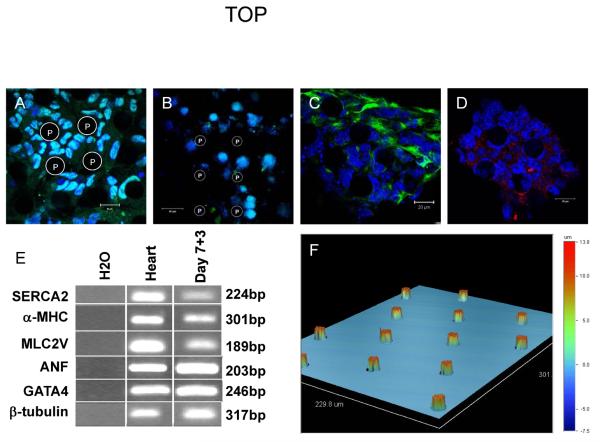
R1 and syNP4 embryonic stem cells were differentiated using a hanging drop technique to allow formation of embryoid bodies (EBs) and generation of cardiomyocytes. Following the initial two day aggregation step, EBs were transferred to suspension culture for 5 days, followed by plating on gelatin coated dishes for four days. Plated EBs generally displayed spontaneously contracting areas within one to two days of plating; however, the majority of cells were not cardiomyocytes. This heterogeneity could be demonstrated through analysis of RNA and cellular proteins. By immunostaining, Oct-4- (pluripotency marker), SSEA-1-(pluripotency and early differentiation marker), nestin- (ectoderm marker) and Brachyury-(mesoderm marker) positive cells were identified in these mixed cultures (Figure 1A–D), and when plated on textured PDMS membranes with the microprojections, shown by profilometry (Figure 1F). By RT-PCR 3–4 days after plating and as previously reported, Brachyury, the skeletal muscle specific marker MyoD, and Sox17 (pan-endoderm marker) were detectable at this time point, but transcripts encoding the neuroectoderm-specific marker Sox1 were not observed (Yamanaka et al, 2008b). Transcripts encoding cardiac-associated proteins (Gata4, atrial natriuretic factor, α-myosin heavy chain, myosin light chain (Mlc) 2a and Mlc2v), and sarcoplasmic reticulum calcium ATPase type 2 and β-tubulin (non-specific cell markers) were also relatively abundant in this non-selected cell populations (Figure 1E). These findings demonstrate that these cultures consist of a highly heterogeneous mix of cells from multiple lineages, including cardiomyocytes, and in various stages of differentiation.
Figure 1. Heterogeneity of unselected ES cell progeny are unaffected by microprojections.
Confocal images (A–D) and RT-PCR (E) showing pluripotentcy and distinctive differentiation lineage markers. Immunochemistry showing pluripotent (A) Oct-4 (green) and (B) SSEA-1 (green) expression, as well as differentiation markers (C) nestin (green) and (D) brachyury (red) on PDMS membranes with microprojections. (E) RT-PCR analysis of selected transcripts in embryoid bodies three days after plating. (F) Optical profilometry of microprojections. (A–D) Nuclei are blue. (A,C,D) Scale bar 20 μm, (B) scale bar 50 μm. (A,C,D) T35-15 PDMS, (B) T75-15 PDMS. P, microprojections.
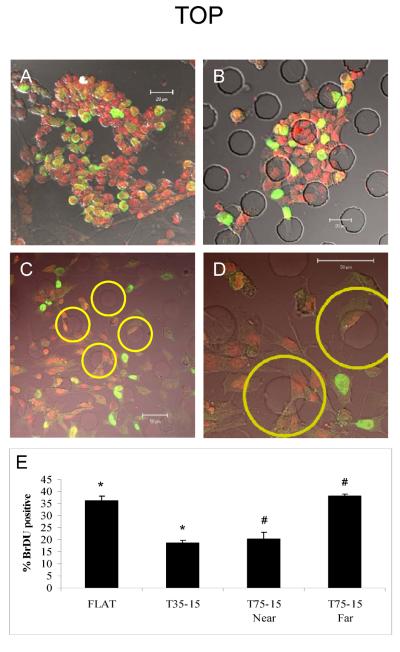
Four days after plating, mixed cell populations were dissociated and transferred to either flat or textured elastomeric membranes. The overall appearance of disassociated mES progeny plated on the 15μm high microtopographies with center-to-center spacing of 35μm (T35-15) or 75μm (T75-15) was similar in all cases. Although diverse, individual living cells were similar in shape and size under all conditions when viewed by phase microscopy; however, an apparent difference in cell density was observed. Although this might be attributable to altered cell adherence, apoptosis or proliferation, we focused on proliferation because of previous publications showing that microtextures could decrease proliferation (Boateng et al. 2003). As an indirect measure of cell proliferation, cells were incubated for one hour with the thymidine analog BrdU (or EdU), which is only incorporated during DNA replication. Following propidium iodide counterstaining, cells that incorporated BrdU were green, while unlabeled cells were red (Figure 2). Using this assay, cells showed marked differences in BrdU incorporation that depended on the texture. BrdU incorporation was significantly (p < 0.005) more abundant in cells cultivated on flat surfaces (Figure 2A) than those cultured on 15μm high microtextured (35μm apart) surfaces (Figure 2B).
Figure 2. Attenuation of mES cell-derived progeny proliferation by 15 μm microprojections.
Merged confocal and phase images of BrdU incorporation in cells on flat (A) or topographied (B–D) PDMS membranes. (A) Flat, no microprojections; (B) T35-15, all cells near a microprojection; (C) T75-15, some cells near microprojections others are far from a microprojection; (D) higher magnification of C. Yellow circles are drawn 15 μm from the edge of all microprojections and used to determine near/far parameter. All cells within yellow circles are considered near a projection and those outside are considered far from a projection (E) Histogram comparing BrdU incorporation in cells on different topographies. Green – BrdU. Red - Propidium Iodide. Data are mean ± SE. *, # p< 0.005
Cells grown on the 75μm spaced microprojections appeared similar to the control flat surfaces and did not show significant differences in overall BrdU incorporation of heterogeneous mES cell progeny (Fig 2C). However, upon further inspection, the cells closer to the microprojections had fewer green nuclei suggesting localized control of proliferation. To quantify this observation of proximity, a circle was drawn with the perimeter 15μm from the microprojections to sort cells as “near” or “far” (Figure 2D). Cells within the yellow circles were considered “near” a microprojection while cells outside of all circles were considered “far” from a microprojection. BrdU incorporation was significantly different depending on proximity. Incorporation was 1.92 ± 0.18 (n = 3, p ≤ 0.01) fold greater in the cells distant from the microprojection compared to those closer. Noting that the flat intervening space beyond the 15μm yellow circles has a flat surface, additional comparisons were made. The proportion of cells proliferating on the flat membranes (35.9 ± 2.02%, n=3) was similar to those distant from the microprojections (38.1 ± 0.08%, n=3) and the cells on the 35μm spaced microprojections (18.5 ± 1.31%, n=3) were similar to those near the microprojections (20.4 ± 2.61%, n=3) (Figure 2E). Note, these results are not due to the method of measuring proliferation since reproducible results are found with both stains. Cells on the flat membranes showed a 1.98 ± 0.25 (n = 3, p ≤ 0.005) and 2.58 ± 0.20 (n = 4, p ≤ 0.001) fold increase in BrdU and EdU incorporation, respectively, compared to the cells grown on the 15μm microprojections spaced 35μm apart.
Heterogeneous mES cell response to height of microprojections
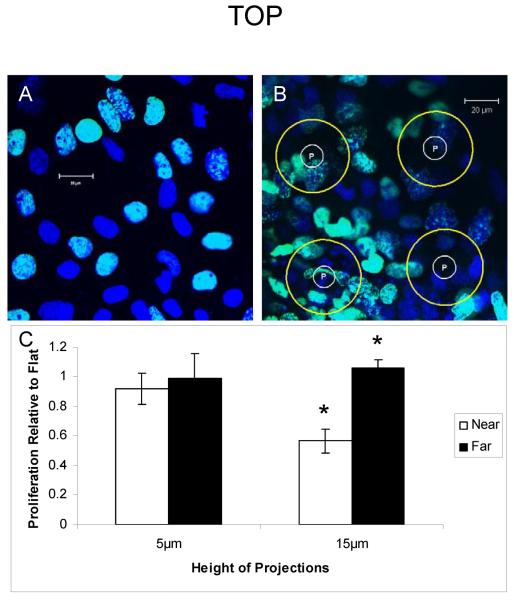
Cells grown on flat and textured PDMS membranes with 75μm tetragonally spaced, 5μm high microprojections (T75-5) are shown (Figure 3). EdU was incorporated into cells and was quantified with respect to proximity to the microprojection in order to determine whether this lower 5μm microprojection height has the same effect as the greater 15μm height. In contrast to the higher microprojections, the decrease in EdU incorporation was absent in cells “near” the microprojections compared to the cells “far” from the microprojection with this lower height. With the lower 5μm high microprojections there was no significant difference in EdU incorporation between cells distant from the microprojection compared to near the microprojection with 35.6 ± 7.6% and 33.2 ± 5.2%, n=3, respectively, p= 0.81, (Figure 3C).
Figure 3. Microprojection height of 5 μm does not attenuate mES cell-derived progeny proliferation.
EdU labeling of mES cells on flat (A) or T75-5 (B) PDMS membranes. P represents microprojection. Yellow circles are drawn 15 μm from microprojection edge and used to determine near/far parameter of cells. (C) Histogram showing influence microprojection height has on proliferation compared to cells grown on flat membranes. Green – EdU. Blue – 4,6-diamidino-2-phenylindole (DAPI). Green+blue gives turquoise color. Data are mean ± SE. * p< 0.01.
Rho kinase inhibition effects on proliferation
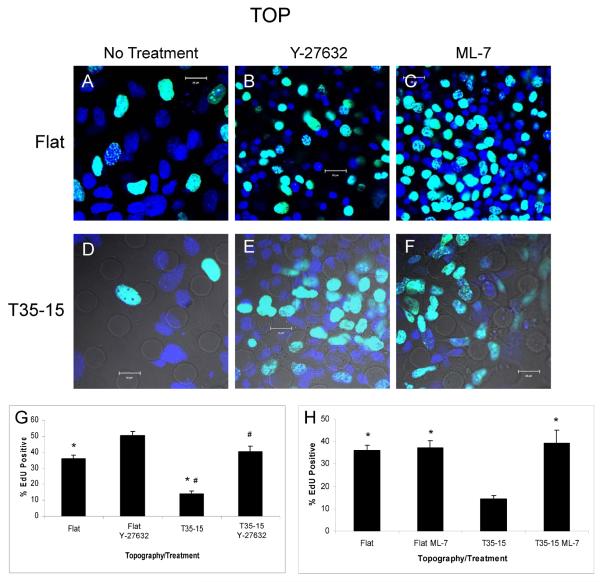
The role of Rho kinase in the attenuation of proliferation due to microprojections was assayed by using the Rho kinase inhibitor, Y-27632 (Figure 4). Cells grown on 15μm high, 35μm spaced microprojections and treated with Y-27632 for 48 hours had more EdU incorporation, detected as a turquoise (blue+green) stain of dividing vs. the blue stain of non proliferating nuclei (Figure 4B and E), than the untreated cells (Figure 4A and D). Quantification of EdU for Y-27632 treatment on 15μm high, 35μm spaced projections (40.42 ± 3.17%, n=3) confirmed a significant, nearly three-fold increase over untreated cells (14.23 ± 1.48%, n=3) (Figure 4G). Interestingly, with the Y-27632 treatment of cells grown on flat surfaces (Figure 4A, B and G) there was also a significant increase in proliferation (flat untreated, 36.1 ± 2.22%, n=4; flat treated with Y-27632, 50.4 ± 2.39%, n=3, p<0.01). However, treated groups of cells on flat and 15μm high microprojections showed no significant difference with both groups being highly proliferative; flat, 50.4 ± 2.39%, n=3, and textured, 40.4 ± 3.17%, n=3 (p=0.06).
Figure 4. Inhibition of stress fiber formation eliminates proliferation blunting effect of microprojections.
Confocal image of EdU labeling in mES cells on flat (A–C) or T35-15 (D–F) PDMS membranes. (A,D) Control cells not treated with inhibitor. (B,E) Cells treated with Rho kinase inhibitor, Y-27632. (C,F) Cells treated with myosin light chain kinase inhibitor, ML-7. (G) Histogram comparing proliferation of mES cell-derived progeny treated with Y-27632 when grown on different topographies. (H) Histogram comparing proliferation of mES cell-derived progeny treated with ML-7 when grown on different topographies. Green – EdU. Blue – DAPI. Green+blue gives turquoise color. Data are mean ± SE. * p< 0.01, # p< 0.05.
Myosin light chain kinase inhibition effects on proliferation
The role of myosin light chain kinase on the blunting of proliferation caused by the microprojections was investigated using the drug ML-7, a myosin light chain kinase inhibitor. EdU incorporation showed that ML-7 also attenuated the influence the microprojections have on proliferation (Figure 4C, F and H). Proliferation increased threefold on the membranes with 15μm high microprojections from 14.2 ± 1.48% in the untreated group to 39.1 ± 6.06% in the ML-7 treated group (n = 4, p ≤ 0.01). Treatment with 5μM of ML-7 had no effect on proliferation of cells grown on flat PDMS as seen by EdU incorporation of 37.2 ± 2.98% with treatment compared to 36.1 ± 2.2% (n = 4, p ≤ 0.8) without treatment. Therefore, the effect the microprojections have on proliferation is eliminated in the presence of ML-7.
Response of cardiomyocytes derived from ES cells to microprojections
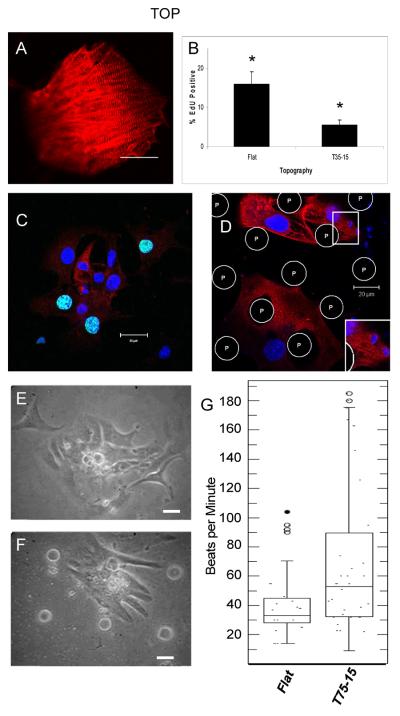
In the mixed cultures of differentiated R1 ES cells, markers of cardiomyocytes were present (Fig 1E) and spontaneous contractions were observed. Similar results were seen with cells from clone syNP4, but through the addition of puromycin, these contracting and transiently proliferating cardiomyocytes could be selectively purified. Following addition of puromycin, contractions continued to be observed and cells were positive for α-actinin (Figure 5A) and other cardiac markers, consistent with our previous report (Yamanaka et al. 2008b). Importantly, the selected and replated cardiomyocytes cultivated on the textured surfaces were different from those grown on identically treated flat PDMS membranes. On the flat surfaces (Figure 5C and E), cells were typically present in larger clusters, while on the textured surfaces (Figure 5D and F) they were distributed into small, medium and large groups. Sarcomeric structures in these early cells are poorly formed, which is typical of relatively immature cells but were slightly more pronounced when plated on textured membranes (Figure 5C,D). However, cardiomyocytes plated on flat versus textured membranes are phenotypically different in proliferation and beating. Cells on flat PDMS membranes were compared with those on 15μm high microprojections spaced 35μm apart to determine if these immature cardiomyocytes were proliferating and whether they too could be blunted by the topography (Figure 5D). Cardiomyocytes were seen proliferating on flat membranes with EdU assay values of 15.9 ± 3.19% (n= 3). Some cardiomyocytes (with red α-actinin staining) were proliferative (EdU positive) but most were not (Figure 5C). This was significantly different from that seen on the textured membranes (Figure 5B) as only 5.4 ± 1.38% (n= 3) were EdU positive, resulting in a fold increase of 3.6 ± 1.6 when cardiomyocytes grown on flat membranes are compared to the microtextured membranes (n = 3, p ≤ 0.05).
Figure 5. Microprojections affect cardiomyocytes derived from mES cells.
(A) Confocal image of more mature, 7+14 day old mES derived cardiomyocytes have a well-defined sarcomeric structure. (B–D) EdU incorporation and α-actinin expression in immature cardiomyocytes derived from mES cells when grown on (C) flat or (D) T35-15 PDMS membranes. (B) Histogram of mES cell derived cardiomyocyte proliferation on different topographies. (E,F) Phase images of mES cell derived cardiomyocytes on (E) flat or (F) T75-15 PDMS membranes. (G) Beating rates of mES cell derived cardiomyocytes grown on flat and T75-15 PDMS membranes displayed as a box plot to graphically show large difference in distribution between groups. Bottom line of box represents the 25th percentile, the line within box the median and the upper line the 75th percentile. Whiskers signify minimum and maximum values with circles representing outliers. * p< 0.05. Dividing nuclei are turquoise with DAPI (blue) and EdU (green). α-actinin (red). P, microprojections. (A) scale bar 50 μm. (C–F) scale bar 20 μm.
Video microscopy was then used to quantify the effect of microprojections on the beating rates of the cardiomyocytes with phase images, (Figure 5E, F, Supplemental online videos 1–4). In most instances the beating rate was steady for the half-minute duration of recording. Arrhythmias were detected in both but more often on the flat membranes. There was no significant difference in the means of the beating rates (flat, 48.3 ± 15.7, textured, 83.8 ± 24.1, n=5, p= 0.26, not significant) with a fold difference of 1.8 ± 0.2 between textured and flat. However, differences in the coefficients of variance were found to vary greatly with values of 0.50 and 0.21 for the textured and flat, respectively indicating there was much more variability in the beating of the variably sized clusters of cells on the textured surface than on the larger groups found on flat ones (Figure 5G).
DISCUSSION
In this study, we have shown that proliferation of heterogeneous mouse ES cell-derived progeny was attenuated by variations in microtopography. This result depended on the height and spacing of the microprojections, as 15μm- but not 5μm-high microprojections inhibited proliferation. Regulation required cell contractility since Rho kinase or myosin light chain kinase inhibition of stem cell progeny reversed the effect of the microprojections. One specific cell lineage, cardiomyocytes, derived from embryonic stem cells was chosen for a more detailed study. Consistent with the observations from the mixed cultures, microprojections attenuated the proliferation of cardiomyocytes, and their beating rates varied more than when grown on flat substrates. Thus, the behavior of heterogeneous stem cell derivatives and one specific cell type appear to be modulated by physical cues in vitro.
Cell proliferation is attenuated by height of microprojections
The proliferation of ES cell-derived progeny is halved when near 15μm-high microprojections. Contact attenuation thus appeared more like adult cells (Boateng et al. 2003, Thakar et al. 2008) than for undifferentiated cultures of ES cell, which are not contact inhibited in vitro (Gammill and Bronner-Fraser 2002). Human embryonic stem cells grown on topography with features 0.6 μm showed alignment but proliferation was not significantly different after 48 hours (Gerecht et al. 2007).
The differential influence of the height of topography has been seen in neonatal cardiomyocytes grown on shallow 2 μm grooves which had partial alignment while full alignment required deeper, 5 μm grooves (Motlagh et al. 2003a). One possibility is that the microprojections alter the distribution or mean area of focal adhesions compared to flat two-dimensional substrates, consistent with the finding that focal adhesion size correlates with generation of cell traction and traction-dependent behaviors (Balaban et al. 2001, Tan et al. 2003). The vertical microprojection may provide a geometry that favors three-dimensional anchorage of contractile elements, such as stress cables and myofibrils, thus enhancing the ability of cells to transmit the force that they generate (Kumar et al. 2006; Motlagh et al. 2003b).
The physical barrier imposed by the microprojections may foster clustering of cells. As seen previously with fibroblasts (Boateng et al. 2003), once a cell attaches to a microprojection it seldom leaves so that cells accumulate near a microprojection. Cluster size could indirectly contribute to the decrease in proliferation by expanding the opportunity for cell adhesion or paracrine/autocrine signaling. Due to the confounding and complicated mechanisms involved in these interactions, additional thorough investigation is needed to sort out the direct role of microtopography from indirect factors like cell clustering.
Role of cell contractility
The differences in proliferation between cells grown on flat or microprojection substrates were reversed when the tension generating ability of the stress fibers was reduced pharmacologically by the Rho kinase (ROCK) inhibitor, Y-27632, or the myosin light chain kinase inhibitor, ML-7. Surprisingly, ES cell progeny on flat membranes actually proliferated more with Rho kinase inhibition, suggesting that the inhibitor, Y-27632, commonly used to study the effect of cellular tension has broader effects. Indeed, Rho kinase is involved in other cell functions such as disassembly of intermediate filaments (Mabuchi et al. 1993), stabilization of actin filaments (Bishop and Hall 2000) and in cytokinesis (Takaishi et al. 1995). Furthermore, Y-27632 has effects on other kinases with numerous subcellular actions, e.g. CaMKII, PKC, and cAMP-dependent kinase (Tamura et al. 2005). Therefore, to further isolate the influence of the tension generating mechanisms a more specific contractility blocker was used. Phosphorylation of myosin light chain activates myosin ATPase activity to generate tension (Kawano et al. 1999), which can be specifically inhibited by ML-7. The hypothesis that tension generation in the third-dimension blunts proliferation is supported by ML-7 inhibition of ES progeny.
Microtopography affects proliferation and beating rates of cardiomyocytes derived from ES cells
The population of ES cell progeny is heterogeneous and contains many cell types, including a small percentage of cardiomyocytes. To determine whether the effects of microtopography on proliferation and cell physiology could be observed in a specific cell lineage, syNP4 ES cell-derived cardiomyocytes were purified through the addition of puromycin. Cardiomyocyte proliferation was then assayed by EdU incorporation into cells actively synthesizing DNA. A nearly threefold reduction in EdU incorporation was observed in cells cultured on 15μm-high, 35μm-spaced microprojections compared to cells plated on flat PDMS surfaces. We had previously shown that pharmacological selection yields early ES cell derived cardiomyocytes that are 98% pure (Yamanaka et al. 2008b). The cells are initially proliferative and more than double in number within 3 days. The proliferative capacity subsequently decreases significantly concomitant with an increase in markers associated with contact inhibition within one week of selection and plating (Yamanaka et al. 2008b). At the time points examined and based on the incorporation of EdU into cells plated on flat membranes, the syNP4-derived cardiomyocytes would be expected to actively proliferate. Based on our previous publication, binucleation should maximally be observed in about 2–7% of the cells. While it is possible that some of the decrease in EdU incorporation observed on PDMS surfaces containing microprojections can be attributed to a loss of binucleation, the three-fold reduction in EdU uptake reported here (see Figure 5) can not be accounted for by this possibility alone. We therefore conclude that the decrease in DNA synthesis is due primarily to a loss of cardiomyocyte cell proliferation, and not to multinucleation or a decrease in DNA synthesis without cytokinesis.
The heterogeneity of beating rates of cardiomyocytes derived from ES cells is puzzling but might be related to cell anchorage given the recent report that ES cell beating rates is related to underlying stiffness of the matrix (Engler et al. 2004, 2008). Rates of cardiomyocytes derived from ES cells typically resemble primary myocardial-like cells early after differentiation. Nodal, atrial, and ventricular-like cells are not found until they are more terminally differentiated (Boheler et al. 2002). Of the terminally differentiated cardiomyocytes, the ventricular-like cells beat the slowest while the nodal and atrial like cells beat more quickly (Antoon et al. 2003). Newly derived cardiomyocytes also do not have fully matured calcium handling machinery which can lead to arrhythmic beating (Fu et al. 2006). It is likely that the cardiomyocytes remain adhered to the microprojections because of the stability provided by the third-dimension as seen for fibroblast by video microscopy (Boateng et al. 2003). Therefore, the high variance in beating rates in the cardiomyocytes cultured on microprojections might result from isolation of cells with highly distinct calcium handling profiles or in some way differentially induced by the microtopography. Conversely, continued migration and adherence permits large clusters of cells that are driven by the fastest, strongest, pace-maker-like cell, among them. Therefore, differences in sizes of cardiomyocyte clusters resulting from the microprojections may be a reason for the differences in proliferation and beating rate distribution and closer investigation of this complicated phenomenon is needed.
Conclusion
Remarkably, microtopography attenuated the overall proliferation of early heterogeneous ES cell progeny, and cardiomyocytes in particular, with higher microprojections being more effective than lower. From the data presented, it remains unclear when ES cell progeny first become sensitive to the physical microenvironment, and future studies will be required to address this issue. Tension-generation in the third dimension within cells is necessary to cause this attenuation of proliferation. This suggests that physical constraints directly, or indirectly by their ability to alter cell clustering, may play a critical role in the loss of proliferative capacity. It is however likely that the loss of pluripotency and acquisition of a more differentiated phenotype is associated with these responses. Irrespective of the mechanism, these data firmly establish that microtopography can affect both the function and phenotype of differentiating cells.
More importantly, both embryonic and adult stem cell derived cardiomyocytes (and other progeny) have been advocated for regenerative medicine to treat heart failure (Yamanaka et al. 2008a). However, the ability of these cells to regenerate damaged myocardium may be limited by physical constraints that actively attenuate proliferation in the local niche environment (Chien et al. 2008). Novel findings herein add support to the notion that microscale topography, in addition to stiffness, can affect stem cell progeny phenotypes. The data furthermore suggest that microscale cues can be exploited to control the stem cell niche for regenerative medicine applications.
EXPERIMENTAL PROCEDURES
Fabrication of Microtextured Substrates
The fabrication of the parylene microtextured molds has been previously reported (Deutsch et al. 2000). Microtextured surfaces with microprojections of different heights and spacing were created using photolithography, with one example shown in figure 1. A 5 μm (SU-8 2005, Microchem) or 15 μm (SU-8 2015) thick layer of photoresist was created by spin coating per manufacturer's instructions. To ensure that the photoresist did not delaminate from the silicon wafer, approximately 1mL of hexamethyldisilazane was spun on a clean silicon wafer at 6000 RPM for 50 seconds prior to addition of photoresist. A layer of parylene was deposited on the patterned photoresist and peeled off, resulting in reusable mold. Liquid polydimethyl-siloxane (PDMS) is spread over the parylene mold, cured, and gently removed, resulting in flat or textured, elastomeric membranes.
Several microtopographies were created in PDMS: 15μm high microprojections with tetragonal spacing of 35μm center to center (T35–15); 15μm high microprojections with tetragonal spacing 75μm center to center (T75–15) or 5μm high (T75–5). The 5 μm height of microprojections allowed cells to extend over the top edge of the microprojections while still providing a vertical substrate for attachment whereas cells were only able to extend up to, but not higher than, the 15 μm microprojections. The microprojections in all designs were circular with a 15μm diameter. Flat two-dimensional sheets of PDMS were made to ensure same surface properties. The PDMS was oxidized using a plasma oxygen treatment. Experiments were then conducted to determine the extracellular matrix protein that performs best with the PDMS membranes. Gelatin, fibronectin, laminin and a combination of the latter two were tested for two parameters: cellular attachment to PDMS and capacity for mES cells to differentiate into cardiomyocytes. Fibronectin at a concentration of 12.5 μg/mL showed excellent cellular attachment and there was no change in cardiomyocyte differentiation compared to the control conditions of 0.01% gelatin on polystyrene dishes.
Mouse Embryonic Stem Cells
Mouse embryonic stem (mES) cells of the R1 line and syNP4 (NCX1-PAC-Resistant) line were cultivated on mitotically inactivated mouse embryonic fibroblast feeder layers and differentiated using the hanging drop technique as previously described (Boheler et al. 2005, Yamanaka et al. 2008b). Hanging drops were used with a beginning concentration of 600 mES cells per 20μL. The resulting embryoid bodies within the hanging drops were washed into Petri dishes coated with PDMS, to prevent adhesion, and incubated in suspension for five days. 24 well plates were coated with gelatin and plated with individual embryoid bodies at day 7. Contraction was seen at 24–48 hours after plating, that is at day 7+1 or 7+2.
The syNP4 cell line has a puromycin resistant cassette incorporated into the promoter for the sodium calcium exchanger (NCX1) gene which is only transcribed in cardiomyocytes, so that when ES cells differentiate, a relatively pure population of cardiomyocytes can be purified by the addition of puromycin (Yamanaka et al. 2008b). Pharmacologic selection of cardiomyocytes from the syNP4 (NCX-PAC-Resistant) line was initiated by addition of 2.5μg/mL puromycin on day 7+2 as described. Whether or not cardiomyocyte selection was induced, on day 7+4 embryoid bodies are scraped from the wells, disassociated with collagenase (Boheler et al. 2005), and the cells were plated onto the desired PDMS membranes coated with fibronectin (12.5μg/mL).
RT-PCR
The relative abundance of RNA transcripts was determined after reverse transcription as described previously (Tarasova et al. 2006). Total cell RNA was extracted using Trizol (Invitrogen) followed by DNAse treatment. cDNA synthesis was performed with 500 ng of total RNA using a High Capacity cDNA Archive Kit (Applied Biosystems). Primer sequences and reaction conditions were taken from Tarasova et al., 2006 and Yamanaka et al., 2008b.
Cardiomyocyte Beating
Video microscopy was used to determine the beating rates of cardiomyocytes on flat or textured surfaces. Cardiomyocytes derived from mES cells were removed from the incubator and placed on a phase microscope under a heat lamp that maintains the microscope stage at 37°C. Videos were recorded of contracting aggregates for approximately 30 seconds before moving to the next group of cells. The dishes were outside the incubator for no longer than 5 minutes, after which, the cells were returned for at least 15 minutes before resuming recording of remaining aggregates.
Contractility Interventions
Cytoskeletal contractility is mediated through the RhoA pathway via interactions with Rho kinase and myosin light chain kinase (Katoh et al. 2007). To disrupt cell contractility, the Rho pathway was interrupted by 5μM addition of a Rho Kinase specific inhibitor, Y-27632 (Cal Biochem) after 2 days in culture. Myosin light chain kinase, also essential for the cell contractility (Fazel et al. 2006), was inhibited by 5μM addition of its specific inhibitor, ML-7 (Sigma Aldrich) after 2 days in culture. The cells were then incubated for two more days before proliferation assays were conducted.
DNA Replication Assessment with BrDU or EdU Incorporation
Cells were cultured for four days after disassociation and plating on PDMS. Two methods were then used to assess cell proliferation: initial experiments used a one-hour incorporation of 5-bromo-2'-deoxy-uridine (BrdU, 10μm, Roche Diagnostics) or a one-hour incorporation of 5-ethynyl-2'-deoxyuridine (EdU, 10μm, Invitrogen Corp.). Once incorporation was complete, the PDMS membranes were removed from each dish, mounted onto glass slides with Vectashield Mounting Medium for nuclear staining with propidium iodide (Vector Labs) for the BrdU marked cells, or with 4',6-diamidino-2-phenylindole (DAPI, Invitrogen) for the EdU method. Similar quantitative results were obtained with both methods but EdU was used in later experiments because of its simplicity.
Imaging
Cells were visualized with confocal microscopy with 25X or 63X objectives (Zeiss LSM 510) using an unbiased raster pattern to acquire images of 500 cells per slide. A series of images in the “Z” plane were taken and stacked to determine the cell height in relation to the microprojections. The images were analyzed using the Cell Counter plug-in for the Image J software program (NIH) in which the number of cells BrdU or EdU positive were compared to the total number of cells.
Immunocytochemistry for differentiation
Mouse ES cells were washed in Ca2+ and Mg2+ free PBS fixed in 4% paraformaldehyde/PBS for ten minutes at room temperature followed by permeabilization in Triton X-100 for 20 minutes. Oct-4 and α-actinin were detected with polyclonal antibodies (Abcam) diluted at 1:400 in 3% BSA/PBS. The cells were rinsed in PBS and blocked in 3% BSA/PBS for one hour prior to incubation with a secondary antibody conjugated with a fluorophore with either 488nm or 568nm emission wavelength at 1:500 dilution. All dishes were rinsed for five minutes three times and mounted to slides with DAPI as described above.
Statistical Analysis
All values are means ± standard error of the mean. Conditions were conducted in multiples of at least three for each variable. All values of significance were calculated using One Way ANOVA. Differences among means were considered significant at p< 0.05. Data are analyzed using Microsoft Exel and GraphPad Prism software.
Supplementary Material
Acknowledgements
The research was supported by NIH (HL 62426), the State of Illinois funds for Regenerative Medicine, the Intramural Research Program of the NIH, National Institute on Aging, and the National Science Foundation. The nestin (Rat-401) and SSEA-1 (MC-480) antibodies developed by Hockfield, S and Solter, D./Knowles, BB., respectively, were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa (Department of Biology, Iowa City, IA 52242).
References
- 1.Antoon F, Moorman M, Christoffels VM. Cardiac Chamber Formation: Development, Genes, and Evolution. Physiol. Rev. 2003;83:1223–1267. doi: 10.1152/physrev.00006.2003. [DOI] [PubMed] [Google Scholar]
- 2.Balaban NQ, Schwartz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001;3(5):466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 3.Bettinger CJ, Orrick B, Misra A, Langer R, Borenstein JT. Microfabrication of poly(glycerolsebacate) for contact guidance applications. Biomaterials. 2006;27:2558–2565. doi: 10.1016/j.biomaterials.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 4.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;248:241–255. [PMC free article] [PubMed] [Google Scholar]
- 5.Boateng SY, Hartman TJ, Ahluwalia N, Vidula H, Desai TA, Russell B. Inhibition of fibroblast proliferation in cardiac myocyte cultures by surface microtopography. Am J. Physiol: Cell. 2003;285:C171–C182. doi: 10.1152/ajpcell.00013.2003. [DOI] [PubMed] [Google Scholar]
- 6.Boheler KR, Crider DG, Tarasova Y, Maltsev VA. Cardiomyocytes derived from embryonic stem cells. Methods Mol Med. 2005;108:417–435. doi: 10.1385/1-59259-850-1:417. [DOI] [PubMed] [Google Scholar]
- 7.Boheler KR, Czyz J, Tweedie D, Yang HT, Anisimov SV, Wobus AM. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res. 2002;91(3):189–201. doi: 10.1161/01.res.0000027865.61704.32. [DOI] [PubMed] [Google Scholar]
- 8.Chen CS, Tan J, Tien J. Mechanotransduction at cell–matrix and cell–cell contacts. Annu Rev Biomed Eng. 2004;6:275–302. doi: 10.1146/annurev.bioeng.6.040803.140040. [DOI] [PubMed] [Google Scholar]
- 9.Cheng CM, LeDuc PR. Micropatterning polyvinyl alcohol as a biomimetic material through soft lithography with cell culture. Mol Biosyst. 2006;2(6–7):299–303. doi: 10.1039/b606496p. [DOI] [PubMed] [Google Scholar]
- 10.Chien KR, Domian IJ, Parker KK. Cardiogenesis and the Complex Biology of Regenerative Cardiovascular Medicine Science. Science. 2008;322(5907):1494–1497. doi: 10.1126/science.1163267. [DOI] [PubMed] [Google Scholar]
- 11.Deutsch J, Motlagh D, Russell B, Desai TA. Fabrication of microtextured membranes for cardiac myocyte attachment and orientation. J Biomed Materials Res. 2000;53(3):267–275. doi: 10.1002/(sici)1097-4636(2000)53:3<267::aid-jbm12>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 12.Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121(pt22):3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166(6):877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specifications. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 15.Engler AJ, Humbert PO, Wehrle-Haller B, Weaver VM. Multiscale modeling of form and function. Science. 2009;324(5924):208–212. doi: 10.1126/science.1170107. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116(7):1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forouhar AS, Liebling M, Hickerson A, Nasiraei-Moghaddam A, Tsai HJ, Hove JR, Fraser SE, Dickinson ME, Gharib M. The embryonic vertebrate heart tube is a dynamic suction pump. Science. 2006;312(5774):751–753. doi: 10.1126/science.1123775. [DOI] [PubMed] [Google Scholar]
- 18.Fu J-D, Li J, Tweedie D, Yu H-M, Chen L, Wang R, Riordon DR, Brugh SA, Wang S-Q, Boheler KR, Yang HT. Crucial Role of the Sarcoplasmic Reticulum in the Developmental Regulation of Ca2+ Transients and Contraction in Cardiomyocytes Derived from Embryonic Stem Cells. FASEB J. 2006;20(1):181–183. doi: 10.1096/fj.05-4501fje. [DOI] [PubMed] [Google Scholar]
- 19.Gammill LS, Bronner-Fraser M. Genomic analysis of neural crest induction. Development. 2002;129:5731–5741. doi: 10.1242/dev.00175. [DOI] [PubMed] [Google Scholar]
- 20.Gerecht S, Bettinger CJ, Shang Z, Borenstein T, Vunjak-Novokovic G, Langer R. The effect of actin disrupting agents on contact guidance of human embryonic stem cells. Biomaterials. 2007;28:4068–4077. doi: 10.1016/j.biomaterials.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingber DE. Mechanical control of tissue morphogenesis during embryological development. Int J Dev Biol. 2006;50(2–3):255–266. doi: 10.1387/ijdb.052044di. [DOI] [PubMed] [Google Scholar]
- 22.Karp JM, Yeh J, Eng G, Fukuda J, Blumling J, Suh KY, Cheng J, Mahdavi A, Vorenstein J, Langer R, Khademhosseini A. Controlling size, shape and homogeneity of embryoid bodies using Poly(ethylene glycol) microwells. Lab on a Chip. 2007;7:786–794. doi: 10.1039/b705085m. [DOI] [PubMed] [Google Scholar]
- 23.Katoh K, Kano Y, Ookawara S. Rho-kinase dependent organization of stress fibers and focal adhesions in cultured fibroblasts. Genes Cells. 2007;12(5):623–638. doi: 10.1111/j.1365-2443.2007.01073.x. [DOI] [PubMed] [Google Scholar]
- 24.Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, Matsumura F, Inagaki M, Kaibuchi K. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J. Cell Biol. 1999;147:1023–1038. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krieg M, Arboleda-Estudillo Y, Puech PH, Kafer J, Graner F, Muller DJ, Heisenberg CP. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;10(4):429–436. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Maxwell IZ, Heisterkamp A, Polte TR, Lele TP, Salanga M, Mazur E, Ingber DE. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys J. 2006;90(10):3762–3773. doi: 10.1529/biophysj.105.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mabuchi I, Hamaguchi Y, Fujimoto H, Morii N, Mishima M, Narumiya S. A rho-like protein is involved in the organisation of the contractile ring in dividing sand dollar eggs. Zygote. 1993;1:325–331. doi: 10.1017/s0967199400001659. [DOI] [PubMed] [Google Scholar]
- 28.Motlagh D, Hartman TJ, Desai TA, Russell B. Microfabricated grooves recapitulate neonatal myocyte connexin43 and N-cadherin expression and localization. J Biomed Mater Res. 2003a;67A:148–157. doi: 10.1002/jbm.a.10083. [DOI] [PubMed] [Google Scholar]
- 29.Motlagh D, Senyo SE, Desai TA, Russell B. Microtextured substrata alter gene expression, protein localization and the shape of cardiac myocytes. Biomaterials. 2003b;24:2436–2476. doi: 10.1016/s0142-9612(02)00644-0. [DOI] [PubMed] [Google Scholar]
- 30.Saez A, Ghibaudo M, Buguin A, Silberzan P, Ladoux B. Rigidity-driven growth and migration of epithelial cells on microstructured anisotropic substrates. Proc Nat Academies Science USA. 2007;104:8281–8286. doi: 10.1073/pnas.0702259104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samarel AM. Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am J Physiol Heart Circ Physiol. 2005;289(6):H2291–2301. doi: 10.1152/ajpheart.00749.2005. [DOI] [PubMed] [Google Scholar]
- 32.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 33.Takaishi K, Sasaki T, Kameyama T, Tsukita S, Tsukita S, Takai Y. Translocation of activated Rho from the cytoplasm to membrane ruffling area, cell±cell adhesion sites and cleavage furrows. Oncogene. 1995;11:39–48. [PubMed] [Google Scholar]
- 34.Tamura M, Nakao H, Yoshizaki H, Shiratsuchi M, Shigyo H, Yamada H, Ozawa T, Totsuka J, Hidaka H. Development of specific Rho-kinase inhibitors and their clinical application. Biochim Biophys Acta. 2005;1754:245–252. doi: 10.1016/j.bbapap.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Nat Academies Science USA. 2003;100(4):1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarasova YS, Riordon DR, Tarasov KV, Boheler KR. In vitro differentiation of mouse ES cells to muscle cells. In: Notarianni E, Evans MJ, editors. Embryonic Stem Cells. Oxford University Press; New York: 2006. pp. 130–168. [Google Scholar]
- 37.Thakar RG, Chown MG, Patel A, Peng L, Kumar S, Desai TA. Contractility-dependent modulation of cell proliferation and adhesion by microscale topographical cues. Small. 2008;4(9):1416–1424. doi: 10.1002/smll.200701302. [DOI] [PubMed] [Google Scholar]
- 38.Timpl R. Macromolecular organization of basement membranes. Curr Opin Cell Biol. 1996;8:618–624. doi: 10.1016/s0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- 39.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. 2000;287(5457):1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 40.Wobus AM, Boheler KR. Embryonic stem cells – Prospects for developmental biology and cell therapy. Physiol Rev. 2005;85(2):635–678. doi: 10.1152/physrev.00054.2003. [DOI] [PubMed] [Google Scholar]
- 41.Yamanaka S, Li J, Kania G, Elliott S, Wersto R, Van Eyk J, Wobus AM, Boheler KR. Pluripotency of Embryonic Stem Cells. Cell Tissue Res. 2008a;331(1):5–22. doi: 10.1007/s00441-007-0520-5. [DOI] [PubMed] [Google Scholar]
- 42.Yamanaka S, Zahanich I, Wersto RP, Boheler KR. Enhanced Proliferation of Monolayer Cultures of Embryonic Stem (ES) Cell-Derived Cardiomyocytes following Acute Loss of Retinoblastoma. PLoS ONE. 2008b;3(12):e3896. doi: 10.1371/journal.pone.0003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yim EK, Reano RM, Pang SW, Yee AF, Chen CS, Leong KW. Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials. 2005;26(26):5405–5413. doi: 10.1016/j.biomaterials.2005.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.